Abstract
Estrogens have direct effects on the brain areas controlling cognition. One of the most studied of these regions is the dorsal hippocampal formation, which governs the formation of spatial and episodic memory formation. In laboratory animals, most investigators report that estrogen enhances synaptic plasticity and improves performance on hippocampal-dependent cognitive behaviors. This review summarizes work conducted in our laboratory and others toward identifying estrogen’s actions in the hippocampal formation, and the mechanisms for these actions. Physiologic and pharmacologic estrogen affects cognitive behavior in mammals, which may be applicable to human health and disease. The effects of estrogen in the hippocampal formation that lead to modulation of hippocampal function include effects on cell morphology, synapse formation, signaling, and excitability that have been studied in laboratory mice, rats, and primates. Finally, estrogen may signal through both nuclear and extranuclear hippocampal estrogen receptors to achieve its downstream effects.
Keywords: estrogen, estrous cycle, synaptic plasticity, hippocampus, cognition, spatial memory, dendritic spine
1. Introduction
Although estrogen’s effects on sex behavior in mammals are well known, its effects on other behaviors including mood and cognition have only recently been recognized. We now know that estrogens have direct effects on the brain areas controlling mood and cognition. One of the most studied of these regions is the dorsal hippocampal formation, which governs the formation of spatial and episodic memory formation. In laboratory animals, most investigators report that estrogen enhances synaptic plasticity and improves performance on hippocampal-dependent cognitive behaviors. This review will detail work conducted in our laboratory and others toward identifying estrogen’s actions in the hippocampal formation, and the mechanisms for these actions. The first section will briefly discuss estrogen’s effects on cognitive behavior in mammals, as well as the physiological relevance of these effects and their applicability to human health and disease. The second section will detail estrogen’s effects in the hippocampus, including effects on cell morphology, synapse formation, signaling, and excitability that have been shown in laboratory mice, rats, and primates. The third section will discuss the role of signaling through estrogen receptors in these effects. Lastly, using estrogen effects on a neurotrophin system as an example, the concluding section will seek to demonstrate how multiple mechanisms may act in concert to produce the final, but as yet incomplete, picture of estrogen effects in the hippocampal formation. Continuing investigations to uncover the mechanisms by which estrogen causes this enhancement are crucial to our ability to apply this information more skillfully in complex clinical settings.
2. Estrogen and behavior
2.1 Estrogen effects on cognitive function
In female mammals, including rodents and nonhuman primates, estrogen historically acts on the brain to elicit reproductive behavior, including solicitation of the opposite sex and mating behavior1. More recently, estrogen effects on non-reproductive behaviors have gained recognition. These include anxiety and depressive-like behaviors, as well as cognitive behaviors. As reviewed recently, 17-beta-estradiol (estradiol) administration to ovariectomized rats decreases anxiety and depressant behavior on standard laboratory tests2. Estrogen effects on cognition depend on the cognitive task and its dependent brain region(s). For instance, while estradiol impairs performance on striatum-dependent tasks in female rats3, 4, it enhances performance on prefrontal cortical-dependent learning in female rats5, aging female rhesus monkeys6, and both young adult and postmenopausal women7, 8. It also enhances performance on hippocampal-dependent tasks in female rats9–12, mice13, 14, and rhesus monkeys6, 15. The hippocampus is a major mediator of spatial learning and memory, and tasks designed to test hippocampal faculties in rodents include place learning in a reward-motivated plus maze, water maze navigation, baited radial maze tests of working and reference memory, active avoidance paradigms, and object placement learning, among others. At least 48 hours of estradiol treatment enhances performance on these tasks. The observed enhancement may be due in part to an improvement in memory consolidation, as estradiol given to ovariectomized female rats after training on spatial memory tasks still enhances performance on subsequent test trials5, 16.
The effects of estrogen on cognition described above have largely been ascribed to its actions on synaptic plasticity in the forebrain, which will be discussed below. However, neuroprotective actions of estrogens have also been described that may contribute to its ability to protect against age-related cognitive decline and neurodegenerative disorders. These actions, which are particularly relevant to hormone replacement therapy in menopausal women, have been recently reviewed17. The neuroprotective (memory preserving) and neurotrophic (memory enhancing) actions of estrogens may share underlying mechanisms, as many of the same hormone actions have been independently linked to both functions.
Even from a behavioral standpoint, the complexity of estrogen actions on the brain is apparent in that its effects are task-dependent, with differences largely stemming from the major brain region mediating the behavior. Behavioral evidence that the estradiol enhancement of “hippocampal” tasks indeed represents effects on hippocampal function comes from studies showing enhanced performance with estradiol infusion directly into the hippocampus, but not other brain regions18, 19. However, estrogen’s effects on cognitive function most likely result from the sum of interacting influences on several brain areas, including hippocampus, striatum, basal forebrain, and prefrontal cortex. Although this review will focus on estrogen effects in the hippocampal formation, it will also address some of the evidence that estrogen modulates hippocampal-dependent behavior through complementary effects on the hippocampal formation and the basal forebrain.
2.2 Physiological relevance of estrogen effects on cognition
Estrous and menstrual cycles
Evidence for the physiological relevance of the behavioral effects of estrogens comes from studies on young adult female rodents and primates at high- and low-estrogen phases of the natural estrous or menstrual cycle that complement the studies of E administration cited above. During the 4–6 day rodent estrous cycle, estradiol levels rise and fall precipitously during the day of proestrus. On the following day, in the estrus phase of the cycle, circulating estradiol is low. It climbs slightly during the diestrus phase, only rising rapidly at the return of proestrus. Performance of laboratory rodents on hippocaumpal-dependent spatial memory tasks fluctuates across the estrous cycle. Rats in proestrus choose a hippocampal-dependent place strategy, and rats in estrus choose a striatal-dependent response strategy, to solve a plus maze task20. These results are consistent with findings using ovariectomized rats treated with estradiol on similar tasks9. In mice, spatial memory memory is enhanced in proestrus relative to estrus21. In women, some cognitive abilities, most notably verbal memory, fluctuate across the menstrual cycle and correlate positively with serum estrogen levels22, 23. In addition, activation of the hippocampal formation correlates with estradiol levels across the female menstrual cycle24. These studies provide evidence that the natural fluctuation of ovarian steroids influences hippocampal-dependent cognition in mammals.
Ovariectomy and hormone replacement
In addition to cyclic fluctuation of cognition, rats and humans with suppressed ovarian function exhibit behavioral deficits on tests of hippocampal- and prefrontal-cortical-dependent memory that can be reversed by exogenous ovarian hormone administration. For example, in rats, ovariectomy impairs object recognition memory, a task which depends on both hippocampal and prefrontal cortical faculties5, 25, and estradiol replacement corrects this deficit3. Similarly, ovariectomy impairs performance of rats on the hippocampal-dependent active avoidance paradigm, a deficit corrected by 5 weeks of estradiol replacement26. Young adult women given a gonadotrophin-releasing hormone agonist to suppress ovarian function exhibit verbal memory deficitis and deficits in task-associated neural activity patterns that can be corrected by administration of exogenous ovarian hormones7, 27. Because of the potentially important differences between effects of pharmacological and physiological doses or hormones, studies in cycling mammals are necessary to corroborate studies of exogenous estradiol administration, to demonstrate the physiological relevance of the findings. Conversely, studies of endocrine gland ablatement (ovariectomy) and hormone replacement (estradiol administration) are needed to provide evidence that the findings of studies on intact cycling animals reflect the effects of particular circulating hormones. Because of this, corresponding evidence will be provided from both types of studies whenever possible in the remainder of this review.
Aging and Reproductive Senescence
Primates experience a period of reproductive senescence at the end of life, when estrous or menstrual cycles cease and circulating levels of estrogen reach a constant low. This state can be mimicked in aging ovariectomized rodents. Evidence in support of the role of gonadal hormones in the maintenance of cognitive behavior comes from the impairments seen in these reproductively senescent or in aging ovariectomized animals compared with age-matched cycling or ovariectomized hormone-replaced controls. For example, cyclic estrogen replacement reverses cognitive impairment in aged ovariectomized monkeys on some tests, including spatial working memory and nonmatching-to-sample object recognition6. This evidence has been corroborated in rats and in humans, where predicted cognitive impairments related to age and natural or surgical menopause can be corrected with various regimens of estrogen replacement23, 28. That estrogen prevented or rescued cognitive decline in some studies suggests a role for estrogen in the prevention of age-related degenerative of cognitive abilities. These neuroprotective actions of estrogens have recently been reviewed17, 29. Several studies suggest that the aging brain responds differently to estrogen treatment than the young brain. Fewer spines on CA1 pyramidal cells contain estrogen receptor alpha (ER alpha) in the aging female compared to the young female rat30. This suggests that aging may be accompanied by a loss of estrogen sensitivity in the rat hippocampus. Indeed, in contrast to young animals, estradiol treatment does not increase CA1 axospinous synapse density in ovariectomized aging rats31, and it fails to alter the distribution of ER alpha in the CA1 of aging rats30. Some investigators have hypothesized that the brain loses its responsivity to estrogen under a period of prolonged hypoestrogenicity, and that estrogen sensitivity can be maintained through timely hormone replacement after the onset of reproductive senescence. This idea, called the “healthy cell bias” of estrogen action, states that estrogen has positive effects on healthy brain structures, but that once the structures are already damaged, estrogen’s effects are detrimental32, 33. This implies that there is a critical window of estrogen sensitivity after ovariectomy or menopause before the effects of long-term hypoestrogenicity become irreversible34. Evidence for this critical period has been shown in rodents, nonhuman primates, and women32, 35–37. These findings may have important implications for postmenopausal hormone replacement in women.
2.3 Estrogen effects on cognition in postmenopausal women
The recent Women’s Health Initiative (WHI) study found that conjugated equine estrogens (CEE) with or without medroxyprogesterone acetate (MPA) somewhat increased the risk of dementia and cognitive decline in postmenopausal women38–40. This study is now a widely cited example of the harm that can be caused by certain regimens of hormone replacement, especially when begun ten or more years after menopause. It is also a reminder of the importance of studying the mechanism of estrogen’s ability to protect and restore cognition in the laboratory. The pitfalls of the WHI study have been reviewed by several authors17, 35. Since the publication of the WHI findings, multiple smaller trials and reviews of laboratory work have provided support for the “healthy cell bias” of estrogen action discussed above. This evidence suggests that women many years postmenopause, such as the majority of the women in the WHI study, will only retain estrogen responsivity if they have began hormone replacement within a few years of the menopausal transition34, 35. Finally, different routes of hormone administration and different forms of estrogen and progesterone have been explored for hormone replacement therapy in women, and they should not be treated equally41. The risks and benefits of the form chosen for the WHI study, oral CEE with or without MPA, should only be strictly applied to the prescription of this form of hormone replacement. Laboratory scientists have begun to investigate the specific biological actions of these hormones and the importance of dose and delivery method42–44.
