Abstract
Because the mechanisms of (n-3) fatty acid–enriched triglyceride-rich particle [(n-3)-TGRP] uptake are not well characterized, we questioned whether (n-3)-TGRP are removed via “nonclassical” pathways, e.g., pathways other than an LDL receptor and/or involving apolipoprotein E (apoE). Chylomicron-sized model (n-3)-TGRP labeled with [3H]cholesteryl ether were injected into wild-type (WT) and CD36 knockout (CD36−/−) mice at low, nonsaturating and high, saturating doses. Blood clearance of (n-3)-TGRP was determined by calculating fractional catabolic rates. At saturating doses, blood clearance of (n-3)-TGRP was slower in CD36−/− mice relative to WT mice, suggesting that in part CD36 contributes to (n-3)-TGRP uptake. To further examine the potential nonclassical clearance pathways, peritoneal-elicited macrophages from WT and CD36−/− mice were incubated with (n-3)-TGRP in the presence of apoE, lactoferrin, and/or sodium chlorate. Cellular (n-3)-TGRP uptake was measured to test the roles of apoE-mediated pathways and/or proteoglycans. ApoE-mediated pathways compensated in part for defective (n-3)-TGRP uptake in CD36−/− cells. Lactoferrin decreased (n-3)-TGRP uptake in the presence of apoE. Inhibition of cell proteoglycan synthesis by chlorate reduced (n-3)-TGRP uptake in both groups of macrophages, and chlorate effects were independent of apoE. We conclude that although CD36 is involved, it is not the primary contributor to the blood clearance of (n-3)-TGRP. The removal of (n-3)-TGRP likely relies more on nonclassical pathways, such as proteoglycan-mediated pathways.
Introduction
Lipids are important macronutrients that provide energy in humans. They are a source of essential fatty acids, carriers of fatsoluble vitamins, and critical for growth and development. Recently, there has been growing interest in increasing intakes of (n-3) fatty acids, especially via triglycerides (TG)7 rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Using chylomicron-sized lipid emulsions as models for triglyceride-rich particles (TGRP), we reported that blood clearance and organ uptake of (n-6) emulsions depended on apolipoprotein E (apoE) and lipoprotein lipase (LpL)-mediated pathways, as well as the LDL receptor (LDLR) (1); these pathways were far less important for (n-3) emulsion tissue delivery. In fact, mechanisms for clearance of (n-3)-fatty acid–enriched triglyceride-rich particles [(n-3)-TGRP] remained poorly defined (1). We thus questioned if “nonclassical” pathways for TGRP could be important for (n-3)-TGRP clearance. Specifically, we examined the potential roles of CD36 and cell surface proteoglycans.
CD36, one of the membrane fatty acid transporters, is an 88-kDa membrane glycoprotein (2). The CD36 gene is located on human chromosome 7q11.2, mouse chromosome 5, and rat chromosome 4 (3-6). CD36 is primarily localized in tissues with high levels of fatty acid oxidation (7). It is highly expressed in heart, adipose tissue, and skeletal muscle with a predominance of oxidative fibers. CD36 functions as the class B scavenger receptor on monocytes/macrophages; it recognizes and internalizes oxidized LDL, leading to foam cell formation in the atherosclerosis process (8,9); and it transports long-chain fatty acids across cell membranes (10-12). Of interest is a possible role of (n-6) and (n-3) long-chain fatty acids in membrane translocation of CD36 (13).
Cell surface proteoglycans, which are abundant in the space of Disse in liver and on the surface of many cells, bind and participate in the capture of remnant lipoproteins by directly mediating uptake or by forming complexes with other receptors, such as LDLR related-protein (14-17). Our previous studies showed that at physiological particle concentrations, cell surface proteoglycans could provide a predominant nonreceptor mechanism for binding and internalizing intermediate-density-lipoprotein–sized TGRP derived from triolein (18). However, the role of proteoglycans in (n-3)-TGRP clearance remains to be elucidated.
To determine the possible roles of CD36 and proteoglycans in (n-3)-TGRP metabolism, we assessed blood clearance of 3H-labeled (n-3)-TGRP after an intravenous injection into wild-type (WT) C57BL/6J and CD36 knockout (CD36−/−) mice and, in in vitro studies, evaluated the mechanisms of cell uptake in elicited peritoneal macrophages from both strains of mice. Our findings indicate that although CD36 is involved, it is not primarily responsible for the blood clearance of (n-3)-TGRP. The removal of (n-3)-TGRP relies more on nonclassical pathways, such as those mediated by proteoglycans.
