Abstract
A group of dicyanodihydrofuran (DCDHF) fluorophores with thiol-reactive maleimide functionality has been synthesized. One of the methods involves aromatic nucleophilic substitution reaction between an arylfluoride containing DCDHF and an amine containing protected maleimide. An alternative and generally useful method involves combination of the Mitsunobu reaction of a DCDHF-OH with a furan or 2-methylfuran protected maleimide and then subsequent retro Diels–Alder reaction.
Single molecule fluorescence imaging is a powerful technique that reveals the subtle characteristics of structure and dynamics in complex condensed phases that are otherwise obscured in ensemble measurements.1 For example, a fluorescent Nile Red dye was recently employed to probe the sequence of conformationally induced polarity changes in the molecular chaperonin GroEL.2 We have identified a new class of DCDHF fluorophores that are particularly well suited for single- molecule studies.3 These dipolar molecules contain an amine donor (usually a dialkylamine, R1R2N), a conjugated π-system (comprised of units including 1,4-benzene, 2,6-naphthalene, 2,5-thiophene, alkene, etc.) and the unique dicyanodihydrofuran DCDHF acceptor (with R3, R4 groups, which are again usually alkyl groups). The generic DCDHF chromophore structure is shown in Figure 1 wherein these three substructures can all be systematically varied to tune a wide range of properties. Additional groups can be appended to any of these substructures to provide additional function. We seek to specifically control and optimize the introduction of the maleimide functional group that is widely used as a Michael addition substrate for thiol groups in proteins.4–6 The maleimide may be directly attached to the fluorophore via the aromatic ring or through an aliphatic spacer.7 Here we describe an efficient method for the introduction of the maleimide through an aliphatic spacer.
Figure 1.
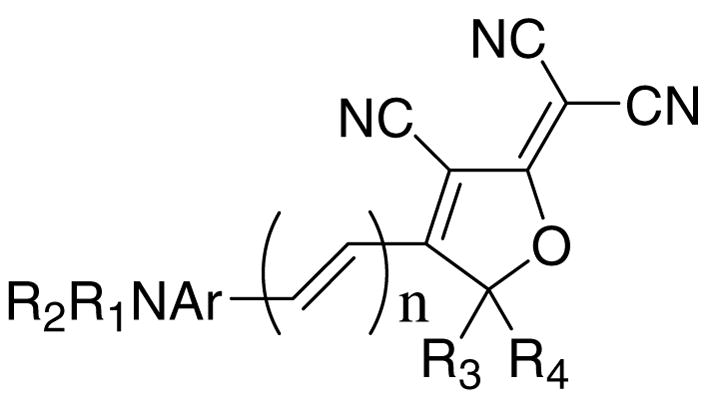
The generic DCDHF chromophore with sites R1–4 and Ar available for synthetic modification.
Several methods have been reported to create the maleimide functional group: (1) Cyclization of the corresponding maleamic acid generated by condensation of amine and maleic anhydride under acidic conditions.8 (2) Alkylation of a furan-protected maleimide with an alkyl halide in the presence of base and subsequent retro-Diels–Alder (retro-D–A) deprotection.9 (3) Mitsunobu reaction of pyrrole-2,5-dione (parent maleimide) or a protected maleimide with an alcohol.10,11
The maleimide unit was first introduced on the amine donor side (R1 or R2) in a DCDHF dye with a benzene-conjugating unit. Since we had synthesized the aryl fluoride intermediate 6 before, and considering the instability of the maleimide to nucleophiles, we adapted a SNAr reaction between a secondary amine with a furan-protected maleimide 5 and the aryl fluoride 6 to obtain the furan-protected maleimide 7.12 The furan-protected maleimide 5 was synthesized starting from 1, the monotosylate of 1,6-hexanediol. The furan-protected maleimide was then alkylated with monotosylate 1 and the remaining alcohol in 3 was subsequently tosylated and treated with N-propylamine to afford dialkylamine 5. After a retro-D–A reaction of 7, the DCDHF fluorophore 8 with maleimide attached on the terminus of the amine donor has been obtained as is summarized overall in Scheme 1.13
Scheme 1.
