Abstract
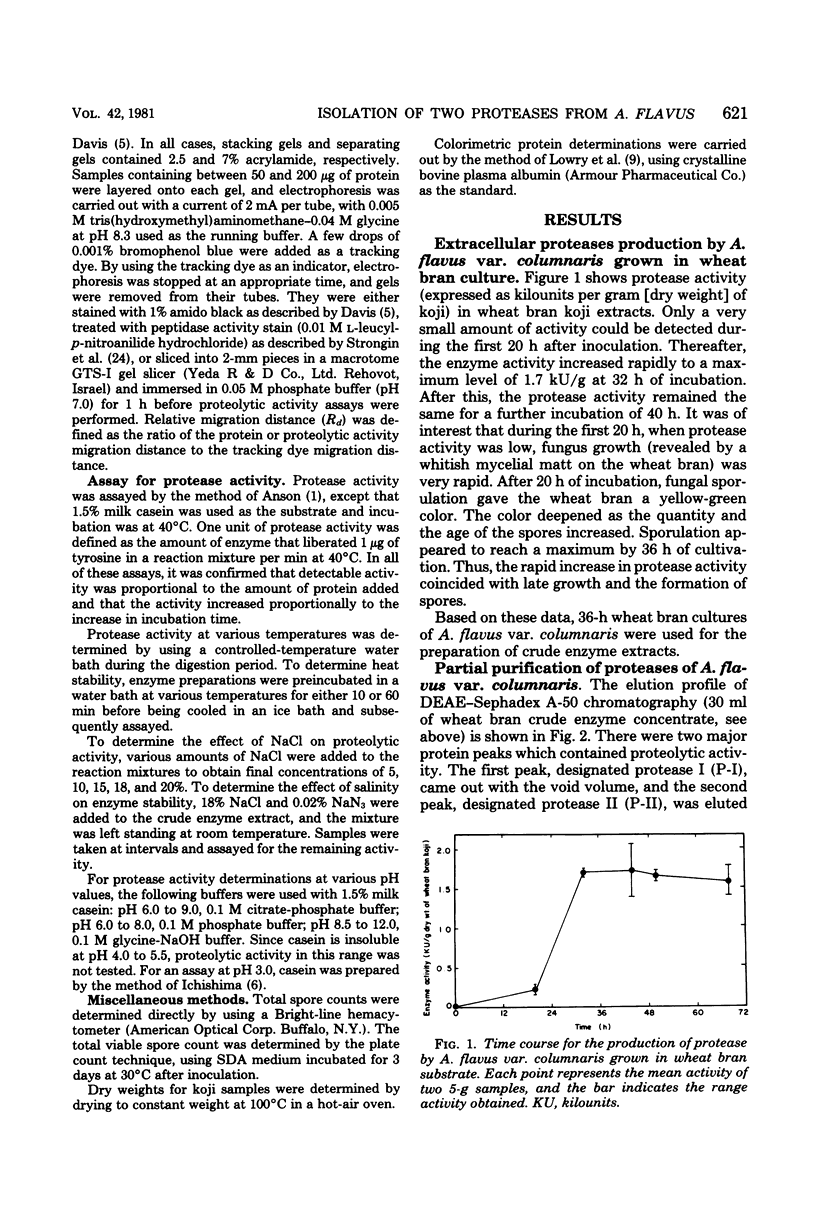
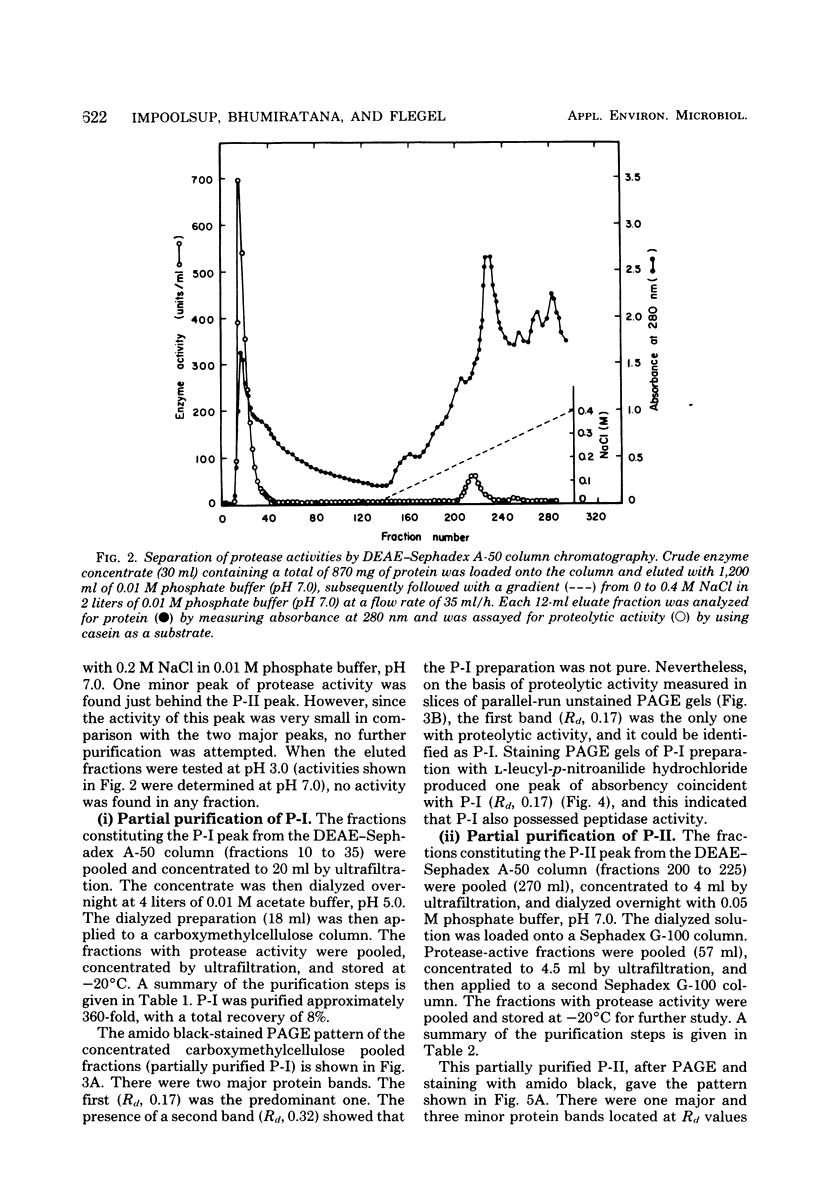
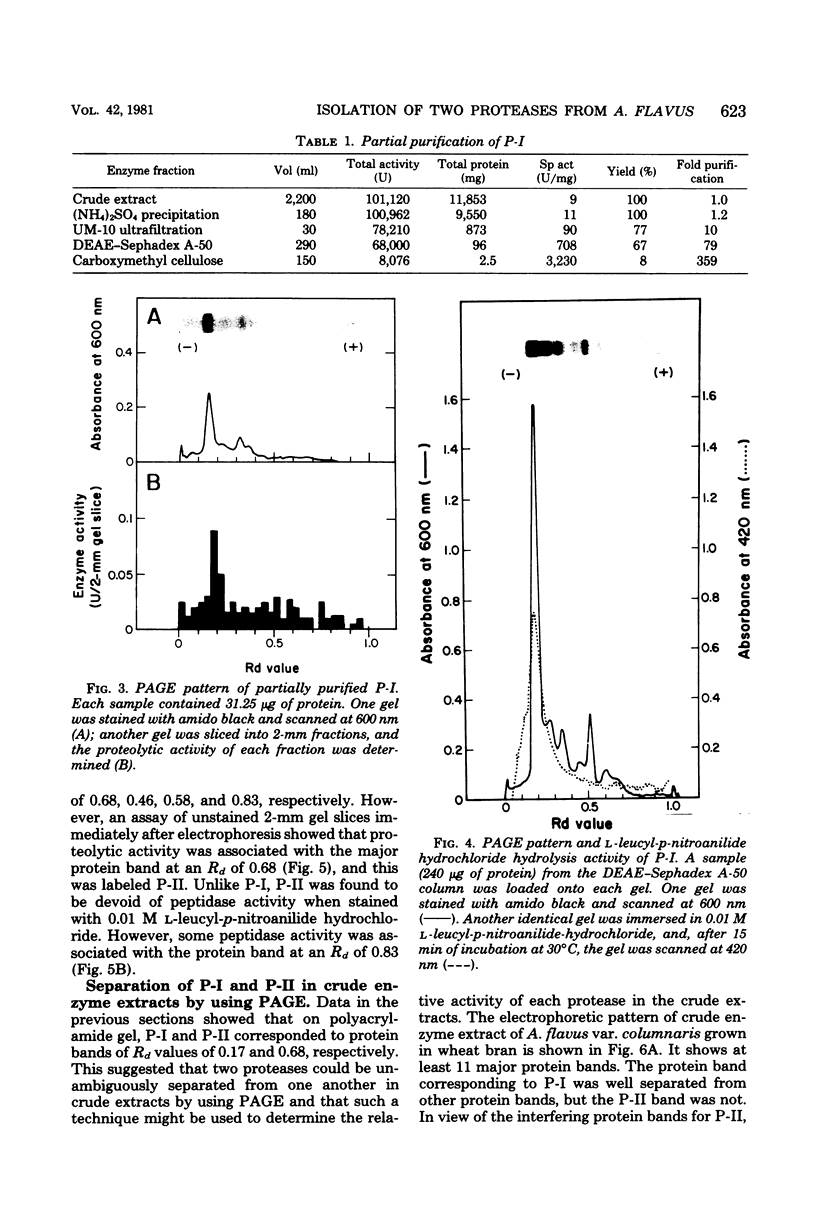
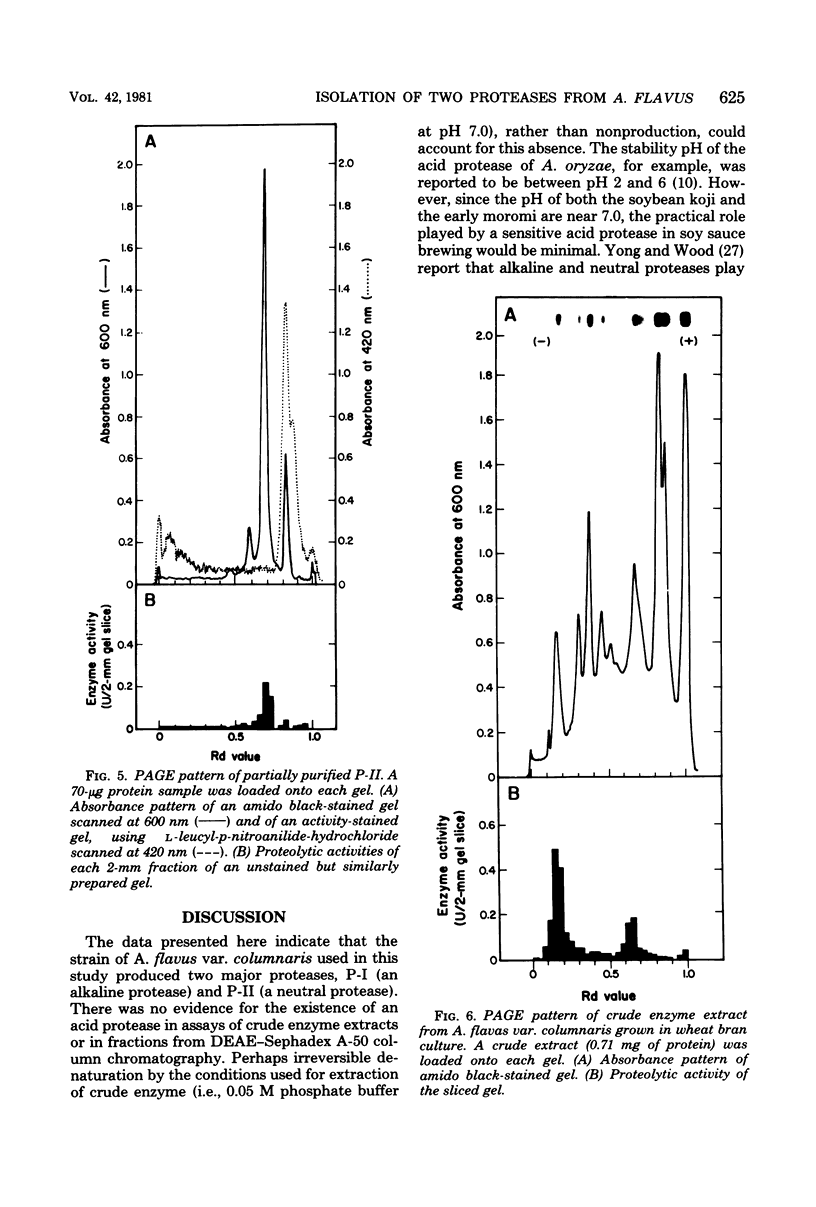
Two different extracellular proteases, protease I (P-I), an alkaline protease, and protease II (P-II) a neutral protease, from Aspergillus flavus var. columnaris were partially purified by using (NH4)2SO4 precipitation, diethylaminoethyl-Sephadex A-50 chromatography, carboxymethylcellulose CM-52 chromatography, and Sephadex G-100 gel filtration. The degree of purity was followed using polyacrylamide gel electrophoresis. The activity of P-I was completely inhibited by 0.1 mM phenylmethylsulfonyl fluoride, and that of P-II was