Abstract
The purpose of this paper was to simultaneously examine changes in urothelial ATP and NO release in normal and spinal cord injured animals as well as in spinal cord injured animals treated with botulinum toxin type A (BoNT-A). Furthermore we correlated changes in transmitter release with functional changes in bladder contraction frequency, and determined the effects of BoNT-A on bladder efferent nerve function. Normal and spinal cord injured rat bladders were injected on day 0 with either vehicle (saline containing bovine serum albumin) or BoNT-A. On day 2, in vitro neurotransmitter release and bladder strip contractility studies as well as in vivo cystometrographic studies were conducted. Resting ATP release was significantly enhanced following spinal cord injury (i.e. 57% increase, p<0.05) and was unaffected by BoNT-A treatment. SCI increased hypo-osmotic evoked urothelial ATP release by 377% (p<0.05). BoNT-A treatment reduced evoked ATP release in SCI bladders by 83% (p<0.05). In contrast, hypo-osmotic stimulation induced NO release was significantly inhibited following SCI (i.e. 50%, p<0.05) but recovered in SCI rats treated with BoNT-A (i.e. 195% increase in NO release in SCI-BTX treated rats compared to SCI controls, p<0.01). Changes in urothelial transmitter release coincided with a significant decrease in non-voiding bladder contraction frequency (i.e. 71%, p<0.05) in SCI-BTX rats compared to SCI rats. While no difference was measured between neurally evoked contractile amplitude between SCI and SCI-BTX animals, atropine (1µM) inhibited contractile amplitude to a greater extent (i.e. 76%, p<0.05) in the SCI-BTX group compared to the SCI group. We hypothesize that alterations in the ratio of excitatory (i.e. ATP) and inhibitory (i.e. NO) urothelial transmitters promote bladder hyperactivity in rat bladders following SCI that can be reversed, to a large extent, by treatment with BoNT-A.
Keywords: Botulinum toxin, Bladder, Nitric Oxide, ATP, Urothelium, Spinal Cord Injury
1. Introduction
ATP levels and P2X receptor mediated responses are enhanced in certain pathologic states (e.g. spinal cord injury, SCI), conditions that are associated with heightened bladder sensory mechanisms. For example, urothelial ATP release is significantly enhanced in chronic SCI rats and in rats subjected to chemically induced chronic bladder inflammation (Khera et al., 2004; Smith et al., 2005). Both of these conditions are associated with bladder hyperreflexia. ATP released from bladder urothelium is thought to activate suburothelially located P2X3 receptors and to increase sensory nerve transmission in a non-synaptic manner, a phenomenon that has been previously described regarding the interaction between efferent nerve terminals (Vizi, 1979). In addition, ATP release within the dorsal lumbosacral spinal cord is also markedly elevated in SCI rats subjected to chemical or pressure bladder stimulation when compared to non-injured rats (Salas et al., 2007).
Bladder urothelium responds to stretch by releasing substances including ATP, nitric oxide (NO) and acetylcholine (Birder et al., 1998; Hanna-Mitchell et al., 2007; Lips et al., 2007; Ferguson et al., 1997). While ATP is viewed primarily as an excitatory transmitter on bladder afferent pathways (Vlaskovska et al., 2001; Cockayne et al., 2005; Pandita and Andersson, 2002), nitric oxide has been shown in many cases to have inhibitory effects on bladder reflex pathways. For example, Ozawa et al (1999) found that intravesical application of NO donors reduced bladder hyperreflexia in a chronic bladder inflammation model. Furthermore, application of oxyhemoglobin, a nitric oxide scavenger, was shown to induce bladder overactivity in normal rats (Pandita and Andersson, 2000). However, while urothelial ATP release is upregulated following SCI (Khera et al., 2004), modulation of urothelial NO release following chronic SCI has not yet been studied.
BoNT-A, well established as an inhibitor of vesicular acetylcholine release from motor nerve terminals, acts by cleaving the SNARE protein SNAP-25 (Schiavo et al., 1993). Recent basic and clinical evidence suggests that BoNT-A has inhibitory effects on sensory pathways unrelated to its actions on efferent nerve terminals (Cui et al., 2004; Smith and Chancellor, 2004). For example, BoNT-A instillation in the bladder has been shown to inhibit ATP release in bladder urothelium as well as reduce bladder overactivity in chronic SCI and chronic bladder inflammation models (Khera et al., 2004, 2005; Smith et al., 2005; Vemulakonda et al., 2005). BoNT-A has also been shown to inhibit stretch-evoked or capsaicin-evoked release of ATP from cultured urothelial cells (Barrick et al., 2004), to reduce bladder activity induced by intravesical ATP (Atiemo et al., 2005), and to decrease P2X3 receptor expression in human patients with neurogenic detrusor overactivity (Apostolidis et al., 2005). Nitric oxide, produced by bladder urothelial nitric oxide synthase (NOS) enzymes, is released by non-vesicular mechanisms (Morris et al., 2001) and, thus, its levels should not be directly impacted by BoNT-A treatment. However, the few reports within the literature addressing this topic demonstrate both positive and negative modulation of NO or NOS activity in tissues treated with BoNT-A (Morris et al., 2001; Olgart et al., 2000; Mariotti et al., 1996; Ellies et al., 2006).
The purpose of this paper was to: 1) simultaneously examine changes in urothelial ATP and NO release in normal and SCI animals as well as in SCI animals treated with BoNT-A; 2) correlate changes in transmitter release with functional changes in bladder contraction frequency and; 3) determine the effects of BoNT-A injection on efferent nerve function by measuring neurally evoked contractions of bladder strips.
2. Experimental procedures
2.1.1. General
All experiments were conducted in accordance with guidelines of the National Institute of Health and the Institutional Animal Care and Use Committee of Baylor College of Medicine regarding the care and use of animals for experimental procedures. Female Sprague-Dawley rats (250 to 300g) were used in all experiments, either as non-injured rats or following spinal cord injury.
2.1.2. SCI Model
Rats were anesthetized with isoflurane and, after laminectomy; the spinal cord was completely transected at the T8–T9 level. The spinal area was surgically closed, the animals received amoxicillin (150mg/kg) for 14 days postoperatively, and the bladders were manually expressed twice daily until spontaneous voiding reappeared. Experiments were performed beginning 21 days after spinal cord injury was induced (i.e. Day 0). A subset of non-injured (normal) rats was also used as a control for in vitro experiments.
