Abstract
Many aspects of photoreceptor metabolism are regulated as diurnal or circadian rhythms. The nature of the signals that drive rhythms in mouse photoreceptors is unknown. Dopamine amacrine cells in mouse retina express core circadian clock genes, leading us to test the hypothesis that dopamine regulates rhythms of protein phosphorylation in photoreceptor cells. To this end, we investigated the phosphorylation of phosducin, an abundant photoreceptor-specific phosphoprotein. In mice exposed to a daily light-dark cycle, robust daily rhythms of phosducin phosphorylation and retinal dopamine metabolism were observed. Phospho-phosducin levels were low during the daytime and high at night, and correlated negatively with levels of the dopamine metabolite 3,4-dihydroxyphenylacetic acid. The effect of light on phospho-phosducin levels was mimicked by pharmacological activation of dopamine D4 receptors. The amplitude of the diurnal rhythm of phospho-phosducin was reduced by more than 50% in D4 receptor knockout mice, due to higher daytime levels of phospho-phosducin. In addition, the daytime level of phospho-phosducin was significantly elevated by L-745,870, a dopamine D4 receptor antagonist. These data indicate that dopamine and other light-dependent processes cooperatively regulate the diurnal rhythm of phosducin phosphorylation. Under conditions of constant darkness, a circadian rhythm of phosducin phosphorylation was observed, which correlated negatively with a circadian rhythm of 3,4-dihydroxyphenylacetic acid level. The circadian fluctuation of phospho-phosducin was completely abolished by constant infusion of L-745,870, indicating that the rhythm of phospho-phosducin level is driven by dopamine. Thus, dopamine release in response to light and circadian clocks drives daily rhythms of protein phosphorylation in photoreceptor cells.
Keywords: Phosducin, retina, circadian clocks, dopamine D4 receptors
Circadian clocks are self-sustaining genetically based molecular machines that impose 24 h rhythmicity on physiology and behavior, and synchronize these functions with the solar day–night cycle. Mammalian retina contains independent circadian oscillators capable of driving circadian rhythms in physiological functions (Tosini and Menaker, 1996; Storch et al., 2007). Many aspects of photoreceptor metabolism are regulated as diurnal or circadian rhythms, including rod outer segment disc shedding, synaptic ribbon dynamics, cAMP-response element binding protein phosphorylation, gene expression, and melatonin biosynthesis (Green and Besharse, 2004; Iuvone et al., 2005). Recently it was shown that the disruption of mouse retinal circadian clocks leads to abnormal retinal transcriptional and electrical responses to light (Storch et al., 2007). However, the significance of circadian rhythms in mammalian photoreceptor physiology is poorly understood and the molecular mechanisms of circadian clock action in photoreceptor cells are unknown. Here we studied the role of dopamine in the daily rhythm of protein phosphorylation in photoreceptor cells.
Dopamine is a neuromodulator secreted from retinal amacrine and interplexiform cells (Dowling, 1991; Witkovsky, 2004). Dopamine synthesis and release are stimulated by light and, in some animals, regulated by circadian clocks [e.g., (Kramer, 1971; Iuvone et al., 1978; Nir et al., 2000; Doyle et al., 2002; Ribelayga et al., 2004)]. Recent studies indicate that mouse retinal dopamine neurons express core circadian clock genes (Witkovsky et al., 2003; Gustincich et al., 2004; Ruan et al., 2006; Dorenbos et al., 2007). Earlier studies in non-mammalian vertebrates identified a photoreceptor melatonin-dopamine feedback loop that regulates retinal circadian rhythms (reviewed in (Besharse et al., 1988; Green and Besharse, 2004; Iuvone et al., 2005)), but the relevance of a similar feedback loop in mammalian photoreceptor rhythms is largely unexplored.
To investigate the potential role of dopamine in the rhythmic regulation of protein phosphorylation in mammalian photoreceptor cells, the phosphorylation of phosducin (Pdc) was investigated. Pdc is an abundant photoreceptor-specific phosphoprotein, which is also expressed the pineal gland (Lee et al., 1987; Kuo et al., 1989; Reig et al., 1990). Phosducin is phosphorylated by cyclic AMP-dependent protein kinase A (PKA) (Lee et al., 1990a; Pagh-Roehl et al., 1995; Wilkins et al., 1996) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Lee et al., 1990b; Thulin et al., 2001) at two distinct serine residues. Light promotes dephosphorylation of Pdc and its consequent binding to transducin βγ subunits (Gtβγ), (Lee et al., 1987; Yoshida et al., 1994; Thulin et al., 2001; Sokolov et al., 2004). Thus, Pdc represents an excellent model protein to study photoreceptor specific phosphorylation by PKA and CaMKII. Proposed functions of Pdc include transducin translocation within photoreceptor cell compartments (Sokolov et al., 2004), protection of Gtβγ from ubiquitination and proteasomal degradation (Zhu and Craft, 1998; Obin et al., 2002), and transcriptional regulation (Zhu and Craft, 2000a; Zhu and Craft, 2000b). In the current study, we found that dopamine contributes to diurnal and circadian rhythms of the phosphorylation state of the protein in mouse photoreceptors, providing direct evidence that dopamine plays a role in regulation of photoreceptor rhythms in mammals.
Materials and methods
Animals
Three strains of mice were used in this study: wild type C57Bl/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME, USA); congenic mice lacking dopamine D4 receptors on a C57Bl/6J background (Drd4−/− mice) (Rubinstein et al., 1997), and C3H/f+/+ mice, a C3H sub-strain that is unaffected by the retinal degeneration (rd1) mutation (Tosini and Menaker, 1998). Animals were kept in a 12-hour light/12-hour dark cycle, with lights on from zeitgeber time (ZT) 0 to ZT 12. Animals were euthanized by cervical dislocation. All manipulations on mice and tissues under conditions of “darkness” were performed under dim red light (No. 92 filter, Eastman Kodak, Rochester, NY, USA). For circadian rhythm experiments mice were kept in total darkness (24 hours per day) for 2 – 3 days before dissection.
