Abstract
The 2007 Summit on “Environmental Challenges to Reproductive Health and Fertility” convened scientists, health care professionals, community groups, political representatives and the media to hear presentations on the impact of environmental contaminants on reproductive health and fertility and to discuss opportunities to improve health through research, education, communication and policy. Environmental reproductive health focuses on exposures to environmental contaminants, particularly during critical periods of development, and their potential effects on future reproductive health, including conception, fertility, pregnancy, adolescent development and adult health. Approximately 87,000 chemical substances are registered for use in commerce in the US, with ubiquitous human exposures to environmental contaminants in air, water, food and consumer products. Exposures during critical windows of susceptibility may result in adverse effects with lifelong and even intergenerational health impacts. Effects can include impaired development and function of the reproductive tract and permanently altered gene expression, leading to metabolic and hormonal disorders, reduced fertility and fecundity and illnesses such as testicular, prostate, uterine and cervical cancers later in life. This executive summary reviews effects of pre- and post-natal exposures on male and female reproductive health and provides a series of recommendations for advancing the field in the areas of research, policy, health care and community action.
Keywords: environmental contaminants, reproductive health, endocrine disrupting chemicals, fertility, fecundity, hormone disruption, sperm quality, reproductive tract development
Background
On Jan 28–30, 2007, a Summit on “Environmental Challenges to Reproductive Health and Fertility” was convened at the Mission Bay Campus of the University of California San Francisco (UCSF). The Summit was the product of a collaboration between the UCSF Program on Reproductive Health and the Environment in the Department of Obstetrics, Gynecology and Reproductive Sciences, the UCSF National Center of Excellence in Women’s Health and the Collaborative on Health and the Environment. This unique gathering coalesced the field of environmental reproductive health by bringing together over 400 scientists, researchers, health care professionals, trainees, health-affected groups, community and political representatives and the media to discuss what is currently known about the impacts of environmental contaminants on reproductive health and fertility. The compelling nature of the collective science, with observations in humans, animal models and wildlife, raised concern for the future health of individuals and families. The Summit also set the stage to improve health through research, education, communication and changes in public health policy. This executive summary presents the highlights from the accompanying Supplement on Environmental Challenges to Reproductive Health and the Environment (1), which summarizes the state of the science presented at the Summit, and outlines the key “next steps” Summit participants recommended for research, policy, health care, community action and safe work.
Defining the Field
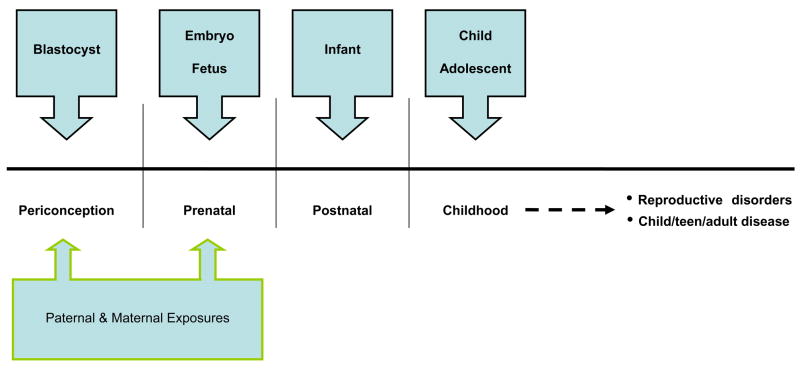
Environmental reproductive health focuses on exposures to environmental contaminants (synthetic chemicals and metals), particularly during critical periods of development (such as prior to conception and during pregnancy), and their potential effects on all aspects of future reproductive health throughout the life course, including conception, fertility, pregnancy, child and adolescent development and adult health. (Figure 1).
Figure 1.
Key Definitions for Environmental Reproductive Health
Environmental Contaminants
Since World War II, there has been a dramatic increase in human exposures to both natural and synthetic chemicals. As of 2006, there are approximately 87,000 chemical substances registered for use in commerce in the U.S. (2). Common environmental pollutants include: pesticides and herbicides such as atrazine and chlorpyrifos; volatile organic compounds such as benzene, toluene and chloroform; heavy metals such as lead, mercury and arsenic; air contaminants such as carbon monoxide, ozone, particulate matter and environmental tobacco smoke; and persistent organic pollutants, such as the dioxins, polychlorinated biphenols (PCBs), the pesticide dichlorodiphenyltrichloroethane (DDT) and its breakdown product dichlorodiphenyldichloroethylene (DDE).
While many environmental contaminants can affect reproductive health (see Table 1), there is an important class of chemicals called endocrine disrupting chemicals (EDCs) that interfere with the production, release, transport, metabolism, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis and the regulation of developmental processes. Some of the common EDCs discussed at the Summit include bisphenol A (BPA), phthalates and certain pesticides (e.g., vinclozolin, dicofol, atrazine). Many of these compounds alter estrogen, androgen, and thyroid signaling, which are essential for normal embryonic development and reproductive activity in all vertebrates studied to date (3–5). They can also alter the synthesis, storage on plasma proteins and hepatic biotransformation and clearance of hormones (6) and disrupt neural and immune signaling pathways (7–9) and the regulation of gene expression (e.g., DNA methylation, RNA stability, protein degradation) (reviewed by (10)). In some cases, altered DNA methylation patterns have been shown to be heritable (11, 12).
Table 1.
Environmental Contaminants: Sources and Selected Health Effects from Developmental and Adult Exposures (Adapted from [254])
| Contaminant | Sources | Examples of health effects associated with exposure during adulthood (animal and/or human data) | Examples of health effects associated with exposure during development (animal and/or human data) | |
|---|---|---|---|---|
| OTHER | Air Pollution | Common air pollutants include carbon monoxide, lead, ground-level ozone, particulate matter, nitrogen dioxide, and sulfur dioxide. Air pollution arises from a variety of sources, including motor vehicles, industrial production, energy (coal) production, wood burning, and small local sources such as dry cleaners. | fetal lossd (254) | low birth weight (254)
preterm delivery (254) |
| Bisphenol A (BPA) | Industrial chemical and building block for polycarbonate plastic and epoxy resins. Found in the lining of metal food and drink cans, plastic baby bottles, pacifiers and baby toys, dental sealants, computers, cell phones, hard plastic water bottles, paints, adhesives, enamels, varnishes, CDs and DVDs, and certain microwavable or reusable food and drink containers. | oocyte (egg) chromosome abnormalities (162)
recurrent miscarriage (160) decreased semen quality (180, 181) |
altered puberty onset (182)
obesity (182) altered prostate development (183, 184) decreased semen quality (181, 185) hormonal changes (185) |
|
| Disinfection by-products | Over 600 compounds formed by the reaction of chemical disinfectants (most often chlorine) with natural organic matter, primarily in surface waters. Most prevalent compounds are trihalomethanes. | menstrual irregularities (131, 186) | fetal growth, IUGR (177–179) | |
| Ethylene oxide | Chemical sterilant used in dental and medical practices. | fetal loss^ (187, 188)
decreased semen quality* (188) miscarriage in female partner (188) |
||
| Glycol ethers | Used in paints, varnishes, thinners, printing inks, electronics, semi-conductor industry, leather, photographic film, varnish, enamels, cosmetics, perfumes, brake fluids, wood stains. | longer mentral cycles (135)
decreased semen quality* (100, 189) reduced fertility‡ (190, 191) fetal loss^ (189, 190) |
||
| Pesticides | Broad category that includes many classes of insecticides, fungicides, herbicides, rodenticides, and fumigants. Pesticides are used on food, in residential and industrial settings. Exposures can occur through food, drinking water, or from in home use. | menstrual irregularities (133, 166)
reduced fertility‡ (147, 148, 188, 192) decreased semen quality* (189, 193–195) miscarriage in female partner (151, 153, 196, 197) sperm chromosome abnormalities (198, 199) hormonal changes (100, 193, 200) |
altered sex ratio (100, 201)
altered puberty onset (202–204) malformations of reproductive tract^ (205–207) reduced fertility (193, 208) fetal growth, IUGR (209–211) |
|
| Phthalates | Plasticizers added to soften plastics like PVC; also found in cosmetics, perfumes, toys, pharmaceuticals, medical devices, lubricans and wood finishers. | altered (earlier) menarche onset (127)
estrous cycle, ovulatory irregularities (187) decreased semen quality* (212) reduced fertility‡ (213) fetal loss^ (187) endometriosis (141, 142) |
shortened anogenital distance (214)
malformations of reproductive tract (215) hormonal changes (215) decreased semen quality* (215) |
|
| Solvents | Benzene, toluene, xylene, styrene, 1-bromopropane, 2-bromopropane, perchloroethylene, trichloroethylene, and others. Solvents include some of the top production volume chemicals in the US Used in plastics, resins, and nylon, synthetic fibers, rubbers, lubricants, dyes, detergents, drugs, pesticides, glues, paints, paint thinners, fingernail polish, lacquers, detergents, printing and leather tanning processes, insulation, fiberglass, food containers, carpet backing, cleaning products, and a component of cigarette smoke. Exposure is primarily through breathing contaminated air. | hormonal changes (100, 187, 216)
menstrual irregularities (187, 188, 193) decreased semen quality* (100, 188, 217, 218) reduced fertility‡ (188, 218–222) fetal loss^ (187, 188, 193, 223) miscarriage in female partner (188) |
||
| Cigarette smoke | Includes active and/or passive smoking | hormonal changes (219, 224)
decreased semen quality* (219) reduced fertility‡ (188, 219) miscarriage (219) early menopause (219) |
IUGR (225)
Low birth weight (225) Preterm delivery (225) decreased semen quality* (124, 226) |
|
| Pharmaceuticals | Examples: DES, ethynylestradiol (birth control pill) | malformations of reproductive tract^ (227, 228)
altered hormone response (228) menstrual irregularities (187, 227) reduced fertility‡ (187, 227) uterine fibroids (227) miscarriage (187) |
||
| Perfluorinated compounds (PFOS, PFOA) | Used to make fabrics and carpets stain-resistant and water-repellant; in coating of cooking pans, floor polish, insecticides, food wrap coatings. Accumulate in the environment and the food chain. | hormonal changes (229)
reduced birth weight (230) fetal loss (230, 231) |
||
| Polybrominat ed Diphenyl Ethers (PBDEs) | Flame retardants found in furniture foam, mattresses, textiles, computers and electronics. Accumulate in the food chain. | decreased semen quality* (232) | ||
| Octylphenol, Nonylphenol | Used to make surfactants (detergents), pesticides, paints, and other formulated products, and also as plasticizers and UV stabilizers in plastics. Primary exposure is through drinking water contaminated by sewage and wet-weather runoff. | hormonal changes (227)
altered puberty onset (233) hormonal changes (185, 234) decreased semen quality* (185, 235) decreased testes size (234, 235) |
||
| CHLORINATED HYDROCARBONS | dioxins/furans | Byproducts of the manufacture and burning of products that contain chlorine. | menstrual irregularities (132, 134, 137, 140, 166, 187, 236)
hormonal changes (138–140, 187, 193, 236) reduced fertility‡ (187, 236) endometriosis (187, 237, 238) fetal loss^ (144, 236, 239) decreased semen quality* (236, 239, 240) altered puberty onset (127, 129, 241) altered menarche onset (126, 128, 241, 242) |
malformations of the reproductive tract^ (236, 243-245)
altered estrous cycle (227) reduced fertility‡ (227) altered sex ratio (100, 186, 187, 236, 246) altered puberty onset (126, 241) decreased semen quality* (91, 243) delayed time to pregnancy (247) |
| Polychlorinated biphenols PCBs | Industrial insulators and lubricants. Banned in the US in 1976. Persist for decades in the environment. Accumulate up the food chain. | |||
| Organochlorine pesticides | Class of pesticides used largely as insecticides. (ex: DDT, chlordane, HCB.) Largely banned in the US Persist for decades in the environment. Accumulate up the food chain. | |||
| pentachlorophenol | Wood preservative for utility poles, railroad ties, and wharf pilings. Formerly used as a pesticide. | |||
| METALS | lead | Used in batteries, ammunition, metal products, X-ray shields. Reduced use in gasoline, paints, ceramic products, caulking, and pipe solder. Most common source of exposure in the US is lead-based paint in older homes, lead-contaminated house dust and soil and vinyl products. | fetal loss^ (187, 217, 248)
reduced fertility‡ (100, 187, 193, 217, 249, 250) hormonal changes (100, 187, 193, 251) menstrual irregularities (130, 187) abnormal sperm (94, 100, 252) altered puberty onset (124–126) |
hormonal changes (227)
altered puberty onset (84, 126) |
| mercury | Used in thermometers, dental fillings, batteries, vaccines and other industries. Air and water contaminated by industrial emissions and the combustion of coal and waste. Accumulates in food chain; most common source of exposure in US is contaminated seafood. | |||
| manganese | Used in the production of batteries, in dietary supplements, and as ingredients in some ceramics, pesticides, and fertilizers. Gasoline additive. | |||
| cadmium | Used in industry and consumer products, mainly batteries, pigments, metal coatings, plastics, and some metal alloys. |
decreased semen quality could include low semen volume, abnormal sperm shapes or motility, decreased sperm counts.