The conundrum of estrogen therapy for women – how to balance the risks against the benefits – stems from a so far inefficient translation of laboratory knowledge into bedside practice. For the most part, the hormone replacement models used in laboratory studies and those used in human trials differ in the estrogenic and progestenic agents, the mode of hormone delivery, temporal aspects of delivery, history of patient or animal hormone exposure, and the endpoint used for evaluation45. This creates difficulty in interpreting the differences between outcomes of animal and human studies. Numerous efforts are now being made to identify the differences between laboratory and clinical study designs and to pinpoint which one(s) are responsible for the dramatic differences in the regimens’ effects on cognition, among other endpoints. Hopefully, the experience of the WHI study and the inapplicability of many past laboratory studies to that clinical setting will encourage more communication between scientists and clinicians before the design of future large-scale clinical trials of hormone replacement and other treatments.
3. Estrogen effects in the hippocampal formation
Decades of work have uncovered cellular and molecular correlates of estrogen’s enhancement of hippocampal function. These include effects on cell morphology, synapse formation, membrane excitability, cell signaling pathways, neurotrophin systems, endogenous opioid systems, and neurogenesis. They will be described in detail in the following sections.
3.1 Spines and synapses
Rats
In female rats, ovariectomy decreases the density of dendritic spines in the CA1 stratum radiatum of the hippocampus, which can be restored by 72 hours of estradiol replacement46–48. Estradiol also increases spine density in rat primary hippocampal neurons in culture, where 48 hours of exposure to 0.1 µg/mL estradiol doubles the dendritic spine density49. A similar fluctuation of spine and synapse density also occurs with the natural estrous cycle: Rats in late proestrus have 30% higher spine densities in the CA1 region than rats in late estrus40. Compared to estrus rats, proestrus rats also have a higher proportion of “mushroom” shaped spines50, widely believed to be a stronger, more mature subset of spines50, 51. This natural fluctuation of spine density and shape during the 4-day rat estrous cycle implies rapid modulation of spine dynamics in response to fluctuating levels of gonadal hormones. The density of dendritic spines on hippocampal CA1 pyramidal neurons has been correlated with the formation of associative memories in both hippocampal and classically non-hippocampal tasks52. Thus estradiol regulation of CA1 spine dynamics may contribute to its ability to enhance and maintain hippocampal function. Consistent with this idea, estradiol increases not just spines but also excitatory-type synaptic contacts examined at the ultrastructural level39, providing evidence for an increased capacity for synaptic plasticity and memory formation. To accomplish this, spines are accommodated presynaptically by an increase in multi-synaptic boutons, which amounts to a 25.5% increase in synapses in the CA1 stratum radiatum with estradiol treatment53.
Recent evidence suggests that the estrogen-mediated increase in spine synapse density is accompanied by comparable increases in molecular components of the spine and synapse apparatus, which may serve to maintain individual synaptic strength. In ovariectomized rats, 48 hours of estradiol treatment increases levels of the presynaptic proteins synaptophysin and syntaxin, and postsynaptic proteins spinophilin and postsynaptic density 95 (PSD-95), in the CA1 region (Figure 1)54–56. Synaptophysin is a presynaptic vesicle protein with unclear function widely used as a marker for presynaptic contacts, while syntaxin is a SNARE protein that mediates neurotransmitter vesicle exocytosis. Spinophilin, a protein phosphatase-1 and actin binding protein localized to the post-synaptic density, plays a role in glutamatergic neurotransmission and dendritic spine morphology57, 58. PSD-95 is a scaffolding protein involved in regulation of synaptic activity at the postsynaptic density59–61. In cultured neurons, estradiol also increases synaptophysin, spinophilin, and PSD-95 immunoreactivity49, 54, 62, 63, suggesting that estradiol also increases synapse density in vitro. Since the expression of these proteins increases predictably with the estrogen-mediated increase in spine and synapse density in CA1, investigators have begun to use them as a marker for estrogen-induced synapse formation.
Figure 1.
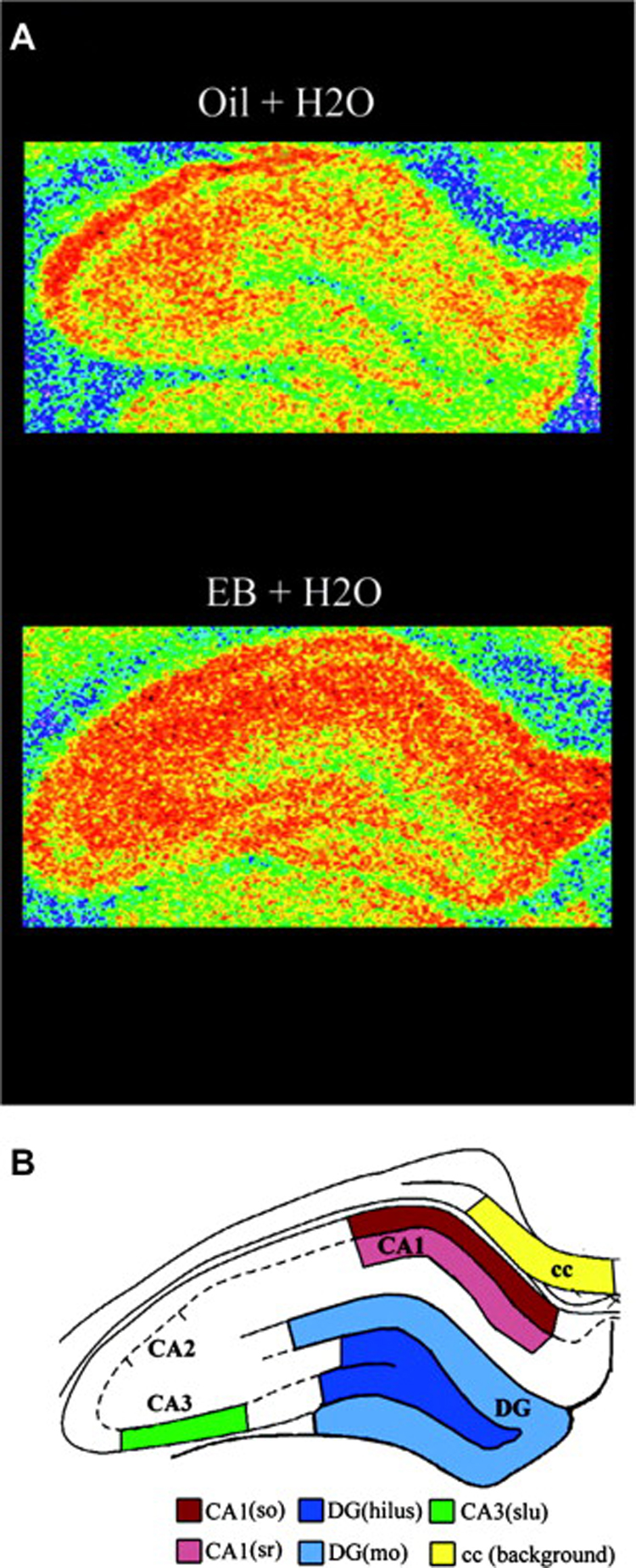
Estradiol increases spinophilin expression in the hippocampal formation of ovariectomized female rats. A, Illustration of autoradiograms using pseudocolor representation of shading densities of spinophilin immunoreactivity in the hippocampal formation of representative estrogen (EB)- and control (Oil)-treated ovariectomized rats (blue < green < yellow < orange). B, Schematic diagram identifying specific hippocampal regions from where measures were taken. cc, corpus callosum; dg, dentate gyrus; (so), stratum oriens; (sr), stratum radiatum; (slu); stratum lucidum; (mo), molecular layer. Reprinted with permission from Brake et al., 2001.
Finally, a few studies suggest that glia may play a role in estradiol effects in the hippocampus. Estradiol treatment increases astrocytic volume in the rat CA164. The importance of neuron-glia interactions in the formation and maintenance of hippocampal spines and synapses is being increasingly recognized65. Conceivably, an estradiol-mediated increase in astrocytic volume could provide necessary trophic support for the formation of new dendritic spines and synapses. Glia have previously been implicated in the neuroprotective effects of estradiol66, but their role in its neurotrophic actions remains to be more thoroughly investigated.
The order and origin of events in the estradiol-mediated effects on synaptic formation in the rat are not yet clear. It is possible that hormone exposure leads to increased dendritic filopodia formation, followed by presynaptic contact and synapse maturation including assembly of pre- and post-synaptic apparati. However, estradiol may also independently mediate each of these stages of synaptic formation. Evidence for direct estradiol effects on filopodia formation, membrane depolarization, and synaptic protein translation will be presented in the next section on estrogen effects in the hippocampal formation.
Mice
Although estrogen enhances hippocampal function similarly in mice and rats, these species differ in the hippocampal response to estradiol as measured by spine density and synaptic protein expression. Exogenous estradiol treatment in OVX mice does not increase dendritic spine density in CA1 stratum radiatum like it does in OVX rats, but only increases the density of spines with mushroom shapes14, 47. Few ultrastructural studies on the effects of estrogen in mice have been conducted, but one study showed that estradiol replacement corrected an ovariectomy-induced decrease in the thickness of the postsynaptic density on pyramidal cell dendrites and the number of synaptic vesicles in the CA1 area13. This suggests that estradiol may regulate spine maturation and synaptic function without increasing spine density per se. Indeed, in ovariectomized mice, estradiol treatment also increases the expression of synaptic proteins PSD-95, spinophilin, and syntaxin14. These increases were apparent not just in the CA1, but in all fields of the hippocampal formation14. Overall, these findings indicate that while in the rat, the CA1 is particularly responsive to estrogen, in the mouse, the response is more widespread, as estrogen increases synaptic protein expression throughout the hippocampal formation with a more subtle morphological phenotype in the CA1 region. While the reason for these two types of response is unclear, they suggest anatomical and qualitative differences in estrogen’s upstream activation of pathways in the mouse and rat hippocampus. The functional relevance of these differences, if any, remains to be clarified.
Nonhuman primates
A few studies using nonhuman primates support the conclusions from rodent studies that estrogen enhancement of hippocampal function correlates with regulation of dendritic spine dynamics. In both young and aged ovariectomized rhesus monkeys, estradiol replacement increases the number of spinophilin-immunoreactive spines in CA1 stratum radiatum by approximately 35%6, 67. Similarly, one month of estradiol treatment increases immunoreactivity for synaptophysin, syntaxin, and spinophilin in the strata radiatum and oriens of ovariectomized female rhesus monkeys68. These findings indicate that some estrogen actions in the hippocampus are conserved from rodents to nonhuman primates, and raise the possibility that they are also conserved in humans.