All experimental protocols meet the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Characterization of α-Ser73-Pdc antibody AN519
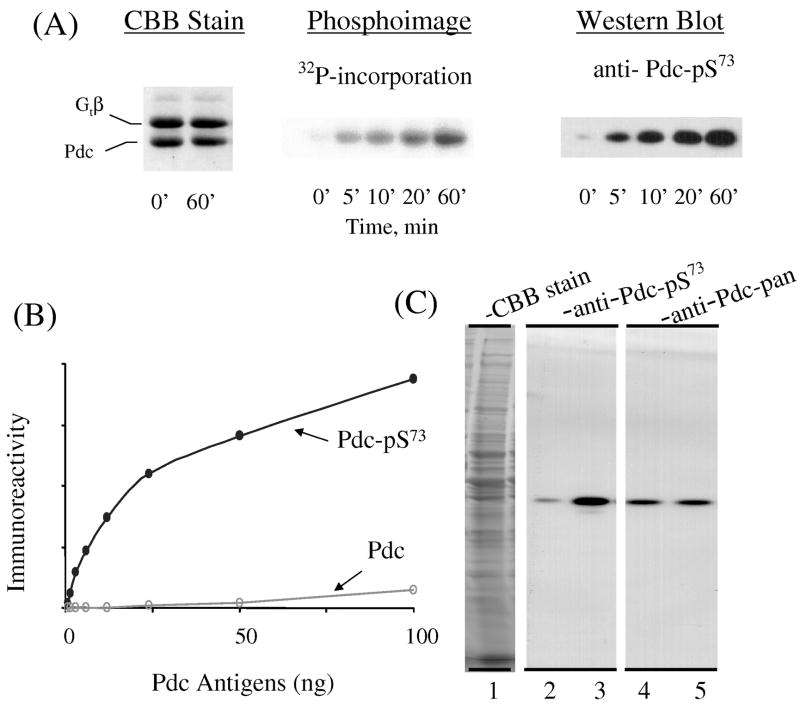
Rabbit polyclonal antibody AN519 was generated against a keyhole limpet hemacyanin-conjugated phosphopeptide, FSRKMpS73VQEYELIHKC, which corresponds to amino acid residues 68–82 of bovine phosducin (GenBank accession number P19632). The antibody was characterized with the following experiments. Time course of phosducin phosphorylation (Fig. 1 A): The Pdc/Gtβγ complex purified from bovine retinas (Lee et al., 1987) was phosphorylated by the catalytic subunit of PKA in the presence of 32P-γ-ATP, as described (Lee et al., 1990a; Chen and Lee, 1997). The time course of phosphate incorporation was monitored by quenching an aliquot of reaction mixture at 0, 5, 10, 20 and 60 min after the start of the reaction and by subjecting the samples to SDS-PAGE and electroblotting to poly vinylidine difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). One membrane which contained 1.5 μg Pdc per lane was stained with Coomassie Brilliant Blue (CBB), and 32P-Pdc was detected by autoradiography. A parallel membrane which contained 0.04 μg Pdc per lane was subjected to Western blot analysis with AN519 (1: 10,000 dilution) and the bound antibodies were detected by chemiluminescence (West-Pico kit, Pierce, Rockford, IL, USA). The intensities of CBB staining, X-ray film and chemiluminescence were captured with a CCD camera (AlphaInnotech ChemImager 5500, San Leandro, CA, USA). Western blot analysis shows a time-dependent increase in anti-pS73-Pdc immunoreactivity that parallels incorporation of 32P into Pdc. The specificity of AN519 for the pS73 site of bovine Pdc (Fig. 1 B): Pdc/Gtβγ samples were incubated for 3 h with high concentrations of PKA with or without 32P-γ-ATP to prepare phosphorylated 32P-labeld Pdc and non-phosphorylated Pdc samples. Following SDS-PAGE and CBB staining, the amounts of Pdc were determined using BSA as standards; the stoichiometry of Pdc phosphorylation (1:1) was established by scintillation counting of the excised 32P-labeled Pdc band, as described (Lee et al., 1990a; Chen and Lee, 1997). To evaluate the specificity of AN519, a series of phosphorylated Pdc (Pdc-pS73) and non-phosphorylated Pdc (Pdc) samples containing 1 to 100 ng of Pdc were subjected to Western blot analysis with AN519 and the immunoreactivity was detected and quantified by AlphaInnotech ChemImager 5500. Based on the slope of dose-response curves, the affinity for pS73-Pdc is at least 50 times higher than for non-phosphorylated Pdc. Phosphorylation of Pdc in mouse retinal homogenate (Fig. 1 C): Two frozen adult C57Bl/6 mouse retinas were homogenized in 500 μL of 10 mM TrisHCl, pH 7.4, containing 2 mM dithiothreitol. The protein concentration of retinal homogenate was determined by CBB binding using BSA as the standards (BioRad binding assay, Hercules, CA, USA). Two aliquots of retinal homogenate (100 μg each) were incubated at 30°C for 20 min with 0.1 mM ATP, 10 mM MgCl2 with (lanes 1, 3, 5) or without (lanes 2, 4) 0.1 mM cyclic AMP. At the end of incubation, multiple sets of retinal samples (20 μg per lane) were separated, in parallel, by SDS-PAGE. After excising a portion of the SDS gel containing one set of sample for CBB staining (lane 1), the remaining gel was electroblotted for Western blot analysis with AN519 (1: 10,000 dilution) (lanes 2, 3) and anti-Pdc-pan (1:50,000 dilution; Lee et al., 1988) (lanes 4,5), respectively. The equal intensities in the immunoreactivity of anti-Pdc-pan (lanes 4, 5) established the presence of the same levels of Pdc in all samples. The detection of a single anti-Pdc-pS73 band which co-migrated with Pdc and showed increasing immunoreactivity by cyclic AMP-activated PKA phosphorylation establishes that AN519 recognizes and is mono-specific for the corresponding PKA phosphorylation site of mouse Pdc, which is Ser71 in the mouse sequence.
Fig. 1. Characterization of antibody AN519 (anti-Pdc-Ser73).
A. Time course of purified bovine Pdc phosphorylation by PKA, analyzed by autoradiography and Western Blotting. B. Specificity of AN519 for phosphorylated bovine Pdc. Increasing amounts of phosphorylated (Pdc-pSer73) or non-phosphorylated (Pdc) bovine phosducin were subjected to Western blot analysis with AN519. C. Phosphorylation of Pdc in mouse retinal homogenate. Mouse retinal extract (40 μg) was incubated with ATP/Mg, okadaic acid, IBMX with (lanes 1, 3, 5) or without cAMP (lanes 2, 4) followed by SDS/PAGE. The proteins were visualized either by Coomassie brilliant blue (CBB) staining or Western blot analysis with AN519 or anti-Pdc-pan. See Materials and methods for details.
Measurement of Ser71- and Ser54 Pdc phosphorylation by immunoblotting
Retinas were homogenized in 100 μl of 10 mM HEPES buffer, pH 7.5, containing 10 mM EDTA, 1mM dithiothreitol, 1 μM microcystin LR (Sigma, St. Louis, MO, USA), and 0.2 mM phenylmethylsulfonyl fluoride. This solution inhibits Pdc phosphorylation/dephosphorylation (Lee et al., 2004). After centrifugation at 15000g for 10 min, protein concentration was measured in supernatant fractions (Lowry et al., 1951), using bovine serum albumin as standard. Protein (20μg) was denatured by sonication for 10 min and separated on 10% Bis-Tris Criterion XT precast gel (BioRad Laboratories, Hercules, CA, USA). After semi-dry transfer of proteins to PVDF membrane, total Pdc, pSer54-Pdc, and pSer71-Pdc were detected by rabbit polyclonal antibodies: anti-Pdc-pan (1:20000) (Lee et al., 1988), which recognizes both unphosphorylated and phospho-Pdc (Chen and Lee, 1997), Pdc54p (1:500), which specifically recognizes mouse pSer54-Pdc (Song et al., 2007), and AN519 (1:5000), which specifically recognizes mouse pSer71-Pdc (Fig. 1).
Protein bands were detected using ECL™ Western blotting detection reagents (Amersham Biosciences, Buckinghamshire, UK). Band densities were quantified using Kodak Molecular Imaging software (Kodak, Rochester, NY, USA). Phospho-Pdc/Pdc ratios were normalized to the average ratio of the control group (vehicle injected and/or wild type animals), which was assigned a value of 1.