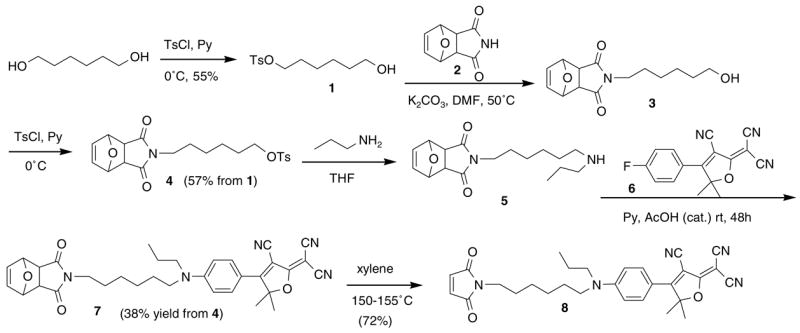
Synthesis of DCDHF with maleimide on the donor side.
This same method cannot be applied if the maleimide is attached on the acceptor side (R3, R4) because DCDHF fluorophores are not compatible with either primary amines, which are the starting material for method (1), or the basic conditions for the alkylation, which are required for method (2). Therefore, as an alternative, the Mitsunobu coupling with pyrrole-2,5-dione appears very attractive. Indeed, using the method of Walker,10 DCDHFs with a maleimide group were isolated but only in moderate (35% for Ar = 1,4-phenyl) to low (7% for Ar = 2,6-naphthyl) yields probably due to the sensitivity of the unprotected maleimide double bond to the nucleophiles existing in the reaction system (Scheme 2).
Scheme 2.
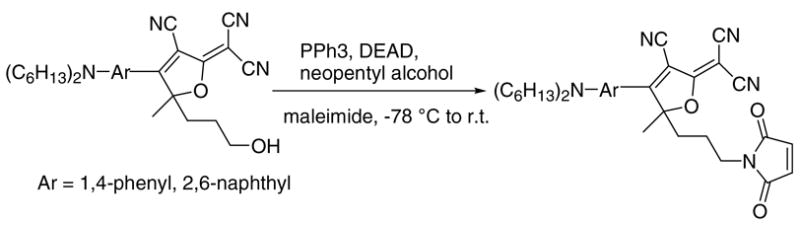
Synthesis of a maleimide substituted DCDHF fluorophore by direct Mitsunobu reaction of pyrrole-2,5-dione.
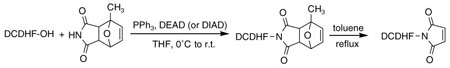
The limited success here prompted us to seek improved methods for synthesis of this kind of molecule. The Mitsunobu reaction of a furan-protected maleimide in combination with the retro-D–A reaction avoids the susceptibility of the maleimide to nucleophilic attack.11 We have examined different pyrrole-2,5-dione adducts of furan, 2-methylfuran, and 2,5-dimethylfuran made by the procedures reported by Kwart and Burchuk.14,15 The pyrrole-2,5-dione adduct of furan has poor solubility in chloroform and other ordinary organic solvents, which limits its use while the 2-methylfuran protected maleimide has much improved solubility. The pure endo, exo isomers or a mixture of both isomers were used in the Mitsunobu reaction and the retro-D–A reaction. The thermal stability of the D–A adducts is furan > 2-methylfuran > 2,5-dimethylfuran and the latter adducts are not convenient to use, as partial retro-D–A reaction was often observed during their handling and purification. We examined the model Mitsunobu reaction between 4-biphenylmethanol and furan or 2-methylfuran protected maleimide and found they are equivalent in terms of the yield of the products (Scheme 3). However, one of the major drawbacks of the Mitsunobu reaction is the necessity to remove hydrazide and phosphine oxide byproducts.16 The availability of the different furan D–A adducts provides versatility for chromatographic separation required for workup of these reactions. The retro-D–A reaction of the 2-methylfuran adduct 14 was run in boiling toluene and the furan adduct was run in xylene or anisole as reported by Clevenger and Turnbull.9
Scheme 3.