Materials and Methods
Materials
[1α, 2α (n)-3H] cholesteryl oleoyl ether ([3H]CEt) was purchased from Amersham Pharmacia Biotech. Avertin (2, 2, 2-tribromoethanol) was purchased from Aldrich Chemical and was solubilized at the concentrations of 125 mg/kg. Heparin sodium, DMEM, and lactoferrin were obtained from Sigma-Aldrich. Penicillin, streptomycin, and glutamine were purchased from Gibco Invitrogen. Sodium chlorate (NaClO3) was purchased from Fischer Scientific. Fatty acid–free bovine serum albumin and bovine calf serum were obtained from Gemini Bio-Products. Purified recombinant apoE3 produced in Escherichia coli was provided by Biotechnology General.
Model (n-3)-TGRP
Chylomicron-sized phospholipid-stabilized therapeutic emulsions of (n-3) fatty acid–enriched-TG (1) served as a model for (n-3)-TGRP and were kindly provided by B. Braun Melsungen AG. The emulsion contained TG with 200 g (n-3) fatty acid–rich TG/L, emulsified by 12 g/L egg yolk lecithin and 25 g/L glycerol. The (n-3)-rich emulsions contained TG with 34.4% EPA [20:5(n-3)] and 20.7% DHA [22:6(n-3)] (wt:wt), as published in detail elsewhere (1).
The (n-3) emulsions were radiolabeled with nondegradable [3H]CEt (0.2 mCi/100 mg TG and 0.05 mCi/100 TG for nonsaturating and saturating levels, respectively, to trace emulsion particle catabolism) (1). These labeling procedures did not change in vitro or in vivo metabolism of TGRP (1,19-21). Briefly, 50 μL of ethanol, 100 μL (nonsaturable amounts), or 25 μL (saturable amounts) of [3H]CEt were transferred to a small brown vial and completely evaporated under a stream of argon gas. Then, 83 μL of the stock emulsion was added immediately to the vial and mixed on a vortex for 10 s and left at room temperature for 25 min. This was repeated 3 times to produce a total of 250 μL emulsion. To incorporate the [3H]CEt into the core of the emulsion particles, the emulsion was sonicated 3 times on ice for 20 s each using a Branson Sonifier Cell Disruptor (model W185). The radiolabeled emulsion was centrifuged, 14,000 × g; 15 s at 4°C, to remove small amounts of titanium debris released from the sonifier probe. The final emulsion was stored under argon gas at 4°C and used for experiments within 7 d, with no appearance of physiochemical changes in the radiolabeled emulsion. There were no differences in the TG/phospholipid ratios or other physical properties, which we previously reported in the sonicated vs. unsonicated nonradiolabeled emulsions (1,19).
Animals
Male WT C57BL/6J mice, aged 10–12 wk, were purchased from Jackson Laboratory and had a 1- to 2-wk recovery period from transportation prior to the experiments. Homozygous CD36−/− founder mice were kindly provided by Dr. Nada A. Abumrad (Washington University, St. Louis, MO) (22) and were used for a breeding colony at Columbia University's Animal Facility. All mice were maintained under a 12-h light/12-h dark cycle and were fed a PioLab Rodent Diet 20 (PMI Nutrition International), with 65, 23, and 14% of energy provided by carbohydrate, protein, and fat, respectively, and consumed water ad libitum. All animal procedures were in compliance and approved by the Institutional Animal Care and Use Committee of Columbia University.
Animal procedures
After anesthetizing the mice with an intraperitoneal injection of Avertin (125 mg/kg), a bolus injection of 50 μL of radiolabeled emulsion [diluted with 0.9% NaCl, containing 400 μg of TG (low or nonsaturating dose) or 4 mg of TG (high or saturating dose)] (1) was performed via a saphenous vein. Retro-orbital blood was taken at 0.5, 2.5, 5, 10, 15, and 25 min via heparinized capillary tubes following the emulsion injection to measure blood emulsion clearance. At the end of the experiments, mice were thoroughly perfused with 0.9% NaCl containing heparin (2 kIU/L). Tritium in blood samples was counted using a Wallac 1409 Liquid Scintillation Counter (PerkinElmer Life Science).