Retina incubation in vitro
To test the effect of quinpirole on phosducin phosphorylation in vitro, mouse retinas were incubated as described elsewhere (Nir et al, 2002) with minor modifications. Retinas were isolated from dark-adapted mice and placed in ice-cold Earle’s saline in an atmosphere of 5% CO2 and 95% O2. After isolation, individual retinas were incubated for 15 min at 37°C in 0.7 ml of Earle’s saline with or without quinpirole (10 μM). Following incubation, retinas were frozen on dry ice and analyzed for phorphorylated and total Pdc.
Immunoprecipitation of Pdc/Gtβγ complexes
Retinas were homogenized in 200 μl of immunoprecipitation buffer (IP buffer) containing 50 mM Tris HCl, 150 mM NaCl, 1% NP-40, 0.1 mM phenylmethylsulfonyl fluoride, 10 μM microcystin LR, 1% of phosphatase inhibitor mixture 1 and 25 μl/retina of protease inhibitor mixture (both mixtures were obtained from Sigma, St. Louis, MO, USA). The supernatant was pre-cleared with 20 μl of protein G Sepharose 4 Fast Flow (Amersham Biosciences, Uppsala, Sweden) for 1h at +4°C. Pdc/Gtβ complex was immunoprecipitated overnight with 50 μL of anti-Gtβ antibody (Gβ1, C-16, Santa Cruz Biotechnologies, Santa Cruz, CA, USA), which binds to a N-terminal epitope of Gtβ and does not interfere with Pdc/Gtβ interactions based on the known structure of the Pdc/Gtβγ complex. (Gaudet et al., 1996; Gaudet et al., 1999), or an equal amount of non-immune rabbit IgG as a control. The antibody/protein complexes were bound to 50 μl of protein G Sepharose 4 Fast Flow beads during 2 h incubation at +4°C and subjected to immunoblotting analysis after multiple washes with immunoprecipitation buffer. Bovine Pdc/Gtβγ complex was purified as described before (Lee et al., 1987) and serial dilutions were loaded together with immunoprecipitate to serve as a standard.
Dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) analysis by high-performance liquid chromatography with coulometric detection
Levels of dopamine and DOPAC in mouse retina were determined by ion-pair reversed-phase high-performance liquid chromatography (HPLC) with coulometric detection (guard cell at 0.6 V, and coulometric analytical cell at 0.3 V) using a modification of the method of Nir et al. (2000). Retinas were homogenized in 100 μl of 0.2 N HClO4 containing 0.01% of sodium metabisulfite and 25 ng/ml of internal standard 3,4-dihydroxybenzylamine hydrobromide. After centrifugation at 15,000 g for 10 minutes, an 80 μl aliquot of supernatant was analyzed. The separation was performed on an Ultrasphere ODS 250 × 4.6 mm column, 5 μm (Beckman Coulter, Fullerton, CA, USA) with a mobile phase containing 0.1 M sodium phosphate, 0.1 mM EDTA, 0.35 mM sodium octyl sulfate, 5.5 % acetonitrile (vol/vol), pH 2.7. External standards of dopamine and DOPAC were analyzed in each experiment.
Chronic delivery of the dopamine D4 receptor antagonist L-745,870 via surgically implanted osmotic pumps
Osmotic pumps model 1003D (ALZET Osmotic Pumps, Cupertino, CA, USA) were loaded with L-745,870 solution (2mg/ml dissolved in 45% (2-hydroxypropyl)-β-cyclodextrin) or vehicle and aseptically implanted on the backs of the C3H/f+/+ mice, subcutaneously slightly posterior to the scapulae. After recovery from anesthesia, mice were placed in constant darkness and retinas were dissected on the second day at circadian time (CT) 6 and CT18. Retinas of right eyes were used for Pdc phosphorylation measurements by immunoblotting; L-745,870 content was estimated in the contralateral retinas as an index of osmotic pump performance.
L-745,870 was measured by HPLC with coulometric detection using the same column and HPLC system as for dopamine and DOPAC. Samples were prepared by precipitation of retinal proteins in 100 μl of 0.2 N HClO4. KH2PO4 (0.05M) with 30 % (vol/vol) of acetonitrile was used as a mobile phase. The potential of the working electrode was set to 0.65 V, which was found to be optimal based on a hydrodynamic voltammogramm constructed for L-745,870. The detection limit of the method was 20 pg (signal-to-noise ratio 3:1).
Chemicals
Quinpirole was purchased from Tocris Bioscience (Ellisville, MO, USA). PD168077, L-745,870, and HPLC standards for dopamine and DOPAC were obtained from Sigma Chemical Co. For intraperitoneal (ip) injections, quinpirole and PD168077 were dissolved in water and L-745,870 in 45% (2-hydroxypropyl)-β-cyclodextrin.
Statistics
Data are expressed as mean ± SEM. Comparisons among groups were made by one-or two-way analysis of variance (ANOVA) with Student-Newman-Keuls multiple comparison test. Comparisons of only two groups were performed using a 2-tailed Student’s t-test.
Results
Diurnal rhythms of Pdc phosphorylation state and dopamine metabolism
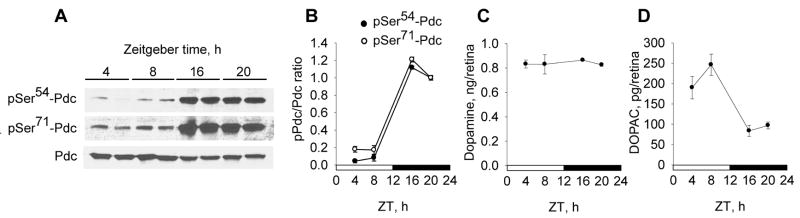
In mice maintained on a 12 h light – 12 h dark cycle, the phosphorylation state of Pdc displayed a robust diurnal rhythm (Fig. 2 A). The levels Pdc phosphorylated at both the CaMKII site (Ser54) and the PKA site (Ser71) were markedly higher at night than during the daytime (p<0.001). Retinal DOPAC levels also showed a diurnal rhythm (p<0.001), but with highest levels during the daytime (Fig. 2 C). Levels of DOPAC and phosphorylated Pdc (pPdc) correlated negatively (r = −0.82 and −0.84 for pSer54-Pdc/DOPAC and pSer71-Pdc/DOPAC pairs, respectively; p<0.001). The steady state level of dopamine did not fluctuate significantly throughout the day (Fig. 2 B).
Fig. 2. Diurnal changes of phosducin phosphorylation state and dopamine and DOPAC levels in mouse retina.
Retinas of C57Bl/6J mice, kept on a 12 hr light – 12 hr dark (LD) cycle, were dissected at the times indicated. From each mouse, one retina was used to measure levels of pSer54-Pdc, pSer71-Pdc, and total (pan) Pdc, and the other retina used to measure dopamine and DOPAC content. One-way ANOVA shows significant diurnal rhythms in pSer54-Pdc, pSer71-Pdc (A, B) and DOPAC (D) (p<0.001) but not pan-Pdc (A) or dopamine (C); n = 4 per time point. A significant negative correlation exists between the degree of Pdc phosphorylation and retinal DOPAC content (r = −0.82 and −0.84 for pSer54-Pdc/DOPAC and pSer71-Pdc/DOPAC pairs, respectively; Pearson correlation, p<0.001).