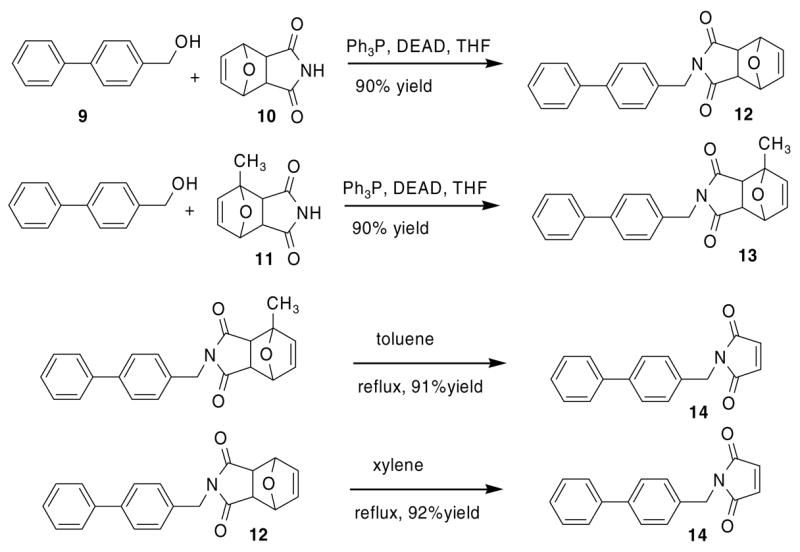
Model reactions of 4-biphenylmethanol with protected maleimide and the corresponding retro Diels–Alder reaction.
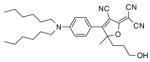
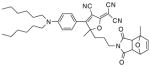
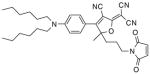
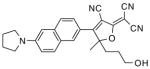
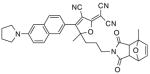
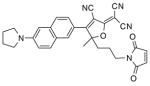
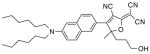
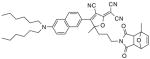
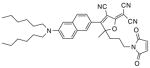
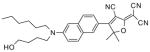
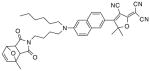
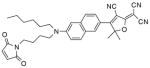
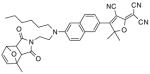
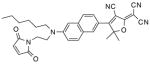
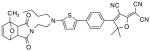
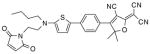
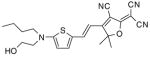
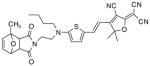
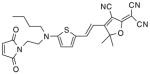
After examination of these model compounds, we focused on the synthesis of the maleimide functionalized DCDHF fluorophores. The detailed synthetic procedures for the DCDHF precursors with hydroxyl functionality (first column of Table 1) will be published elsewhere. These hydroxyl functionalized DCDHF were reacted with the 2-methylfuran protected maleimide to obtain a protected DCDHF-maleimide. Either individual pure exo or endo isomers or a mixture of both isomers of the 2-methylfuran protected maleimide are useful for this purpose.
Table 1.
Synthesis of maleimide functionalized DCDHFs via the 2-methylfuran protected maleimide and retro Diels–Alder reaction17
 | ||
|---|---|---|
| DCDHF-OH | DCDHF-protected maleimide | DCDHF-maleimide |
1
|
15
|
22
|
2
|
16
|
23
|
3
|
17
|
24
|
4
|
18
|
25
|
| 5
|
19
|
26
|
| 6
|
20
|
27
|
7
|
21
|
28
|
In contrast to the problems we encountered with the Mitsunobu reaction of pyrrole-2,5-dione, the reactions and purifications involving the protected maleimide were straightforward. The reaction is not sensitive to an excess of triphenylphosphine and DEAD or DIAD and so typically 20–50% of excess of the reagents could be used. Seven DCDHF-maleimides (22–28) have been synthesized via their corresponding 2-methylfuran protected DCDHF-maleimides (15–21). The conjugation linkages for DCDHFs include phenyl (22), naphthalene (23–26), Th-Ph (27), and Th-V (28) and maleimide was installed on either the donor side or acceptor side of the molecule. The detailed photophysical properties of these new DCDHF chromophores and their applications as biolabels will be described elsewhere.