3.2 Neurotransmitter Systems and Electrophysiology
Excitability and long-term potentiation
Estrogen’s ability to increase the excitability and electrical potentiation of neural synapses may be one mechanism by which it enhances hippocampal-dependent learning and memory. The first report on the modulation of hippocampal electrical activity by physiologic gonadal hormones was published almost forty years ago, describing electrical changes in the limbic system across the estrous cycle of the female rat69. Since then, other groups have demonstrated that estradiol increases hippocampal excitability in the rat70, 71. Estradiol also has a well-demonstrated ability to enhance long-term potentiation (LTP) in vivo in awake rats72 and cultured hippocampal slices73. Recent follow-ups on the original finding of electrical fluctuation across the estrous cycle have also found that hippocampal excitability and LTP at CA3-CA1 synapses are enhanced during proestrus compared to other cycle stages in anesthetized female rats74–76. The lasting increase in synaptic strength characterizing LTP is believed to be a hallmark of synaptic plasticity in neurons. LTP also has also been considered an electrical manifestation of learning at individual synapses; indeed, learning induces LTP in the hippocampal formation77. Thus by increasing the capacity for LTP in the hippocampus, estradiol effectively increases the synaptic plasticity of this brain region in a way that correlates with learning.
N-methyl-D-Aspartate (NMDA) Receptor-Mediated Neurotransmission
A number of reports have implicated NMDA receptor-mediated neurotransmission in estrogen’s ability to regulate hippocampal spine density. Ovariectomy causes a decrease NMDA receptor binding and expression of the NR1 and NR2B receptor subunits in the rat hippocampus78, 79, and estradiol replacement increases these measures80, 81 82, 83. These effects on NMDA receptor binding and subunit expression correlate with increased NMDA receptor-mediated input73 that may modulate dendritic spine density. Indeed, in hippocampal slices, the estradiol-induced increase in CA1 spine density correlates with sensitivity to NMDA-mediated synaptic input57. NMDA receptor input is also necessary for the estradiol-mediated increase in CA1 spine density both in vivo and in hippocampal slice cultures49, 81, 84. Since NMDA receptors are the major mediators of neuronal LTP, the ability of estradiol to increase NMDA receptor-mediated input may account for at least some of its rapid effects on neuronal excitability and LTP85, 86. Of note, although the above findings suggest that estradiol enhances NMDA-mediated neurotransmission in hippocampal pyramidal cells, this may not always be the case. For example, in models of excitotoxic brain injury, estrogen protects against hippocampal cell death by suppressing NMDA receptor input onto pyramidal cells29. This suggests that estrogen effects on NMDA-mediated neurotransmission may depend on context.
GABA and inhibition
Estradiol may also promote hippocampal synaptic plasticity through modulation of inhibitory input onto pyramidal cells. Indeed, estrogen effects have been described both in vitro and in vivo on expression of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD), and on electrophysiological GABAergic inhibition. These effects are more complex than those on the NMDA receptor system. In vitro experiments implicate an estradiol-induced decrease in GABAergic inhibition in the increase in spine density on CA1 pyramidal cell dendrites87. Ultrastructural evidence supporting this hypothesis of estradiol-induced disinhibition showed that estradiol decreases the number of synaptic vesicles adjacent to the presynaptic membrane at inhibitory presynaptic boutons on CA1 pyramidal cells. However, ovariectomy decreases, and estradiol increases, GAD protein expression in the CA1 pyramidal cell layer88, suggesting that estradiol facilitates an increase in the synthesis and therefore availability of GABA neurotransmitter. These seemingly inconsistent results may be reconciled by a study showing that in hippocampal slices from estradiol-treated rats, a transient decrease in GABAergic inhibition is followed by a recovery of the inhibition and an increase in GAD expression89. This recovery may indicate that homeostatic mechanisms are in place to control estrogen-mediated excitation of pyramidal cells that could otherwise lead to excitotoxicity and even seizures. Indeed, the necessity for this kind of control mechanism is apparent in human epilepsy disorders and animal models, in which estrogen lowers seizure threshold by increasing excitability of pyramidal neurons71.
Finally, recent work at the ultrastructural level suggests that estradiol may specifically target different subtypes of GABAergic neurons. In CA1 interneurons expressing neuropeptide Y (NPY), estradiol mobilizes vesicles to the presynaptic membrane and increases NPY expression90–92. NPY acts to inhibit glutamate release at the synapse93. By increasing the availability of NPY in addition to presynaptic vesicles at inhibitory synapses, estradiol may enhance inhibitory input onto CA1 pyramidal cells from this subset of NPY-expressing GABAergic interneurons. On the other hand, additional ultrastructural evidence in the CA1 showed that on average, estradiol decreases the number of vesicles adjacent to the presynaptic membrane at inhibitory presynaptic boutons onto pyramidal cells94. Therefore, estradiol may decrease total GABAergic activity at pyramidal cell synapses in the CA1 while enhancing inhibitory transmission from the subset of interneurons expressing NPY. Although the reason for this is not clear, it may indicate that estradiol itself has opposing effects on different interneuronal subtypes. These effects could serve on the one hand, to facilitate increased NMDA receptor input and on the other hand, to keep that excitation in check. The complex involvement of GABAergic neurons in estradiol effects in the CA1 has recently been reviewed by members of our laboratory93.
Acetylcholine
Initial demonstrations of estrogen effects on the cholinergic system in the brain came from our laboratory in 1975, showing that estradiol upregulates the activity of the acetylcholine-synthesizing enzyme, choline acetyltransferase (ChAT), in the preoptic area and corticomedial amygdala of ovariectomized female rats95. The involvement of this system in estrogen effects on hippocampal function was not demonstrated until 2001, with a study showing that the muscarinic cholinergic system mediates estrogen effects on memory and NMDA binding in the hippocampus96. This report showed that delivery of an M2 receptor antagonist blocks the estradiol-induced enhancement of NMDA binding and working memory in ovariectomized rats, and that increasing cholinergic tone by delivering an acetylcholinesterase inhibitor mimics the effects of estradiol. Later studies showed that estrogen increases potassium-stimulated acetylcholine release in the hippocampus97, 98, suggesting that increased cholinergic stimulation may mediate some estrogen effects in this brain region. Furthermore, ultrastructural studies that examined estrogen receptor alpha and vesicular acetylcholine transporter (VAChT) in the hippocampal CA1 region showed that VAChT is present in ER alpha-labeled terminals and in terminals affiliated with ER alpha-labeled dendritic spines99. This suggests that estrogen bound to these ERs may affect local acetylcholine release and reuptake to influence NMDA receptor expression and hippocampal function. Interestingly, in the dentate gyrus, cholinergic afferents selectively target NPY-containing interneurons, rather than parvalbumin-containing interneurons100. As described in the previous section, estradiol selectively targets NPY-containing neurons in the CA1 region. Although the relationship between cholinergic terminals and NPY-containing interneurons in the CA1 region remains to be investigated, these findings from the dentate gyrus suggest that estradiol may selectively affect NPY-containing interneurons in the hippocampal formation in part through direct actions on septal cholinergic terminals that contact these interneurons.
Estradiol may also affect acetylcholine release by acting directly on cholinergic projections to the hippocampus. These projections originate in the basal forebrain. Indeed, lesions of the basal forebrain partially attenuate estrogen-mediated disinhibition of hippocampal pyramidal cells in female rats, demonstrating a key role for the basal forebrain in estrogen’s effects on the hippocampus101. In addition, estrogen effects on ChAT expression in the basal forebrain have been reported102, 103. Finally, estradiol activates similar signaling pathways in basal forebrain cholinergic and hippocampal neurons in vitro104, suggesting that estrogen may have synergistic effects on basal forebrain and hippocampal neurons that culminate in enhanced hippocampal-dependent learning and memory. Estradiol activation of these signaling pathways in the hippocampal formation will be discussed in the next section.
In conclusion, estrogen may influence not only excitatory NMDA and inhibitory GABAergic neurotransmission, but also the cholinergic system, in both the basal forebrain and its hippocampal afferents. These effects may be important for estrogen’s ability to enhance hippocampal-dependent learning and memory behavior.
3.3 Signaling Pathways
Not only can estrogens rapidly affect neuronal excitability, but they can also activate neuronal signaling pathways in neurons. Several of these signaling pathways have been implicated in estrogen-mediated structural and functional plasticity in the hippocampal formation, and they will be discussed in this section.
Akt
The PI3K/Akt pathway has been implicated in estrogen effects on synaptogenesis and spine number and morphology. Rapid estradiol activation of Akt activates downstream pathways leading to actin remodeling, filopodia growth, and translation of postsynaptic protein PSD-95 in the neuroblastoma cell line NG108-1563, 105. Immunoreactivity for pAkt is increased in proestrus and estradiol-replaced rats relative to ovariectomized, estrus, and diestrus animals106. In cultured midbrain and cortical neurons, estrogen rapidly activates Akt107, 108, suggesting that estrogen’s ability to rapidly activate Akt is a direct action of estrogen on neurons from multiple brain regions. In addition to its actions on protein translation that may promote translation of new spine and synaptic proteins upon estrogen stimulation, the Akt pathway plays a well-known role in cell survival, and has been implicated in estrogen’s neuroprotective actions109.
MAPK/ERK and CREB
Estradiol causes phosphorylation of the cyclic AMP response element binding protein (CREB) in both cultured neurons and in vivo in the rat85, 110–112. CREB phosphorylation has been implicated in estrogen-induced spine formation and upregulation of synaptic protein expression in primary cultured hippocampal neurons62, 111. In vivo, estradiol activation of CREB occurs in the CA1 region and also in the CA3112. This action is not surprising considering estrogen also activates CREB in neurons from the hippocampus110, 113, basal forebrain104, and cortex108, and it suggests that the CA3 may also play a role in estrogen’s effects on hippocampal function. In these neurons, CREB phosphorylation depends upon estrogen activation of mitogen-activated protein kinases (MAPK) 104, 108, 110, 113. Several recent studies demonstrate that estrogen activation of MAPK/CREB in hippocampal neurons is mediated by rapid calcium influx, within minutes of estrogen exposure114, 115. The mechanism of estrogen’s rapid effects on calcium influx is unknown. Estrogen activation of MAPKs and CREB may be crucial for its downstream enhancement of hippocampal learning and memory. Though this hypothesis has not yet been tested, MAPKs, or extracellular-signal regulated kinases (ERKs), play important roles in brain synaptic plasticity and memory that have recently been reviewed116.