Dopamine and dopamine receptor agonists reduce the Pdc phosphorylation state
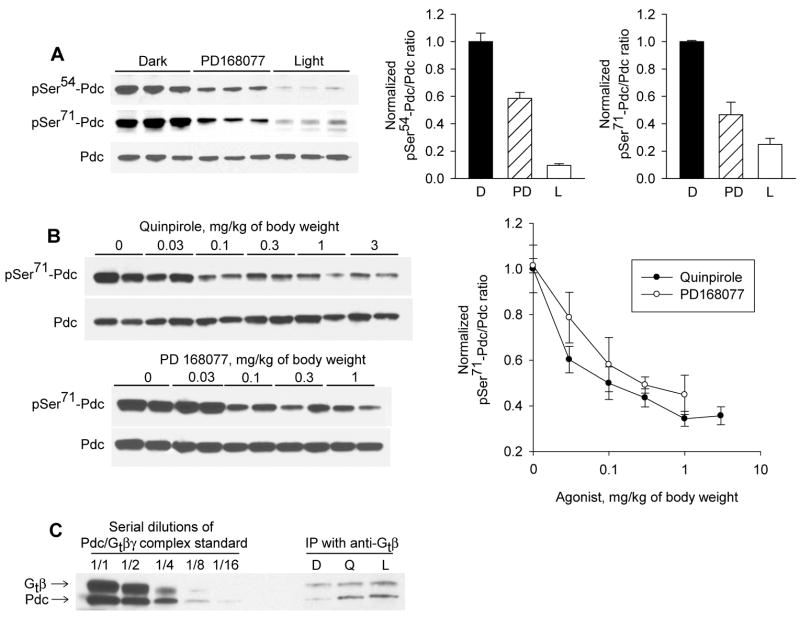
Injection of PD168077 (1 mg/kg), a selective dopamine D4 receptor agonist, reduced pSer54-Pdc/Pdc and pSer71-Pdc/Pdc ratios in the dark-adapted retina (Fig. 3 A; p<0.001), mimicking the effect of light exposure (30 min; 100 μW/cm2). The decrease in phosphorylation state in response to light, was significantly greater than that of PD168077 (Fig. 3 A; p<0.001 for pSer54-Pdc/Pdc; p<0.05 for pSer71-Pdc/Pdc ). Further characterization of the role of dopamine in the regulation of Pdc phosphorylation state focused on Ser71 because both phosphorylation sites responded similarly to PD168077. Quinpirole, an agonist of the D2 family of dopamine receptors, which includes the dopamine D4 receptor, also reduced the Pdc phosphorylation state in dark-adapted retinas (Fig. 3 B; p<0.001). The effect of both drugs was dose dependent (Fig. 3B; p<0.001). Quinpirole (10 μM) reduced pSer71-Pdc in retinas dissected from dark-adapted mice and incubated in darkness in vitro (pSer71-Pdc/Pdc: control 1.0 ± 0.08; quinpirole 0.65 ± 0.06; n=10; t-test, p<0.01), demonstrating a direct effect of dopamine receptor activation in the retina.
Fig. 3. Agonists of dopamine D4 receptors induce dephosphorylation of Pdc in the retina and promote Pdc/ Gtβ interaction.
C57Bl/6J mice, which had been dark adapted for 14 h beginning at ZT 12, were injected intraperitoneally (ip) with PD168077 (A, B), quinpirole (B, C), vehicles, or were exposed to light for 30 min (100 μW/cm2) (A, C). Retinas were dissected 30 min after injection or the beginning of light exposure. A. PD168077 (1 mg/kg of body weight) caused dephosphorylation of both Ser54 and Ser71 of Pdc mimicking the effect of light (n=3; ANOVA p<0.001). B. The effects of PD168077 and quinpirole on Ser71-Pdc were dose dependent (n= 4; ANOVA p<0.001 for both drugs) C. Proteins from dark-adapted (D), dark-adapted quinpirole-treated (Q, 1 mg/kg b.w. for 30 min) or light-treated (L, 30 min, 100μW/cm2) retinas were subjected to immunoprecipitation with anti-Gtβ antibody (Gβ1, C-16) or non-immune rabbit IgG. Precipitated proteins were analyzed by immunoblotting (anti-Pdc-pan, 1:20,000 and Gβ1, 1:1,000 for Pdc and Gtβ, respectively). Results shown are representative of 3 independent experiments. Quinpirole and light promoted the interaction of Pdc with Gtβ.
Dephosporylated Pdc binds to Gtβγ subunits (Lee et al., 1987; Yoshida et al., 1994; Thulin et al., 2001; Sokolov et al., 2004). Treatment of dark adapted mice with quinpirole or light increased the amount of Pdc that co-immunoprecipitated with Gtβ, using an antibody against the transducin subunit (Fig. 3 C). This observation provides additional evidence, independent of the phosphospecific antibodies, that dopamine receptor activation decreases the phosphorylation state of Pdc.
We tested the effects of quinpirole administration (Fig. 4 A) in dark-adapted wild type and Drd4−/− mice. Quinpirole induced a rapid decrease in pSer71-Pdc/Pdc in wild type mice (n=3–4; two-way ANOVA, p<0.001), but had no significant effect on pSer71-Pdc/Pdc in Drd4−/− mice (Fig. 4 A), indicative of the involvement of dopamine D4 receptors in Pdc dephosphorylation.
Fig. 4. Role of dopamine D4 receptors in the regulation of Pdc Ser71 phosphorylation.
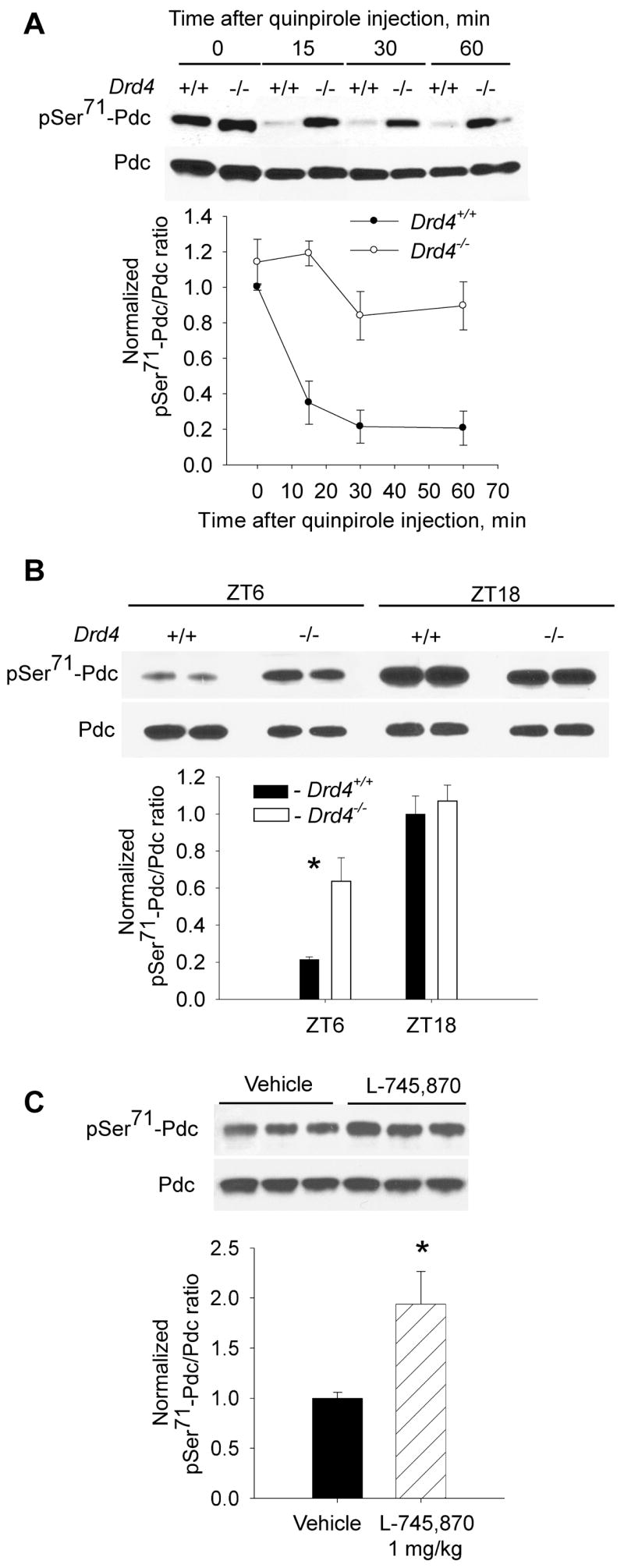
Pdc Ser71 phosphorylation was studied in dark-adapted wild type (Drd4+/+) and dopamine D4 receptor knock-out (Drd4−/−) mice on a C57Bl/6 background. A. Mice were injected ip with quinpirole (1 mg/kg body weight [b.w.]) and retinas were dissected in darkness 15, 30 and 60 min after drug administration. Two-way ANOVA indicated significant effects of quinpirole (p<0.001), genotype (p<0.001), and a significant interaction of quinpirole and genotype (p=0.011); n=4 / group. B. pSer71-Pdc/Pdc was measured in Drd4+/+ and Drd4−/− mice, maintained on a regular 12 h light/12h dark schedule, at ZT6 (middle of the day) and ZT18 (middle of the night). Both time of day (p<0.001) and genotype (p=0.02) significantly affected phosphorylation of Pdc Ser71, and a significant interaction of time and genotype was observed (p=0.005); n=4 / group. C. The dopamine D4 receptor antagonist L-745,870 (1 mg/kg b.w.) increases the amount of pSer71-Pdc during the daytime (ZT6). n=4 / group, t-test, * -p<0.05). Representative immunoblots and densitometry data are shown for each experiment.