To determine the percent recovery of the injected dose, total blood volume was calculated assuming that it represented 4.9% of body weight (1). Typically, 50–70% of the injected dose was recovered from the circulation. The radioactivity in the blood was expressed as a percentage of the injected radiolabeled emulsion remaining in the blood at each time point. The fractional catabolic rate (FCR) was calculated on the basis of first-order linear kinetics during the first 10 min when clearance followed first-order kinetics (1,23).
Lipid analyses
Concentrations of TG, total cholesterol, and free fatty acids were measured in the plasma of mice that had been food-deprived overnight, using commercial enzymatic colorimetric assays (TG/glycerol blanked kit and Cholesterol kit, Roche Diagnostics, and NEFA C kit, Wako Diagnostics, respectively).
Cell uptake of lipid emulsions in vitro
A total of 72 h after the intraperitoneal injection of 1 mL of 10% thioglycollate in WT and CD36−/− mice, thioglycollate-elicited macrophages were obtained by intraperitoneal lavage, as described (8,24-26). Briefly, cells were pelleted by centrifugation (Beckman TJ-6 Centrifuge), 134 × g; 10 min. After removing the supernatant fluid, pellet cells were resuspended in DMEM. Then, cells were plated and grown in a humidifier incubator (5% CO2) at 37°C for 4 h in DMEM containing 10% bovine calf serum and other standard supplements. To remove nonadherent cells, cells were washed with DMEM, and only macrophages remained attached to the plates. Mouse peritoneal macrophages were further grown in the previous growth media. On the next day, in some studies, macrophages were preincubated in growth media supplemented with 50 mmol/L NaClO3 for 24 h to inhibit sulfation of cell proteoglycan. Incubations of macrophages from WT and CD36−/− mice in the presence or absence of apoE were used as the control conditions for other perturbations. On the day of the experiment, cells were incubated with (n-3) rich-TG emulsion (200 mg TG/L) containing 100 nmol/L lactoferrin, or for those cells preincubated with NaClO3, 50 mmol/L NaClO3, with and without apoE (20:1 TG:apoE ratio, wt:wt) for 4 h. At the end of the experiment, cells were washed and lysed in 0.1 mol/L NaOH to measure the amount of TG, calculated from [3H]CEt-specific radioactivity taken up by cells and normalized by cell protein as described (18).
Statistical analysis
Data are expressed as means ± SEM. A 2-way ANOVA was performed to analyze FCR and macrophage uptake of (n-3)-TGRP in the presence or absence of apoE and the CD36 gene. A 3-way ANOVA was used for analysis of macrophage uptake of (n-3)-TGRP in the presence or absence of lactoferrin, apoE, and the CD36 gene, and similarly for NaClO3 in place of lactoferrin. A Bonferroni correction was used for the post hoc multiple comparisons in evaluating differences in FCR. All tests of statistical significance were defined as P < 0.05, and analyses were conducted with SPSS software (version 11.5).
Results
Plasma lipids and clearance of (n-3)-TGRP
Plasma, cholesterol, and free fatty acid concentrations in food-deprived CD36−/− mice were greater, and that of TG tended to be greater (P = 0.06), than in WT mice (Table 1), similar to earlier reports (27,28).
TABLE 1.
Plasma lipid concentrations in food-deprived CD36−/− and WT mice1
| Plasma lipid | WT | CD36−/− | P-value |
|---|---|---|---|
| mmol/L | |||
| TG | 0.54 ± 0.09 | 0.86 ± 0.11 | 0.06 |
| Cholesterol | 2.83 ± 0.11 | 3.39 ± 0.09 | <0.01 |
| Nonesterified fatty acid | 0.97 ± 0.05 | 1.27 ± 0.05 | <0.01 |
Values are means ± SEM, n = 7 or 8 (WT) or 4 (CD36−/−).