Acknowledgments
Support from DOE (DG-FG02-04ER63777), NIH (1P20HG003638-01), and the Ohio Board of Regents is acknowledged.
References and notes
- 1.(a) Moerner WE, Orrit M. Science. 1999;283:1670– 1676. doi: 10.1126/science.283.5408.1670. [DOI] [PubMed] [Google Scholar]; (b) Weiss S. Science. 1999;283:1676–1683. doi: 10.1126/science.283.5408.1676. [DOI] [PubMed] [Google Scholar]
- 2.Kim SY, Semyonov AN, Twieg RJ, Horwich AL, Frydman J, Moerner WE. J Phys Chem B. 2005;109:24517–24525. doi: 10.1021/jp0534232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Willets KA, Nishimura SY, Schuck PJ, Twieg RJ, Moerner WE. Acc Chem Res. 2005;38:549–556. doi: 10.1021/ar0401294. [DOI] [PubMed] [Google Scholar]; (b) Willets KA, Ostroverkhova O, He M, Twieg RJ, Moerner WE. J Am Chem Soc. 2003;125:1174– 1175. doi: 10.1021/ja029100q. [DOI] [PubMed] [Google Scholar]; (c) Nishimura SY, Lord SJ, Klein LO, Willets KA, He M, Lu Z, Twieg RJ, Moerner WE. J Phys Chem B. 2006;110:8151–8157. doi: 10.1021/jp0574145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Silva AP, Gunaratne HQN, Gunnlaugsson T. Tetrahedron Lett. 1998;39:5077–5080. [Google Scholar]
- 5.Zhang X, Li ZC, Li KB, Du FS, Li FM. J Am Chem Soc. 2004;126:12200–12201. doi: 10.1021/ja0487527. [DOI] [PubMed] [Google Scholar]
- 6.Girouard S, Houle MH, Grandbois A, Keillor JW, Michnick SW. J Am Chem Soc. 2005;127:559–566. doi: 10.1021/ja045742x. [DOI] [PubMed] [Google Scholar]
- 7.Corrie JET. J Chem Soc, Perkin Trans 1. 1994:2975– 2982. [Google Scholar]
- 8.Reddy PY, Kondo S, Fujita S, Toru T. Synthesis. 1998:999–1002. [Google Scholar]
- 9.Clevenger RC, Turnbull KD. Synth Commun. 2000;30:1379–1388. [Google Scholar]
- 10.Walker MA. J Org Chem. 1995;60:5352–5355. [Google Scholar]
- 11.Farha OK, Julius RL, Hawthorne MF. Tetrahedron Lett. 2006;47:2619–2622. [Google Scholar]
- 12.He M, Twieg RJ, Ostroverkhova O, Gubler U, Wright D, Moerner WE. Proc SPIE. 2002;4802:9–20. [Google Scholar]
- 13.DCDHF 8: 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 9.5 Hz, 2H), 6.73 (d, J = 9.5 Hz, 2H), 6.72 (s, 2H), 3.55 (t, J = 7.2 Hz, 2H), 3.45–3.37 (m, 4H), 1.85 (s, 6H), 1.73–1.59 (m, 6H), 1.40–1.37 (m, 4H), 1.01 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 177.0, 173.6, 170.9, 152.8, 134.1, 132.5, 113.2, 113.0, 112.0, 97.2, 90.8, 54.1, 53.0, 51.3, 37.6, 28.3, 27.7, 27.1, 26.4, 26.3, 20.6, 11.3. HRMS (m/z): [M+Na] calcd for C29H31N5O3Na, 520.2325; found, 520.2332.