The diurnal fluctuation of Pdc phosphorylation state was also affected by the absence of dopamine D4 receptors. In wild type animals, the amplitude of the midday-midnight difference in pSer71-Pdc/Pdc was approximately 5-fold, with low levels during the daytime in light (Fig. 4 B). The amplitude of the difference was significantly lower in retinas of Drd4−/− mice, due to a 3 fold higher level of pSer71-Pdc/Pdc at midday in Drd4−/− mice compared to wild type controls (Fig. 4 B; p<0.001). The pSer71-Pdc/Pdc levels in Drd4−/− mice and wild type controls were not significantly different at midnight. The diurnal fluctuation of pSer71-Pdc/Pdc in Drd4−/− mice was smaller than in controls, but still highly significant (p<0.001). These results indicate that activation of dopamine D4 receptors during the daytime contributes to the diurnal rhythm of pSer71-Pdc/Pdc level, but that other light-dependent processes also contribute to its generation. In support of this conclusion, pharmacological antagonism of dopamine D4 receptors with L-745,870 also significantly increased pSer71-Pdc/Pdc levels during the middle of the day (Fig. 4 C; p<0.05).
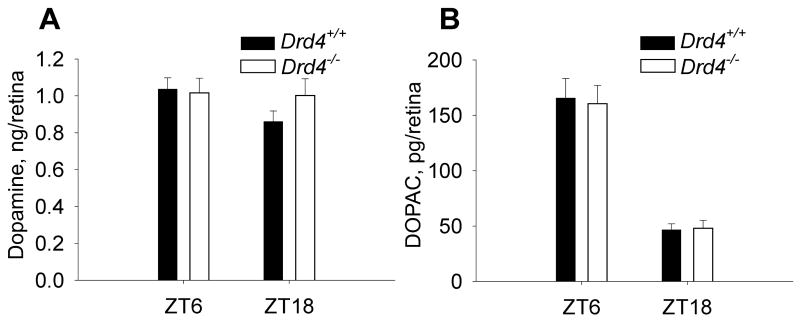
No differences in retinal concentrations of dopamine and its metabolite DOPAC were found in Drd4−/− mice when compared to wild type animals exposed to a light-dark cycle (Fig. 5), which indicates that the observed deficiency in Pdc phosphorylation regulation is unlikely due to changes in dopaminergic activity in the knockout mice. The concentration of retinal DOPAC was significantly higher in the middle of the day (in light) than the middle of the night (in darkness) in both genotypes of mice (p<0.001; Fig. 5 B).
Fig. 5. Diurnal changes of retinal dopamine and DOPAC in Drd4+/+ and Drd4−/− mice.
Retinas from wild type and mutant mice were dissected at ZT6 during the daytime (in light) and at ZT18 at night and dopamine and DOPAC levels were measured by HPLC with electrochemical detection. n = 5 / group. No significant differences in dopamine or DOPAC levels between genotypes were found (two-way ANOVA, p>0.05).
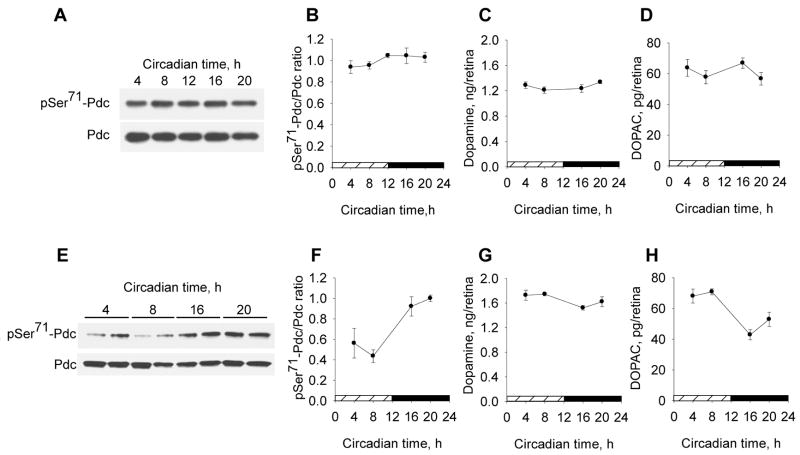
Circadian rhythms of pSer71-Pdc/Pdc, dopamine, and DOPAC in the retinas of C57Bl/6J and C3H/f+/+ mice
The circadian regulation of Pdc phosphorylation was examined in two strains of mice: C3H/f+/+ strain, in which retinal dopamine is subject to both light-driven and circadian regulation, and the C57Bl/6J strain, in which retinal dopamine is regulated only by light (Doyle et al., 2002). We confirmed that the levels of retinal dopamine and its primary metabolite DOPAC in C57Bl/6J mice are not rhythmic in constant (24 hr/day) darkness (Fig. 6 C,D; p>0.05). In contrast, retinas of C3H/f+/+ mice display a robust rhythm in DOPAC levels (Fig. 6 H, p<0.001), and a less prominent rhythm in dopamine concentration (Fig. 6 G, p<0.05). Accordingly, a circadian rhythm of pSer71-Pdc/Pdc was found in C3H/f+/+ mice (Fig. 6 E-F, p<0.001), but not in C57Bl/6J animals (Fig. 6 A–B). Significant interactions between factors “circadian time” and “genotype” (two-factor ANOVA, p<0.05 for pSer71-Pdc/Pdc ratio and dopamine, p<0.001 for DOPAC) indicate that retinal circadian rhythms in phosducin, dopamine and DOPAC are dependent on genotype. A significant negative correlation between retinal DOPAC concentration and pSer71-Pdc/Pdc (r = −0.77, p<0.001) suggests that the circadian rhythm in Pdc phosphorylation is regulated by retinal dopamine.