Actin remodeling pathways
Dendritic spine formation and retraction in neurons necessitates actin remodeling117. Therefore, since estrogen increases dendritic spine density in cultured hippocampal neurons, it must affect actin remodeling in the dendrites of these neurons. Initial investigations on this topic were performed in our laboratory in the NG108-15 neuroblastoma cell line105. These cells express ERs alpha and beta and show increased filopodial density with estrogen treatment105. In these cultures, several hours of estradiol treatment stimulates phosphorylation of cofilin, an actin depolymerizing facor that is inactivated in the phosphorylated form. This estradiol-mediated cofilin inactivation requires the Rho-GTPase, Rac1, the upstream activator of Akt, PI3-kinase, and the actin-binding kinase that directly phosphorylates cofilin, LIM kinase-1. This suggests that by mediating cofilin phosphorylation, estradiol favors actin polymerization in NG108-15 cells, allowing for increased filopodia density. Continued work has shown that estradiol also mediates LIMK phosphorylation in vivo in the rat hippocampal CA1 region118. Estrogen-mediated LIMK phosphorylation could be important for the downstream increase in spine density and enhancement of hippocampus-dependent memory in vivo, as studies of a LIMK-1 knockout mouse have implicated LIMK in spine morphology and hippocampal function119. In summary, through activation of the PI3K/Akt pathway, estradiol activates actin polymerizing pathways previously implicated in dendritic spine formation in vivo, suggesting a mechanism for the estrogen-mediated increase in dendritic spine density.
Calcium/Calmodulin-Dependent Protein Kinases
The calcium/calmodulin-dependent protein kinase II (CamKII) plays a well-investigated role in synaptic plasticity and learning and memory120. A few recent studies have implicated CamKII in estrogen’s rapid effects on the hippocampus. In primary cultured hippocampal neurons, estrogen activation of CREB depends not only on MAPK, but also on CamKII113. In vivo, CamKII is activated in mouse hippocampal formation just one hour after peripheral estradiol injection121. Thus estrogen rapidly activates CamKII in vivo, and in vitro studies implicate this kinase in the downstream activation of pathways necessary for estradiol-induced dendritic spine formation. One potential CamKII target involved in estrogen effects in the hippocampus is the dendritic spine protein spinophilin, whose expression is increased with estrogen treatment. CamKII-mediated spinophilin phosphorylation results in spinophilin binding to actin and increases its synaptic membrane localization58. Perhaps estrogen activation of CamKII also causes spinophilin phosphorylation. This hypothesis, and the possible role of spinophilin phosphorylation and actin binding in estrogen effects on actin remodeling and spine formation, has yet to be investigated. Finally, one study reported that estradiol treatment increased the expression of another CamK, CamKIV, in the CA1 and CA3 of the hippocampus in ovariectomized rats112. CamKIV facilitates hippocampal synaptic plasticity by acting upstream of CREB phosphorylation122. The role of CamKIV in estradiol-induced CREB phosphorylation has not yet been investigated.
3.4 Neurotrophins
Neurotrophins mediate neuronal growth, survival, and plasticity in the central and peripheral nervous system123. These roles raise the possibility that neurotrophins may mediate some of estrogen’s neuroprotective, neurotrophic, and neuromodulatory functions. Indeed, many investigators have demonstrated estrogen effects on neurotrophin systems, primarily on the expression of neurotrophins and their receptors. This section will focus on estrogen actions on the NGF/TrkA and BDNF/TrkB neurotrophin/receptor systems in the hippocampus, and one of its projecting brain areas, the basal forebrain.
NGF/TrkA
Nerve growth factor (NGF) signals through a high-affinity interaction with its specific receptor tyrosine receptor kinase A (TrkA), as well as through a low-affinity interaction with the pan-neurotrophin receptor, p75 (P75NTR). The effects of estrogen that have been reported on NGF and TrkA protein and mRNA in the hippocampus and the basal forebrain are somewhat conflicting. One study compared levels of TrkA mRNA in the basal forebrain and NGF mRNA in the hippocampus across the estrous cycle and with acute hormone replacement in ovariectomized rats124. This work showed that TrkA mRNA in the basal forebrain fluctuates across the estrous cycle, with the highest levels on the morning of diestrus, and that TrkA mRNA increases 24 hours after estrogen administration. NGF mRNA showed no significant changes. In contrast, earlier work showed different effects of slightly longer estrogen administration (2 days to 2 weeks) in ovariectomized rats102. This regimen of estrogen replacement showed decreased NGF in the hippocampus and TrkA in the basal forebrain. Furthermore, another study showed an increase in hippocampal NGF protein in ovariectomized mice following estrogen administration125. Differences between these study designs, including species, route of hormone delivery, and time of hormone exposure make the different results difficult to interpret. One recent study showed that TrkA immunoreactivity fluctuates across the estrous cycle, although this group saw the highest level in estrus rats126. This study used conditions in which the TrkA immunoreactivity was largely observed in astrocytes127, suggesting that astrocytes may mediate estrogen effects on TrkA in the hippocampus.
BDNF/TrkB
Brain-derived neurotrophic factor (BDNF) signals through high-affinity interaction with its specific receptor, tyrosine kinase receptor B (TrkB), and low-affinity interaction with P75NTR. The numerous similarities between the effects of estrogen and BDNF on hippocampal physiology and behavior have recently been reviewed74, 92, 128, 129, and have led many investigators to hypothesize a role for BDNF in estrogen effects on this brain region. Exogenous estradiol administration to ovariectomized rats increases BDNF mRNA and protein expression in vivo hippocampus74, 112, 124, 130. In addition, levels of BDNF mRNA and protein fluctuate across the estrous cycle in female rats, with the highest levels of mRNA during late diestrus124, and the highest levels of protein during proestrus and the morning of estrus74. Activation of TrkB is involved in spatial memory formation in rats131, raising the plausible hypothesis that TrkB signaling may mediate the effects of E-induced BDNF expression on memory. Indeed, signaling through TrkB leads to Akt and LIMK activation123, 132, and postsynaptic knockout of TrkB decreases a mature subset of spines in the mouse hippocampal CA1 region133, suggesting that TrkB activation could mediate estrogen effects on cell signaling and spine maturation. Additionally, ultrastructural localization of full-length TrkB in rat hippocampus shows immunoreactivity in dendritic spines of pyramidal cells134. TrkB is therefore appropriately positioned to carry out local effects of estrogen on LIMK and Akt activation within dendritic spines of pyramidal cells.
A few reports provide evidence for TrkB signaling as a mediator of estrogen effects in the brain. In the hypothalamus of male rats, an estradiol-induced upregulation in TrkB is necessary for estradiol’s effects on axonal outgrowth135, 136. In the hippocampus, BDNF expression and pyramidal cell excitability fluctuate in concert with the rat estrous cycle, increasing during the high-estrogen phase of proestrus74. The proestrus phase hyperexcitability is blocked by exposure to a receptor tyrosine kinase inhibitor and mimicked by the addition of BDNF, suggesting that BDNF is necessary for the fluctuation of neuronal excitability across the rat estrous cycle74. Estrogen upregulation of BDNF expression, followed by increased BDNF signaling through TrkB, could therefore mediate estrogen-induced hyperexcitability in the hippocampus. To assess whether signaling through TrkB fluctuates with hippocampal BDNF levels across the estrous cycle of the female rat, we used an antibody raised against the phosphorylated TrkB receptor, pTrkB (Figure 2, Spencer J.L., unpublished). pTrkB immunoreactivity is found in pyramidal cell processes, and is eliminated by preadsorption of the antibody with a pTrkB blocking peptide. Immunoreactivity for pTrkB fluctuates across the estrous cycle. Peroxidase labeling and densitometry were conducted according to previously described methods, and data analyzed as an analysis of variance (ANOVA) with post-hoc comparisons106. Significant differences among proestrus, estrus, and diestrus rats were seen in the tip and hilus of the dentate gyrus, and the CA3 stratum radiatum, but not the CA3 stratum lucidum or CA1 stratum radiatum. Post-hoc comparisons revealed increased pTrkB immunoreactivity in proestrus compared to diestrus rats in these areas. These findings suggest that physiological fluctuations of gonadal hormones mediate fluctuations of TrkB activation in the dentate gyrus and CA3 stratum radiatum of the rat hippocampus, with the highest level of activation occurring during proestrus, when estrogen levels are highest. As expected, pTrkB density was highest during the phase of reportedly highest BDNF protein expression, proestrus. As BDNF expression is highest in the dentate and CA3 area of the hippocampus, the limitation of the observed effects on TrkB signaling to these areas is not surprising. Estrogen effects on dentate and CA3 neurons could indirectly impact upon the CA1 layer through Schaffer collateral projections to CA1 pyramidal cells.
Figure 2.
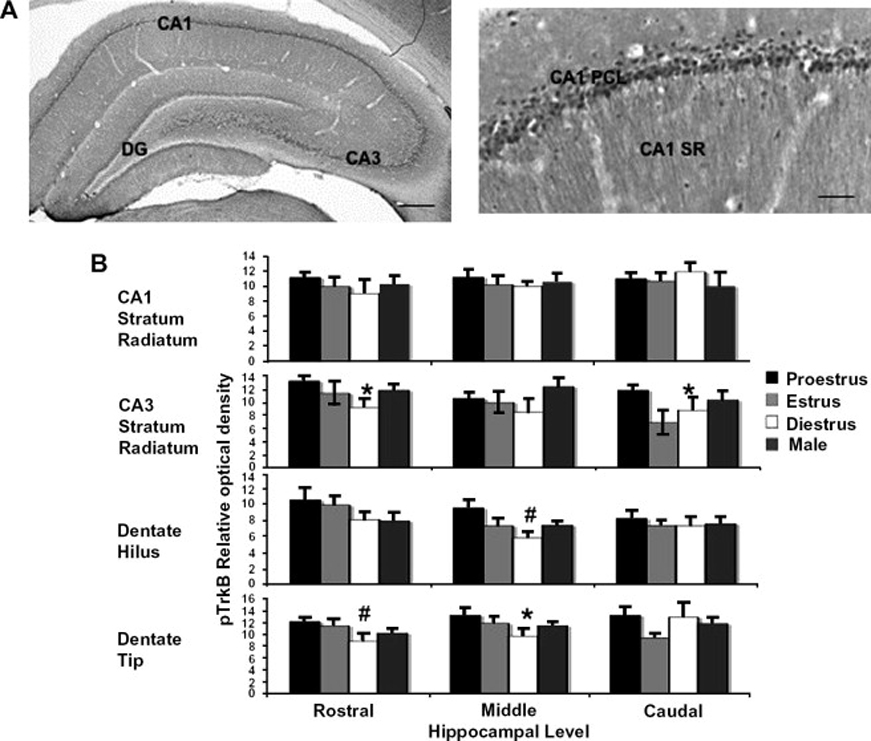
pTrkB immunoreactivity (IR) fluctuates across the estrous cycle in the dorsal hippocampal formation of the female rat. A, pTrkB IR in the rat dorsal hippocampal formation. The antibody raised in rabbits against pTrkB was a gift of Moses Chao. IR can be seen in principal cells and processes of the CA1 and CA3 regions of the hippocampus and dentate gyrus (DG). Preadsorption of the antibody with pTrkB blocking peptide eliminates all staining (not shown). B, Matched sections from three different levels of dorsal hippocampus were incubated with rabbit anti-pTrkB at a concentration 1:3000 for 72 hours, labeled by the avidin-biotin complex peroxidase method, and analyzed by densitometry with normalization to corpus callosum staining. Proestrus, n = 7, Estrus, n = 4, Diestrus, n = 4. *p<0.05, #p<0.01 relative to diestrus. Sections correspond to Plates 29 (rostral), 33 (middle), and 36 (caudal) of Paxinos and Watson (Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego, 1998). Scale Bars, 100 µm. DG, dentate gyrus; PCL, pyramidal cell layer; SR, stratum radiatum.