- 14.Kwart H, Burchuk I. J Am Chem Soc. 1952;74:3094– 3097. [Google Scholar]
- 15.Representative method for prep of a furan-protected maleimide 10: In a 5 mL vial were added pyrrole-2,5- dione (98 mg, 1.0 mmol) and 3 mL diethyl ether. The pyrrole-2,5-dione was dissolved with ultrasound and furan (0.3 mL) was added. After 5 days the diethyl ether was evaporated until only 1 mL, and white crystals were filtered o. (133 mg, ~75% is endo and 25% exo). 1H NMR (100 MHz, CDCl3) (mixture) 1H 400 Hz, 8.49 (s, br, 0.25H), 8.08 (s, br, 0.75H), 6.55–6.52 (m, 3H), 5.37–5.32 (m, 2H), 3.60–3.58 (m, 1.5H), 2.91 (s, 0.5H). 13C NMR (100 MHz, CDCl3) (mixture) δ 176.4, 175.0, 136.6, 134.6, 81.0, 79.4, 48.7, 47.4. Representative method for prep of a 2-methylfuran protected maleimide 11: In a 500 mL flask were added pyrrole-2,5-dione (5.82 g, 60 mmol) and 300 mL diethyl ether. The pyrrole-2,5-dione was dissolved with ultrasound, and 2-methylfuran (7.38 g, 90 mmol) was added. The mixture was permitted to stand for 3 days and diethyl ether was removed by distillation until only 50 mL was left. Crystals were filtered o. to obtain 2.3 g white solid (this sample is >98% endo isomer). The diethyl ether solution was evaporated to dryness (this residue is >95% exo isomer). endo isomer 1H NMR (400 Hz, CDCl3) 7.59 (s, br, 1H), 6.51 (dd, J = 1.7, 5.7 Hz, 1H), 6.34 (d, J = 5.7 Hz, 1H), 5.24 (dd, J = 1.7, 5.6 Hz, 1H), 3.70 (ddd, J = 5.6, 7.6 Hz, 1H), 3.17 (d, J = 7.6 Hz, 1H), 1.84 (s, 3H). exo isomer 1H NMR (400 Hz, CDCl3) 8.85 (s, br, 1H), 6.51 (dd, J = 1.5, 5.5 Hz, 1H), 6.31 (d, J = 5.7 Hz, 1H), 5.24 (d, J = 1.5 Hz, 1H), 3.02 (ddd, J = 6.4 Hz, 1H), 2.76 (d, J = 6.4 Hz, 1H), 1.75 (s, 3H).
- 16.Hughes DL. Org React. 1992;42:335–656. [Google Scholar]
- 17.General procedure (prep of 22 as an example): In a 100 mL dry round bottom flask were added 3-cyano- 2-dicyanomethylen-5-methyl-5-(3-hydroxypropyl)-4-(4-dihexylaminophenyl)- 2,5-dihydrofuran (100 mg, 0.20 mmol), 1-methyl-10-aza-tricyclo[5.2.1.02,6]dec-8-ene-3,5- dione (endo) (40.3 mg, 0.225 mmol) Ph3P (80.5 mg, 0.31 mmol), and 4 mL of anhydrous THF. The resulting solution was cooled in an ice-water bath. DEAD (62 mg, 0.31 mmol) was added over 2 min. The reaction mixture was stirred for 3 h and the bath was permitted to warm to room temperature. THF was removed by rotary evaporation and the residue was purified by flash column chromatography eluting with dichloromethane and dichloromethane/ethyl acetate (9:1) to give 128 mg (96% yield) of 15 as an orange-red solid. 