Fig. 6. Circadian rhythms of pSer71-Pdc/Pdc, dopamine, and DOPAC in the retinas of C57Bl/6J and C3H/f+/+ mice.
Retinal concentrations of pSer71-Pdc, total Pdc, dopamine and the dopamine metabolite DOPAC were measured in two strains of mice, C57Bl/6J (A–D) and C3H/f+/+ (E–H), on the second/third day of constant (24h/day) darkness. Two-factor ANOVA shows a significant effect of “circadian time” for C3H/f+/+ mice but not C57Bl/6J mice for pSer71-Pdc/Pdc (n=4–5; p<0.001), dopamine (n=5; p<0.05) and DOPAC (n=5; p<0.001); and significant interactions between factors circadian time and genotype (p<0.05 for pSer71-Pdc/Pdc ratio and dopamine, p<0.001 for DOPAC). A significant negative correlation existed between retinal DOPAC content and pSer71-Pdc/Pdc ratio (Pearson correlation coefficient r = −0.77, p<0.001).
To further test the hypothesis that the circadian rhythms of Pdc phosphorylation state were driven by dopamine, the effect of constant infusion of the dopamine D4 receptor antagonist, L-745,870, was examined (Fig. 7). In C3H/f+/+ mice implanted with osmotic mini-pumps containing vehicle, a significant circadian fluctuation in the degree of both Ser54-and Ser71-Pdc phosphorylation was observed on the second day of constant darkness (p<0.001), with highest levels at night. In contrast, no day-night difference in the phosphorylation state of either Ser54 or Ser71 was detected in C3H/f+/+ mice implanted with mini-pumps releasing L-745,870. Retinas in drug-treated mice had high levels of phosphorylation during the day and night.
Fig. 7. Abolition of the circadian rhythm of Pdc phosphorylation state by the dopamine D4 receptor antagonist L-745,870.
C3H/f+/+ mice were implanted with osmotic pumps releasing L-745,870 or vehicle and transferred to constant darkness. On the second day of constant darkness, retinas were dissected in the middle of subjective day (circadian time 6 (CT6)) and the middle of subjective night (CT18). From each mouse, one retina was used to measure levels of pSer54-Pdc, pSer71-Pdc and total (pan) Pdc and the other retina to measure the content of L-745,870. The levels of L-745,870 was 156±26 and 129±21 pg / retina at CT6 and CT18, respectively (n=5, t-test, p=0.47) in drug treated mice. The degree of phosphorylation of both Ser54 and Ser71 exhibited circadian rhythms in vehicle treated mice (two-way ANOVA, Student-Newman-Keuls test, p<0.001, n=5) that were eliminated by the dopamine D4 receptor antagonist (p>0.05).
Discussion
Our data indicate that dopamine, released from the inner retinal neurons in response to light and/or circadian clocks and acting on dopamine D4 receptors, regulate rhythms of protein phosphorylation in mammalian photoreceptor cells. The data show that the photoreceptor-specific protein Pdc undergoes diurnal and circadian fluctuations in the degree of phosphorylation of its PKA (Ser71) and CaMKII (Ser54) consensus sites. The involvement of dopamine in the generation of these rhythms is supported by several lines of evidence. The level of Pdc phosphorylation is lowest during the daytime in light, when dopamine synthesis and metabolism are highest [e.g., (Kramer, 1971; Iuvone et al., 1978; Nir et al., 2000; Doyle et al., 2002; Ribelayga et al., 2004)]. Pharmacological activation of retinal dopamine D4 receptors results in dephosphorylation of Pdc and promotes Pdc binding to Gtβγ. Genetic ablation of the gene encoding dopamine D4 receptors or pharmacological blockade of D4 receptors increases the level of phosphorylated Pdc during the daytime, reducing the amplitude of the diurnal fluctuation. A circadian rhythm of Pdc phosphorylation state is observed in a mouse strain (C3H/f+/+) that also expresses a circadian rhythm in dopamine metabolism, but not in a strain that does not (C57Bl/6J). Lastly, the circadian fluctuation in Pdc phosphorylation in photoreceptors of C3H/f+/+ mice is completely eliminated by dopamine D4 receptor antagonism.
The partial reduction in amplitude of the diurnal rhythm of Pdc phosphorylation state in Drd4−/− mice suggest that dopamine and other light-dependent processes within the photoreceptors act cooperatively to generate the rhythm in intact mice. The effect of light on Pdc phosphorylation state may be mediated by activation and translocation of phosphoprotein phosphatase (Brown et al., 2002). Reductions in protein kinase activities may also contribute to the light and dopamine responses. The observation that both the PKA- and CaMKII-phosphorylation sites are regulated similarly by light and dopamine D4 receptor activation suggest that both kinases may be involved. Light-induced reductions in PKA activity occur subsequent to decreased cAMP, and light and dopamine D4 receptor activation appear to reduce the same pool of cAMP in mouse photoreceptor cells (Cohen and Blazynski, 1990; Cohen et al., 1992), but by different mechanisms (Nir et al., 2002). A reduction in CaMKII in photoreceptors may occur subsequent to light- or dopamine-induced decreases of intracellular Ca2+. Light rapidly decreases intracellular Ca2+ levels by closing cGMP-gated and voltage-gated channels (Krizaj and Copenhagen, 2002). Activation of dopamine receptors also decreases intracellular Ca2+ in photoreceptors (Thoreson et al., 2002; Ivanova et al., 2008), but the mechanisms involved are less well established.
Circadian rhythms in retinal dopamine synthesis and metabolism have been observed in rats, birds, fish, amphibians, and some strains of mice (Wirz-Justice et al., 1984; Adachi et al., 1998; Pozdeyev and Lavrikova, 2000; Doyle et al., 2002; Ribelayga et al., 2004; Bartell et al., 2007). A circadian rhythm in Pdc was observed in C3H/f+/+ mice, which show robust circadian rhythms of dopamine metabolism. The highly significant negative correlation between retinal DOPAC levels and pSer71-Pdc/Pdc, coupled with our data showing regulation of Pdc phosphorylation by dopamine D4 receptors and the abolition of the circadian rhythm of Pdc phosphorylation state by a D4 receptor antagonist, strongly supports the conclusion that clocks generate a circadian rhythm in Pdc phosphorylation through rhythmic changes in dopaminergic neuronal activity.