2.1.3. Bladder Wall Injection
On day 0, spinal cord injured and normal rats underwent isoflurane anesthesia, followed by midline laparotomy and exposure of the urinary bladder. Animals undergoing in vivo cystometrogram studies had a suprapubic bladder catheter placed followed by baseline cystometry studies (Day 0). All animals underwent bladder injection either with botulinum toxin [2 units of Botox®, diluted in 25µl of vehicle (saline containing 0.5% bovine serum albumin)] or with vehicle alone (25µl total). Injections were distributed in 5 locations using a 30-gauge needle connected to an infusion pump to ensure equal volume injections into the bladder wall.
2.2. In vitro experiments
Two experimental groups were used in these experiments; normal rats that were treated with vehicle (NL) and spinal cord injured rats that were treated either with vehicle alone or with BoNT-A+vehicle (SCI-BTX).
2.2.1. Hypo-osmotic shock
On day 2, normal and SCI rats were euthanized with CO2 inhalation and the bladders were removed and placed on a Silgard® covered Petri dish containing oxygenated Krebs solution. The bladder was cut open longitudinally and half of the bladder was mounted in the Ussing chamber (WPI, Sarasota, FL) while the other half was used for the contractility experiments. The bladder, mounted in this way, is separated into two hemi-chambers where the urothelial and serosal sides are isolated from each other. Both halves of the chamber were perfused with oxygenated preheated 37 °C Krebs solution (mm/l; NaCl 113, KCL 4.7, CaCl2 1.25, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11.5) at a rate of 1.0 mL/min. After 30 min of equilibration, 2 min effluents were collected using a fraction collector (Gilson, Middleton, WI) from the urothelial side of the chamber. At the 6th min of the perfusion, a bolus of hypo-osmotic Krebs solution was perfused for 6 min on the urothelial side of the chamber. The hypo-osmotic solution was created by reducing the concentration of NaCl by 40% from 113 to 67.8mM. A 500uL and 50uL aliquots of each 2 min urothelial effluent were analyzed for purine content by HPLC and nitric oxide (NO) content by a nitric oxide analyzer (Sievers, Boulder, CO), respectively.
2.2.2. HPLC-Based Assays for Adenine Nucleotides and ADO
Purines (ATP, ADP, AMP, and ADO) were analyzed as described previously by Todorov et al. (1996). After performing precolumn derivatization with chloroacetyl aldehyde the resulting etheno-purine derivatives were separated on a gradient HPLC system equipped using a Resolve Radial Pack Cartridge (8NV Ph 4 µm; 8 × 10 mm) (Waters, Milford, MA) connected to a fluorescent detector (Dynamax FL-1, Rainin, Woburn, MA) at an excitation wavelength of 230 nm and an emission wavelength of 420 nm. Buffer solutions consisted of 40 mM phosphate (KH2PO4, pH 6.0) (buffer A) and 75% 40 mM phosphate and 25% methanol (buffer B). The adenine nucleotides and ADO were separated using a linear gradient in which the concentration of buffer B was increased to 100% from 1–8 min and decreased to 0% from 10–13 min. The HPLC pumps were controlled by the Unipoint LC system (Gilson, Middleton WI). The same software system was used for data collection as well as for peak evaluation. Identification of individual peaks in chromatograms was carried out using the retention times of the etheno-derivatives of purine standards, and the individual purine amount was calculated by comparing the area of peaks in the samples with that of the standards. The released amount of ATP was represented as the amount of total purine within each sample (Todorov et al., 1996).
2.2.3. Nitric Oxide measurement
The concentration of nitric oxide metabolites (i.e. NO2 and NO3) in the samples was measured with a nitric oxide analyzer (Sievers, Boulder, CO). An aliquot of 10uL of each sample was injected into a reaction chamber containing vanadium chloride as a reducing agent at 95°C. The NO formed in the reaction chamber was measured based on a chemo-luminescent reaction with ozone that was then converted by a photomultiplier to voltage change. The resulting voltage in the samples was compared to sodium nitrate standards and the amounts of NO were calculated back to the total sample volume.
2.2.4. Bladder Strip Contractility
Two longitudinal strips each weighing approximately 20 mg were prepared from the bladders of SCI rats on day 2 and were mounted in a double-jacketed organ bath at 35°C in Krebs solution that was constantly bubbled with a mixture of 95% O2 and 5% CO2. The initial tension was set at 10 mN and isometric contractions were measured with strain-gauge transducers (FT-100, WPI Sarasota) and recorded with a computerized data acquisition program (Windaq, DATAQ Instruments, Akron, OH). The amplitude of the stimulation-evoked contractions was computed with the WindaqEx program (DATAQ Instruments, Inc.).
Electrical field stimulation using maximal voltage at a frequency of 20Hz and 0.25 ms stimulus duration was delivered with a Grass S88 stimulator through platinum electrodes inserted from the top and bottom of the organ bath and separated by 4cm. The fatigue stimulation paradigm was established as described previously (Smith et al., 2003) and included trains of 100 shocks at 20Hz with maximal voltage every 20 s for a period of 10 min after which the strips were allowed to recover for 10 min by applying trains every 100 s (Fig. 1). The same pattern of stimulation was repeated 15 min after application of the muscarinic blocker, atropine (1µM). Potassium chloride (100mM) was applied at the end of each experiment to determine the effect of direct muscle stimulation. Mean amplitude before and after fatigue stimulation and before and after atropine administration were calculated either as a percentage of mean KCl induced contractile amplitude or as an absolute value (mN/cm2) calculated from the cross-sectional area ofeach strip (Somogyi et al., 2002).
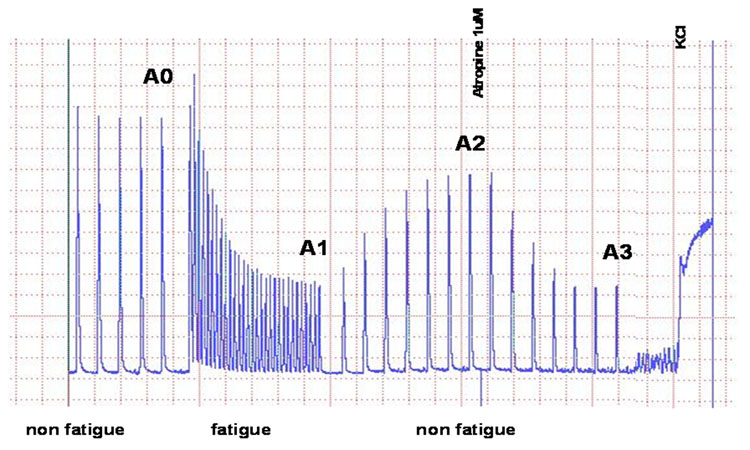
Figure 1.