- reduced fertility could include both infertility and increased time to pregnancy (reduced fecundity).
menstrual irregularities could include short or long menstrual cycles, missed periods, abnormal bleeding, anovulation.
malformations of the reproductive tract: In males, could include shortened ano-genital distance in animals or hypospadias (humans), undescended testicles (cryptorchidism), small testicles (hypoplasia), and structural abnormalities of the epididymis. In females, could include small ovaries, reduced number of follicles (eggs), and structural abnormalities of the oviducts, uterus, cervix, and/or vagina.
IUGR=intrauterine growth retardation; DDT=dichlorodiphenyltrichloroethane; DES=diethylstilbestrol; HCB=hexachlorobenzene.
Studying the effects of EDCs on the reproductive system is a natural area of inquiry, as EDCs can interact with the hormonal system, which regulates development and maintenance of the reproductive system. However, since EDCs also target the neuroendocrine system, which plays regulatory and homeostasis roles in the control of human physiology, exposure to EDCs has broader implications for health.
Exposure to Multiple Chemicals
Humans are exposed daily to a mixture of environmental contaminants in air, water and food. In a recent biomonitoring study of over 150 contaminants, the U.S. Centers for Disease Control and Prevention (CDC) reported that all 150 chemicals were detected in some portion of the U.S. population and that several of the chemicals, such environmental tobacco smoke, lead, mercury and phthalates, are detected in nearly all of the population (13). These and similar biomonitoring efforts improve our understanding of current body burdens of environmental contaminants. With this knowledge comes a need for better science on the health risks associated with current patterns of exposure, including increased risks resulting from exposures to multiple chemicals. For example, the majority of studies and regulatory focus has been on exposures to individual phthalates, which may underestimate the actual risks, as recent studies have found that simultaneous prenatal exposure to both di(n-butyl) phthalate (DBP) and di(2-ethylhexyl)phthalate (DEHP) produced reproductive malformations in the offspring in a cumulative, dose-additive manner (14). Finally, biomonitoring data indicate that more effort is needed toward approaches that identify and mitigate exposure to harmful chemicals prior to measuring harmful contaminants in people.
Susceptible Populations
Environmental chemicals can cause a broad spectrum of effects, which depend not only on route of exposure and dose, but on the susceptibility of the individual to the compound. Age, gender and genotype can influence susceptibility to disorders, anatomic abnormalities and diseases from exposures. For example, we know that children are not small adults; they have different behaviors, metabolism and responses to infectious and environmental challenges. The elderly may also be a population at special risk to environmental chemicals.
Critical and Sensitive Windows of Susceptibility
A critical window of susceptibility is a time-sensitive interval during development when exposures to environmental contaminants can disrupt or interfere with the physiology of a cell, tissue or organ (15, 16). It is a period characterized by marked cellular proliferation and development and numerous changing metabolic capabilities in the developing organism (16, 17). Exposures to environmental contaminants during this window may result in adverse, permanent and irreversible effects that can have lifelong and even intergenerational impacts on health.
Researchers have suggested the need to also define sensitive windows of susceptibility. Exposures during sensitive windows of susceptibility may still affect development or result in eventual adult disease, but with reduced magnitude compared to the effect of exposure during the critical window of susceptibility (16, 18). For example, DES exposure reprograms the expression of estrogen responsive genes in Eker rats exposed on post-natal days 3–5 or 10–12 (critical window of susceptibility), leading to increased incidence of uterine leiomyoma. In contrast, rats exposed on post-natal days 17–19 (sensitive window of susceptibility) did not experience this developmental programming and had a rate of uterine leiomyoma that was elevated but not statistically different from control animals (19).
Given that development continues after birth, critical and sensitive windows occur periconceptually (prior to, during and shortly after the fertilization of the egg) and during pregnancy; infancy, childhood and puberty (Figure 2).
Figure 2. Windows of Susceptibility to Environmental Insults.
Adapted from (253).
Developmental Programming and Fetal Origins of Adult Disease
Studies from the 1990’s found that adverse effects on the fetal environment, such as poor maternal nutrition, can result in an increased risk of adult onset of chronic conditions such as coronary heart disease (20–22). These findings led to the fetal origins of disease hypothesis (commonly known as the “Barker” hypothesis), which proposes that exposures to adverse insults during critical or sensitive windows of development can permanently reprogram normal physiological responses, and thus give rise to illnesses and metabolic and hormonal disorders later in life (23–28).
The DES Example
Prenatal exposure to diethylstilbestrol (DES), a synthetic estrogen and thus EDC, provides an unfortunate example of developmental programming. DES was given to U.S. pregnant women between 1938 and 1971 under the erroneous assumption that it would prevent pregnancy complications. In fact, in utero exposure to DES alters the normal programming of gene families, such as Hox and Wnt, that play important roles in reproductive tract differentiation (28–31). As a result, female offspring exposed to DES in utero are at increased risk of clear cell adenocarcinoma of the vagina and cervix, structural reproductive tract anomalies, infertility and poor pregnancy outcomes, while male offspring have an increased incidence of genital abnormalities and a possibly increased risk of prostate and testicular cancer (32). These observed human effects have been confirmed in numerous animal models which have also provided information on the toxic mechanisms of DES. Animal experiments have also predicted changes later found in DES-exposed humans, such as oviductal malformations (33), increased incidence of uterine fibroids (34–36) and second-generational effects (37, 38) such as increased menstrual irregularities (39) and possibly ovarian cancer (40) in DES-granddaughters and increased hypospadias in DES-grandsons (41, 42).
DES is but one example of how exposure to EDCs can disrupt developing organ systems and cause abnormalities, many of which only appear much later in life or in the subsequent generation (43), such as endometriosis, fibroids and breast, cervical and uterine cancer in women; poor sperm quality and increased incidence of cryptorchidism and hypospadias in men; and subfertility and infertility in men and women (28),
Signals from Wildlife
For over a century, wildlife and laboratory animals have been used to predict the human health effects of various environmental contaminants. Although each species has its unique attributes, a growing literature indicates that substantial conservation exists in the underlying molecular, cellular and physiological systems associated with vertebrate reproduction (44). For example, estrogen, androgen and thyroid signaling are essential for normal embryonic development and reproductive activity in all vertebrates studied to date (3–5). Furthermore, wildlife studies demonstrate the effects of levels and mixtures of exposures in our environment in genetically diverse populations (44). Therefore observations from wildlife are directly relevant to assessing potential environmental influences on human reproduction.
In the early 1990s, studies began to associate environmental contamination with altered reproductive performance in wild populations of fish, amphibians, reptiles and birds (45). For example, studies in fish demonstrate increased rates of feminized male phenotype and reduced fertility from environmental exposures to: ethynylestradiol, a synthetic estrogen found in birth control pills and increasingly in treated sewage effluent; tributyltin, an anti-fouling agent used on boats; BPA; tetrabromobisphenol A, a widely used flame retardant; and nitrate, a common fertilizer (44). Studies in alligators inhabiting pesticide-contaminated lakes report reduced fertility and increased occurrence of multioocyte follicles (ovarian follicles with multiple rather than the normal single oocyte) (46); alterations in folliculogenesis resulting in multioocyte follicles have been associated with infertility and early embryonic loss in DES-treated mice (47, 48). Exposure of reptilian embryos to endogenous (estradiol-17β), pharmaceutical (e.g., ethynylestradiol, diethylstilbestrol) or industrial (e.g., DDT, DDE, BPA, trans-nonachlor) estrogens during a critical window of development induces sex reversal at male incubation temperatures, leading to increased female sex ratios (49–52). And, exposure to even lower concentrations of these contaminants alters steroidogenesis of the ovary or testis in neonates and juveniles (53). Fish and amphibians also experience effects following exposure to endocrine-active compounds, including aberrant gonadal morphology (e.g., the presence of oocytes in the testis, alterations in Leydig and Sertoli cell morphology or number) (54, 55). This literature documents the endocrine-disruptive effects of a wide array of commercial chemicals and byproducts, including: pesticides; sewage contaminants, such as surfactants (e.g., octylphenol and nonylphenol) and pharmaceutical agents; plasticizers (e.g., phthalates); flame retardants (e.g., PCBs, polybrominated diphenol ethers, tetrabromobisphenol A) and industrial pollutants (e.g., heavy metals, dioxin, polycyclic aromatic hydrocarbons) (for reviews, see (3, 6, 56–58)). Furthermore, these effects were caused by exposure to levels of chemicals found in the environment.
Concerning Trends
There have been a number of concerning trends in human reproductive health. The incidence of testis cancer, primarily a disease of young men, has increased in Europe, with a lifetime risk approaching 1% (59). In addition, young men born today in Europe have remarkably low average sperm counts and a high prevalence (~1 in 6) of abnormally low sperm counts likely to cause fertility problems (60). New data in three cities (Boston, U.S., Copenhagen, Denmark and Turku, Finland) demonstrate a significant secular trend in serum testosterone (61–63). The details vary somewhat, but together these studies suggest that testosterone has declined about 1% per year for the past 40–50 years. This decline is consistent with the reduction in sperm concentration reported by Carlsen in 1992 (64) and these two trends taken together increase the plausibility of a significant decrease in male reproductive function. For girls in the U.S., there has been a reported decline in age of onset of breast development and menarche over the last 30 years (65). Rapid changes in health endpoints are of concern because they suggest environmental and lifestyle, and thus avoidable, causes..
Compelling New Science: Moving Beyond Genetic Determinants
Genetic mutations are known to alter gene expression and lead to disease. Environmental exposures have typically been thought of as influencing genetics and health by causing mutations. For example, it has long been known that radiation leads to genetic mutations and increased risk of disease, such as cancer.
However, research during the past decade has revealed that many environmental exposures also act through modification of the epigenome (the collection of biochemical reactions that determine the gene expression) of cells, leading to either immediate or latent adverse effects on reproduction. For example, recent epigenetic research has revealed a possible mechanism by which in utero exposure to BPA heightens susceptibility to prostate cancer in adult rats: BPA altered the normal process of silencing, through hypermethylation, the phosphodiesterase type 4 variant 4 gene that occurs with aging, thus elevating gene expression (66). BPA also permanently alters expression of HOXA10, a gene necessary for uterine development (67). Epigenetic studies have also shown that DES causes alterations in uterine tissue architecture and morphology and heightens susceptibility to uterine adenocarcinoma by inducing permanent changes in several estrogen-responsive uterine genes (28) These are but a few examples of how the field of epigenetics has and will continue to contribute to our mechanistic understanding of the impact of environmental contaminants on reproductive health.
Environmental Contaminants and Effects in Males
Reproductive Effects of Early Life Exposures
Testicular Development and the Environment
Over the past 10–15 years, the central role that deficient androgen production and action during fetal testis development may play in the origin of disorders has been well documented and is reviewed in Sharpe and Skakkebaek (68).