1H NMR (400 MHz, CDCl3) δ 7.95–7.92 (m, 2H), 6.70–6.68 (m, 2H), 6.39 (dd, J = 1.7, 5.7 Hz, 0.5H), 6.32 (dd, J = 1.7, 5.7 Hz, 0.5H), 6.23 (d, J = 5.7 Hz, 0.5H), 6.15 (d, J = 5.7 Hz, 0.5H), 5.21–5.18 (m, 1H), 3.63–3.58 (1H), 3.40 (t, J = 7.7 Hz, 4H), 3.28 (t, J = 6.7 Hz, 2H), 3.11–3.07 (m, 1H), 2.15–1.97 (m, 2H), 1.82–1.81 (s, 3H), 1.79 (s, 3H), 1.69–1.57 (m, 4H), 1.45–1.18 (m, 14H), 0.92 (t, J = 6.8 Hz, 6H). In a 100 mL long neck round bottom flask were placed 30 mL of toluene, the protected (endo) maleimide DCDHF (128 mg, 0.197 mmol) and a stirbar. A nitrogen flow was introduced to the surface of the solution through a pipette and the system was heated to reflux. After 30 min, TLC indicated the retro Diels–Alder reaction was complete and the system was cooled down with nitrogen flow. The mixture was directly transferred onto a flash silica gel column and the product was eluted with dichloromethane containing 5% of ethyl acetate to obtain 100 mg (91% yield) of the maleimide DCDHF 22. 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 9.5 Hz, 2H), 6.70 (s, 2H), 6.71 (d, J = 9.5 Hz, 2H), 3.51–3.47 (m, 2H), 3.42 (t, J = 7.9 Hz, 4H), 2.26–2.05 (m, 2H), 1.81 (s, 3H), 1.69– 1.60 (m, 4H), 1.60–1.40 (m, 14H), 0.94 (t, J = 6.9 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 177.2, 171.7, 170.5, 152.9, 134.2, 132.1, 113.11, 113.07, 112.8, 112.06, 111.9, 99.0, 91.5, 54.1, 51.4, 37.6, 37.0, 31.6, 27.3, 26.9, 26.7, 22.6, 14.2. HRMS (m/z): [M+Na] calcd for C34H41N5O3Na, 590.3107; found, 590.3101. Compound 23. 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 2.0 Hz, 1H), 7.80–7.76 (m, 2H), 7.61 (d, J = 9.0 Hz, 1H), 7.06 (dd, J = 2.4, 9.0 Hz, 1H), 6.72 (d, J = 2.4 Hz, 1H), 6.53 (s, 2H), 3.53–3.45 (m, 6H), 2.30–2.23 (m, 1H), 2.17–2.09 (m,1H), 1.90 (s, 3H), 1.63–1.54 (m, 1H), 1.42– 1.36 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 176.5, 173.8, 170.5, 149.4, 138.5, 133.9, 132.1, 127.0, 124.9, 124.2, 118.6, 117.3, 112.3, 112.2, 111.3, 104.6, 100.2, 96.4, 56.2, 48.0, 37.1, 36.8, 26.8, 25.5, 22.6. HRMS (m/z): [M+Na] calcd for C30H25N5O3Na, 526.1855; found, 526.1866. Compound 24. 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 2.1 Hz, 1H), 7.80 (dd, J = 9.0, 2.1 Hz, 1H), 7.78 (d, J = 9.3 Hz, 1H), 7.61 (d, J = 9.0 Hz, 1H), 7.13 (dd, J = 9.3, 2.5 Hz, 1H), 6.78 (d, J = 2.5 Hz, 1H), 6.57 (s, 2H), 3.55–3.40 (m, 6H), 2.33–2.10 (m, 2H), 1.92 (s, 3H), 1.73–1.55 (m, 6H), 1.45–1.33 (m, 12H), 0.94 (t, J = 7.0 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 176.5, 173.8, 170.5, 150.0, 138.6, 134.0, 132.1, 131.8, 127.1, 124.7, 124.2, 118.7, 116.7, 112.2, 112.1, 111.2, 104.4, 100.1, 96.5, 56.3, 51.3, 37.1, 36.8, 31.7, 27.3, 26.81, 26.75, 22.7, 14.1. HRMS (m/z): [M+Na] calcd for C38H43N5O3Na, 640.3264; found, 640.3283. Compound 25. 