This illustration depicts the stimulation paradigm used for bladder strip contractility experiments. Non-fatigue stimulation (i.e. trains of 100 shocks at 20Hz with maximal voltage every 100 s) was applied at A0, followed by 10 minutes of fatigue stimulation (i.e. trains of 100 shocks at 20Hz with maximal voltage every 20 s; A1) after which the muscle strips were allowed to recover for 10 minutes during non-fatigue stimulation. Point A2 depicts the time period when atropine (1µM) was applied to the baths during non-fatigue stimulation while point A3 displays the inhibitory effect of atropine on contractile amplitude. Potassium chloride (100mM KCl) was given at the end of each experiment to determine the effect of direct muscle stimulation.
2.3. In vivo cystometrography experiments
Prior to (Day 0) and 2 days after bladder wall injection (Day 2), conscious SCI rats were gently immobilized, and open cystometry was performed by perfusing saline through the suprapubic catheter at a rate of 0.04ml/min with a syringe pump (KD Scientific, New Hope, PA). The bladder pressure was monitored with a pressure transducer (WPI, Sarasota, FL) connected to a bridge amplifier (TBM4, WPI), and the data were collected with a computerized data acquisition system (DATAQ, Akron, OH). Following a 60 min equilibration period, three consecutive voiding cycles were recorded. Voiding bladder contractions were differentiated from non-voiding bladder contractions by computerized measurement of the volume of the voided urine with a strain gauge system. Changes in bladder contraction frequency of non-voiding and voiding contractions were analyzed at day 0 and day 2. We chose 48 hours as an optimal time point to measure BoNT-A’s effect based on prior results carried out in our lab (Khera et al., 2005; Vemulakonda et al., 2005).
2.4. Statistical Analysis
All data was expressed as the mean ± SEM. Data from urothelial release and bladder strip contractility experiments were compared using the unpaired t-test when two groups were compared and the one-way analysis of variance test followed by Newman-Keuls multiple comparisons test or the Kruskal-Wallis test followed by Dunn multiple comparisons test when three groups were compared. CMG data was analyzed by the student t-test. A level of P < 0.05 was considered statistically significant.
2.5. Drugs
BoNT-A (Botox®) was provided by Allergan Inc. (Irvine, CA). Atropine, amoxicillin, vanadium chloride, ATP, ADP, AMP, adenosine, chloroacetaldehyde, and all constituents of Krebs and HPLC solutions were purchased from Sigma Aldrich (St. Louis, MO).
3. Results
3.1. Transmitter release from bladder urothelium
3.1.1. ATP release
Basal release
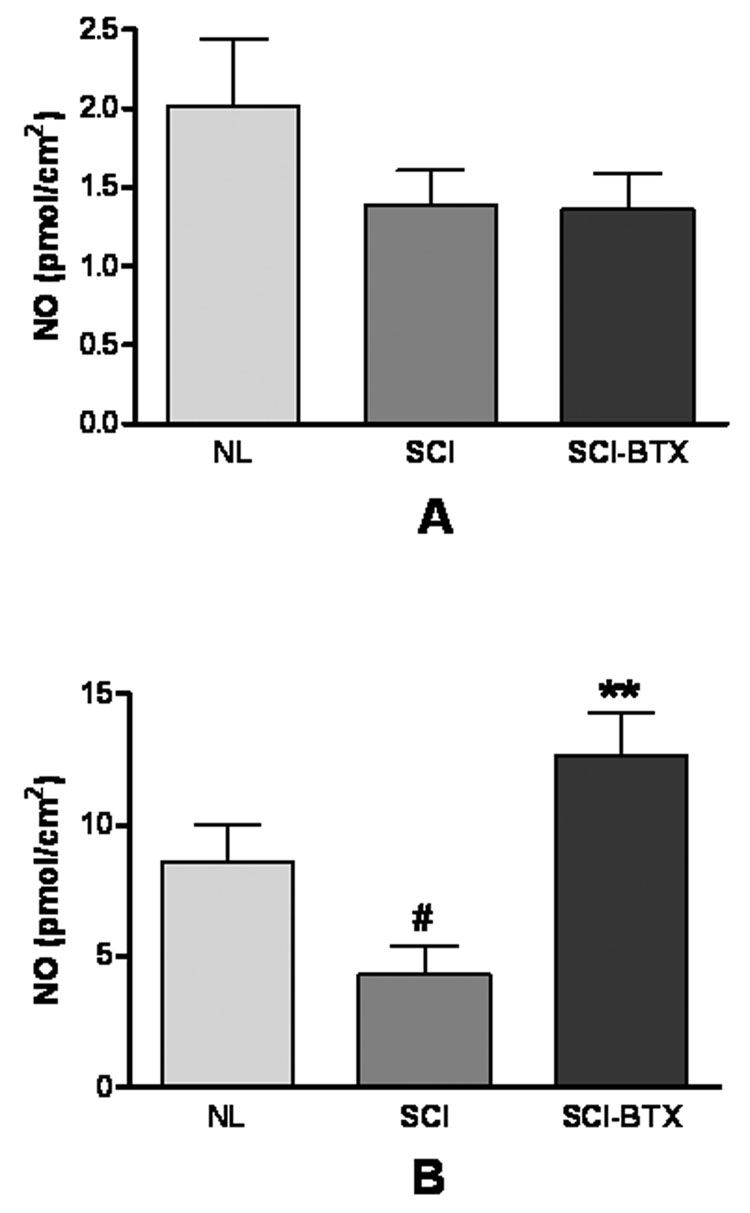
Figure 2A demonstrates the effect of spinal cord injury and BoNT-A treatment on ATP release from bladder urothelium under basal conditions. This figure shows that resting ATP release was significantly enhanced following spinal cord injury (i.e. 57% increase, p<0.05). BoNT-A treatment did not change basal release of ATP in SCI bladders.
Figure 2.