There is a relatively high incidence of male reproductive disorders that manifest at birth (cryptorchidism, hypospadias) or in young adulthood (testicular germ cell cancer (TGCC) and infertility) (69, 70). These four disorders are increasing in prevalence in the West (69). They are risk factors for each other and they share several, pregnancy-related risk factors (68–70). Skakkebaek hypothesizes that TGCC, cryptorchidism and some cases of hypospadias and low sperm count comprise a testicular dysgenesis syndrome (TDS) with a common origin in fetal life (69). The hypothesis proposes that “abnormal testis development (dysgenesis), which could have numerous primary causes, leads secondarily to hormonal or other malfunctions of the Leydig and Sertoli cells during male sexual differentiation, leading to increased risk of reproductive disorders of the testicular system” (Figure 3) (68–70).
Figure 3.
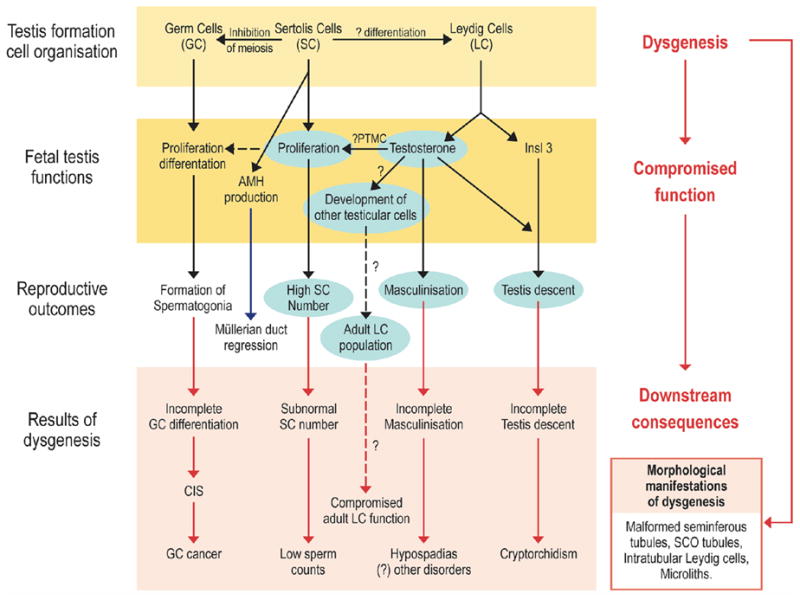
Schematic diagram to illustrate how dysgenesis of the early fetal testis is thought to lead to abnormalities of somatic cell function, resulting in hormonal changes and the downstream disorders that comprise testicular dysgenesis syndrome (TDS). The central role of testosterone is highlighted by the blue boxes. Dashed lines show pathways that are hypothesized but unproven (68).
Abbreviations: PTMC=peritubular myoid cell; Insl3=insulin-like factor 3; AMH=anti-mullerian hormone; CIS=carcinoma-in-situ; SCO=Sertoli cell only.
This hypothesis has been supported by findings in an animal model of TDS involving fetal exposure to the phthalate DBP as well as by new clinical studies described in Sharpe and Skakkebaek (68). Exposure of rats in utero to DBP induces a TDS-like syndrome in the male offspring (71–73); this is manifest as dose-dependent induction of cryptorchidism, hypospadias and impaired spermatogenesis and infertility. Focal dysgenesis (73, 74), subnormal fetal Leydig cell function (71–73) and subnormal Sertoli cell proliferation (75) and possibly function (73), consistent with changes predicted in the TDS hypothesis, are also demonstrated (69). Furthermore, the characteristics of the focal dysgenesis induced by fetal DBP exposure in rats (73, 74) – malformed seminiferous cords, Sertoli cell-only tubules with immature-appearing Sertoli cells and the abnormal occurrence of intratubular Leydig cells – are all also reported in testes of men with TGCC (76–78).
A particularly important recent development is the observation that inhibition of androgen production or action in rodents, resulting from transgenesis (79), DBP exposure (75) or flutamide treatment (80), reduces Sertoli cell number substantially in the perinatal period and leads to downstream TDS disorders. Thus, androgens appear to play a determining role during the most important periods of Sertoli cell proliferation (fetal and early postnatal life) (68, 81) (Figure 3). This finding is consistent with data in humans showing that Sertoli cell number increases during fetal life (when testosterone levels are high) and during the period of the neonatal testosterone rise (81, 82). Since Sertoli cell number in adulthood is the primary determinant of sperm production and counts in men (81), it is hypothesized that reduction in testosterone levels in the fetal testis, as a secondary consequence of dysgenesis leading to reduced Sertoli cell numbers, could lead to low sperm counts in adulthood (Figure 3). This is an important finding because Sertoli cells in the fetal testis in all species so far examined do not express androgen receptors. Therefore, anti-androgens appear to exert toxic effects on male reproductive development through multiple pathways (75)
The TDS syndrome is further supported by studies that induce hypospadias in CD1 mice through exposure to EDCs during the critical period of urethral development. These chemicals include 17α estradiol, pesticides such as vinclozolin, pharmaceutical products such as the antihistamine loratadine and the flame retardant, benzophenone-2 (83). A recent human study by Swan et al. found in utero exposure to phthalates associated with shortened (and thus less masculine) male anogenital distance, which has been observed in animal studies (84).
Based on the increasing prevalence of TDS disorders and recent evidence for declining testosterone levels in men, endocrine disrupting chemicals in our environment are likely to become ever more important in shaping the reproductive health of young men in the present and next generation.
Prostate Development and the Environment
Similar to the testis, male accessory sex glands and organs are also vulnerable to environmental EDCs, with adverse effects in adulthood. The developing prostate gland is particularly sensitive to estrogens and high-dose exposures during a critical developmental window results in prostatic intraepithelial neoplasia (PIN) in adult rodent models (85). Early life exposure to estrogenic substances could sensitize the developing prostate to later risks from increasing estrogen levels that occurs in the aging male. A study of rats treated neonatally to BPA followed by hormones that mimic the aging male in adulthood showed a significantly higher PIN incidence and score compared to controls (rats exposed only to BPA neonatally or those given only the aging hormones in adulthood) (86). As discussed above, this heightened predisposition to prostate carcinogenesis results from permanent alterations to the prostate epigenome (66).
Reproductive Effects of Adult Exposures
Hauser and Sokol (87) review human and animal evidence on exposure to several classes of environmental contaminants during adulthood and adverse male reproductive outcomes. In the past two decades, numerous animal and clinical studies have provided evidence that a variety of chemicals can disrupt the hypothalamic-pituitary-testicular axis by acting as hormonal antagonists or agonists or by disrupting the biochemical processes regulating hormone secretion and sperm function (87).
Consistent with the effects of prenatal exposure discussed above, rodent models of pubertal and adult exposure to phthalate esters report testicular toxicity characterized by testicular atrophy, reduced sperm counts, altered Leydig cell structure and function, Sertoli cell toxicity and increased germ cell apoptosis (68). These studies indicate an age-dependent sensitivity to exposure, with prenatal exposure causing the most, and adult exposures the least, severe effects. Studies of phthalate exposure and male reproductive health in humans are limited and inconsistent. For example, certain phthalate metabolites (MBP and MBzP) were associated with decreased sperm quality among U.S. (88) but not among Swedish men (89). The differences across studies, such as the ages of the population (older in the U.S.) or the source of the men (general population in Sweden and infertile couples in the U.S.), may account for some of the differences in study results, but may also suggest that a subpopulation of men may have increased susceptibility to phthalate exposure (87).
PCBs are another industrial contaminant for which data on prenatal and adult exposures in humans are available. For example, epidemiologic studies of high-dose exposures from accidental food contamination report abnormal sperm morphology, higher oligozoospermia rates and reduced hamster oocyte penetration 20 years after exposure (90). Effects on sperm quality resulting from prenatal exposure were similar: abnormal morphology, decreased motility and reduced hamster oocyte penetration (91). Studies to date of lower-dose, environmental exposures to PCBs support an association with reduced semen quality, specifically reduced sperm motility (92).
Heavy metals such as lead were among the first recognized human reproductive toxicants (93). Animal, clinical and epidemiologic studies have demonstrated that exposure to lead disrupts all levels of the reproductive axis, with the central nervous system and testis appearing to be the most sensitive organs and puberty a critical window of susceptibility (94–96). Epidemiologic studies report a dose-related suppression of spermatogenesis, normal or decreased serum testosterone and inappropriately normal urinary gonadotropins in the face of low testosterone levels in men with higher blood lead levels (97). Recent findings suggest that lead may also induce chromosomal abnormalities and lead to infertility by interfering with the acrosome reaction in spermatozoa (98). Human studies evaluating other heavy metals suggest that cadmium, mercury and boron may also disrupt male reproduction (99).
Dibromochloropropane (DBCP) is the most characterized agricultural chemical with respect to male reproductive toxicity. Occupational exposure to DBCP produced: azoospermia and oligospermia, damaged germinal epithelium, genetic alterations in sperm (such as double Y-bodies), reduced male fertility, increased rates of spontaneous abortions in wives of exposed workers, hormonal imbalances and altered sex ratio in offspring (100). Reversibility of effects following cessation of exposure are variable (101, 102). The reproductive toxicity of other agricultural chemicals such as organophosphate pesticides, vinclozolin and DDT is less well characterized in humans; nevertheless, animal and human studies demonstrate these chemicals to have adverse effects on semen quality as well as anti-androgen properties (100).
Additional classes of chemicals that are of particular interest due to widespread human exposure and animal evidence of reproductive toxicity, but for which human data are lacking or minimal, include those used in consumer products, such as BPA, parabens and phthalates, pyrethroid pesticides and air pollution (87).
Environmental Contaminants and Effects in Females
Reproductive Effects of Early Life Exposures
Prenatal exposure to environmental factors can modify normal cellular and tissue development and function through developmental programming, such that women may have a higher risk of reproductive pathologies and metabolic and hormonal disorders later in life (23–27). Woodruff and Walker (28) review new research on the effects of environmental estrogen exposure during key developmental windows on normal reproductive development of the ovaries and the uterus and the link to specific disease states in the adult.
Ovarian Follicular Development and the Environment
The ovarian follicle is the functional unit of the ovary and is comprised of an oocyte surrounded and supported by the somatic granulosa and theca cells (28). The health of the follicle can impact the health of the woman as well as the health of her offspring. For example, decreased numbers of follicles, multiple eggs per follicle and incomplete follicular development can all result in decreased fertility. The precise mechanisms involved in early ovarian follicle formation are not known, but are essential in organizing the fetal ovary and establishing the postnatal follicle number that will provide the female with sufficient oocytes for a lifetime of fertility (28).
Estrogen and activin are two known factors that play an important role in regulating oocyte and follicle development and function (103–112) and aberrant development and ovarian pathologies are observed in mice exposed to neonatal estrogen or activin. Neonatal exposure of rats to estradiol benzoate has been show to delay follicle and interstitial development (113). Neonatal exposure to DES or the natural estrogen estradiol (E2) results in lack of corpora lutea in adult mice (114), suggesting that these effects persist beyond reproductive tract development and impact fertility in the adult. Neonatal exposure to DES, E2, or the phytoestrogen genistein also induces formation of multi-oocytic follicles in mice (115–117) – an effect that is also reported in alligators exposed to environmental estrogenic contaminants (see above) (46). Additionally, activin administered during the critical, postnatal period of primordial follicle formation changes the number of postnatal follicles (28, 118). Current mechanistic studies are exploring whether neonatal estrogen exposure alters activin signaling in the ovary; preliminary findings of decreased activin subunit gene expression and impacted activin signaling in the mouse ovary support this hypothesis (28)
Uterus Development and the Environment
Women exposed to DES in utero during critical periods of reproductive tract development developed several types of reproductive tract abnormalities, as well as an increased incidence of cervical-vaginal cancer later in life (118). Animal studies that simulate the human DES experience have since shown that exposure of the developing reproductive tract of CD-1 mice to DES imparts a permanent estrogen imprint that alters reproductive tract morphology, induces persistent expression of the lactoferrin and c-fos genes and induces a high incidence of uterine adenocarcinoma (119–121). Experiments in rats have shown exposure to DES during the critical window of uterine development leaves a hormonal imprint on the developing uterine myometrium in rats that were genetically predisposed to uterine leiomyoma (28), increasing the risk for adult uterine leiomyoma from 65% to greater than 90% and increasing tumor multiplicity and size (35). DES-induced developmental programming appears to require the estrogen receptor α (122), suggesting that signaling through this receptor is crucial for establishing developmental programming.