Possible mechanisms by which estradiol may interact with the BDNF/TrkB system will be further explored in the concluding section of this review, as an example of the convergence of various mechanisms of estrogen signaling to affect hippocampal synaptic maturation and plasticity.
3.5 Opioids
Endogenous opioid peptides and receptors are expressed in the hippocampus and dentate gyrus137. Opioid peptides include enkephalins, which bind to mu and delta opiod receptors, and dynorphins, which bind to kappa opioid receptors. A few reports have examined the effects of exogenous estrogens and the estrous cycle on opioid receptor binding and peptide expression. The evidence gathered so far suggests that estradiol may enhance signaling through mu opiod receptors (MOR)137. For example, estradiol increases MOR binding in the hippocampus of ovariectomized rats138. Estradiol treatment also increases MOR-immunoreactive particles in dendrites of parvalbumin-containing interneurons in the dentate gyrus, where MORs are abundant139. Immunoreactivity for the leu-enkephalin ligand for these receptors increases in the dentate hilus and hippocampal CA3 in proestrus compared to estrus rats, and in ovariectomized rats 24 hours after estradiol injection140, 141. Because MORs have largely disinhibitory effects in the hippocampus137, estrogen’s upregulation of MOR and enkephalin expression may contribute to the observed enhancement of excitatory neurotransmission in the hippocampus.
The investigation of the interaction between opiods and estrogens in the hippocampus is young, but the results cited above suggest that endogenous gonadal steroids and opioid systems interact in the hippocampus. If these interactions have real physiological and behavioral consequences, there may also be important interactions between pharmacological estrogens and exogenous opioids, the opiates, which are widely used as pain relievers and drugs of abuse.
3.6 Neurogenesis
Given its neurotrophic and neuroprotective effects on neurons in vivo and in cell culture described above, several investigators have hypothesized that estrogens may influence the processes by which new neurons are born, proliferate, and survive in the subgranular zone and dentate gyrus of the mammalian brain. Indeed, estradiol increases proliferation of neurons in the adult female rat142, and the survival of new neurons in the female vole143. These effects may be rapid, as estradiol increases proliferation within four hours of administration in the rat143. The effects of gonadal steroids on neurogenesis in the rat and vole brain have recently been reviewed144. Although correlational links between neurogenesis in this brain region and hippocampal-dependent learning have been demonstrated, their relationship, if any, is still unclear145. One recent report suggested that the effect of estradiol on neurogenesis may be species-specific. Estradiol treatment did not affect neurogenesis in mice as it did in rats146, similar to its effect on dendritic spine density. This suggests that estradiol effects on the rate of neurogenesis, including the proliferation and survival of new neurons, are not necessary for the estradiol-induced enhancement of hippocampal learning that is seen in both mice and rats. Whether estradiol influences more subtle aspects of neurogenesis in the mouse hippocampal formation, such as the integration of new neurons into the hippocampal network, remains to be investigated.
Summary
Several of estrogen’s effects in the hippocampal formation may account for the hormone’s enhancing effects on hippocampal learning and memory. Estradiol rapidly activates signaling pathways leading to synaptic protein translation and modification, actin remodeling, and dendritic spine and synapse formation. In addition, estradiol rapidly induces calcium currents through L-type calcium channels, increases NMDA-mediated excitation, and decreases GABA-mediated inhibition on hippocampal pyramidal cells. Estrogen also influences the expression of multiple ligands and their receptors in the hippocampus, including neurotrophins and endogenous opioid peptides. Finally, estrogen may directly activate signaling pathways, neurotrophin and neurotransmitter systems in the basal forebrain, which sends cholinergic projections to the hippocampus to indirectly impact hippocampal function. This complex picture of estrogen’s actions in the hippocampus has emerged from extensive in vitro and parallel in vivo studies, but numerous questions remain. For example, few studies have attempted to integrate estrogen effects on signaling pathways, neurotransmitter and neurotrophin systems into an integrative model of estrogen’s impact on hippocampal function. Additionally, how estrogen activates signaling pathways and the expression of specific proteins in neurons is as yet unclear.
4. Estrogen receptors in the hippocampal formation
Numerous studies have provided evidence that estrogen effects on the hippocampal formation depend on estrogen receptors. Both “genomic” and “nongenomic” types of signaling through the ER have been reported147, 148. In the genomic or classical mode of estrogen action, nuclear estrogen-receptor complexes bind estrogen response elements (EREs) in the DNA to influence gene transcription. In nongenomic hormone signaling, extranuclear estrogen receptors activate cell-signaling pathways upon estrogen binding. The evidence gathered thus far points to both genomic and non-genomic effects of estrogen mediated by these receptors in a number of hippocampal cell types shown to mediate estrogen effects. These include excitatory CA1 pyramidal cells, inhibitory interneurons, and presynaptic cholinergic and GABAergic terminals.
4.1 Role of ER subtypes
Both types of classical estrogen receptor, ER alpha and ER beta, are expressed in the rodent and primate hippocampal formation149–152. In different studies, knockout of either the ER alpha or ER beta gene impaired the performance of female mice on hippocampal-dependent learning tasks including inhibitory avoidance and spatial navigation153–155. Knockout of ER beta impairs synaptic plasticity in the CA1 region of female mice154. Finally, both ER alpha and ER beta have been implicated in steroid-sensitive hippocampal neurogenesis156 and spinogenesis157, 158, and pharmacological ER antagonism blocks estrogen-mediated pyramidal cell disinhibition and spinogenesis in vivo101, 159.
Using two different methods in mice and rats, members of our laboratory have investigated the role of ER alpha and ER beta in estradiol upregulation of synaptic proteins in the hippocampal CA1 region. Figure 3 shows spinophilin-immunoreactive puncta in ER alpha-knockout (ERKO) and ER beta-knockout (BERKO) mice in the CA1 stratum radiatum of the hippocampus (Romeo R.D. et al., unpublished). Young female mice were ovariectomized and two weeks later treated with two injections of 5 µg estradiol benzoate or oil vehicle 24 hours apart and sacrificed 24 hours after the last injection. Mice were then perfused with 4% paraformaldehyde and tissue sections stained for spinophilin using silver-enhanced immunocytochemistry as previously described14, 54. The rabbit anti-spinophilin antibody was a gift of Patrick Allen at Yale University. Data analyzed by two-way Analysis of Variance (ANOVA) showed only an effect of treatment in the BERKO mice, with E-treated mice having a higher density of spinophilin immunoreactive (IR) puncta than vehicle-treated mice. This indicates that developmental knockout of ER beta in mice does not mitigate estradiol’s ability to upregulate spinophilin expression. In the ERKO mice, two-way ANOVA revealed a significant effect of both treatment and genotype, and a significant treatment X genotype interaction. Estradiol increased spinophilin-IR only in the wild type mice, with no effect in the ERKOs. This indicates that in mice, developmental knockout of ER alpha disrupts estradiol upregulation of spinophilin in the CA1 stratum radiatum, suggesting that this ER mediates estradiol’s enhancement of spinophilin expression.
Figure 3.
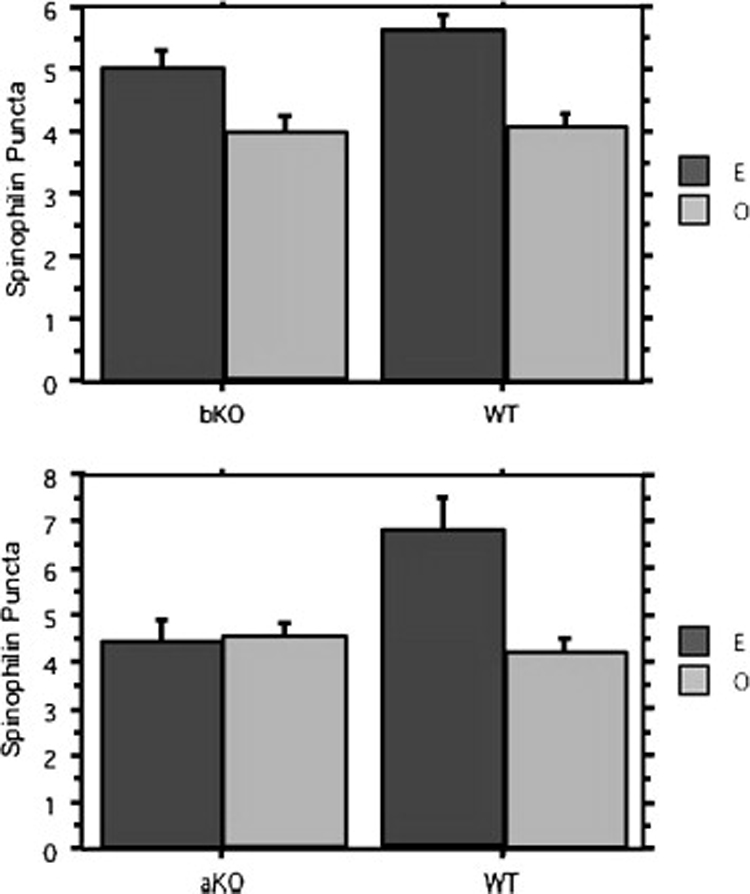
Estradiol increases spinophilin expression in ovariectomized ER beta knockout mice, but not ER alpha knockout (KO) mice. Ovariectomized female mice were treated with 5 µg estradiol benzoate or oil vehicle for 48 hours and then perfused with 4% paraformaldehyde. Tissue sections stained were for spinophilin using silver-enhanced immunocytochemistry. Data analyzed by two-way Analysis of Variance (ANOVA) showed only an effect of treatment in the ER beta KO mice, with E-treated mice having a higher density of spinophilin immunoreactive puncta than vehicle-treated mice. In the ER alpha KO mice, two-way ANOVA revealed a significant effect of both treatment and genotype, and a significant treatment X genotype interaction. bKO, n = 15 (8 O, 7 E); WT, n = 10 (6 O, 4 E). aKO, n = 11 (6 O, 5 E); WT, n = 7 (3 O, 4 E). O, oil; E, estradiol benzoate; aKO, ER alpha KO; bKO, ER beta KO; WT, wild type; IR, immunoreactive puncta. *p<0.05 relative to O.