This figure shows bladder urothelial ATP release (i.e. representing total purine release) in normal and spinal cord injured bladders treated with bovine serum albumin [NL (n=8), and SCI (n=6), respectively], and in spinal cord injured bladders treated with BoNT-A (SCI-BTX, n=6) under basal (i.e. figure 2A) and hypoosmotic (i.e. figure 2B) conditions. This figure demonstrates a significant increase in both basal and hypoosmotic evoked urothelial ATP release following spinal cord injury. BoNT-A, however, was only effective in inhibiting hypoosmotic evoked and not basal ATP release in spinal cord injured bladders. *denotes p<0.05 between NL and SCI groups while #denotes p<0.05 between SCI and SCI-BTX groups using the Kruskal-Wallis non-parametric test with Dunn multiple comparison test
Hypoosmotic evoked release
As shown in figure 2B, SCI dramatically increased hypoosmotic evoked urothelial ATP release when compared to normal rat bladders (i.e. 377% increase, p<0.05). In contrast to its lack of effect on basal ATP release, BoNT-A treatment significantly reduced evoked ATP release in SCI bladders by 83% (p<0.05).
3.1.2. NO release
Basal release
No significant changes in resting NO release were demonstrated either after SCI or in SCI bladder subjected to BoNT-A treatment (Figure 3A).
Figure 3.
The figure displays urothelial nitric oxide release in normal and spinal cord injured bladders treated with bovine serum albumin [NL (n=7), and SCI (n=5), respectively], and in spinal cord injured bladders treated with BoNT-A (SCI-BTX, n=5) under basal (i.e. figure 3A) and hypoosmotic (i.e. figure 2B) conditions. There was no significant change in basal nitric oxide release following spinal cord injury (i.e. figure 3A). In contrast, hypoosmotic evoked nitric oxide release decreased following spinal cord injury (i.e. figure 3B). In addition, while BoNT-A had no effect on basal nitric oxide release, it significantly enhanced hypoosmotic nitric oxide release in spinal cord injured bladders (figure 3B). #denotes p<0.05 between NL and SCI groups while **denotes p<0.01 between SCI and SCI-BTX groups using the one way analysis of variance test with Newman-Keuls multiple comparison test.
Hypoosmotic evoked release
Figure 3B demonstrates that hypoosmotic stimulation induced NO release was significantly inhibited following SCI (i.e. 50%, p<0.05) but recovered in SCI rats treated with 2 units of BoNT-A (i.e. 195% increase in NO release in SCI-BTX treated rats compared to SCI controls, p<0.01).
3.2. Effect of BoNT-A on electrically evoked contractions
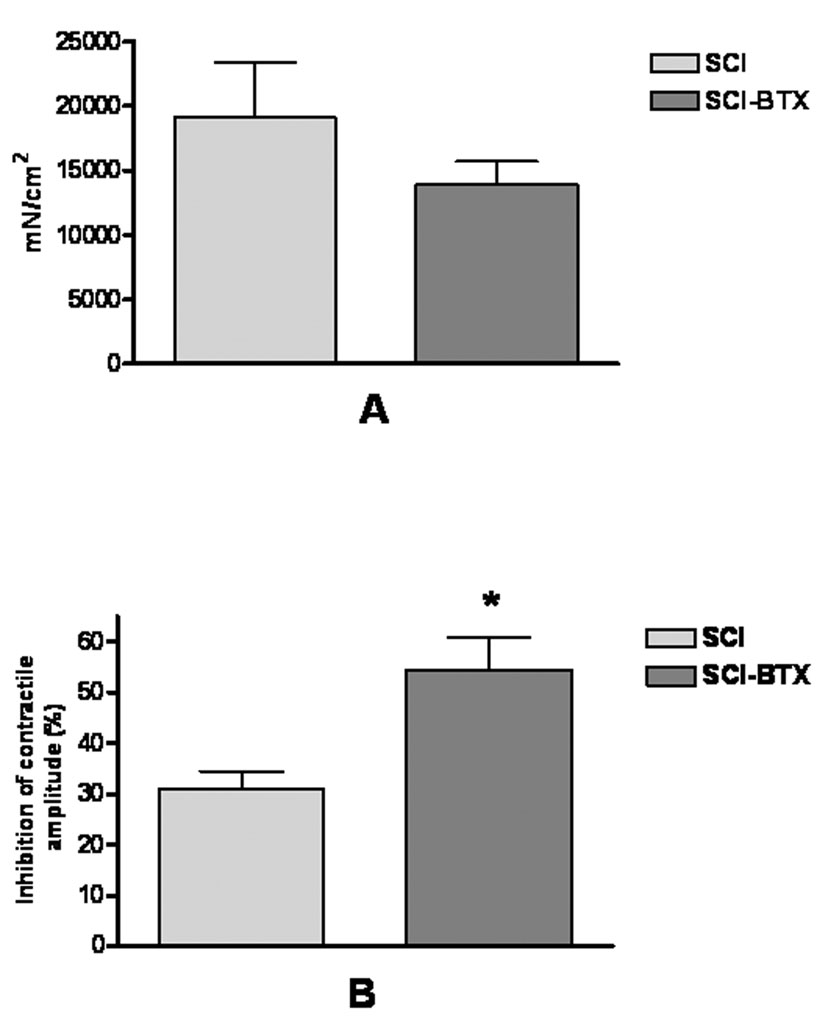
Bladder strips from vehicle or BoNT-A treated SCI rats were tested for changes in contractile amplitude induced by non-fatigue and fatigue stimulation (see Methods) as well as following administration of atropine (1µM). Figure 4A displays the mean baseline contractile response induced by non-fatigue stimulation (i.e. A0 in figure 1) standardized to the cross-sectional area of each bladder strip. This figure shows that no significant difference in contractile amplitude was found between vehicle and BoNT-A treated rat bladders. In fact, BoNT-A did not change contractile amplitude when compared to vehicle treated rats before, during, or after fatigue stimulation (i.e. A0, A1, or A2 in figure 1) even when standardized to cross-sectional area or to the KCL evoked bladder contraction (data not shown).
Figure 4.
This graphic depicts results of in vitro bladder strip contractility experiments in spinal cord injured bladders injected with bovine serum albumin or BoNT-A [SCI (n=5), and SCI-BTX (n=6), respectively). Figure 4A demonstrates the mean contractile amplitude standardized to the cross-sectional area of each bladder strip during non-fatigue stimulation (i.e. point A0 defined in figure 1). BoNT-A treatment had no effect on the baseline contractile amplitude of spinal cord injured bladder strips. Figure 4B shows the mean inhibitory effect of atropine (1µM) on contractile amplitude during non-fatigue stimulation (i.e. point A3 defined in figure 1). Atropine had a significantly greater inhibitory effect in spinal cord injured bladders treated with BoNT-A. *denotes p<0.05 between SCI and SCI-BTX groups using the two-tailed unpaired t-test.