Studies have now been extended beyond DES to demonstrate that other environmental estrogens reprogram gene expression in the uterus (28): exposure to genistein and BPA during the period of maximum sensitivity to developmental programming induces the expression of the estrogen-responsive genes calbindin and progesterone receptor. Neonatal BPA exposure attenuated estrogen-responsive genes whereas genistein exposure induced an even higher level of estrogen responsiveness than DES exposure. In contrast to DES, exposure to these environmental estrogens does not disrupt ovarian function in adult females, which continue to cycle normally.
Reproductive Effects of Adult Exposures
Mendola et al. (123) review the growing body of epidemiologic and occupational studies demonstrating that environmental exposures can interfere with all developmental stages of reproductive function in adult females, including puberty, menstruation and ovulation, fertility and fecundity and menopause.
Puberty
Environmental contaminants can accelerate or delay pubertal development. Lead exposure delays puberty in girls, even at very low levels (<5 micrograms per deciliter) (124–126). Earlier age at puberty has been observed with phthalate exposure (127) and correlates with serum DDT (128), DDE (129) and PCBs (126).
Menstrual and Ovarian Function
Variations in menstrual and ovarian function have been observed following consumption of drinking water disinfection byproducts (DBPs) and fish contaminated with PCBs and other pollutants; similar associations were noted in studies using biological markers of 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD), DDT, DDE and PCBs (123). These studies generally describe functional variations (e.g., long or short cycles, changes in luteal or follicular phase) that indicate an underlying perturbation of hormones rather than the development of clinical disorders, although long-term effects are not known.
Shorter cycles have been observed for occupational exposure to lead (130) and to chlordibromoethane in drinking water (131). Longer cycles have been observed in studies of EDCs such as TCDD (132), hormonally-active pesticides (133), serum PCBs (134) and multiple industrial chemicals (e.g., ethylene glycol ethers) used in the semi-conductor industry (135). Menstrual disorders such as missed periods and abnormal uterine bleeding were also observed (130, 133, 134). Other studies found menstrual abnormalities, such as abnormal menstrual bleeding with no change in cycle length, associated with PCBs or metal exposure (136, 137). Follicle-stimulating hormone is decreased in women exposed to pentachlorophenol (138). Progesterone and estrogens are reduced in women exposed to DDT and DDE (139, 140).
Endometriosis has been widely studied in relation to environmental exposures. Most studies considering PCBs have found increased serum levels among endometriosis cases, compared with controls (123). Phthalate esters have also been associated with endometriosis among some women (141, 142).
Fertility and Fecundity
Fertility and fecundity studies include time-to pregnancy and spontaneous abortion outcomes as well as studies of infecundity (lack of fecundity) and other measures of subfertility (123). Lead is consistently observed to be a reproductive toxicant, causing decreased fertility and increased pregnancy loss (130, 143). Pregnancy loss has also been found to be associated with DDE in most studies (144–146).
Working with or applying pesticides, primarily in agricultural and horticultural settings, appears to consistently reduce fertility and fecundability (147–152). Preconception exposure, but not exposure during pregnancy (153), appears to elevate risk for spontaneous abortion (154). Pesticides are detrimental to both fecundity and fertility in the limited number of animal studies conducted to date (155, 156).
Additional environmental exposures, including solvents, radiation and other compounds, are also associated with decrements in female fertility, but the literature is limited or inconclusive (123). In particular, studies on solvent exposure in a variety of settings (157–159) suggest decreases in fertility. One study in humans found an increase in recurrent miscarriage associated with BPA (160), a finding that is consistent with the disruption of oogenesis through meiotic disruption and aneuploidy in mice exposed to environmentally relevant levels of BPA (161, 162).
Menopause
Menopause has not been extensively studied, but earlier age at menopause has been observed with exposure to serum dioxin (163), DDT, DDE and other pesticides (164–166). Animal studies report disruption of folliculogenesis in mice exposed to lead (167) as well as follicle destruction after exposures to mancozeb, dibromoacetic acid, polycyclic aromatic hydrocarbons, cyclophosphamide and 4-vinylcyclohexene diepoxide (168–173), suggesting possible mechanisms relevant to human disorders associated with these exposures.
Environmental Exposures During Pregnancy and Adverse Birth Outcomes
Windham and Fenster (174) review the epidemiologic literature on exposure to certain environmental contaminants during pregnancy and adverse birth outcomes, such as low birth weight, intrauterine growth retardation (IUGR), preterm delivery and stillbirth.
Exposure to ETS reduces mean birth weight (or slightly increasing the risk of IUGR), with suggestive evidence of an effect on preterm delivery as well (175, 176). Studies of water disinfection byproducts (DBPs) support an association between DBP exposure and IUGR, with little consistent effect on preterm delivery (174, 177–179). The weight of epidemiologic evidence also suggests that high levels of exposure to DDT or DDE is associated with adverse fetal growth outcomes and preterm delivery (174). Studies of organophosphate exposure and reproductive outcomes have suffered from lack of a standard validated measure of exposure. However, despite inconsistencies in study results, the weight of evidence and precautionary principle suggest that exposure to organophosphates should be avoided during pregnancy (174).
Moving Forward
At the Summit, participants from research, academic, health care, government, advocacy and community sectors identified the most important needs and directions for advancing reproductive environmental health through research, health care, policy community action and occupational health.
Research
Participants in the research breakout group focused on identifying the critical research directions and key needs for advancing the science database on environmental reproductive health. They identified priority actions in two main areas: communication and research priorities that will benefit from continued dialogue among government agencies, basic scientists, epidemiologists, clinicians and the general public, who all have critical voices in the discussion.
1. There is a need for better communication to foster collaborations
To enhance collaborations among researchers and between researchers and granting agencies, the group proposed the following:
Fostering technologies that encourage collaboration, such as listservs and web-based databases of tissue banks.
Working with government agencies and universities to promote collaboration among researchers, such as broadening the definition of a principal investigator to include project leaders in a program project or center grant.
Developing opportunities for researchers to meet and discuss collaborations in
environmental reproductive health research, such as at professional society meetings.
2. Critical research directions in environmental reproductive health
The following priorities were identified:
Human and animal studies that are longitudinal and take into account the full life cycle, including prenatal exposures (e.g. The National Children’s Health Study);
Leveraging existing mechanisms of data collection to incorporate semen analysis into the CDC’s NHANES study.
Biological measurement collection and banking should be incorporated into
epidemiological study designs for future research.
Development of biomarkers of exposure and pre-clinical indicators of disease in animals and humans, and better biomarkers of human fertility.
Regulatory obstacles need to be addressed, such as interpreting and working with the Health Insurance Portability and Accountability Act rules.
Increased funding for emerging areas of research for individual chemicals and mixtures and their effects on epigenome; fetal programming and transgenerational effects; low-dose effects; non-traditional dose-response curves; and cross talk among endocrine systems and receptors.
How do we identify new emerging contaminants?
Health Professional Communication
Participants in this break out group, comprised primarily of health professionals and health-affected groups or patient advocates, discussed what clinicians need to educate and advocate for patients. Participants agreed that:
Clinicians need to be well-informed about the sources and effects of environmental and workplace contaminant exposures, especially in relation to periconceptional, prenatal, early infancy and childhood windows of susceptibility.
Due to the complexity of analyzing exposures and difficulty in predicting precise health effects in a given individual, the clinician must address uncertainty when communicating with patients on these issues.
Health professionals need to take a precautionary stance and provide patients specific advice on avoiding exposures.
Clinicians and scientists can help interpret complex scientific research for legislators and the public to support better regulation of contaminants, leading to reduced exposures.
Some important needs of health care professionals include:
Clear, simple-to-use health information tools that list contaminants and sources of exposure and steps to take to reduce exposures and health effects of specific exposures. Tools need to be developed collaboratively by scientists, clinicians and advocacy and community groups in order to be relevant and appropriate to a diversity of populations.
Education on reproductive environmental health should be included in health professional and public education
Health care professionals should take a work history and inquire about patients’ exposures, ideally before pregnancy. This is not the current standard of practice.
Examples of Health Information Tools available to Health Professionals
The Pediatric Environmental Health Tool Kit provides easy to use, anticipatory, age-appropriate guidance on how to minimize harmful pediatric environmental exposures (http://psr.igc.org/ped-env-hlth-toolkit-project.htm). The Hazard Evaluation System and Information Service is comprised of informational materials, training and a workplace hazard helpline for workers and health professionals for a number of workplace reproductive and developmental hazards (thttp://www.dhs.ca.gov/ohb/HESIS/hesispubs.htm.)
Policy
Participants from all sectors represented at the Summit identified four key policy needs:
1) Advance models for comprehensive chemicals evaluation at local, state and national levels and develop effective chemical regulation
Because there is such a lack of data on chemicals that are already on the market, comprehensive testing should be required for chemicals remaining on the market and pre-market testing should include reproductive environmental health outcomes. The testing should: evaluate effects on both the environment and human health; assess exposures at different stages of development; and identify cumulative and synergistic impacts. The review of the testing results needs to include mechanisms for reducing, limiting or removing chemicals that pose reproductive environmental health risks.
2) Improve the science base: increase resources and improve methods to enhance research on reproductive environmental health
Key areas include improving research design to: better identify developmental effects that can occur from exposures during important reproductive windows: track impacts that can be passed on through multiple generations; assess low dose effects and effects from multiple exposures to chemicals; and develop improved and faster screening technologies to more quickly identify potentially harmful chemicals.
3) Improve the use of science in decision making
Participants noted that there are a number of steps between development of scientific findings and then using those findings to make decisions in a policy context. The process for doing this can be complicated and highly technical. Further efforts should focus on: acknowledging uncertainty in the science and allowing for action in the face of this uncertainty; increasing steps to limit undue influence or bias in the review process; and incorporating low dose effects and exposure to multiple chemicals into decision making and risk assessment.
4) Right to know: improve information given to consumers and workers on environmental contaminants in products
Participants identified the need to address the inadequacies of consumer product labeling and Material Safety Data Sheets, as well as the obstacles that trade secret protections place on accessing information on consumer product ingredients.
Community Action
Summit participants gathered to talk about the science in the context of environmental justice, occupational health and reproductive justice. Participants noted that learning about potentially hazardous chemicals in everyday products and in the workplace and their effects on babies in utero are powerful personal motivators toward further education and activism. However, placing the responsibility on women to avoid everyday toxins such as mercury in fish or hazardous chemicals in common household products is not an effective strategy for protecting reproductive health. Efforts by community members, scientists, epidemiologists, clinicians, activists, communications strategists and spokespeople will be more successful if they work towards a reformed and improved public health policy that adequately regulates chemicals and reduces exposures.
Safe Work
Participants in the Safe Work break out group discussed the implications of the science and key needs for improving worker health and safety. The group noted that more attention needs to be paid to workers' exposures within the area of environmental health. Their discussion also echoed themes from some of the groups, such as the need for better communication of the science, and improved methods for making decisions in the face of uncertainty that consider worker health. They also identified some unique needs of workers and proposed the following:
Reduce permissible exposure levels to chemicals that harm reproduction and development so that they are more in line with environmental exposure limits. In addition, permissible exposure limits should reflect the toxicity of exposure to mixtures of chemicals used in the workplace, rather than exposure to chemicals individually.
Exposure assessment and monitoring in occupational settings should be expanded.
Expand occupational health researchers' access to workers so that health consequences can be identified and corrected.