We previously demonstrated in vitro that at low concentrations, cell uptake of almost all (n-6)-TGRP was primarily cleared by the LDLR, whereas proteoglycan-mediated pathways played critical roles at higher TGRP concentrations (18). Thus, we examined in vivo whether blood clearance of an (n-3)-TG lipid emulsion can be influenced by the amount injected (Fig. 1). Clearance of (n-3)-TGRP from the blood after injection of nonsaturating doses did not differ between WT and CD36−/− mice (Fig. 1A). After injection of saturating doses, however, clearance of the injected dose was slower compared with nonsaturating doses, and the decrease in clearance was greater (P < 0.01) in CD36−/− mice, as shown by the FCR (Fig. 1B). The FCR did not differ between WT and CD36−/− mice after they were given a nonsaturating bolus of 400 μg of (n-3)-TGRP. In contrast, the saturating dose of 4 mg of (n-3)-TGRP was cleared from blood faster in WT compared with CD36−/− mice (P < 0.01). Although FCR of both WT and CD36−/− mice were substantially (P < 0.05) lower at high doses than at low doses, the difference was greater (P = 0.003) in CD36−/− mice (67%) than in WT mice (43%) (Fig. 1B). The FCR was affected by the interaction between the genotype and injected dose (P < 0.01), suggesting that CD36 facilitates (n-3)-TGRP clearance only after classical clearance routes are saturated.
FIGURE 1.
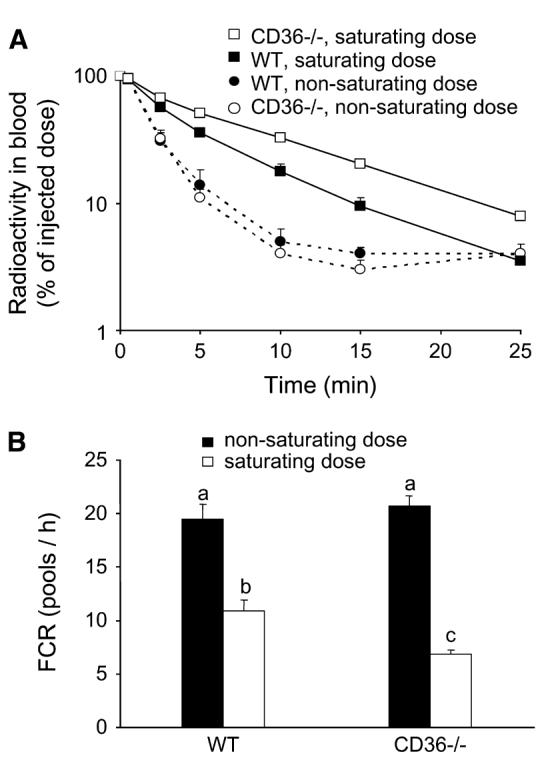
Blood clearance (A) and FCR (B) of nonsaturating and saturating doses of (n-3)-TGRP lipid emulsion in WT and CD36−/− mice. (A) Values are means ± SEM, n = 4 or 5. The effect of the dose (P < 0.001) and the dose × genotype interaction (P < 0.01), but not the effect of genotype, were significant. Means without a common letter differ, P < 0.01.
Uptake of (n-3)-TGRP in WT and CD36−/− macrophages
Uptake of (n-3)-TGRP in CD36−/− macrophages was 28 and 18% lower than in WT macrophages in the absence and presence of apoE, respectively (P < 0.05) (Fig. 2). Although apoE increased macrophage uptake of (n-3)-TGRP in both CD36−/− and in WT to 1.2- to 1.4-fold of the control (P < 0.05), these increases were far less than the 5-fold previously reported for (n-6)-TGRP (1,18,29).
FIGURE 2.
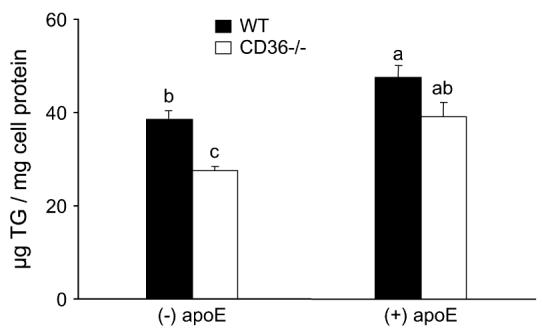
Effects of apoE on (n-3)-TGRP uptake in WT and CD36−/− peritoneal macrophages. Values are means ± SEM, n = 4 or 5 from a representative experiment. The effect of genotype (P < 0.001) and of apoE (P < 0.001) were significant; but not the effect of genotype and apoE interaction on (n-3)-TGRP uptake values. Means without a common letter differ, P < 0.05. To convert μg TG to μmol, divide by 946.