However, atropine had a 76% greater inhibitory effect on contractile amplitude (A3 in figure 1) in BoNT-A treated SCI bladders compared to vehicle treated bladders (i.e. Figure 4B, p<0.05). This finding suggests that BoNT-A treated bladders have a more prominent cholinergic contractile component than vehicle treated bladders.
3.3. Effect of BoNT-A on reflex bladder activity by cystometry
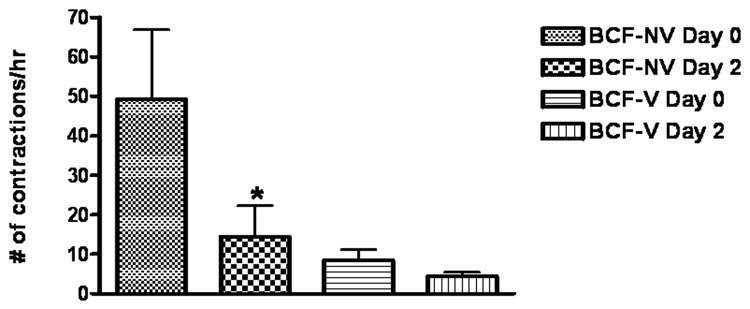
Bladder contraction frequency (i.e. BCF) was utilized as an indirect measure of bladder afferent activity. In SCI rats not all bladders contractions are followed by voiding because of inherent variability in the intensity of bladder contractile force. Therefore, one can categorize bladder contractions as either voiding on non-voiding. In this paper, BCF was represented as either non-voiding bladder contractions (i.e. BCF-NV) or as voiding contractions (i.e. BCF-V). Figure 5 demonstrates that the majority of bladder contractions after SCI were non-voiding in nature. More importantly, figure 5 shows the significant decrease in BCF-NV (i.e. 71%, p<0.05) induced by BoNT-A treatment. No difference in BCF was detected in vehicle treated animals (data not shown).
Figure 5.
The figure demonstrates the bladder contraction frequency (i.e. BCF) measured during in vivo cystometrographic experiments in spinal cord injured rats (n=6) before (i.e. day 0) and after (i.e. day 2) bladder injection with BoNT-A. Voiding and non-voiding bladder contractions were distinguished by measuring whether urine was expelled during each contraction. The frequency of bladder contractions per hour were categorized as “non-voiding bladder contractions” (i.e. BCF-NV) and as “voiding bladder contractions” (i.e. BCF-V). While BoNT-A had no effect on the frequency of voiding bladder contractions, it significantly inhibited bladder contraction frequency of non-voiding bladder contractions (i.e. BCF-NV). *denotes p<0.05 between day 0 and day 2 groups using the two-tailed student t-test.
3. Discussion
The major findings of these experiments are that: 1) SCI induces a significant increase in urothelial total purine release and a significant decrease in urothelial release of NO; 2) BoNT-A has a dramatic effect toward normalization of SCI induced changes in urothelial transmitter release (i.e. NO (increase) and ATP (decrease); 3) The inhibition of ATP release and stimulation of NO release following BoNT-A treatment are associated with a decrease in reflex bladder activity during in vivo cystometrographic studies and; 4) Bladder wall injection with BoNT-A preferentially targets sensory over motor nerve fibers in the rat SCI bladder.
SCI induced a dramatic increase in both basal and hypoosmotic evoked release of ATP measured with HPLC, a finding consistent with prior investigations measuring ATP directly with luminometry (Khera et al., 2004). Hypoosmotic stress was used as a stimulus to imitate the response of urothelium during normal bladder filling. We previously demonstrated the utility of Ussing Chamber experiments to document changes in ATP release induced by SCI and following BoNT-A treatment (Khera et al., 2004). Total purine release was expressed as ATP because of prior research demonstrating that released ATP is rapidly metabolized by nucleotidases in periopheral tissues innervated by cholinergic and adrenergic neurons (Nitahara et al., 1995; Todorov et al., 1996).
In contrast to changes in ATP release, hypoosmotic induced NO release from bladder urothelium was dramatically decreased in SCI rats when compared to normal rats (i.e. reduced by 50%, p<0.05). The differential effects of SCI on urothelial NO (i.e. inhibitory) and ATP (i.e. stimulatory) release suggest that changes in these transmitter levels may contribute to the bladder hyperactivity associated with chronic SCI. Nitric oxide is produced constitutively in tissues by neuronal and endothelial nitric oxide synthase enzymes (e.g. nNOS and eNOS, respectively) and can also be produced during specific conditions (e.g. inflammation) by inducible NOS (e.g. iNOS) (Frostermann et al., 1994). Birder and colleagues demonstrated that the major sites of NO release in the rat bladder are urothelium and afferent nerves (Birder 1997, 1998, 2001). Rat urothelium expresses eNOS and iNOS but not nNOS and can be stimulated by B-adrenoceptor agonists to release NO (Birder et al., 2002b). Nitric oxide can also be released from bladder urothelium in response to stretch (Birder et al. 2002a) and has been shown to have modulatory effects on bladder reflex activity (de Groat, 2006).
In concert with these findings, intravesically administered NO donors reduced bladder contraction frequency in experimental models of bladder hyperactivity (Ozawa et al., 1999). Conversely, intravesical administration of the NO scavenger oxyhemoglobin induced bladder hyperactivity in normal rats (Pandita and Andersson., 2000). Furthermore, Ca2+ channel activity in dorsal root ganglion neurons innervating the urinary bladder were inhibited by exogenously applied NO (Yoshimura et al, 2001). Thus, these findings suggest an inhibitory action of NO on bladder hyperactivity. In addition, clinical studies have shown that some patients with interstitial cystitis (IC), a condition characterized by urinary frequency, urgency, and pain, have decreased urine NOS activity (Smith et al., 1997). Moreover, oral treatment with the NO donor, L-arginine, increases NOS levels and activity and leads to a statistically significant improvement in IC symptoms (Wheeler et al., 1997; Korting et al., 1999; Cartledge et al., 2000).