Develop alliances that can improve health across different sectors.
For example, making the connections between worker safety and hospital patient safety (concerning phthalates) and fostering alliances between environmental health groups and labor and worker groups.
Conclusions
The Summit provided a view of critical scientific information that underscored the need for further efforts in areas to improve reproductive health. One common theme throughout the Summit was communication and collaboration. Scientists bring unique and important contributions to studying the impact of environmental contaminants on reproductive health. A goal of moving forward from the Summit is to bring together epidemiologists, basic scientists, clinicians and clinician researchers to approach the study of environmental contaminants on reproductive health in an integrated way. However, such research is most valuable and could be of highest benefit for human health if it is conducted in collaboration with health-affected and community based groups that can facilitate focusing research questions on the most pressing issues of the most affected constituencies.. Communication across scientific disciplines and among scientists, health care providers, health-affected groups and the public, as well as efforts in research, education and policy, are key to reducing the adverse impacts of environmental contaminants and enhancing the reproductive health of this and future generations.
Acknowledgments
We would like to thank all of the speakers, the break out group leaders (Sarah Janssen, Andrea Gore, Mark Miller, Julia Quint, Miriam Gordon, Tracey Woodruff, Rivka Gordon, Kirsten Moore, Amanda Hawes, Catherine Porter) and the staff from the Center of Excellence in Women’s Health at UCSF and the Collaborative on Health and the Environment. We would also like to acknowledge our funding sources: Adeza Biomedical; Anonymous/Private Foundation; Center for Environmental Health; Collaborative on Health and the Environment; Compton Foundation, Inc.; Fred Gellert Family Foundation; Global Community Monitor/Tides ; The John Merck Fund; National Institute of Environmental Health Sciences; New York Community Trust; UCSF Department of Obstetrics, Gynecology and Reproductive Sciences; UCSF Institute for Health Policy Studies; UCSF National Center of Excellence in Women’s Health; UCSF Obstetrics and Gynecology Research and Education Foundation; U.S. Environmental Protective Agency, Office of Children's Health Protection; U.S. Environmental Protective Agency, Reproductive Toxicology Division; Women’s Foundation of California. Tracey Woodruff is on sabbatical from the U.S. Environmental Protection Agency; the views here express those of the author and not of U.S. Environmental Protection Agency. Finally, we would like to acknowledge the contributors the Supplement on Environmental Challenges to Reproductive Health and the Environment in Fertility and Sterility, as this executive summary draws upon their work.
Abbreviations
- AMH
anti-mullerian hormone
- BPA
bisphenol A
- CDC
US Centers for Disease Control and Prevention
- CIS
carcinoma-in-situ
- DBCP
dibromochloropropane
- DBP
di(n-butyl phthalate
- DBPs
disinfection byproducts
- DDE
dichlorodiphenyldi-chloroethylene
- DDT
dichlorodiphenyl-trichloroethane
- DEHP
di(2-ethylhexyl phthalate
- DES
diethylstilbestrol
- E2
estradiol
- EDCs
endocrine disrupting chemicals
- ETS
environmental tobacco smoke
- HCB
hexachlorobenzene
- Insl3
insulin-like factor 3
- IUGR
intrauterine growth retardation
- PCBs
polychlorinated biphenols
- PIN
prostatic intraepithelial neoplasia
- PTMC
peritubular myoid cell
- SCO
Sertoli cell only
- TCDD
2,3,7,8 tetrachlorodibenzo-p-dioxin
- TDS
testicular dysgenesis syndrome
- TGCC
testicular germ cell cancer
- UCSF
University of California San Francisco
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tracey J. Woodruff, Program on Reproductive Health and the Environment, National Center of Excellence in Women’s Health, Department of Obstetrics, Gynecology and Reproductive Sciences, UC San Francisco.
Alison Carlson, Collaborative on Health and the Environment.
Jackie M. Schwartz, Program on Reproductive Health and the Environment, National Center of Excellence in Women’s Health, Department of Obstetrics, Gynecology and Reproductive Sciences, UC San Francisco.
Linda C. Giudice, Department of Obstetrics, Gynecology and Reproductive Sciences, UC San Francisco.
References
- 1.Fertil Steril Supplement. In Press.
- 2.U.S. EPA. What is the TSCA Chemical Substance Inventory. Vol. 2007. US Environmental Protection Agency; 2006. [Google Scholar]
- 3.McLachlan JA. Environmental Signaling: What embryos and evolution teach us about endocrine disrupting chemicals. Endocrine Rev. 2001;22:319–41. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- 4.Zoeller RT, Dowling ALS, Herzig CTA, Iannacone EA, Gauger KJ, Bansal R. Thyroid hormone, brain development, and the environment. Environ Health Perspec. 2002;110(Suppl 3):355–61. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. International Journal of Andrology 29:96–104. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 6.Guillette LJ, Jr, Gunderson MP. Alterations in the development of the reproductive and endocrine systems of wildlife exposed to endocrine disrupting contaminants. Reproduction. 2001;122:857–64. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- 7.Fournier M, Brousseau P, Tryphonas H, Cyr D. Biomarkers of immunotoxicity: An evolutionary perspective. In: Guillette LJ Jr, Crain DA, editors. Endocrine Disrupting Contaminants: An Evolutionary Perspective. Philadelphia: Francis and Taylor Inc; 2000. pp. 182–215. [Google Scholar]
- 8.Guillette LJ., Jr Endocrine disrupting contaminants – beyond the dogma. Environ Health Perspect. 2006;114(Suppl 1):9–12. doi: 10.1289/ehp.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osteen KG, Sierra-Rivera E. Does disruption of immune and endocrine systems by environmental toxins contribute to development of endometriosis? Sem Reprod Endocrinol. 1997;15:301–8. doi: 10.1055/s-2008-1068760. [DOI] [PubMed] [Google Scholar]
- 10.Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ Health Perspect. 2007 doi: 10.1289/ehp.9951. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and mate fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, et al. Transgenerational epigenetic imprints on mate preference. Proc Nat Acad Sci. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. Third national report on human exposure to environmental chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 14.Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE., Jr Cumulative Effects of dibutyl phthalate and diethylhexyl phthalate on Male Rat Reproductive Tract Development: Altered Fetal Steroid Hormones and Genes. Toxicol Sci. 2007 doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- 15.Morford LL, Henck JW, Breslin WJ, DeSesso JM. Hazard identification and predictability of children's health risk from animal data. Environ Health Perspect. 2004;112:266–71. doi: 10.1289/ehp.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis RH. Periconception Window: Advising the Pregnancy Planning Couple. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.043. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabrese EJ. Sex differences in susceptibility to toxic industrial chemicals. Br J Ind Med. 1986;43:577–9. doi: 10.1136/oem.43.9.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–93. [PubMed] [Google Scholar]
- 19.Cook JD, Davis BJ, Goewey JA, Berry TD, Walker CL. Identification of a Sensitive Period for Developmental Programming That Increases Risk for Uterine Leiomyoma. Eker Rats. 2007;14:121–36. doi: 10.1177/1933719106298401. [DOI] [PubMed] [Google Scholar]
- 20.Barker DJ. Fetal origins of coronary heart disease. Bmj. 1995;311:171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson R. The fetal origins of adult disease. Bmj. 2001;322:375–6. doi: 10.1136/bmj.322.7283.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson M, Gluckman P, Bier D, Challis J, Fleming T, Forrester T, et al. Report on the 2nd World Congress on Fetal Origins of Adult Disease, Brighton, U.K., June 7–10, 2003. Pediatr Res. 2004;55:894–7. doi: 10.1203/01.PDR.0000115682.23617.03. [DOI] [PubMed] [Google Scholar]
- 23.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 24.Couzin J. Quirks of fetal environment felt decades later. Science. 2002;296:2167–9. doi: 10.1126/science.296.5576.2167. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–8. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 26.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 27.Hattersley AT, Tooke JE. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet. 1999;353:1789–92. doi: 10.1016/S0140-6736(98)07546-1. [DOI] [PubMed] [Google Scholar]
- 28.Woodruff TK, Walker CL. Fetal and Early Postnatal Environmental Exposures and Reproductive Health Effects in the Female. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.029. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat Genet. 1998;20:228–30. doi: 10.1038/3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlova A, Boutin E, Cunha G, Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development. 1994;120:335–45. doi: 10.1242/dev.120.2.335. [DOI] [PubMed] [Google Scholar]
- 31.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–45. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 32.Schrager S, Potter BE. Diethylstilbestrol exposure. Am Fam Physician. 2004;69:2395–400. [PubMed] [Google Scholar]
- 33.Newbold RR, Tyrey S, Haney AF, McLachlan JA. Developmentally arrested oviduct: a structural and functional defect in mice following prenatal exposure to diethylstilbestrol. Teratology. 1983;27:417–26. doi: 10.1002/tera.1420270316. [DOI] [PubMed] [Google Scholar]
- 34.Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20:81–4. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Cook JD, Davis BJ, Cai SI, Barrett JC, Conti CJ, Walker CL. Interaction between genetic susceptibility and early -life environmental exposure determines tumor-suppressor-gene penetrance. Proceedings of the National Acad of Scientists USA. 2005;102:8644–9. doi: 10.1073/pnas.0503218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Research. 1980;40:3988–99. [PubMed] [Google Scholar]
- 37.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–63. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- 38.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–63. [PubMed] [Google Scholar]
- 39.Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES) Int J Epidemiol. 2006;35:862–8. doi: 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- 40.Blatt J, Van Le L, Weiner T, Sailer S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J Pediatr Hematol Oncol. 2003;25:635–6. doi: 10.1097/00043426-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod. 2006;21:666–9. doi: 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- 42.Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359:1102–7. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- 43.Colborn T, Dumanoski D, Myers JP. Our Stolen Future. Penguin Books USA, Inc; 1996. [Google Scholar]
- 44.Guillette LJ, Jr, Edwards TM. Environmental Influences on Fertility: Can We Learn Lessons from Studies of Wildlife? Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.019. In Press. [DOI] [PubMed] [Google Scholar]
- 45.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspec. 1993;101:378–84. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspec. 1994;102:680–8. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guillette LJJ, Moore BC. Contaminants, fertility and multioocytic follicles: A lesson from wildlife? Sem Reprod Med. 2006;24:134–41. doi: 10.1055/s-2006-944419. [DOI] [PubMed] [Google Scholar]
- 48.Iguchi T. Cellular effects of early exposure to sex hormones and antihormones. Intn Rev Cytol. 1992;139:1–57. doi: 10.1016/s0074-7696(08)61409-6. [DOI] [PubMed] [Google Scholar]