We determined whether lactoferrin, a compound that inhibits apoE-mediated uptake pathways, had different effects on (n-3)-TGRP uptake in WT and CD36−/− macrophages. Compared with incubations in the absence of apoE, lactoferrin (P < 0.01) lowered uptake of (n-3)-TGRP in both types of macrophages in the presence of apoE (Fig. 3). Lactoferrin decreased uptake of (n-3)-TGRP by 14 and 25% (P < 0.05 and P < 0.01, respectively) in WT macrophages in the absence and presence of apoE, respectively. Similarly, lactoferrin decreased (n-3)-TGRP uptake by 10 and 31% (P < 0.01 and P < 0.01, respectively) in CD36−/− macrophages in the absence and presence of apoE, respectively. Thus, lactoferrin decreased (n-3)-TGRP uptake via apoE-mediated mechanisms in both types of cells, but again, effects were less than previously reported for (n-6)-TGRP (1).
FIGURE 3.
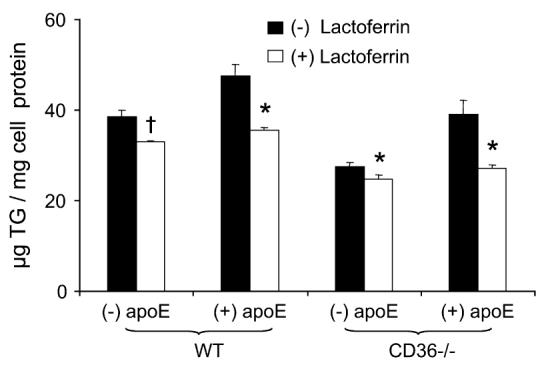
Effects of lactoferrin and apoE on (n-3)-TGRP uptake in WT and CD36−/− peritoneal macrophages. Values are means ± SEM, n = 3 to 5 from a representative experiment. The effects of genotype (P < 0.001), apoE (P < 0.001), and lactoferrin (P < 0.001), and the interaction between apoE and lactoferrin (P < 0.01), were significant, but there was no significant interaction among genotype, apoE, and lactoferrin on the (n-3)-TGRP uptake values. † P < 0.05; *, P < 0.01 for comparison of presence vs. absence of lactoferrin in each genotype-apoE combination. To convert μg TG to μmol, divide by 946.
We preincubated macrophages with growth media supplemented with 50 mmol/L NaClO3 for 24 h to inhibit synthesis by blocking sulfation of all cell surface proteoglycans. There were major, significant reductions of (n-3)-TGRP uptake after NaClO3 incubation in both the presence and absence of apoE in both lines of macrophages (Fig. 4). The extent of inhibition was similar in the 2 cell lines under all incubation conditions and ranged from 56 to 67%. Thus, cell surface proteoglycans were important contributors to (n-3)-TGRP uptake both in the presence and absence of apoE.
FIGURE 4.
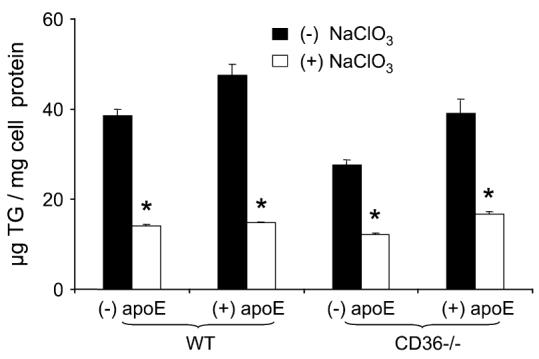
Effect of NaClO3 and apoE on (n-3)-TGRP uptake in WT and CD36−/− peritoneal macrophages. Values are means ± SEM, n = 3 to 5 from a representative experiment. The effects of genotype (P < 0.001), apoE (P < 0.001), and NaClO3 (P < 0.001) between the genotype and NaClO3 (P < 0.01) and between apoE and NaClO3 (P < 0.01) were significant, but there was no significant interaction among genotype, apoE, and NaClO3 on the (n-3)-TGRP uptake values. *, P < 0.001 for comparison of presence vs. absence of NaClO3 in each genotype-apoE combination. To convert μgTG to μmol, divide by 946.