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 2.1 Hz, 1H), 7.88 (dd, J = 8.9, 2.1 Hz, 1H), 7.79 (d, J = 9.2 Hz, 1H), 7.66 (d, J = 8.9 Hz, 1H), 7.13 (dd, J = 9.2, 2.4 Hz, 1H), 6.81 (s, br, 1H), 6.73 (s, 2H), 3.61 (t, J = 6.4 Hz, 2H), 3.51 (t, J = 7.5 Hz, 2H), 3.46 (t, J = 7.5 Hz, 2H), 1.93 (s, 6H), 1.75–1.63 (m, 6H), 1.43–1.32 (m, 6H), 0.94 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.4, 175.6, 170.8, 149.7, 138.4, 134.2, 132.0, 127.1, 124.8, 124.5, 119.0, 116.7, 112.4, 112.3, 111.4, 104.7, 98.3, 95.7, 65.2, 51.3, 50.6, 35.4, 31.6, 27.5, 27.2, 26.7, 26.1, 24.4, 22.6, 14.0. HRMS (m/z): [M+Na] calcd for C34H35N5O3Na, 584.2638; found, 586.2644. Compound 26. 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 2.1 Hz, 1H), 7.88 (dd, J = 9.1, 2.1 Hz, 1H), 7.82 (d, J = 9.3 Hz, 1H), 7.72 (d, J = 9.1 Hz, 1H), 7.22 (dd, J = 9.2, 2.5 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.70 (s, 2H), 3.82 (t, J = 6.6 Hz, 2H), 3.69 (t, J = 7.6 Hz, 2H), 3.46 (t, J = 7.6 Hz, 2H), 1.94 (s, 6H), 1.72–1.65 (m, 2H), 1.43–1.30 (m, 6H), 0.94 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.2, 175.8, 170.5, 149.4, 138.3, 134.3, 132.0, 131.8, 127.4, 125.1, 124.5, 119.5, 116.6, 112.2, 112.1, 111.2, 105.2, 98.4, 96.5, 56.6, 51.0, 48.4, 34.6, 31.6, 27.4, 27.3, 26.6, 22.6, 14.0. HRMS (m/z): [M+Na] calcd for C32H31N5O3Na, 556.2325; found, 556.2327. Compound 27. 1H NMR (400 MHz, CDCl3) δ 7.91 (ddd, J = 8.9, 2.4, 2.0 Hz, 2H), 7.54 (ddd, J = 8.9, 2.4, 2.0 Hz, 2H), 7.33 (d, J = 4.0 Hz, 1H), 6.71 (s, 2H), 6.02 (d, J = 4.0 Hz, 1H), 3.82 (t, J = 6.6 Hz, 2H), 3.56 (t, J = 6.6 Hz, 2H), 3.36 (t, J = 7.5 Hz, 2H), 1.88 (s, 6H), 1.72–1.64 (m, 2H), 1.43–1.37 (m, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.0, 174.5, 170.5, 160.5, 141.7, 134.3, 130.0, 128.5, 123.7, 123.6, 122.1, 112.1, 111.9, 111.1, 104.1, 98.2, 96.7, 56.9, 53.6, 50.5, 34.6, 29.1, 27.2, 20.1, 13.9. HRMS (m/z): [M+Na] calcd for C30H27N5O3SNa, 560.1732; found, 560.1730. Compound 28 1H NMR (400 MHz, CDCl3) δ 7.81 (d, br, J = 14.6 Hz, 1H), 7.35 (d, J = 4.6 Hz, 1H), 6.76 (s, 2H), 6.21 (d, J = 4.6 Hz, 1H), 6.00 (d, br, J = 14.6 Hz, 1H), 3.85 (t, J = 6.6 Hz, 2H), 3.67 (t, J = 6.6 Hz, 2H), 3.47 (t, J = 7.7 Hz, 2H), 1.78–1.67 (m, 8H), 1.49–1.39 (m, 2H), 1.03 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.9, 172.6, 170.2, 168.2, 143.0, 140.3, 134.5, 125.1, 113.7, 112.9, 112.8, 107.7, 105.0, 95.8, 54.7, 51.8, 50.7, 34.4, 29.7, 26.8, 20.1, 13.8. HRMS (m/z): [M+Na] calcd for C26H25N5O3SNa, 510.1576; found, 510.1579.