- 49.Matter JM, Crain DA, Sills-McMurry C, Pickford DB, Rainwater TR, Reynolds KD, et al. Effects of endocrine-disrupting contaminants in reptiles: Alligators. In: Kendall R, Dickerson R, Giesy J, Suk W, editors. Principles and Processes for Evaluating Endocrine Disruption in Wildlife. Pensacola, FL: SETAC Pr; 1998. pp. 267–89. [Google Scholar]
- 50.Milnes MR, Bryan TA, Medina JG, Gunderson MP, Guillette LJ., Jr Developmental alterations as a result of in ovo exposure to the pesticide metabolite p,p′-DDE in Alligator mississippiensis. Gen Comp Endocrin. 2005;144:257–63. doi: 10.1016/j.ygcen.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Stoker C, Rey F, Rodriguez H, Ramos JG, Sirosky P, Larriera A, et al. Sex reversal effects on Caiman latirostris exposed to environmentally relevant doses of the xenoestrogen bisphenol A. Gen Comp Endocrinol. 2003;133:287–96. doi: 10.1016/s0016-6480(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 52.Willingham E, Crews D. Sex reversal effects of environmentally relevant xenobiotic concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination. Gen Comp Endocrinol. 1999;113:429–35. doi: 10.1006/gcen.1998.7221. [DOI] [PubMed] [Google Scholar]
- 53.Willingham E, Rhen T, Sakata JT, Crew D. Embryonic treatment with xenobiotics disrupts steroid hormone profiles in hatchling red-eared slider turtles (Trachemys scripta elegans) Environ Health Perspec. 2000;108:329–32. doi: 10.1289/ehp.00108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jobling S, Beresford N, Nolan M, Rodgers-Gray TP, Brighty G, Sumpter J, et al. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol Repro. 2002;66:272–81. doi: 10.1095/biolreprod66.2.272. [DOI] [PubMed] [Google Scholar]
- 55.Mylchreest E, Sar M, Wallace DG, Foster PMD. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 56.Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: A critical review of the evidence. Crit Rev Toxicol. 1998;28:319–61. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- 57.Iguchi T, Watanabe H, Katsu Y. Developmental effects of estrogenic agents on mice, fish and frogs: a mini-review. Horm Behav. 2001;40:248–51. doi: 10.1006/hbeh.2001.1675. [DOI] [PubMed] [Google Scholar]
- 58.Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin IV, et al. Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res. 2006;100:3–17. doi: 10.1016/j.envres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer. 2006;118:3099–111. doi: 10.1002/ijc.21747. [DOI] [PubMed] [Google Scholar]
- 60.Jorgensen N, Asklund C, Carlsen E, Skakkebaek NE. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl. 2006;29:54–61. doi: 10.1111/j.1365-2605.2005.00635.x. discussion 105–8. [DOI] [PubMed] [Google Scholar]
- 61.Andersson Trends in Leydig cell function in Danish men. Human Reproduction. 2005;20:i26–7. [Google Scholar]
- 62.Perheentupa A, Laatikainen T, Vierula M, Skakkebaek NE, Andersson AM, Toppari T. Clear Birth Cohort Effect in Serum Testosterone and SHBG Levels in Finnish Men. Endocrine Society Meeting 2006; 2006. [DOI] [PubMed] [Google Scholar]
- 63.Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- 64.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herman-Giddens ME. Recent data on pubertal milestones in Unite States children: the secular trend toward earlier development. Int J Androl. 2006;29:241–6. doi: 10.1111/j.1365-2605.2005.00575.x. discussion 86–90. [DOI] [PubMed] [Google Scholar]
- 66.Russo VEA, Martienssen RA, Riggs AD, editors. Epigenetic Mechanisms of Gene Regulation. Plainview; NY: 1996. [Google Scholar]
- 67.Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. Faseb J. 2007;21:239–46. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- 68.Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.026. In Press. [DOI] [PubMed] [Google Scholar]
- 69.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 70.Sharpe RM, Skakkebaek NE. Male reproductive disorders and the role of endocrine disruption: Advances in understanding and identification of areas for future research. Pure and Applied Chemistry. 2003;75:2023–38. [Google Scholar]
- 71.Mylchreest E, Wallace DG, Cattley RC, Foster PM. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci. 2000;55:143–51. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- 72.Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–49. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 73.Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human 'testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–94. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- 74.Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–23. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- 75.Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, et al. Role of Androgens in Fetal Testis Development and Dysgenesis. Endocrinology. 2007;148:2027–36. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- 76.Hoei-Hansen CE, Holm M, Rajpert-De Meyts E, Skakkebaek NE. Histological evidence of testicular dysgenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol. 2003;200:370–4. doi: 10.1002/path.1372. [DOI] [PubMed] [Google Scholar]
- 77.Nistal M, Gonzalez-Peramato P, Regadera J, Serrano A, Tarin V, De Miguel MP. Primary testicular lesions are associated with testicular germ cell tumors of adult men. Am J Surg Pathol. 2006;30:1260–8. doi: 10.1097/01.pas.0000213361.10756.08. [DOI] [PubMed] [Google Scholar]
- 78.Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, et al. The role of androgens in sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology. 2005;146:2674–83. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 80.Atanassova NN, Walker M, McKinnell C, Fisher JS, Sharpe RM. Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol. 2005;184:107–17. doi: 10.1677/joe.1.05884. [DOI] [PubMed] [Google Scholar]
- 81.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 82.Cortes D, Muller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10:589–96. doi: 10.1111/j.1365-2605.1987.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 83.Baskin LS. Can We Prevent Hypospadias? Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prins GS, Tang WY, Belmonte J, Ho SM. Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: epigenetic mode of action is implicated. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.023. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauser R, Sokol R. Science Linking Environmental Contaminant Exposures with Fertility and Reproductive Health Impacts in the Adult Male. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.033. In Press. [DOI] [PubMed] [Google Scholar]
- 88.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 89.Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–93. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- 90.Hsu PC, Huang W, Yao WJ, Wu MH, Guo YL, Lambert GH. Sperm changes in men exposed to polychlorinated biphenyls and dibenzofurans. Jama. 2003;289:2943–4. doi: 10.1001/jama.289.22.2943. [DOI] [PubMed] [Google Scholar]
- 91.Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- 92.Hauser R. The environment and male fertility: recent research on emerging chemicals and semen quality. Semin Reprod Med. 2006;24:156–67. doi: 10.1055/s-2006-944422. [DOI] [PubMed] [Google Scholar]
- 93.Cunningham M. Chronic occupational lead exposure: the potential effect on sexual function and reproductive ability in male workers. Aaohn J. 1986;34:277–9. [PubMed] [Google Scholar]
- 94.Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update. 2000;6:107–21. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- 95.Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45–53. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Apostoli P, Kiss P, Porru S, Bonde JP, Vanhoorne M. Male reproductive toxicity of lead in animals and humans. ASCLEPIOS Study Group. Occup Environ Med. 1998;55:364–74. doi: 10.1136/oem.55.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lancranjan I, Popescu HI, O GA, Klepsch I, Serbanescu M. Reproductive ability of workmen occupationally exposed to lead. Arch Environ Health. 1975;30:396–401. doi: 10.1080/00039896.1975.10666733. [DOI] [PubMed] [Google Scholar]
- 98.Winder C. Reproductive and chromosomal effects of occupational exposure to lead in the male. Reprod Toxicol. 1989;3:221–33. doi: 10.1016/0890-6238(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 99.Sheiner EK, Sheiner E, Hammel RD, Potashnik G, Carel R. Effect of occupational exposures on male fertility: literature review. Ind Health. 2003;41:55–62. doi: 10.2486/indhealth.41.55. [DOI] [PubMed] [Google Scholar]
- 100.Figa-Talamanca I, Traina ME, Urbani E. Occupational exposures to metals, solvents and pesticides: recent evidence on male reproductive effects and biological markers. Occup Med (Lond) 2001;51:174–88. doi: 10.1093/occmed/51.3.174. [DOI] [PubMed] [Google Scholar]
- 101.Potashnik G, Porath A. Dibromochloropropane (DBCP): a 17-year reassessment of testicular function and reproductive performance. J Occup Environ Med. 1995;37:1287–92. doi: 10.1097/00043764-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Lantz GD, Cunningham GR, Huckins C, Lipshultz LI. Recovery from severe oligospermia after exposure to dibromochloropropane. Fertil Steril. 1981;35:46–53. doi: 10.1016/s0015-0282(16)45257-x. [DOI] [PubMed] [Google Scholar]
- 103.Britt KL, Findlay JK. Estrogen actions in the ovary revisited. J Endocrinol. 2002;175:269–76. doi: 10.1677/joe.0.1750269. [DOI] [PubMed] [Google Scholar]
- 104.Palter SF, Tavares AB, Hourvitz A, Veldhuis JD, Adashi EY. Are estrogens of import to primate/human ovarian folliculogenesis? Endocr Rev. 2001;22:389–424. doi: 10.1210/edrv.22.3.0433. [DOI] [PubMed] [Google Scholar]
- 105.Mather JP, Moore A, Li RH. Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Proc Soc Exp Biol Med. 1997;215:209–22. doi: 10.3181/00379727-215-44130. [DOI] [PubMed] [Google Scholar]
- 106.Findlay JK, Drummond AE, Dyson M, Baillie AJ, Robertson DM, Ethier JF. Production and actions of inhibin and activin during folliculogenesis in the rat. Mol Cell Endocrinol. 2001;180:139–44. doi: 10.1016/s0303-7207(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 107.Pangas SA, Rademaker AW, Fishman DA, Woodruff TK. Localization of the activin signal transduction components in normal human ovarian follicles: implications for autocrine and paracrine signaling in the ovary. J Clin Endocrinol Metab. 2002;87:2644–57. doi: 10.1210/jcem.87.6.8519. [DOI] [PubMed] [Google Scholar]
- 108.Ethier JF, Findlay JK. Roles of activin and its signal transduction mechanisms in reproductive tissues. Reproduction. 2001;121:667–75. doi: 10.1530/rep.0.1210667. [DOI] [PubMed] [Google Scholar]
- 109.Woodruff TK, Lyon RJ, Hansen SE, Rice GC, Mather JP. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990;127:3196–205. doi: 10.1210/endo-127-6-3196. [DOI] [PubMed] [Google Scholar]
- 110.Xiao S, Robertson DM, Findlay JK. Effects of activin and follicle-stimulating hormone (FSH)-suppressing protein/follistatin on FSH receptors and differentiation of cultured rat granulosa cells. Endocrinology. 1992;131:1009–16. doi: 10.1210/endo.131.3.1505447. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura M, Nakamura K, Igarashi S, Tano M, Miyamoto K, Ibuki Y, et al. Interaction between activin A and cAMP in the induction of FSH receptor in cultured rat granulosa cells. J Endocrinol. 1995;147:103–10. doi: 10.1677/joe.0.1470103. [DOI] [PubMed] [Google Scholar]
- 112.Sadatsuki M, Tsutsumi O, Yamada R, Muramatsu M, Taketani Y. Local regulatory effects of activin A and follistatin on meiotic maturation of rat oocytes. Biochem Biophys Res Commun. 1993;196:388–95. doi: 10.1006/bbrc.1993.2261. [DOI] [PubMed] [Google Scholar]
- 113.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Neonatal estrogen exposure inhibits steroidogenesis in the developing rat ovary. Dev Dyn. 2001;221:443–53. doi: 10.1002/dvdy.1162. [DOI] [PubMed] [Google Scholar]
- 114.Forsberg JG. Treatment with different antiestrogens in the neonatal period and effects in the cervicovaginal epithelium and ovaries of adult mice: a comparison to estrogen-induced changes. Biol Reprod. 1985;32:427–41. doi: 10.1095/biolreprod32.2.427. [DOI] [PubMed] [Google Scholar]