Discussion
We previously demonstrated that (n-6)- and (n-3)-TGRP were cleared by disparate pathways both in vitro and in vivo. Although (n-6)-TGRP uptake was mediated by LDLR, LpL, and other apoE-mediated pathways, these were not important for (n-3)-TGRP clearance from the circulation (1). In fact, we have more information on how (n-3)-TGRP were not cleared, rather than on how they were cleared from blood. In these studies, we found a contributing role for CD36 in (n-3)-TGRP clearance after other clearance pathways were saturated in vivo, and from our in vitro cell studies, we suggest an important role for cell surface proteoglycans.
We found slower blood clearance for (n-3)-TGRP at high, saturating injected doses than at low, nonsaturating doses. This was expected because of similar findings in many situations or conditions where metabolic pathways were saturated. What was unexpected was that clearance was slower after injection of (n-3)-TGRP into CD36−/− mice than in WT mice. CD36 served not only as a receptor but also as an important cofactor in the uptake of a number of molecules (30). For example, it recognized oxidized phospholipids to enhance uptake of lipoprotein particles containing these products (31). In ongoing studies, we have found that, in CD36−/− mice, blood clearance of (n-6)-TGRP with nonsaturating dose injections is ∼15–25% slower than in control WT mice, in keeping with our findings that clearance of (n-6)-TGRP relied on a number of interacting mechanisms, e.g., the LDLR and related apoE-mediated pathways, LpL, and now, to a minor extent, CD36 (Ton MN, Abumrad NA, Goldberg IJ, and Deckelbaum RJ, unpublished data). One consideration is that CD36 would recognize oxidized lipids in (n-3)-TGRP; however, in previous studies, we found that (n-3) lipid emulsions, similar to those we have used in the studies herein, did not have increased levels of reactive oxidant species (Carpentier YA, unpublished data). Also, we think that the higher plasma lipid concentrations in food-deprived CD36−/− mice were not confounders of our data because, at the low, non-saturating doses of injected (n-3)-TGRP, no differences in blood clearance occurred. We estimate that at the high, injected dose of 4 mg TG, plasma TG concentrations should have increased to ∼4.2 mmol/L, a value much greater than the differences in plasma TG concentrations in food-deprived CD36−/− mice compared with WT mice (Table 1). Although the role of CD36 as a potential (n-3)-TGRP receptor needs to be further defined, we suggest, based on these studies, that it contributes to (n-3)-TGRP blood clearance when other, more dominant pathways are saturated: a situation that might occur during postprandial lipemia after a meal rich in (n-3) fatty acids.
Both the effects of adding apoE to (n-3)-TGRP and of inhibiting their uptake pathways by lactoferrin were considerably less than those previously described for (n-6) soy oil or triolein particles, when lactoferrin inhibited the uptake pathways by 50% or more, in vitro and in vivo (1,18,26). This diminished effect of apoE could relate to the fact that (n-3)-TGRP do not depend nearly as much as (n-6)-TGRP on the LDLR for clearance in vivo, and in cell uptake in vitro (1). We suggest, although not directly proven from our current data, that lactoferrin in the experiments herein are modulating cell uptake of (n-3)-TGRP by interfering with the ability of these particles to bind to cell surface proteoglycans.
We previously have found that cell surface proteoglycans contribute to cell uptake of model (n-6)-TGRP, and that these low-affinity, but high-capacity, pathways only begin to contribute after receptor-mediated pathways have been saturated (18). Because (n-3)-TGRP seem to rely little on the LDLR (1), it is likely that these proteoglycan pathways contribute to cellular uptake of (n-3)-TGRP, even at lower particle concentrations. In these studies, we only performed experiments with the pre-incubation of NaClO3 and did not treat cells with heparinase or heparitinase as we have previously done in studies with human fibroblasts (18). This is because we found that heparan sulfate proteoglycans are not highly expressed or synthesized in macrophages compared with other cell types, such as HepG2 cells or fibroblasts. Thus, proteoglycans other than heparan sulfate proteoglycans also can contribute to (n-3)-TGRP clearance.