Recent findings support the hypothesis that dopaminergic amacrine cells contain an autonomous circadian clock that drives dopamine release and metabolism (Witkovsky et al., 2003; Gustincich et al., 2004; Ruan et al., 2006; Dorenbos et al., 2007). However, it must be noted that retinal dopamine content and metabolism are circadian in mice that rhythmically synthesize melatonin, including C3H/f+/+ mice, but not in mice that are genetically incapable of synthesizing melatonin (Tosini and Menaker, 1998; Nir et al., 2000; Doyle et al., 2002). In C57Bl/6 mice, which do not synthesize melatonin, a circadian rhythm of dopamine metabolism can be induced by daily injections of melatonin (Doyle et al., 2002). Similarly, the circadian rhythms of dopamine release in fish, lizard, and bird retinas appear to be dependent on melatonin receptor activation (Adachi et al., 1998; Ribelayga et al., 2004; Bartell et al., 2007). It is tempting to speculate that melatonin released from photoreceptor cells is required to entrain the circadian oscillators in dopamine amacrine cells to the daily light-dark cycle. Our new data suggest that melatonin in the mouse retina may contribute to circadian rhythms in photoreceptors, including rhythms of protein phosphorylation, by regulating dopamine release, and indicate that the retinal dopamine-melatonin feedback loop previously described in non-mammalian vertebrates [reviewed in (Besharse et al., 1988; Green and Besharse, 2004; Iuvone et al., 2005)] plays an important role in the circadian organization of mammalian retina.
In rat retina, a circadian oscillator that drives melatonin synthesis rhythms is localized to photoreceptor cells, which also express transcripts of the core circadian clock genes Per1, Per3, Cry1, Cry2, Clock, Bmal1, Rev-erbα, and Rora, (Tosini et al., 2007), albeit at lower levels than in the inner retina. However, in mouse retina, the full complement of core clock gene transcripts was not detected by single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) (Ruan et al., 2006; Dorenbos et al., 2007). It remains to be determined if this represents a fundamental difference in circadian organization of mouse and rat retina or if clock genes in mouse photoreceptors are expressed at levels below the detection limit of single-cell RT-PCR.
Our present study and earlier published data provide the basis for the following working hypothesis for the rhythmic control of protein phosphorylation in photoreceptor cells (Fig. 8). In darkness, cyclic GMP levels in photoreceptors are high, leading to Na+ and Ca2+ influx through cyclic nucleotide-gated cation channels, depolarization of the plasma membrane, and activation of voltage-gated Ca2+ channels [reviewed in (Iuvone et al., 2005)]. The increase of intracellular Ca2+ activates CaMKII and stimulates cyclic AMP formation, activating PKA, resulting in increased protein phosphorylation by these kinases. Activation of PKA also stimulates nocturnal melatonin formation in a circadian clock-dependent manner (Fukuhara et al., 2004). Melatonin acts on dopamine neurons to suppress dopamine synthesis and release at night (Dubocovich, 1983; Adachi et al., 1998; Ribelayga et al., 2004; Bartell et al., 2007) and, possibly, to entrain the dopaminergic circadian clock. Light exposure during the daytime reverses these effects. Light decreases cyclic GMP, Ca2+ and cyclic AMP in photoreceptor cells, reducing protein kinase activity. These effects combined with increased phosphoprotein phosphatase activity (Brown et al., 2002) result in a decrease in the phosphorylation state of the kinase substrates. In addition, light exposure, acting via photoreceptors and retinal circuitry, stimulates dopamine release, with dopamine acting on D4 receptors to further reduce Ca2+ and cyclic AMP levels, and the activities of the respective protein kinases. The effects of light and darkness on photoreceptors combined with the effects of dopamine drive the robust diurnal rhythms of phosphorylation of PKA and CaMKII substrates in photoreceptor cells. We propose that in animal species capable of circadian synthesis of melatonin and containing circadian oscillators in the dopamine cells, the day-night rhythm in photoreceptor protein phosphorylation state persists in constant darkness, albeit with lower amplitude, due to the reciprocal circadian release of melatonin and dopamine, with activation of photoreceptor dopamine D4 receptors during the subjective day.
Fig. 8. A working model for the diurnal and circadian control of photoreceptor protein phosphorylation state.
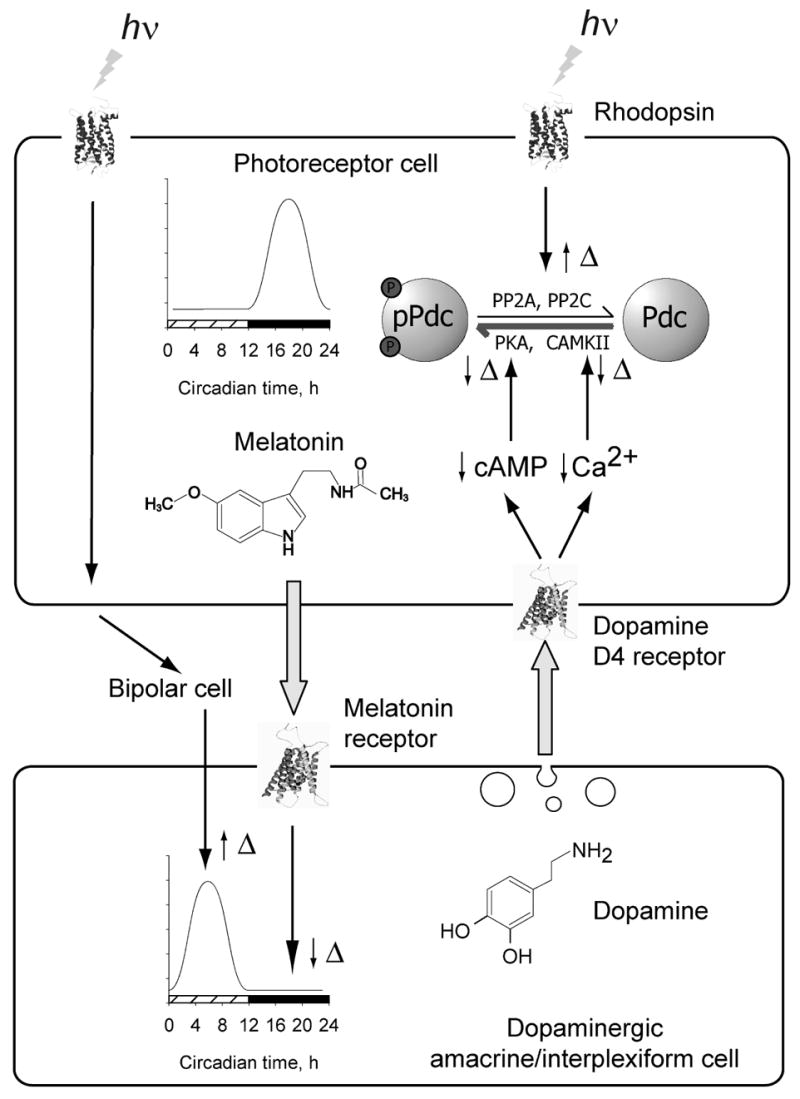
See text for details.
In conclusion, our data demonstrate a role for dopamine in the regulation of diurnal and circadian rhythms of protein phosphorylation state in photoreceptor cells. The role of dopamine in other aspects of circadian photoreceptor metabolism remains to be investigated.
Acknowledgments
The authors are grateful to Amy Visser, Holly Zhou for assistance genotyping the mice, to David Grandy and Malcolm Low (Oregon Health Sciences University) for providing the Drd4−/− breeder mice, and Maxim Sokolov (West Virginia University) for providing the pSer54-Pdc antibody. This study was supported by NIH grants EY004864 and EY014764 (PMI), NS43459 (GT), and a VA Merit Award (RHL). NP and SR were supported by a grant RUB1-2637 from the U.S. Civilian Research & Development Foundation.