However, others have demonstrated increased levels of NO and neuronal nitric oxide synthase production in the urothelium of cats with naturally occurring chronic bladder inflammation (i.e. interstitial cystitis, IC) (Birder et al., 2005) and increased levels of inducible nitric oxide synthase in SCI human bladders associated with chronic indwelling catheters and bladder inflammation (Wall et al., 2001). In the latter case, iNOS was localized to suburothelial macrophages and was probably induced by the presence of a chronic foreign body (i.e. catheter) as much as from the underlying bladder pathophysiology (i.e. neurogenic bladder). Some investigators theorize that elevated NO levels increase urothelial permeability and lead to enhanced activation of bladder sensory mechanisms (Birder et al 2005).
The complex nature of NO signaling is magnified by a study showing that inhibition of endothelial nitric oxide synthase (i.e. eNOS) or neuronal nitric oxide synthase (nNOS) activity in the urothelium of cat bladders has opposing effects on bladder activity: inhibition of eNOS increases bladder contraction frequency while inhibition of nNOS reduces bladder contraction frequency (Theobald, 2003). Thus, the effects of NO on bladder activity (i.e. stimulatory or inhibitory) may be dose dependent (Colasanti and Suzuki, 2001), species dependent (i.e. rat vs. cat), pathology dependent (i.e. normal vs. SCI vs. chronic bladder inflammation) or may be dependent on the enzyme producing NO (i.e. eNOS vs. nNOS, vs. iNOS) or the target tissue that is modulated by released NO (i.e. urothelium, afferent nerve, smooth muscle). Moreover, evidence for “cross-talk” between constitutive (i.e. eNOS and nNOS) and inducible NOS enzymes suggest that each, when active, suppresses the level and the activity of the other enzyme class (Persichini et al., 2006).
Our present findings demonstrate that treatment with BoNT-A results in an 82% decrease in urothelial ATP release in SCI rats. These results corroborate prior experiments demonstrating that intravesical instillation of BoNT-A significantly reduces urothelial release of ATP in SCI rat bladders (Khera et al., 2004). Moreover, our experiments show that detrusor wall injection of BoNT-A, which simulates the clinical treatment paradigm used in humans, can modulate the release of transmitters from the mucosal surface of urothelium. The fact that urothelial release of ATP was inhibited to a greater extent by detrusor wall injection of BoNT-A than by intravesical instillation of BoNT-A (i.e. 86% in our present experiments vs. 53% in Khera et al., 2004) could suggest that: 1) Two units of BoNT-A injected in the detrusor wall achieves a higher urothelial tissue concentration of BoNT-A than 20 units instilled intravesically or; 2) The direct inhibitory effect of BoNT-A on bladder tissues other than urothelium (i.e. afferent or efferent nerves) strongly contributes to the reduction of urothelial ATP release observed following hypoosmotic stimulation.
The marked decrease in urothelial ATP release in BoNT-A treated SCI rats directly contrasts with BoNT-A’s strong stimulation of NO release in SCI rat bladders subjected to hypoosmotic stimulation (i.e. 1.95 fold increase). Nitric oxide is released by diffusion and not packaged in vesicles dependent on SNARE proteins as evidenced by prior studies demonstrating that BoNT-A or BoNT-B treatment fails to inhibit nerve stimulation evoked release of NO from the guinea pig autonomic nervous system (Morris et al., 2001; Olgart et. al, 2000). Thus, BONT-A should not have a direct effect on NO release from bladder urothelium. However, BoNT could indirectly affect NO levels through actions on NOS activity. For example, studies have shown that BoNT-A injection of facial motor neurons induced an increase in NOS activity that paralleled muscle paralysis (Mariotti and Bentivoglio, 1996). In contrast, other investigations found no change in nNOS immunoreactivity or nNOS expression following BoNT-A treatment in guinea pig nasal mucosa and rat spinal motor neurons, respectively (Rorhbach et al., 2002; Jung et al., 1997). In addition, Ellies and colleagues showed that nNOS immunoreactivity in the rat parotid gland was decreased following BoNT-A treatment (Ellies et al., 2006). Thus, BoNT-A appears to have variable effects on NOS activity all of which could have an impact on local tissue NO levels. In the future, we will examine whether SCI ± BoNT-A treatment induces changes in NOS activity that could account for differences in NO levels observed in this study.
Similar to our prior studies utilizing intravesical BoNT-A instillation, bladder contraction frequency (BCF), an indirect measure of bladder afferent activity and a parameter known to be increased following SCI, was significantly reduced by BoNT-A injection. Our unique findings regarding urothelial ATP and NO release suggest that inverse changes in the levels of these transmitters following SCI and in SCI bladders after BoNT-A treatment drive the functional changes observed in bladder contraction frequency. The fact that BoNT-A had an inhibitory effect on non-voiding bladder contractions but no measurable impact on voiding contractions suggests that BoNT-A may selectively inhibit specific bladder afferent pathways.
Previous work has demonstrated that non-voiding contractions in SCI rats are predominantly mediated by capsaicin-sensitive C-fibers, while A-delta afferents mediate voiding contractions (Cheng et al., 1995). Thus, BoNT-A appears to preferentially target C-fibers, at least in SCI rat bladders. This finding could be a result of native differences in BoNT-A receptor expression (i.e. SV2) between these two sensory nerve populations (Dong et al., 2006). Alternatively, BoNT-A intoxication can be enhanced in both animal and human models by increasing nerve activity, suggesting that binding and endocytosis of BoNT-A within nerve terminals is dependent on nerve terminal exocytosis (Hughes and Walker, 1962; Simpson, 1982; Hesse et al., 1995; Kim et al., 2003). In this regard, C-fibers may be more sensitive to the effects of BoNT-A because they are more active than A-delta afferents in the rat SCI bladder (i.e. non-voiding bladder contractions more numerous than voiding contractions) (Cheng et al., 1995). We previously have demonstrated the use-dependence of BoNT-A intoxication in fatigue bladder muscle strip experiments that examined the effects of BoNT-A on bladder efferent pathways (Smith et al., 2003).
The fact that bladder hyperactivity was reduced without any measurable effect on bladder strip contractility suggests that BoNT-A’s predominant effect was on afferent rather than efferent nerve pathways. We cannot exclude any effect of BoNT-A on efferent nerve conduction but we assume the effects were minimal given the lack of functional consequences (i.e. urinary retention, lack of inhibition of bladder contractility to electrical stimulation). We found that BoNT-A treated SCI strips had a greater inhibition to atropine application following fatigue stimulation. These results are consistent with results demonstrated during in vitro fatigue stimulation experiments in normal rat bladders (Smith et al., 2003) and imply that BoNT-A treated bladders have a stronger cholinergic contractile component than control bladders.