In our experiments, we used model (n-3)-TGRP, in which EPA plus DHA contributed to about half of the total TG fatty acids. This raised the question of whether our data are of physiological relevance. We contend that they are because, in a recent study on lipid emulsions containing only 5% of the total TG fatty acids as EPA and DHA (32), levels comparable to postprandial TGRP after an (n-3)-rich meal, we found that even these small concentrations of (n-3)-TG in TGRP led to blood clearance and cell uptake properties more similar to pure fish oil (n-3)-TGRP than to soy oil (n-6)-TGRP (1,32).
More studies need to be performed in animal models to explore further the potential role of proteoglycans in (n-3)-TGRP clearance in vivo. Nevertheless, the results of the current studies suggest that, in addition to the contributing role of CD36 to (n-3)-TGRP clearance, cell surface proteoglycans also contribute substantially to (n-3)-TGRP clearance. We predict that these 2 pathways will prove important in understanding how (n-3) fatty acid–enriched TG are cleared from plasma and delivered to tissues.
Acknowledgments
We thank Mrs. Fannie Kayserman and Ms. Inge Hansen for excellent technical assistance. We thank Dr. Nada Abumrad for providing the CD36−/− mice for establishing our breeding colony.
Footnotes
Supported by NIH Grant HL40404, and by a grant from the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (N.D.).
Author disclosures: N. Densupsoontorn, Y. A. Carpentier, R. Racine, F. M. Murray, T. Seo, R. Ramakrishnan, and R. J. Deckelbaum, no conflicts of interest.
Abbreviations used: apoE, apolipoprotein E; CD36−/−, CD36 knockout; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FCR, fractional catabolic rate; [3H]CEt, [1α, 2α (n)-3H] cholesteryl oleoyl ether; LDLR, LDL receptor; LpL, lipoprotein lipase; (n-3)-TGRP, (n-3) fatty acid–enriched triglyceride-rich particle; NaClO3, sodium chlorate; TG, triglyceride; TGRP, triglyceride-rich particles; WT, wild type.
Literature Cited
- 1.Qi K, Seo T, Al-Haideri M, Worgall TS, Vogel T, Carpentier YA, Deckelbaum RJ. Omega-3 triglycerides modify blood clearance and tissue targeting pathways of lipid emulsions. Biochemistry. 2002;41:3119–27. doi: 10.1021/bi015770h. [DOI] [PubMed] [Google Scholar]
- 2.Harmon CM, Abumrad NA. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol. 1993;133:43–9. doi: 10.1007/BF00231876. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Ruiz E, Armesilla AL, Sanchez-Madrid F, Vega MA. Gene encoding the collagen type I and thrombospondin receptor CD36 is located on chromosome 7q11.2. Genomics. 1993;17:759–61. doi: 10.1006/geno.1993.1401. [DOI] [PubMed] [Google Scholar]
- 4.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 5.Blake JA, Eppig JT, Richardson JE, Davisson MT. The Mouse Genome Database (MGD): expanding genetic and genomic resources for the laboratory mouse. The Mouse Genome Database Group. Nucleic Acids Res. 2000;28:108–11. doi: 10.1093/nar/28.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverstein RL, Febbraio M. CD36 and atherosclerosis. Curr Opin Lipidol. 2000;11:483–91. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–8. [PubMed] [Google Scholar]
- 8.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–56. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakata A, Nakagawa Y, Nishida M, Nozaki S, Miyagawa J, Nakagawa T, Tamura R, Matsumoto K, Kameda-Takemura K, et al. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–9. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–6. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- 11.Nozaki S, Tanaka T, Yamashita S, Sohmiya K, Yoshizumi T, Okamoto F, Kitaura Y, Kotake C, Nishida H, et al. CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Mol Cell Biochem. 1999;192:129–35. [PubMed] [Google Scholar]