- 115.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–84. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 116.Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34:29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- 117.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–96. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 118.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–81. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 119.Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog. 2003;38:78–84. doi: 10.1002/mc.10147. [DOI] [PubMed] [Google Scholar]
- 120.Newbold RR, Hanson RB, Jefferson WN. Ontogeny of lactoferrin in the developing mouse uterus: a marker of early hormone response. Biol Reprod. 1997;56:1147–57. doi: 10.1095/biolreprod56.5.1147. [DOI] [PubMed] [Google Scholar]
- 121.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50:7677–81. [PubMed] [Google Scholar]
- 122.Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, et al. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001;238:224–38. doi: 10.1006/dbio.2001.0413. [DOI] [PubMed] [Google Scholar]
- 123.Mendola P, Messer LC, Rappazzo R. Science Linking Environmental Contaminant Exposures with Fertility and Reproductive Health Impacts in the Adult Female. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.036. In Press. [DOI] [PubMed] [Google Scholar]
- 124.Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348:1527–36. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- 125.Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Environ Health Perspect. 2003;111:737–41. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP. Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics. 2005;115:e127–34. doi: 10.1542/peds.2004-1161. [DOI] [PubMed] [Google Scholar]
- 127.Colon I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect. 2000;108:895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ouyang F, Perry MJ, Venners SA, Chen C, Wang B, Yang F, et al. Serum DDT, age at menarche, and abnormal menstrual cycle length. Occup Environ Med. 2005;62:878–84. doi: 10.1136/oem.2005.020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krstevska-Konstantinova M, Charlier C, Craen M, Du Caju M, Heinrichs C, de Beaufort C, et al. Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Hum Reprod. 2001;16:1020–6. doi: 10.1093/humrep/16.5.1020. [DOI] [PubMed] [Google Scholar]
- 130.Tang N, Zhu ZQ. Adverse reproductive effects in female workers of lead battery plants. Int J Occup Med Environ Health. 2003;16:359–61. [PubMed] [Google Scholar]
- 131.Windham GC, Waller K, Anderson M, Fenster L, Mendola P, Swan S. Chlorination byproducts in drinking water and menstrual cycle function. Environ Health Perspect. 2003;111:935–41. doi: 10.1289/ehp.5922. discussion A409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eskenazi B, Warner M, Mocarelli P, Samuels S, Needham LL, Patterson DG, Jr, et al. Serum dioxin concentrations and menstrual cycle characteristics. Am J Epidemiol. 2002;156:383–92. doi: 10.1093/aje/kwf046. [DOI] [PubMed] [Google Scholar]
- 133.Farr SL, Cooper GS, Cai J, Savitz DA, Sandler DP. Pesticide use and menstrual cycle characteristics among premenopausal women in the Agricultural Health Study. Am J Epidemiol. 2004;160:1194–204. doi: 10.1093/aje/kwi006. [DOI] [PubMed] [Google Scholar]
- 134.Cooper GS, Klebanoff MA, Promislow J, Brock JW, Longnecker MP. Polychlorinated biphenyls and menstrual cycle characteristics. Epidemiology. 2005;16:191–200. doi: 10.1097/01.ede.0000152913.12393.86. [DOI] [PubMed] [Google Scholar]
- 135.Hsieh GY, Wang JD, Cheng TJ, Chen PC. Prolonged menstrual cycles in female workers exposed to ethylene glycol ethers in the semiconductor manufacturing industry. Occup Environ Med. 2005;62:510–6. doi: 10.1136/oem.2004.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X, Tian J. Health risks related to residential exposure to cadmium in Zhenhe County, China. Arch Environ Health. 2004;59:324–30. doi: 10.3200/AEOH.59.6.324-330. [DOI] [PubMed] [Google Scholar]
- 137.Yu ML, Guo YL, Hsu CC, Rogan WJ. Menstruation and reproduction in women with polychlorinated biphenyl (PCB) poisoning: long-term follow-up interviews of the women from the Taiwan Yucheng cohort. Int J Epidemiol. 2000;29:672–7. doi: 10.1093/ije/29.4.672. [DOI] [PubMed] [Google Scholar]
- 138.Gerhard I, Frick A, Monga B, Runnebaum B. Pentachlorophenol exposure in women with gynecological and endocrine dysfunction. Environ Res. 1999;80:383–8. doi: 10.1006/enrs.1998.3934. [DOI] [PubMed] [Google Scholar]
- 139.Perry MJ, Ouyang F, Korrick SA, Venners SA, Chen C, Xu X, et al. A prospective study of serum DDT and progesterone and estrogen levels across the menstrual cycle in nulliparous women of reproductive age. Am J Epidemiol. 2006;164:1056–64. doi: 10.1093/aje/kwj329. [DOI] [PubMed] [Google Scholar]
- 140.Windham GC, Lee D, Mitchell P, Anderson M, Petreas M, Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005;16:182–90. doi: 10.1097/01.ede.0000152527.24339.17. [DOI] [PubMed] [Google Scholar]
- 141.Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, et al. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod. 2003;18:1512–5. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- 142.Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. Bjog. 2006;113:515–20. doi: 10.1111/j.1471-0528.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 143.Chang SH, Cheng BH, Lee SL, Chuang HY, Yang CY, Sung FC, et al. Low blood lead concentration in association with infertility in women. Environ Res. 2006;101:380–6. doi: 10.1016/j.envres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Venners SA, Korrick S, Xu X, Chen C, Guang W, Huang A, et al. Preconception serum DDT and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol. 2005;162:709–16. doi: 10.1093/aje/kwi275. [DOI] [PubMed] [Google Scholar]
- 145.Longnecker MP, Klebanoff MA, Dunson DB, Guo X, Chen Z, Zhou H, et al. Maternal serum level of the DDT metabolite DDE in relation to fetal loss in previous pregnancies. Environ Res. 2005;97:127–33. doi: 10.1016/S0013-9351(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 146.Korrick SA, Chen C, Damokosh AI, Ni J, Liu X, Cho SI, et al. Association of DDT with spontaneous abortion: a case-control study. Ann Epidemiol. 2001;11:491–6. doi: 10.1016/s1047-2797(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 147.Greenlee AR, Arbuckle TE, Chyou PH. Risk factors for female infertility in an agricultural region. Epidemiology. 2003;14:429–36. doi: 10.1097/01.EDE.0000071407.15670.aa. [DOI] [PubMed] [Google Scholar]
- 148.Abell A, Juul S, Bonde JP. Time to pregnancy among female greenhouse workers. Scand J Work Environ Health. 2000;26:131–6. doi: 10.5271/sjweh.522. [DOI] [PubMed] [Google Scholar]
- 149.Curtis KM, Savitz DA, Weinberg CR, Arbuckle TE. The effect of pesticide exposure on time to pregnancy. Epidemiology. 1999;10:112–7. [PubMed] [Google Scholar]
- 150.Idrovo AJ, Sanin LH, Cole D, Chavarro J, Caceres H, Narvaez J, et al. Time to first pregnancy among women working in agricultural production. Int Arch Occup Environ Health. 2005;78:493–500. doi: 10.1007/s00420-005-0615-9. [DOI] [PubMed] [Google Scholar]
- 151.Crisostomo L, Molina VV. Pregnancy outcomes among farming households of Nueva Ecija with conventional pesticide use versus integrated pest management. Int J Occup Environ Health. 2002;8:232–42. doi: 10.1179/107735202800338812. [DOI] [PubMed] [Google Scholar]
- 152.Garry VF, Harkins M, Lyubimov A, Erickson L, Long L. Reproductive outcomes in the women of the Red River Valley of the north. I. The spouses of pesticide applicators: pregnancy loss, age at menarche, and exposures to pesticides. J Toxicol Environ Health A. 2002;65:769–86. doi: 10.1080/00984100290071333. [DOI] [PubMed] [Google Scholar]
- 153.Arbuckle TE, Savitz DA, Mery LS, Curtis KM. Exposure to phenoxy herbicides and the risk of spontaneous abortion. Epidemiology. 1999;10:752–60. [PubMed] [Google Scholar]
- 154.Arbuckle TE, Lin Z, Mery LS. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ Health Perspect. 2001;109:851–7. doi: 10.1289/ehp.01109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bindali BB, Kaliwal BB. Anti-implantation effect of a carbamate fungicide mancozeb in albino mice. Ind Health. 2002;40:191–7. doi: 10.2486/indhealth.40.191. [DOI] [PubMed] [Google Scholar]
- 156.Greenlee AR, Ellis TM, Berg RL. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ Health Perspect. 2004;112:703–9. doi: 10.1289/ehp.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Plenge-Bonig A, Karmaus W. Exposure to toluene in the printing industry is associated with subfecundity in women but not in men. Occup Environ Med. 1999;56:443–8. doi: 10.1136/oem.56.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wennborg H, Bodin L, Vainio H, Axelsson G. Solvent use and time to pregnancy among female personnel in biomedical laboratories in Sweden. Occup Environ Med. 2001;58:225–31. doi: 10.1136/oem.58.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sallmen M, Baird DD, Hoppin JA, Blair A, Sandler DP. Fertility and exposure to solvents among families in the Agricultural Health Study. Occup Environ Med. 2006;63:469–75. doi: 10.1136/oem.2005.021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20:2325–9. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 161.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A Exposure In Utero Disrupts Early Oogenesis in the Mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–53. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 163.Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, et al. Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005;113:858–62. doi: 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Akkina J, Reif J, Keefe T, Bachand A. Age at natural menopause and exposure to organochlorine pesticides in Hispanic women. J Toxicol Environ Health A. 2004;67:1407–22. doi: 10.1080/15287390490483845. [DOI] [PubMed] [Google Scholar]
- 165.Cooper GS, Savitz DA, Millikan R, Chiu Kit T. Organochlorine exposure and age at natural menopause. Epidemiology. 2002;13:729–33. doi: 10.1097/00001648-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 166.Farr SL, Cai J, Savitz DA, Sandler DP, Hoppin JA, Cooper GS. Pesticide exposure and timing of menopause: the Agricultural Health Study. Am J Epidemiol. 2006;163:731–42. doi: 10.1093/aje/kwj099. [DOI] [PubMed] [Google Scholar]
- 167.Taupeau C, Poupon J, Nome F, Lefevre B. Lead accumulation in the mouse ovary after treatment-induced follicular atresia. Reprod Toxicol. 2001;15:385–91. doi: 10.1016/s0890-6238(01)00139-3. [DOI] [PubMed] [Google Scholar]
- 168.Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14:1903–7. doi: 10.1093/humrep/14.7.1903. [DOI] [PubMed] [Google Scholar]
- 169.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, et al. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–81. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 170.Kao SW, Sipes IG, Hoyer PB. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999;13:67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 171.Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167:191–8. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- 172.Bodensteiner KJ, Sawyer HR, Moeller CL, Kane CM, Pau KY, Klinefelter GR, et al. Chronic exposure to dibromoacetic acid, a water disinfection byproduct, diminishes primordial follicle populations in the rabbit. Toxicol Sci. 2004;80:83–91. doi: 10.1093/toxsci/kfh135. [DOI] [PubMed] [Google Scholar]
- 173.Baligar PN, Kaliwal BB. Induction of gonadal toxicity to female rats after chronic exposure to mancozeb. Ind Health. 2001;39:235–43. doi: 10.2486/indhealth.39.235. [DOI] [PubMed] [Google Scholar]
- 174.Windham G, Fenster L. Environmental Contaminants and Pregnancy Outcomes. Fertil Steril. doi: 10.1016/j.fertnstert.2007.12.041. In Press. [DOI] [PubMed] [Google Scholar]
- 175.National Cancer Institute. Health Effects of Exposure to Environmental Tobacco Smoke: The Report of the California Environmental Protection Agency, Smoking and Tobacco Control Monograph no. 10: US Department of Health and Human Services, National Institutes of Health, 1999.