In rats, without the option of a developmental gene knockout, members of our laboratory used ER alpha- and ER beta-specific pharmacological agonists to investigate the role of ER subtypes in estradiol regulation of synaptic protein expression (E.M. Waters, unpublished). Young adult female rats were ovariectomized and after four days of recovery, they were treated with subcutaneous injections of either oil vehicle, 10 µg estradiol benzoate, or 1 mg/kg of an ER alpha selective agonist, 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)tris-phenol (PPT), or ER beta selective agonist, 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN). Two injections were given 24 hours apart, and rats were sacrificed 48 hours after the last injection. Brains were collected fresh frozen and tissue collected from the CA1 region using a 400 µm micropuncher on a freezing microtome. Tissue lysates were separated by SDS-PAGE and synaptic proteins were detected by Western Blotting using antibodies to synaptophysin (anti-mouse, Sigma Aldrich) and spinophilin (anti-rabbit, Upstate Biotechnologies). The optical density of the bands was compared by ANOVA. Estradiol, DPN, and PPT upregulated the expression of both synaptic proteins, suggesting that in this model, both ERs alpha and beta are sufficient for estradiol upregulation of synaptic protein expression.
Several differences between the studies pictured in Figure 3 and Figure 4 may explain the discrepant results. First, steroid hormones have organizing effects in the brain during development160, and developmental knockout of the estrogen receptor could disrupt these effects. In this case, studies of the adult knockout animal such as the mouse study in Figure 3 may lead to misled conclusions about the role of the missing receptor in the adult, intact animal. Second, species differences between estradiol effects in the mouse and rat hippocampus were discussed above. The results shown in Figure 3 and Figure 4 may indicate that ER subtypes differentially regulate estradiol effects in mouse and rat hippocampus. Third, in the absence of developmental organizing effects, the presence or absence of ER beta during exposure to an ER alpha agonist may influence the response to the agonist, and vice versa. This is possible given the effects of ER alpha-beta dimmers and ligand-independent hormone receptor agonists, and may contribute to the differential results seen in in Figure 3 and Figure 4. Finally, important differences between the experimental protocols, aside from species, include time between ovariectomy and estradiol administration (10 days versus 4 days), time of estradiol treatment (48 hours versus 72 hours), and protein detection method (immunohistochemistry versus Western Blotting). Members of our laboratory are currently investigating these possibilities.
Figure 4.
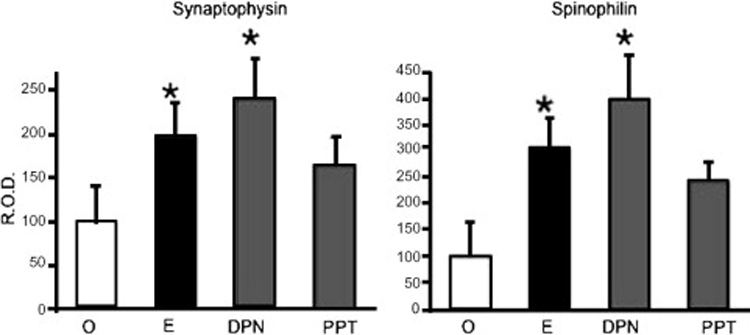
ER alpha and beta agonists increase synaptic protein expression in ovariectomized female rats. Ovariectomized female rats were treated for 72 hours with oil vehicle, 10 µg estradiol benzoate, or 5 µg of either PPT (ER alpha agonist) or DPN (ER beta agonist). Tissue lysates from CA1 micropunches were separated by SDS-PAGE and synaptophysin and spinophilin were detected by Western Blotting. The optical density of synaptic protein bands normalized to actin expression was expressed as a percent of the control group. n = 3 animals per group. R.O.D., relative obtical density; EB, estradiol benzoate; O, oil vehicle. *p<0.05 compared to oil-treated group.
Despite the evidence that estrogen acts through estrogen receptors to influence hippocampal function, much about the mechanism of these actions remains to be explored. The sections below will review the evidence for estrogen effects in the hippocampus through classical genomic and rapid nongenomic mechanisms of estrogen acting through hormone receptors. The final section will use the BDNF neurotrophin system to illustrate several plausible mechanisms through which estrogen may achieve one downstream effect. As just one of many downstream systems influenced by estrogen, the BDNF example will serve to highlight the breadth of current knowledge about the actions of steroid hormones, and to underscore the enormous amount of work that remains to clearly define the mechanisms by which estrogen acts on downstream systems to influence hippocampal function.
4.2 Genomic effects
If the actions of estrogens on the hippocampus described above occur through the classical action of hormone on hippocampal cells, then these cells must express nuclear estrogen receptors. Work by our laboratories has shown that nuclear estradiol binding and ER alpha-immunoreactivity is found in scattered inhibitory GABAergic interneurons in the hippocampus161, 162. This suggests that the estrogen modulation of GABAergic neurotransmission described above may take place through classical action of estrogens on gene transcription. As noted above, estrogen effects on transcription of the GABA-synthesizing GAD enzymes have been demonstrated87, 88, although no estrogen response elements have been described in the promoter of these genes. Nuclear ER beta also localizes to astrocytes in the hippocampus163. The role of astrocytes in hippocampal estrogen effects has been poorly studied, but one report of fluctuation in astrocytic TrkA expression across the rat estrous cycle126 indicates that astrocytes may be sensitive to ovarian hormones. Finally, nuclear estradiol binding has been demonstrated in the basal forebrain, which projects to the hippocampal formation and plays an important role in estradiol effects on hippocampal function, as described above164. Despite the above evidence, to our knowledge no group has provided strong evidence for the functionality of these nuclear hippocampal and basal forebrain receptors, or for their importance in estradiol effects on hippocampal function.
4.3 Nongenomic Effects
Recent work has identified nonclassical estrogen signaling through classical estrogen receptors located outside the nucleus148, 165. In cells from various different tissues, these extranuclear ERs can be found at the plasma membrane, in mitochondria, and in the cytoplasm148. They act by coupling to G proteins, growth factor receptors, and intracellular kinases to initiate both rapid hormone actions and more delayed effects on gene transcription through the activation of signal transduction cascades108, 148. In the rodent hippocampal formation, estradiol binding and ultrastructural studies have identified extranuclear ERs in neurons, where they are well positioned to play direct roles in some already described estrogen effects162, 166, 167, 150, 168–170. For example, ERs can be found in axons and axon terminals making both inhibitory- and excitatory-type synapses168, 170. These ERs, by activating signal transduction cascades leading to cytoskeletal rearrangements or protein translation, may directly affect presynaptic function by regulating neurotransmitter release or synaptic protein expression. ERs are also located in dendritic spines, sometimes affiliated with the spine apparatus168, 170, where they could similarly activate signal pathways to affect neurotransmitter receptor expression, actin remodeling, postsynaptic protein expression, and electrophysiological properties of the postsynaptic membrane. Extranuclear ERs alpha and beta have similar, yet distinct distributions in the hippocampal formation162. Extranuclear ER beta is more extensively found than extranuclear ER alpha. Extranuclear ER alpha is found mostly in axons and axon terminals and to a lesser degree in dendritic spines, while extranuclear ER beta is mainly affiliated with the membrane of somata and dendrites of principle cells. These distinct distributions may reflect different roles of ER subtypes in the maintenance of hippocampal function.
Several elegant electron microscopy studies using double immunolabeling and comparisons before and after estrogen treatment have provided further evidence for the role of these hippocampal extranuclear receptors in estrogen effects. For example, ultrastructural relationships between ERs and components of estrogen-sensitive systems suggest local modulation of these systems by extranuclear ERs. ER alpha may directly regulate cholinergic neurotransmission, as presynaptic terminals expressing the VAChT are affiliated with both presynaptic and postsynaptic ER alpha99. ERs may also directly modulate opioid signaling, as ER alpha and MORs both localize to cholinergic terminals in the hippocampus171, and ER beta colocalizes with enkephalin and dynorphin peptide at mossy fiber terminals137. Furthermore, estradiol may modulate the localization of its own receptor, as estradiol treatment increases the synaptic localization of ER alpha in ovariectomized rats30. Finally, estrogen effects on GABAergic interneurons may also be mediated through extranuclear ER alpha. ER alpha is associated with presynaptic vesicles in NPY-expressing interneurons94 in the CA1 region that are mobilized towards the presynaptic membrane upon estradiol treatment.
Several pieces of evidence suggest that nongenomic estrogen signaling partially mediates estrogen enhancement of hippocampal function. First, many estrogen effects on signal transduction, synaptic potentiation, and calcium influx in hippocampal neurons occur too quickly for classical genomic effects on gene transcription. Indeed, estrogen enhancement of spatial memory can be achieved by administration immediately, but not two hours, after training, suggesting a rapid effect of the hormone on memory consolidation during this narrow two-hour time window following training5, 16. Second, the estrogen-mediated increase in PSD-95, spinophilin, and MOR protein expression is post-transcriptional, suggesting estrogen effects on protein translation54, 63, 138, 172. Translational control is a well-known mechanism of rapid and local synaptic plasticity in neurons173, and its role in estrogen effects should be more thoroughly investigated. Finally, compounds thought to be antagonists at nuclear estrogen receptors in the brain have in fact shown some estrogenic properties. For example, the selective estrogen receptor modulators (SERMs) CI628, tamoxifen, and raloxifene, have estrogenic activity on expression and/or activity of the ChAT enzyme in the brain174–176. In addition, the estrogen receptor antagonist ICI 182,780 acts as an agonist to enhance spatial learning when infused directly into the hippocampus of female rats18. Another study showed agonistic activity of this compound on rapid calcium influx in hippocampal neurons177. Finally, low doses of 17-alpha estradiol that have no effect on lipid metabolism or uterine weight have been shown to rapidly enhance spatial memory and increase CA1 spine synapse density in ovariectomized rats, within four hours of subcutaneous injection178, 179. Although still inconclusive, this evidence suggests that these compounds act through mechanisms other than the classical nuclear hormone effects on gene transcription to achieve these estrogen agonistic effects in the brain. In particular, the rapidity of the effects of ICI 182,780 and 17-alpha estradiol support this hypothesis.
Aside from classical ERs, the role of novel membrane ERs such as the “ER-X,180” and the G-protein receptor, GPR30181, in estrogen effects on the hippocampus remains to be investigated. Finally, which G proteins and growth factor receptors couple to classical membrane ERs in the hippocampus is unknown. Investigation of these ER partners will be an important step in delineating the role of nongenomic signaling in estrogen’s effects on the hippocampal formation.