- 12.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 13.Alexander Aguilera A, Hernandez Diaz G, Lara Barcelata M, Angulo Guerrero O, Oliart Ros RM. Induction of Cd36 expression elicited by fish oil PUFA in spontaneously hypertensive rats. J Nutr Biochem. 2006;17:760–5. doi: 10.1016/j.jnutbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mahley RW. Heparan sulfate proteoglycan/low density lipoprotein receptor-related protein pathway involved in type III hyperlipoproteinemia and Alzheimer's disease. Isr J Med Sci. 1996;32:414–29. [PubMed] [Google Scholar]
- 15.Mahley RW, Ji ZS, Brecht WJ, Miranda RD, He D. Role of heparan sulfate proteoglycans and the LDL receptor-related protein in remnant lipoprotein metabolism. Ann N Y Acad Sci. 1994;737:39–52. doi: 10.1111/j.1749-6632.1994.tb44300.x. [DOI] [PubMed] [Google Scholar]
- 16.Ji ZS, Brecht WJ, Miranda RD, Hussain MM, Innerarity TL, Mahley RW. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–7. [PubMed] [Google Scholar]
- 17.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 18.Al-Haideri M, Goldberg IJ, Galeano NF, Gleeson A, Vogel T, Gorecki M, Sturley SL, Deckelbaum RJ. Heparan sulfate proteoglycan-mediated uptake of apolipoprotein E-triglyceride-rich lipoprotein particles: a major pathway at physiological particle concentrations. Biochemistry. 1997;36:12766–72. doi: 10.1021/bi9631024. [DOI] [PubMed] [Google Scholar]
- 19.Ton MN, Chang C, Carpentier YA, Deckelbaum RJ. In vivo and in vitro properties of an intravenous lipid emulsion containing only medium chain and fish oil triglycerides. Clin Nutr. 2005;24:492–501. doi: 10.1016/j.clnu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res. 1999;40:565–74. [PubMed] [Google Scholar]
- 21.Treskova E, Carpentier YA, Ramakrishnan R, Al-Haideri M, Seo T, Deckelbaum RJ. Blood clearance and tissue uptake of intravenous lipid emulsions containing long-chain and medium-chain triglycerides and fish oil in a mouse model. JPEN J Parenter Enteral Nutr. 1999;23:253–7. doi: 10.1177/0148607199023005253. [DOI] [PubMed] [Google Scholar]
- 22.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–62. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 23.Berman M. Kinetic analysis of turnover data. Prog Biochem Pharmacol. 1979;15:67–108. [PubMed] [Google Scholar]
- 24.Michl J, Pieczonka MM, Unkeless JC, Silverstein SC. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979;150:607–21. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn ZA, Benson B. The differentiation of mononuclear phagocytes. Morphology, cytochemistry, and biochemistry. J Exp Med. 1965;121:153–70. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabas I, Boykow GC, Tall AR. Foam cell-forming J774 macrophages have markedly elevated acyl coenzyme A:cholesterol acyl transferase activity compared with mouse peritoneal macrophages in the presence of low density lipoprotein (LDL) despite similar LDL receptor activity. J Clin Invest. 1987;79:418–26. doi: 10.1172/JCI112828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Out R, Kruijt JK, Rensen PC, Hildebrand RB, de Vos P, Van Eck M, Van Berkel TJ. Scavenger receptor BI plays a role in facilitating chylomicron metabolism. J Biol Chem. 2004;279:18401–6. doi: 10.1074/jbc.M401170200. [DOI] [PubMed] [Google Scholar]
- 28.Out R, Hoekstr M, de Jager SC, de Vos P, van der Westhuyzen DR, Webb NR, Van Eck M, Biessen EA, Van Berkel TJ. Adenovirus-mediated hepatic overexpression of scavenger receptor class B type 1 accelerates chylomicron metabolism in C57BL/6J mice. J Lipid Res. 2005;46:1172–81. doi: 10.1194/jlr.M400361-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Granot E, Schwiegelshohn B, Tabas I, Gorecki M, Vogel T, Carpentier YA, Deckelbaum RJ. Effects of particle size on cell uptake of model triglyceride-rich particles with and without apoprotein E. Biochemistry. 1994;33:15190–7. doi: 10.1021/bi00254a030. [DOI] [PubMed] [Google Scholar]
- 30.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–91. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–23. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 32.Qi K, Seo T, Jiang Z, Carpentier YA, Deckelbaum RJ. Triglycerides in fish oil affect the blood clearance of lipid emulsions containing long- and medium-chain triglycerides in mice. J Nutr. 2006;136:2766–72. doi: 10.1093/jn/136.11.2766. [DOI] [PubMed] [Google Scholar]