Rat bladders are known to have an increase in the cholinergic component of the bladder contraction following SCI (Somogyi et al., 1998). Thus, the fact that BoNT-A made rat SCI bladders even more cholinergic dominant suggests two possible explanations: 1. BoNT-A inhibits efferent nerve terminal release of ATP to a greater extent than ACh release in rat SCI bladders or; 2). Chemodenervation with BoNT-A alters post-junctional bladder smooth muscle response (i.e. upregulate muscarinic receptors) creating a more prominent cholinergic contractile component. Previous work in a cryoinjury bladder model demonstrated that injuring the bladder wall shifts the bladder contraction to one with a greater cholinergic contribution (Somogyi et al., 2002). Thus, BoNT-A could create a bladder contraction with a more robust cholinergic contribution because of its direct inhibitory effects on efferent nerve release of ATP or by indirectly up-regulating post-junctional muscarinic responses as a result of its chemodenervating effects on parasympathetic nerve terminals. Further studies will directly explore the effects of BoNT-A on pre- and post-junctional sites within the normal and SCI rat bladder.
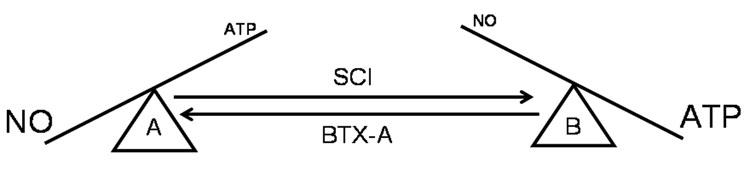
In summary, bladder wall injection with BoNT-A in the rat SCI bladder significantly reduces ATP release while simultaneously increasing NO release from bladder urothelium. These differential effects on urothelial transmitter release are functionally manifested by significant reductions in bladder hyperactivity without compromising efferent nerve function. We hypothesize that alterations in the ratio of excitatory (i.e. ATP) and inhibitory (i.e. NO) urothelial transmitters promote bladder hyperactivity in rat bladders following SCI. Moreover, we theorize that BoNT-A reduces bladder hyperactivity, in large part, by restoring both the levels and the ratios of ATP and NO released from urothelium (Figure 6).
Figure 6.
This diagram depicts our proposed hypothesis that changes in excitatory (i.e. ATP) and inhibitory (i.e. nitric oxide, NO) urothelial transmitters promote bladder hyperactivity following spinal cord injury and how BoNT-A injection, by normalizing the amount and ratio of released ATP to NO, significantly reduces C-fiber mediated bladder activity. “A” represents normal bladder urothelium characterized by large amounts of NO released and relatively small amounts of ATP released. “B” represents bladder urothelium after spinal cord injury, represented by enhanced release of ATP and decreased release of NO. Botulinum toxin A (BoNT-A) restores urothelial transmitter release to near normal levels.
4. Acknowledgements
This study was supported by a research grant from Allergan, Inc., Irvine, CA, and NIH grant RO1-DK-069988.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005 Sep;174(3):977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- Atiemo H, Wynes J, Chuo J, Nipkow L, Sklar GN, Chai TC. Effect of botulinum toxin on detrusor overactivity induced by intravesical adenosine triphosphate and capsaicin in a rat model. Urology. 2005 Mar;65(3):622–626. doi: 10.1016/j.urology.2004.10.057. [DOI] [PubMed] [Google Scholar]
- Barrick S, de Groat WC, Birder LA. Regulation of chemical and mechanical-evoked ATP release from urinary bladder urothelium by botulinum toxin. A. Soc. Neurosci Abstract Viewer. 541:5. [Google Scholar]
- Birder L, Apodaca G, de Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am. J. Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol. 1997 Nov;158(5):1989–1995. doi: 10.1016/s0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001 Nov 6;98(23):13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002a Sep;5(9):856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002b Sep 15;22(18):8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Wolf-Johnston A, Buffington CA, Roppolo JR, de Groat WC, Kanai AJ. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol. 2005 Feb;173(2):625–629. doi: 10.1097/01.ju.0000145900.22849.1d. [DOI] [PubMed] [Google Scholar]
- Cartledge JJ, Davies AM, Eardley I. A randomized double-blind placebo-controlled crossover trial of the efficacy of L-arginine in the treatment of interstitial cystitis. BJU Int. 2000 Mar;85(4):421–426. doi: 10.1046/j.1464-410x.2000.00490.x. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995 Apr 24;678(1–2):40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci. 2000 Jul;21(7):249–252. doi: 10.1016/s0165-6147(00)01499-1. [DOI] [PubMed] [Google Scholar]
- Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004 Jan;107(1–2):125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006 Feb;147 Suppl 2:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006 Apr 28;312(5773):592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Ellies M, Schutz S, Quondamatteo F, Laskawi R. Immunohistochemical investigations of the influence of botulinum toxin A on the immunoreactivity of nNOS in the parotid gland of the rat. J Oral Maxillofac Surg. 2006 Mar;64(3):397–401. doi: 10.1016/j.joms.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol. 1997 Dec 1;505(Pt 2):503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007 May 30;80(24–25):2298–2302. doi: 10.1016/j.lfs.2007.02.010. Epub 2007 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Jahnke MT, Luecke D, Mauritz KH. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett. 1995 Dec 1;201(1):37–40. doi: 10.1016/0304-3940(94)12124-9. [DOI] [PubMed] [Google Scholar]
- Hughes R, Walker BC. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Clostridium Botulinum type toxin A. J. Physiol. 1962;160:221–233. doi: 10.1113/jphysiol.1962.sp006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HH, Lauterburg T, Burgunder JM. Expression of neurotransmitter genes in rat spinal motoneurons after chemodenervation with botulinum toxin. Neuroscience. 1997 May;78(2):469–479. doi: 10.1016/s0306-4522(96)00596-9. [DOI] [PubMed] [Google Scholar]
- Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–993. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology. 2005 Jul;66(1):208–212. doi: 10.1016/j.urology.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Kim HS, Hwang JH, Jeong ST, Lee YT, Lee PK, Suh YL, Shim JS. Effect of muscle activity and botulinum toxin dilution volume on muscle paralysis. Dev Med Child Neurol. 2003 Mar;45(3):200–206. doi: 10.1017/s0012162203000380. [DOI] [PubMed] [Google Scholar]
- Korting GE, Smith SD, Wheeler MA, Weiss RM, Foster HE., Jr A randomized double-blind trial of oral L-arginine for treatment of interstitial cystitis. J Urol. 1999 Feb;161(2):558–565. [PubMed] [Google Scholar]
- Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007 Apr;51(4):1042–1053. doi: 10.1016/j.eururo.2006.10.028. Epub 2006 Oct 27. [DOI] [PubMed] [Google Scholar]
- Mariotti R, Bentivoglio M. Botulinum toxin induces nitric oxide synthase activity in motoneurons. Neurosci Lett. 1996 Nov 15;219(1):25–28. doi: 10.1016/s0304-3940(96)13167-0. [DOI] [PubMed] [Google Scholar]
- Morris JL, Jobling P, Gibbins IL. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am J Physiol Heart Circ Physiol. 2001 Nov;281(5):H2124–H2132. doi: 10.1152/ajpheart.2001.281.5.H2124. [DOI] [PubMed] [Google Scholar]
- Nitahara K, Kittel A, Liang SD, Vizi ES. A1-receptor-mediated effect of adenosine on the release of acetylcholine from the myenteric plexus: role and localization of ecto-ATPase and 5′-nucleotidase. Neuroscience. 1995 Jul;67(1):159–168. doi: 10.1016/0306-4522(94)00585-s. [DOI] [PubMed] [Google Scholar]
- Olgart C, Gustafsson LE, Wiklund NP. Evidence for nonvesicular nitric oxide release evoked by nerve activation. Eur J Neurosci. 2000 Apr;12(4):1303–1309. doi: 10.1046/j.1460-9568.2000.01021.x. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999 Dec;162(6):2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002 Sep;168(3):1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000 Aug;164(2):545–550. [PubMed] [Google Scholar]
- Persichini T, Cantoni O, Suzuki H, Colasanti M. Cross-talk between constitutive and inducible NO synthase: an update. Antioxid Redox Signal. 2006 May–Jun;8(56):949–954. doi: 10.1089/ars.2006.8.949. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Olthoff A, Laskawi R, Gotz W. Neuronal nitric oxide synthase-immunoreactivity. A neuromodulating system independent of peripheral nasal gland denervation in guinea pig nasal mucosal tissue after treatment with botulinum toxin type A. ORL J Otorhinolaryngol Relat Spec. 2002 Sep-Oct;64(5):330–334. doi: 10.1159/000066087. [DOI] [PubMed] [Google Scholar]
- Salas NA, Somogyi GT, Gangitano DA, Boone TB, Smith CP. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int. 2007 Jan;50(2):345–350. doi: 10.1016/j.neuint.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993 Nov 29;335(1):99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Simpson LL. Kinetic studies on the interaction between Botulinum toxin type A and the cholinergic neuromuscular junction. J. Pharmacol. Exp. Ther. 1982;212:16–21. [PubMed] [Google Scholar]
- Smith CP, Boone TB, de Groat WC, Chancellor MB, Somogyi GT. Effect of stimulation intensity and botulinum toxin isoform on rat bladder strip contractions. Brain Res Bull. 2003 Jul 15;61(2):165–171. doi: 10.1016/s0361-9230(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Smith CP, Chancellor MB. Emerging role of botulinum toxin in the management of voiding dysfunction. J Urol. 2004 Jun;171(6 Pt 1):2128–2137. doi: 10.1097/01.ju.0000127725.48479.89. Review. [DOI] [PubMed] [Google Scholar]
- Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin. A. Neurochem Int. 2005 Sep;47(4):291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Smith SD, Wheeler MA, Foster HE, Jr, Weiss RM. Urinary nitric oxide synthase activity and cyclic GMP levels are decreased with interstitial cystitis and increased with urinary tract infections. J Urol. 1996 Apr;155(4):1432–1435. [PubMed] [Google Scholar]
- Somogyi GT, Yokoyama T, Szell EA, Smith CP, de Groat WC, Huard J, Chancellor MB. Effect of cryoinjury on the contractile parameters of bladder strips: a model of impaired detrusor contractility. Brain Res Bull. 2002 Oct 15;59(1):23–28. doi: 10.1016/s0361-9230(02)00833-x. [DOI] [PubMed] [Google Scholar]
- Somogyi GT, Zernova GV, Yoshiyama M, Yamamoto T, de Groat WC. Frequency dependence of muscarinic facilitation of transmitter release in urinary bladder strips from neurally intact or chronic spinal cord transected rats. Br J Pharmacol. 1998 Sep;125(2):241–246. doi: 10.1038/sj.bjp.0702041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald RJ., Jr Differing effects of N(G)-monomethyl L-arginine and 7-nitroindazole on detrusor activity. Neurourol Urodyn. 2003;22(1):62–69. doi: 10.1002/nau.10064. [DOI] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Craviso GL, Bjur RA, Westfall DP. Evidence for the differential release of the cotransmitters ATP and noradrenaline from sympathetic nerves of the guinea-pig vas deferens. J Physiol (Lond) 1996;496:731–748. doi: 10.1113/jphysiol.1996.sp021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemulakonda VM, Somogyi GT, Kiss S, Salas NA, Boone TB, Smith CP. Inhibitory effect of intravesically applied botulinum toxin A in chronic bladder inflammation. J Urol. 2005 Feb;173(2):621–624. doi: 10.1097/01.ju.0000143189.19835.f3. [DOI] [PubMed] [Google Scholar]
- Vizi ES. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(34):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neuroscience. 2001;21(15):5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BM, Dmochowski RR, Malecha M, Mangold T, Bobal MA, Cooke CR. Inducible nitric oxide synthase in the bladder of spinal cord injured patients with a chronic indwelling urinary catheter. J Urol. 2001 May;165(5):1457–1461. [PubMed] [Google Scholar]
- Wheeler MA, Smith SD, Saito N, Foster HE, Jr, Weiss RM. Effect of long-term oral L-arginine on the nitric oxide synthase pathway in the urine from patients with interstitial cystitis. J Urol. 1997 Dec;158(6):2045–2050. doi: 10.1016/s0022-5347(01)68150-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001 Jul;86(1):304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]