Multiple Mechanisms
Most likely, estrogen alters hippocampal function through a combination of nongenomic and genomic effects on different brain regions, subregions, and cell types. Different effects may require nongenomic activation of actin remodeling pathways, nongenomic activation of protein translation, or classical nuclear hormone-dependent gene transcription. This raises the possibility that estrogen effects in the hippocampus might be pharmacologically separable through the use of selective estrogen response modulators. 17-alpha estradiol may be one such compound. This compound acts as an agonist for only some estrogen effects in the hippocampus. As described above, 45 µg/kg subcutaneously injected 17-alpha estradiol increases CA1 spine synapse density in rats, with no effect on lipid metabolism or uterine weight. To determine whether 17-alpha estradiol also has estrogenic effects on hippocampal synaptic protein expression, members of our laboratory delivered two injections of 10 µg estradiol benzoate, 15 or 45 µg 17-alpha estradiol, or oil vehicle by subcutaneous injection to ovariectomized rats. Ovariectomy, hormone administration, tissue collection and Western Blotting, densitometry and data analysis were conducted as described above for Figure 4. Figure 5 shows the expression of the synaptic proteins spinophilin and synaptophysin, as well as syntaxin (ant-mouse, Sigma Aldrich) and PSD-95 (anti-mouse, Sigma Aldrich) in these animals, measured as optical density. Although estradiol increased the expression of all four of these proteins as previously reported, neither the low nor the high dose of 17-alpha estradiol affected synaptic protein expression. Thus 17-alpha-estradiol, despite its ability to rapidly increase spine synapse density within 4.5 hours of administration, does not increase synaptic protein expression in ovariectomized female rats. This suggests that either the effect of 17-alpha estradiol is transient, as opposed to the more sustained effect of the 17-beta isomer, or that 17-alpha estradiol initiates actin remodeling and spine formation in the absence of any effect on synaptic protein expression. The dissociation between estrogenic effects on spine formation and synaptic protein expression is striking, and it implies that different mechanisms mediate these downstream effects of estrogen. Because the effects of 17-alpha estradiol on spine formation are rapid (within 4.5 hours in vivo), this compound may specifically activate nongenomic estrogen signaling. This hypothesis remains to be tested, but the current findings suggest that multiple mechanisms of hormone action, which can be dissociated pharmacologically, mediate estrogen effects on hippocampal function.
Figure 5.
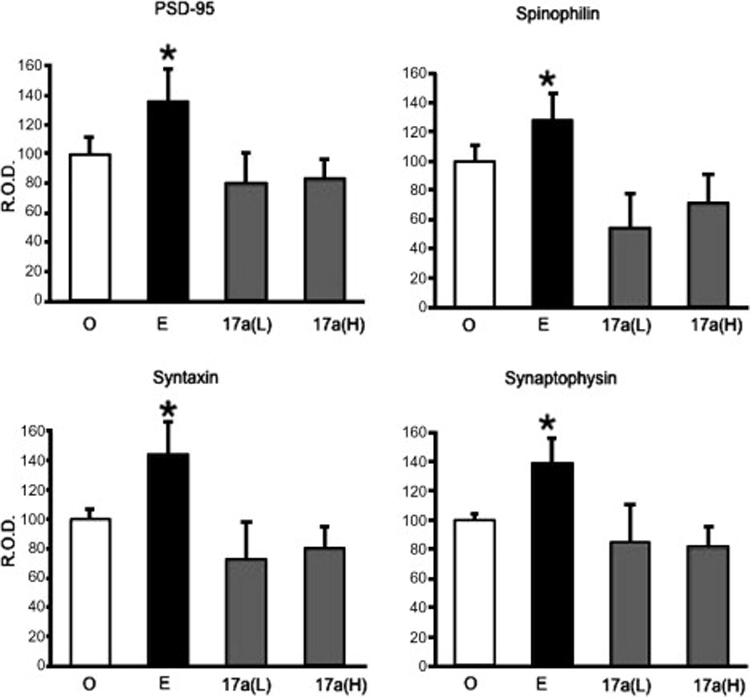
17-alpha estradiol does not increase synaptic protein expression in ovariectomized female rats. Rats were treated with 72 hours of 10 µg estradiol benzoate, 15 or 45 µg 17-alpha estradiol, or oil vehicle. Western Blots from punches of hippocampal CA1 area for spinophilin, synaptophysin, syntaxin, and PSD-95 were conducted. Optical density was expressed as a percent of the control (oil) group and normalized to actin expression. O, n = 4; E, n = 4; 17a(L), n = 3; 17a(H), n = 4. O, Oil; E, estradiol benzoate; 17a(L), 17-alpha estradiol, 15 µg; 17a(H), 17-alpha estradiol, 45 µg; R.O.D., relative optical density. *p<0.05 compared to oil.
5. Mechanism of estrogen-BDNF interaction in the hippocampus
Much about the mechanisms by which estrogen affects the expression of specific proteins and anatomically distinct synapse formation in the hippocampus remains to be elucidated. However, the extensive literature on basic hormone biology enables the development of testable hypotheses regarding these mechanisms. As an example, three mechanisms by which estrogen may influence the BDNF/TrkB system in the hippocampus are outlined below (Figure 6). They include nuclear estrogen receptor induction of BDNF expression, extranuclear estrogen receptor induction of BDNF expression, and estrogen interaction with the TrkB receptor independent of BDNF.
Figure 6.
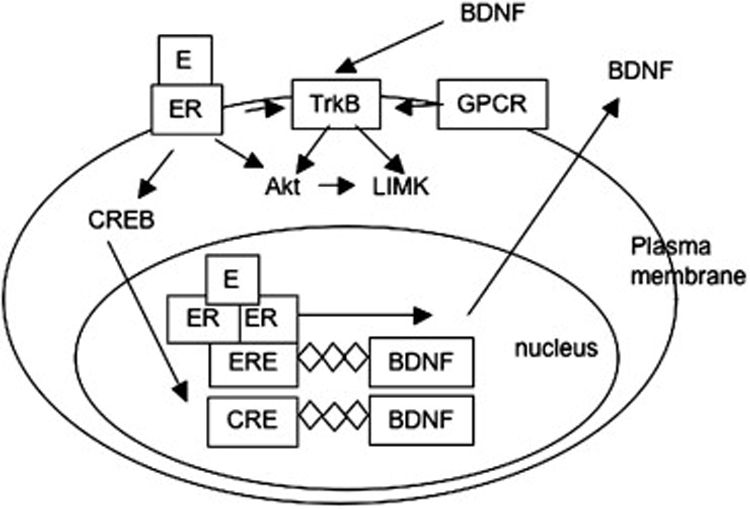
Estrogen may influence signaling through the TrkB receptor by at least three different mechanisms. Estrogen may induce BDNF transcription by classical action of nuclear hormone receptor through an estrogen response element (ERE) in the BDNF promoter. Alternatively, estrogen actions at extranuclear estrogen receptors (ER) may activate signaling pathways leading to phosphorylation of the CREB protein, leading to transcription through a cAMP response element (CRE) in the BDNF promoter. Finally, estrogen activation at extranuclear ERs may transactivate the TrkB receptor, possibly by coupling to G-protein signaling.
1. Nuclear estrogen receptors influence BDNF gene expression
As discussed above, ER alpha is found in selected interneurons in the pyramidal cell layer of the rat hippocampus. A putative estrogen response element (ERE) has been identified in the rat BDNF gene182 whose sequence is preserved in mouse and human that binds estrogen receptor-ligand complexes in vitro. Classical genomic hormone signaling through nuclear ER alpha could in this way activate transcription of the BDNF gene in ER alpha-expressing hippocampal interneurons. Although the functionality of this ERE has not been demonstrated, estrogen induction of BDNF mRNA in the hippocampus, cerebral cortex, and olfactory bulb has been reported74, 112, 124, 130,182.
2. Extranuclear estrogen receptors mediate BDNF gene expression
Estrogen induction of BDNF mRNA could also occur via the actions of the transcription factor CREB. CREB is phosphorylated by estradiol in vitro and in vivo in neurons104, 113 and acts at CRE sites in DNA, one of which is contained in the BDNF promoter region183. Nongenomic estrogen activation of CREB could therefore activate BDNF gene transcription via its CRE site. The operation of this mechanism could be investigated by observing the effects of disrupting the CREB pathway on estradiol induction of BDNF.
3. Estrogen co-opts BDNF signaling pathways to affect synaptic plasticity
Finally, estrogen could co-opt BDNF signaling pathways to enhance synaptic plasticity without mediation of the BDNF neurotrophin. Estrogen could accomplish this by transactivating the TrkB receptor independent of BDNF. Transactivation of TrkB receptors by G-protein coupled receptors has been demonstrated184, and a novel G-protein coupled estrogen receptor has recently been identified181 that could mediate TrkB transactivation by estrogen. Alternatively, membrane estrogen receptors may be coupled to TrkB as they are coupled to other growth factor receptor tyrosine kinases including the IGF-1 and EGF receptors148. Finally, extranuclear estrogen receptors could interact with tyrosine kinase or G-protein receptors distinct from TrkB, but leading to downstream activation of similar signaling pathways.
Conclusions
Major research problems surrounding estrogen effects on hippocampal function include the relative importance of nongenomic and/or genomic mechanisms, and the relative contribution of different hippocampal cell types and projecting brain areas. The continued investigation of these questions will provide insight not only into the mechanisms of steroid hormone effects on the brain, but also into which pathways, cell types and brain regions are the most responsive to pharmacological manipulation of hippocampus-dependent learning and memory. Although this review focuses on estrogen effects in young, healthy brains, extending these findings to aging or damaged brain tissue is of course crucial185. By refining the knowledge of hormone actions in the healthy and unhealthy brain, targeted therapies can be developed for cognitive impairments at all stages of health and aging. The development of testable hypotheses such as those presented above for BDNF, and the careful design of experiments aimed to test those hypotheses, will bring us ever closer to understanding the multitude of estrogen effects that converge to enhance hippocampal-dependent learning and memory.