- 176.U.S. Dept. of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: USDHHS, Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- 177.Bove F, Shim Y, Zeitz P. Drinking water contaminants and adverse pregnancy outcomes: a review. Environ Health Perspect. 2002;110(Suppl 1):61–74. doi: 10.1289/ehp.02110s161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup Environ Med. 2000;57:73–85. doi: 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tardiff RG, Carson ML, Ginevan ME. Updated weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection byproducts. Regul Toxicol Pharmacol. 2006;45:185–205. doi: 10.1016/j.yrtph.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 180.Al-Hiyasat AS, Darmani H, Elbetieha AM. Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci. 2002;110:163–7. doi: 10.1034/j.1600-0722.2002.11201.x. [DOI] [PubMed] [Google Scholar]
- 181.Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, Hayashi Y, et al. Bisphenol-A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–90. [Google Scholar]
- 182.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 183.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102:7014–9. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224:61–8. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 185.Herath CB, Jin W, Watanabe G, Arai K, Suzuki AK, Taya K. Adverse effects of environmental toxicants, octylphenol and bisphenol A, on male reproductive functions in pubertal rats. Endocrine. 2004;25:163–72. doi: 10.1385/ENDO:25:2:163. [DOI] [PubMed] [Google Scholar]
- 186.del Rio Gomez I, Marshall T, Tsai P, Shao YS, Guo YL. Number of boys born to men exposed to polychlorinated byphenyls. Lancet. 2002;360:143–4. doi: 10.1016/s0140-6736(02)09386-8. [DOI] [PubMed] [Google Scholar]
- 187.Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertil Steril. 1998;70:613–22. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 188.Hruska KS, Furth PA, Seifer DB, Sharara FI, Flaws JA. Environmental factors in infertility. Clin Obstet Gynecol. 2000;43:821–9. doi: 10.1097/00003081-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 189.LaDou J, editor. Occupational and Environmental Medicine. 3. Stamford, Connecticut: Lange Medical/McGraw Hill; 2004. [Google Scholar]
- 190.Correa A, Gray RH, Cohen R, Rothman N, Shah F, Seacat H, et al. Ethylene glycol ethers and risks of spontaneous abortion and subfertility. Am J Epidemiol. 1996;143:707–17. doi: 10.1093/oxfordjournals.aje.a008804. [DOI] [PubMed] [Google Scholar]
- 191.Eskenazi B, Gold EB, Samuels SJ, Wight S, Lasley BL, Hammond SK, et al. Prospective assessment of fecundability of female semiconductor workers. Am J Ind Med. 1995;28:817–31. doi: 10.1002/ajim.4700280614. [DOI] [PubMed] [Google Scholar]
- 192.Fuortes L, Clark MK, Kirchner HL, Smith EM. Association between female infertility and agricultural work history. Am J Ind Med. 1997;31:445–51. doi: 10.1002/(sici)1097-0274(199704)31:4<445::aid-ajim11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 193.Schettler T, Solomon G, Valenti M, Huddle A. Generations at Risk. Reproductive Health and the Environment. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 194.Oliva A, Spira A, Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16:1768–76. doi: 10.1093/humrep/16.8.1768. [DOI] [PubMed] [Google Scholar]
- 195.Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–84. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goldsmith JR. Dibromochloropropane: epidemiological findings and current questions. Ann N Y Acad Sci. 1997;837:300–6. doi: 10.1111/j.1749-6632.1997.tb56882.x. [DOI] [PubMed] [Google Scholar]
- 197.Savitz DA, Arbuckle T, Kaczor D, Curtis KM. Male pesticide exposure and pregnancy outcome. Am J Epidemiol. 1997;146:1025–36. doi: 10.1093/oxfordjournals.aje.a009231. [DOI] [PubMed] [Google Scholar]
- 198.Recio R, Robbins WA, Borja-Aburto V, Moran-Martinez J, Froines JR, Hernandez RM, et al. Organophosphorous pesticide exposure increases the frequency of sperm sex null aneuploidy. Environ Health Perspect. 2001;109:1237–40. doi: 10.1289/ehp.011091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sanchez-Pena LC, Reyes BE, Lopez-Carrillo L, Recio R, Moran-Martinez J, Cebrian ME, et al. Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol Appl Pharmacol. 2004;196:108–13. doi: 10.1016/j.taap.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 200.Garry VF, Holland SE, Erickson LL, Burroughs BL. Male reproductive hormones and thyroid function in pesticide applicators in the Red River Valley of Minnesota. J Toxicol Environ Health A. 2003;66:965–86. doi: 10.1080/15287390306399. [DOI] [PubMed] [Google Scholar]
- 201.Garry VF, Schreinemachers D, Harkins ME, Griffith J. Pesticide appliers, biocides, and birth defects in rural Minnesota. Environ Health Perspect. 1996;104:394–9. doi: 10.1289/ehp.96104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. The effects of atrazine on female wistar rats: an evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol Sci. 2000;58:366–76. doi: 10.1093/toxsci/58.2.366. [DOI] [PubMed] [Google Scholar]
- 203.Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE., Jr Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol Ind Health. 1999;15:65–79. doi: 10.1177/074823379901500107. [DOI] [PubMed] [Google Scholar]
- 204.Stoker TE, Guidici DL, Laws SC, Cooper RL. The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol Sci. 2002;67:198–206. doi: 10.1093/toxsci/67.2.198. [DOI] [PubMed] [Google Scholar]
- 205.Kristensen P, Irgens LM, Andersen A, Bye AS, Sundheim L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology. 1997;8:537–44. doi: 10.1097/00001648-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 206.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–15. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 207.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–64. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 208.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–32. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–5. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Levario-Carrillo M, Amato D, Ostrosky-Wegman P, Gonzalez-Horta C, Corona Y, Sanin LH. Relation between pesticide exposure and intrauterine growth retardation. Chemosphere. 2004;55:1421–7. doi: 10.1016/j.chemosphere.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 212.Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–77. [PubMed] [Google Scholar]
- 213.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–45. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Couse JF, Korach KS. Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology. 2004;205:55–63. doi: 10.1016/j.tox.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 215.Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 216.Svensson BG, Nise G, Erfurth EM, Nilsson A, Skerfving S. Hormone status in occupational toluene exposure. Am J Ind Med. 1992;22:99–107. doi: 10.1002/ajim.4700220109. [DOI] [PubMed] [Google Scholar]
- 217.Winker R, Rudiger HW. Reproductive toxicology in occupational settings: an update. Int Arch Occup Environ Health. 2006;79:1–10. doi: 10.1007/s00420-005-0011-5. [DOI] [PubMed] [Google Scholar]
- 218.Ichihara G. Neuro-reproductive toxicities of 1-bromopropane and 2-bromopropane. Int Arch Occup Environ Health. 2005;78:79–96. doi: 10.1007/s00420-004-0547-9. [DOI] [PubMed] [Google Scholar]
- 219.Younglai EV, Holloway AC, Foster WG. Environmental and occupational factors affecting fertility and IVF success. Hum Reprod Update. 2005;11:43–57. doi: 10.1093/humupd/dmh055. [DOI] [PubMed] [Google Scholar]
- 220.Sallmen M, Lindbohm ML, Kyyronen P, Nykyri E, Anttila A, Taskinen H, et al. Reduced fertility among women exposed to organic solvents. Am J Ind Med. 1995;27:699–713. doi: 10.1002/ajim.4700270506. [DOI] [PubMed] [Google Scholar]
- 221.Beliles RP. Concordance across species in the reproductive and developmental toxicity of tetrachloroethylene. Toxicol Ind Health. 2002;18:91–106. doi: 10.1191/0748233702th137oa. [DOI] [PubMed] [Google Scholar]
- 222.Eskenazi B, Wyrobek AJ, Fenster L, Katz DF, Sadler M, Lee J, et al. A study of the effect of perchloroethylene exposure on semen quality in dry cleaning workers. Am J Ind Med. 1991;20:575–91. doi: 10.1002/ajim.4700200502. [DOI] [PubMed] [Google Scholar]
- 223.Kyyronen P, Taskinen H, Lindbohm ML, Hemminki K, Heinonen OP. Spontaneous abortions and congenital malformations among women exposed to tetrachloroethylene in dry cleaning. J Epidemiol Community Health. 1989;43:346–51. doi: 10.1136/jech.43.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vine MF. Smoking and male reproduction: a review. Int J Androl. 1996;19:323–37. doi: 10.1111/j.1365-2605.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 225.U.S. Dept. of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Google Scholar]
- 226.Jensen TK, Jorgensen N, Punab M, Haugen TB, Suominen J, Zilaitiene B, et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol. 2004;159:49–58. doi: 10.1093/aje/kwh002. [DOI] [PubMed] [Google Scholar]
- 227.Miller KP, Borgeest C, Greenfeld C, Tomic D, Flaws JA. In utero effects of chemicals on reproductive tissues in females. Toxicol Appl Pharmacol. 2004;198:111–31. doi: 10.1016/j.taap.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 228.Storgaard L, Bonde JP, Ernst E, Spano M, Andersen CY, Frydenberg M, et al. Does smoking during pregnancy affect sons' sperm counts? Epidemiology. 2003;14:278–86. [PubMed] [Google Scholar]
- 229.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198:231–41. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 230.Butenhoff JL, Kennedy GL, Jr, Frame SR, O'Connor JC, York RG. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196:95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 231.Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ, Butenhoff JL. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology. 2005;215:126–48. doi: 10.1016/j.tox.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 232.Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–54. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect. 1995;103:1136–43. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sweeney T, Nicol L, Roche JF, Brooks AN. Maternal exposure to octylphenol suppresses ovine fetal follicle-stimulating hormone secretion, testis size, and sertoli cell number. Endocrinology. 2000;141:2667–73. doi: 10.1210/endo.141.7.7552. [DOI] [PubMed] [Google Scholar]
- 235.Bogh IB, Christensen P, Dantzer V, Groot M, Thofner IC, Rasmussen RK, et al. Endocrine disrupting compounds: effect of octylphenol on reproduction over three generations. Theriogenology. 2001;55:131–50. doi: 10.1016/s0093-691x(00)00451-9. [DOI] [PubMed] [Google Scholar]
- 236.Toft G, Hagmar L, Giwercman A, Bonde JP. Epidemiological evidence on reproductive effects of persistent organochlorines in humans. Reprod Toxicol. 2004;19:5–26. doi: 10.1016/j.reprotox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 237.Buck Louis GM, Weinter JM, Whitcomb BW, Sperrazza R, Schisterman EF, Lobdell DT, et al. Environmental polychlorinated biphenyl exposure and risk of endometriosis. Obstet gynecol surv. 2005;60:243–4. doi: 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- 238.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, et al. Serum dioxin concentrations and endometriosis: a cohort study in Seveso, Italy. Environ Health Perspect. 2002;110:629–34. doi: 10.1289/ehp.02110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT) Lancet. 2005;366:763–73. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- 240.Hauser R, Chen Z, Pothier L, Ryan L, Altshul L. The relationship between human semen parameters and environmental exposure to polychlorinated biphenyls and p,p′-DDE. Environ Health Perspect. 2003;111:1505–11. doi: 10.1289/ehp.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Den Hond E, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, Vandermeulen C, et al. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek's hypothesis revisited. Environ Health Perspect. 2002;110:771–6. doi: 10.1289/ehp.02110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Axmon A. Menarche in women with high exposure to persistent organochlorine pollutants in utero and during childhood. Environ Res. 2006;102:77–82. doi: 10.1016/j.envres.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 243.Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol Sci. 1999;51:259–64. doi: 10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- 244.Hosie S, Loff S, Witt K, Niessen K, Waag KL. Is there a correlation between organochlorine compounds and undescended testes? Eur J Pediatr Surg. 2000;10:304–9. doi: 10.1055/s-2008-1072381. [DOI] [PubMed] [Google Scholar]
- 245.Hamm JT, Chen CY, Birnbaum LS. A mixture of dioxins, furans, and non-ortho PCBs based upon consensus toxic equivalency factors produces dioxin-like reproductive effects. Toxicol Sci. 2003;74:182–91. doi: 10.1093/toxsci/kfg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ryan JJ, Amirova Z, Carrier G. Sex ratios of children of Russian pesticide producers exposed to dioxin. Environ Health Perspect. 2002;110:A699–701. doi: 10.1289/ehp.021100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cohn BA, Cirillo PM, Wolff MS, Schwingl PJ, Cohen RD, Sholtz RI, et al. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet. 2003;361:2205–6. doi: 10.1016/S0140-6736(03)13776-2. [DOI] [PubMed] [Google Scholar]
- 248.Klaassen CD, editor. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 6. New York: McGraw Hill; 2001. [Google Scholar]
- 249.Sallmen M, Anttila A, Lindbohm ML, Kyyronen P, Taskinen H, Hemminki K. Time to pregnancy among women occupationally exposed to lead. J Occup Environ Med. 1995;37:931–4. doi: 10.1097/00043764-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 250.Choy CM, Lam CW, Cheung LT, Briton-Jones CM, Cheung LP, Haines CJ. Infertility, blood mercury concentrations and dietary seafood consumption: a case-control study. Bjog. 2002;109:1121–5. doi: 10.1111/j.1471-0528.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- 251.Jurasovic J, Cvitkovic P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals. 2004;17:735–43. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 252.Bonde JP, Joffe M, Apostoli P, Dale A, Kiss P, Spano M, et al. Sperm count and chromatin structure in men exposed to inorganic lead: lowest adverse effect levels. Occup Environ Med. 2002;59:234–42. doi: 10.1136/oem.59.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Louis RH. Environmental Influences on Female Reproductive Health. Presentation to the Association of Reproductive Health Professionals; 2005. [Google Scholar]
- 254.CHE. CHE Toxicant and Disease Database. Collaborative on Health and the Environment. http://database.healthandenvironment.org [accessed 16 October 2007]