completely inhibited by 1 mM ethylenediaminetetraacetate. By using these inhibitors with extracts of wheat bran koji, the proportions of total activity that could be assigned to P-I and P-II were 80 and 20%, respectively. This compared favorably with activities estimated by using polyacrylamide gel electrophoresis slices (82 and 18%, respectively). Extracts from factory-run soybean koji gave comparable results. Both enzymes demonstrated maximum activity at 50 to 55°C and only small changes in activity between pH 6 and 11. For P-I, activity was somewhat higher from pH 8.0 to 11.0, whereas for P-II it was somewhat higher from pH 6 to 9. In the presence of 18% NaCl, the activities of both P-I and P-II dropped by approximately 90 and 85%, respectively. P-I was inferred to possess aminopeptidase activity since it could hydrolyze l-leucyl-p-nitroanilide hydrochloride. P-II was devoid of such activity. The ramifications of the results for factory-produced soy sauce koji are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhumiratana A., Flegel T. W., Glinsukon T., Somporan W. Isolation and analysis of molds from soy sauce koji in Thailand. Appl Environ Microbiol. 1980 Feb;39(2):430–435. doi: 10.1128/aem.39.2.430-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kundu A. K., Manna S. Purification and characterization of extracellular proteinases of Aspergillus oryzae. Appl Microbiol. 1975 Oct;30(4):507–513. doi: 10.1128/am.30.4.507-513.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lysenkov N. V., Tsyperovich A. S. Vlastyvosti proteazy Aspergillus flavus. Ukr Biokhim Zh. 1969;41(2):157–162. [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Comparison of the specificities of various serine proteinases from microorganisms. Arch Biochem Biophys. 1969 Feb;129(2):620–634. doi: 10.1016/0003-9861(69)90223-9. [DOI] [PubMed] [Google Scholar]
- SUBRAMANIAN A. R., KALNITSKY G. THE MAJOR ALKALINE PROTEINASE OF ASPERGILLUS ORYZAE, ASPERGILLOPEPTIDASE B. I. ISOLATION IN HOMOGENEOUS FORM. Biochemistry. 1964 Dec;3:1861–1867. doi: 10.1021/bi00900a012. [DOI] [PubMed] [Google Scholar]