Abbreviations
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CT
circadian time
- DOPAC
3,4-dihydroxyphenylacetic acid
- HPLC
high-performance liquid chromatography
- Pdc
phosducin
- PKA
cyclic AMP-dependent protein kinase
- ZT
zeitgeber time
Reference List
- Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 1998;792:361–369. doi: 10.1016/s0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- Bartell PA, Miranda-Anaya M, McIvor W, Menaker M. Interactions between dopamine and melatonin organize circadian rhythmicity in the retina of the green iguana. J Biol Rhythms. 2007;22:515–523. doi: 10.1177/0748730407308167. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Iuvone PM, Pierce ME. Regulation of rhythmic photoreceptor metabolism: A role for post-receptoral neurons. Progress in Retinal Research. 1988;7:21–61. [Google Scholar]
- Brown BM, Carlson BL, Zhu X, Lolley RN, Craft CM. Light-driven translocation of the protein phosphatase 2A complex regulates light/dark dephosphorylation of phosducin and rhodopsin. Biochemistry. 2002;41:13526–13538. doi: 10.1021/bi0204490. [DOI] [PubMed] [Google Scholar]
- Chen F, Lee RH. Phosducin and betagamma-transducin interaction I: effects of post-translational modifications. Biochem Biophys Res Commun. 1997;233:370–374. doi: 10.1006/bbrc.1997.6460. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990;4:43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci USA. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991;7:87–97. doi: 10.1017/s0952523800010968. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–4. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–11. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- Gaudet R, Savage JR, McLaughlin JN, Willardson BM, Sigler PB. A molecular mechanism for the phosphorylation-dependent regulation of heterotrimeric G proteins by phosducin. Mol Cell. 1999;3:649–660. doi: 10.1016/s1097-2765(00)80358-5. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Gustincich S, Contini M, Gariboldi M, Puopolo M, Kadota K, Bono H, LeMieux J, Walsh P, Carninci P, Hayashizaki Y, Okazaki Y, Raviola E. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc Natl Acad Sci USA. 2004;101:5069–5074. doi: 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Alonso-Gomez AL, Iuvone PM. Dopamine D4 receptors regulate intracellular calcium concentration in cultured cone photoreceptor cells: relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain Res. 2008 doi: 10.1016/j.brainres.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer SG. Dopamine: A retinal neurotransmitter. I Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol. 1971;10:438–452. [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Calcium regulation in photoreceptors. Front Biosci. 2002;7:d2023–d2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CH, Akiyama M, Miki N. Isolation of a novel retina-specific clone (MEKA cDNA) encoding a photoreceptor soluble protein. Brain Res Mol Brain Res. 1989;6:1–10. doi: 10.1016/0169-328x(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Lee BY, Thulin CD, Willardson BM. Site-specific phosphorylation of phosducin in intact retina. Dynamics of phosphorylation and effects on G protein beta gamma dimer binding. J Biol Chem. 2004;279:54008–54017. doi: 10.1074/jbc.M405669200. [DOI] [PubMed] [Google Scholar]
- Lee RH, Brown BM, Lolley RN. Protein kinase A phosphorylates retinal phosducin on serine 73 in situ. J Biol Chem. 1990a;265:15860–15866. [PubMed] [Google Scholar]
- Lee RH, Fowler A, McGinnis JF, Lolley RN, Craft CM. Amino acid and cDNA sequence of bovine phosducin, a soluble phosphoprotein from photoreceptor cells. J Biol Chem. 1990b;265:15867–15873. [PubMed] [Google Scholar]
- Lee RH, Lieberman BS, Lolley RN. A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry. 1987;26:3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- Lee RH, Whelan JP, Lolley RN, McGinnis JF. The photoreceptor-specific 33 kDa phosphoprotein of mammalian retina: generation of monospecific antibodies and localization by immunocytochemistry. Exp Eye Res. 1988;46:829–840. doi: 10.1016/s0014-4835(88)80035-6. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obin M, Lee BY, Meinke G, Bohm A, Lee RH, Gaudet R, Hopp JA, Arshavsky VY, Willardson BM, Taylor A. Ubiquitylation of the transducin betagamma subunit complex. Regulation by phosducin. J Biol Chem. 2002;277:44566–44575. doi: 10.1074/jbc.M205308200. [DOI] [PubMed] [Google Scholar]
- Pagh-Roehl K, Lin D, Su L, Burnside B. Phosducin and PP33 are in vivo targets of PKA and type 1 or 2A phosphatases, regulators of cell elongation in teleost rod inner-outer segments. J Neurosci. 1995;15:6475–6488. doi: 10.1523/JNEUROSCI.15-10-06475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev NV, Lavrikova EV. Diurnal changes of tyrosine, dopamine, and dopamine metabolites content in the retina of rats maintained at different lighting conditions. J Mol Neurosci. 2000;15:1–9. doi: 10.1385/JMN:15:1:1. [DOI] [PubMed] [Google Scholar]
- Reig JA, Yu L, Klein DC. Pineal transduction. Adrenergic----cyclic AMP-dependent phosphorylation of cytoplasmic 33-kDa protein (MEKA) which binds beta gamma-complex of transducin. J Biol Chem. 1990;265:5816–5824. [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci USA. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Strissel KJ, Leskov IB, Michaud NA, Govardovskii VI, Arshavsky VY. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem. 2004;279:19149–19156. doi: 10.1074/jbc.M311058200. [DOI] [PubMed] [Google Scholar]
- Song H, Belcastro M, Young EJ, Sokolov M. Compartment-specific phosphorylation of phosducin in rods underlies adaptation to various levels of illumination. J Biol Chem. 2007;282:23613–23621. doi: 10.1074/jbc.M701974200. [DOI] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the Mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL, Jr, Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci. 2002;19:235–247. doi: 10.1017/s0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulin CD, Savage JR, McLaughlin JN, Truscott SM, Old WM, Ahn NG, Resing KA, Hamm HE, Bitensky MW, Willardson BM. Modulation of the G protein regulator phosducin by Ca2+/calmodulin-dependent protein kinase II phosphorylation and 14-3-3 protein binding. J Biol Chem. 2001;276:23805–23815. doi: 10.1074/jbc.M101482200. [DOI] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Wilkins JF, Bitensky MW, Willardson BM. Regulation of the kinetics of phosducin phosphorylation in retinal rods. J Biol Chem. 1996;271:19232–19237. doi: 10.1074/jbc.271.32.19232. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Da Prada M, Rem{ C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D, Silver R. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci. 2003;23:7670–7676. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Willardson BM, Wilkins JF, Jensen GJ, Thornton BD, Bitensky MW. The phosphorylation state of phosducin determines its ability to block transducin subunit interactions and inhibit transducin binding to activated rhodopsin. J Biol Chem. 1994;269:24050–24057. [PubMed] [Google Scholar]
- Zhu X, Craft CM. Interaction of phosducin and phosducin isoforms with a 26S proteasomal subunit, SUG1. Mol Vis. 1998;4:13. [PubMed] [Google Scholar]
- Zhu X, Craft CM. Modulation of CRX transactivation activity by phosducin isoforms. Mol Cell Biol. 2000a;20:5216–5226. doi: 10.1128/mcb.20.14.5216-5226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Craft CM. The carboxyl terminal domain of phosducin functions as a transcriptional activator. Biochem Biophys Res Commun. 2000b;270:504–509. doi: 10.1006/bbrc.2000.2414. [DOI] [PubMed] [Google Scholar]