Acknowledgments
This work was supported by NIH grants DA 08259, HL18974 (T.A.M.), and NS 007080 (B.S.M.). The authors would like to acknowledge Brad Rosenberg for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson RJ. An Introduction to Behavioral Endocrinology. sunderland, MA: sinauer associates; 2005. [Google Scholar]
- 2.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 6.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman KF, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen's effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 9.Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- 10.Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 12.Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Li C, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 16.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 17.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- 20.Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27:835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- 23.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 24.Dreher JC, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006 doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- 27.Sherwin BB, Tulandi T. "Add-back" estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 28.Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 30.Adams MM, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki S, et al. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 34.Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 38.Craig MC, Maki PM, Murphy DG. The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 2005;4:190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- 39.Rapp SR, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 40.Shumaker SA, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 41.Prestwood KM, Unson C, Kulldorff M, Cushman M. The effect of different doses of micronized 17beta-estradiol on C-reactive protein, interleukin-6, and lipids in older women. J Gerontol A Biol Sci Med Sci. 2004;59:827–832. doi: 10.1093/gerona/59.8.m827. [DOI] [PubMed] [Google Scholar]
- 42.Chen S, Nilsen J, Brinton RD. Dose And Temporal Pattern Of Estrogen Exposure Determines Neuroprotective Outcome In Hippocampal Neurons: Therapeutic Implications. Endocrinology. 2006 doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol's effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- 45.Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M. Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci Lett. 2005;379:52–54. doi: 10.1016/j.neulet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 51.Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Lee SJ, et al. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004;127:983–988. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 55.Brake WG, et al. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- 56.Waters EM, Spencer JL, Bloss EB, McEwen B. Society for Neuroscience Annual Meeting. Atlanta, GA: 2006. [Google Scholar]
- 57.Feng J, et al. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grossman SD, et al. Spinophilin is phosphorylated by Ca2+/calmodulin-dependent protein kinase II resulting in regulation of its binding to F-actin. J Neurochem. 2004;90:317–324. doi: 10.1111/j.1471-4159.2004.02491.x. [DOI] [PubMed] [Google Scholar]
- 59.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 60.Beique JC, et al. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 63.Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klintsova A, Levy WB, Desmond NL. Astrocytic volume fluctuates in the hippocampal CA1 region across the estrous cycle. Brain Res. 1995;690:269–274. doi: 10.1016/0006-8993(95)00642-4. [DOI] [PubMed] [Google Scholar]
- 65.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 66.Sortino MA, et al. Glia mediates the neuroprotective action of estradiol on beta-amyloid-induced neuronal death. Endocrinology. 2004;145:5080–5086. doi: 10.1210/en.2004-0973. [DOI] [PubMed] [Google Scholar]
- 67.Hao J, et al. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 68.Choi JM, et al. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–4738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- 69.Terasawa E, Timiras PS. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- 70.Kim MT, et al. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 71.Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- 73.Foy MR, et al. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 74.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- 76.Good M, Day M, Muir JL. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur J Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 77.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 78.Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology. 2001;25:242–257. doi: 10.1016/S0893-133X(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 79.Cyr M, et al. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- 80.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- 81.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- 84.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 1999;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- 86.Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weiland NG. Glutamic acid decarboxylase messenger ribonucleic acid is regulated by estradiol and progesterone in the hippocampus. Endocrinology. 1992;131:2697–2702. doi: 10.1210/endo.131.6.1446611. [DOI] [PubMed] [Google Scholar]
- 89.Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: a potential role for neuropeptide Y. Neuroscience. 2005;136:357–369. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 91.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharfman HE, Maclusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006 doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakamura NH, Akama KT, Yuen GS, McEwen BS. Thinking outside the pyramidal cell: unexplored contributions of interneurons and neuropeptide Y to estrogen-induced synapse formation in the hippocampus. Rev Neurosci. 2007;18:1–13. doi: 10.1515/revneuro.2007.18.1.1. [DOI] [PubMed] [Google Scholar]
- 94.Ledoux VA, Woolley CS. Evidence that disinhibition is associated with a decrease in number of vesicles available for release at inhibitory synapses. J Neurosci. 2005;25:971–976. doi: 10.1523/JNEUROSCI.3489-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 1975;86:293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- 96.Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–322. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 99.Towart LA, et al. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- 100.Dougherty KD, Milner TA. Cholinergic septal afferent terminals preferentially contact neuropeptide Y-containing interneurons compared to parvalbumin-containing interneurons in the rat dentate gyrus. J Neurosci. 1999;19:10140–10152. doi: 10.1523/JNEUROSCI.19-22-10140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 103.Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- 104.Szego EM, et al. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuen G. Laboratory of Neuroendocrinology. Vol. 138. New York: The Rockefeller University; 2006. [Google Scholar]
- 106.Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ivanova T, Mendez P, Garcia-Segura LM, Beyer C. Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. J Neuroendocrinol. 2002;14:73–79. doi: 10.1046/j.0007-1331.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 108.Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du B, et al. Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J Endocrinol. 2004;183:605–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- 110.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 111.Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci U S A. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee SJ, et al. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 114.Zheng FF, Wu RC, Smith CL, O'Malley BW. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol. 2005;25:8273–8284. doi: 10.1128/MCB.25.18.8273-8284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 116.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 117.Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- 118.Yildrim M, et al. Society for Neuroscience Annual Meeting. Washington, D.C.: 2005. [Google Scholar]
- 119.Meng Y, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 120.Colbran RJ. Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J Neurosci. 2004;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sawai T, et al. Estrogen induces a rapid increase of calcium-calmodulin-dependent protein kinase II activity in the hippocampus. Brain Res. 2002;950:308–311. doi: 10.1016/s0006-8993(02)03186-4. [DOI] [PubMed] [Google Scholar]
- 122.Minichiello L, et al. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- 123.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 124.Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;810:294. doi: 10.1016/s0006-8993(98)00945-7. [DOI] [PubMed] [Google Scholar]
- 125.Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCarthy JB, Barker-Gibb AL, Alves SE, Milner TA. TrkA immunoreactive astrocytes in dendritic fields of the hippocampal formation across estrous. Glia. 2002;38:36–44. doi: 10.1002/glia.10060. [DOI] [PubMed] [Google Scholar]
- 127.Barker-Gibb AL, Dougherty KD, Einheber S, Drake CT, Milner TA. Hippocampal tyrosine kinase A receptors are restricted primarily to presynaptic vesicle clusters. J Comp Neurol. 2001;430:182–199. doi: 10.1002/1096-9861(20010205)430:2<182::aid-cne1024>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 128.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: Implications for neurodegenerative diseases. Front Neuroendocrinol. 2006 doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 130.Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- 131.Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem. 2003;10:108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakravarthy S, et al. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proc Natl Acad Sci U S A. 2006;103:1071–1076. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carrer HF, Cambiasso MJ, Brito V, Gorosito S. Neurotrophic factors and estradiol interact to control axogenic growth in hypothalamic neurons. Ann N Y Acad Sci. 2003;1007:306–316. doi: 10.1196/annals.1286.029. [DOI] [PubMed] [Google Scholar]
- 136.Brito VI, Carrer HF, Cambiasso MJ. Inhibition of tyrosine kinase receptor type B synthesis blocks axogenic effect of estradiol on rat hypothalamic neurones in vitro. Eur J Neurosci. 2004;20:331–337. doi: 10.1111/j.1460-9568.2004.03485.x. [DOI] [PubMed] [Google Scholar]
- 137.Drake CT, Chavkin CI, Milner TA. In: Progress in Brain Research. Scharfman H, editor. Elsevier B.V.: 2007. pp. 245–266. [DOI] [PubMed] [Google Scholar]
- 138.Piva F, et al. Effects of steroids on the brain opioid system. J Steroid Biochem Mol Biol. 1995;53:343–348. doi: 10.1016/0960-0760(95)00072-8. [DOI] [PubMed] [Google Scholar]
- 139.Torres-Reveron A, Williams TJ, Prasad P, Drake CT, Milner TA. Society for Neuroscience Annual Meeting. San Diego, CA: 2007. [Google Scholar]
- 140.Torres-Reveron A, Khalid S, Drake CT, Milner TA. International Narcotics Research Conference; St. Paul, MN. 2006. [Google Scholar]
- 141.Torres-Reveron A, Khalid S, Drake CT, Milner TA. Society for Neuroscience Annual Meeting. Atlanta, GA: 2006. [Google Scholar]
- 142.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 143.Ormerod BK, Lee TT, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 144.Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- 145.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 146.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 147.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 148.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 150.Mitra SW, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 151.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 152.Register TC, Shively CA, Lewis CE. Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res. 1998;788:320–322. doi: 10.1016/s0006-8993(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 153.Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 154.Day M, Sung A, Logue S, Bowlby M, Arias R. Beta estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav Brain Res. 2005;164:128–131. doi: 10.1016/j.bbr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 155.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mazzucco CA, et al. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 157.Mukai H, et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 158.Szymczak S, et al. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- 159.McEwen BS, Tanapat P, Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a nonsteroidal estrogen antagonist in female rats. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- 160.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 161.Loy R, Gerlach JL, McEwen BS. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Brain Res. 1988;467:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- 162.McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- 164.Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- 165.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 166.Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 167.Shughrue PJ, Merchenthaler I. Estrogen is more than just a "sex hormone": novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- 168.Milner TA, et al. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 169.Kalita K, Szymczak S, Kaczmarek L. Non-nuclear estrogen receptor beta and alpha in the hippocampus of male and female rats. Hippocampus. 2005;15:404–412. doi: 10.1002/hipo.20066. [DOI] [PubMed] [Google Scholar]
- 170.Milner TA, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 171.Kaplan TJ, Skyers PR, Tabori NE, Drake CT, Milner TA. Ultrastructural evidence for mu-opioid modulation of cholinergic pathways in rat dentate gyrus. Brain Res. 2004;1019:28–38. doi: 10.1016/j.brainres.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 172.Quinones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW. Estrogen regulation of mu-opioid receptor mRNA in the forebrain of female rats. Brain Res Mol Brain Res. 1997;47:134–138. doi: 10.1016/s0169-328x(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 173.Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- 174.Luine VN, McEwen BS. Effects of an estrogen antagonist on enzyme activities and [3H]estradiol nuclear binding in uterus, pituitary and brain. Endocrinology. 1977;100:903–910. doi: 10.1210/endo-100-4-903. [DOI] [PubMed] [Google Scholar]
- 175.McMillan PJ, LeMaster AM, Dorsa DM. Tamoxifen enhances choline acetyltransferase mRNA expression in rat basal forebrain cholinergic neurons. Brain Res Mol Brain Res. 2002;103:140–145. doi: 10.1016/s0169-328x(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 176.Wu X, et al. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 1999;847:98–104. doi: 10.1016/s0006-8993(99)02062-4. [DOI] [PubMed] [Google Scholar]
- 177.Zhao L, O'Neill K, Brinton RD. Estrogenic Agonist Activity of ICI 182,780 (Faslodex) in Hippocampal Neurons: Implications for Basic Science Understanding of Estrogen Signaling and Development of Estrogen Modulators with a Dual Therapeutic Profile. J Pharmacol Exp Ther. 2006;319:1124–1132. doi: 10.1124/jpet.106.109504. [DOI] [PubMed] [Google Scholar]
- 178.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 179.Lundeen SG, Carver JM, McKean ML, Winneker RC. Characterization of the ovariectomized rat model for the evaluation of estrogen effects on plasma cholesterol levels. Endocrinology. 1997;138:1552–1558. doi: 10.1210/endo.138.4.5083. [DOI] [PubMed] [Google Scholar]
- 180.Toran-Allerand CD, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bologa CG, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 182.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 184.Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 185.Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front Neuroendocrinol. 2005;26:51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]


