Abstract
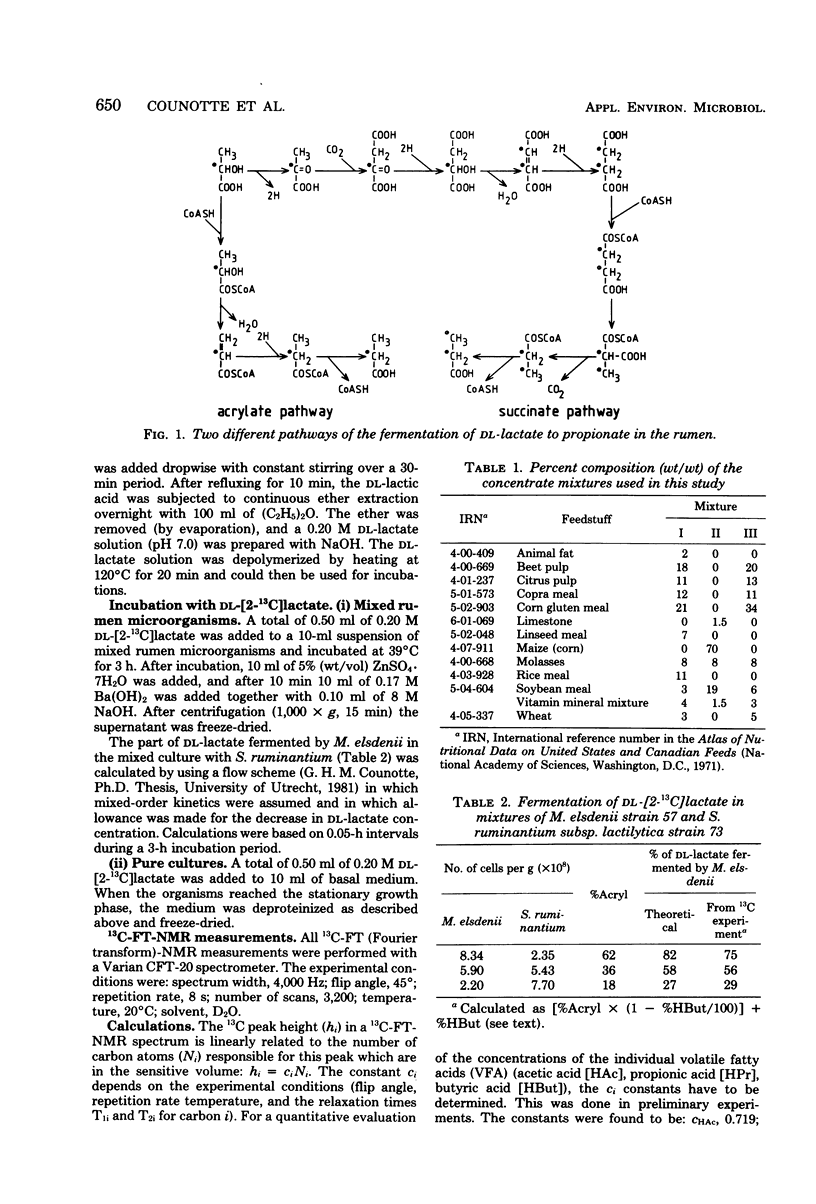
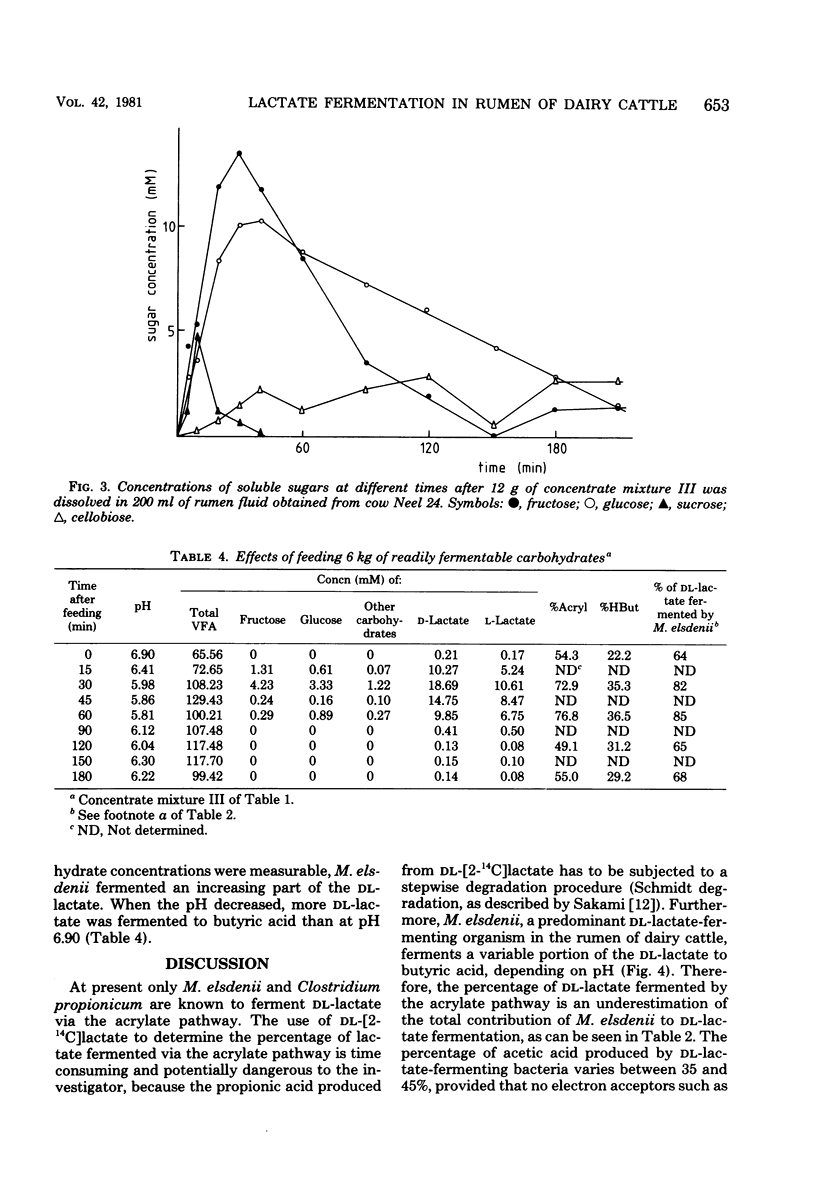
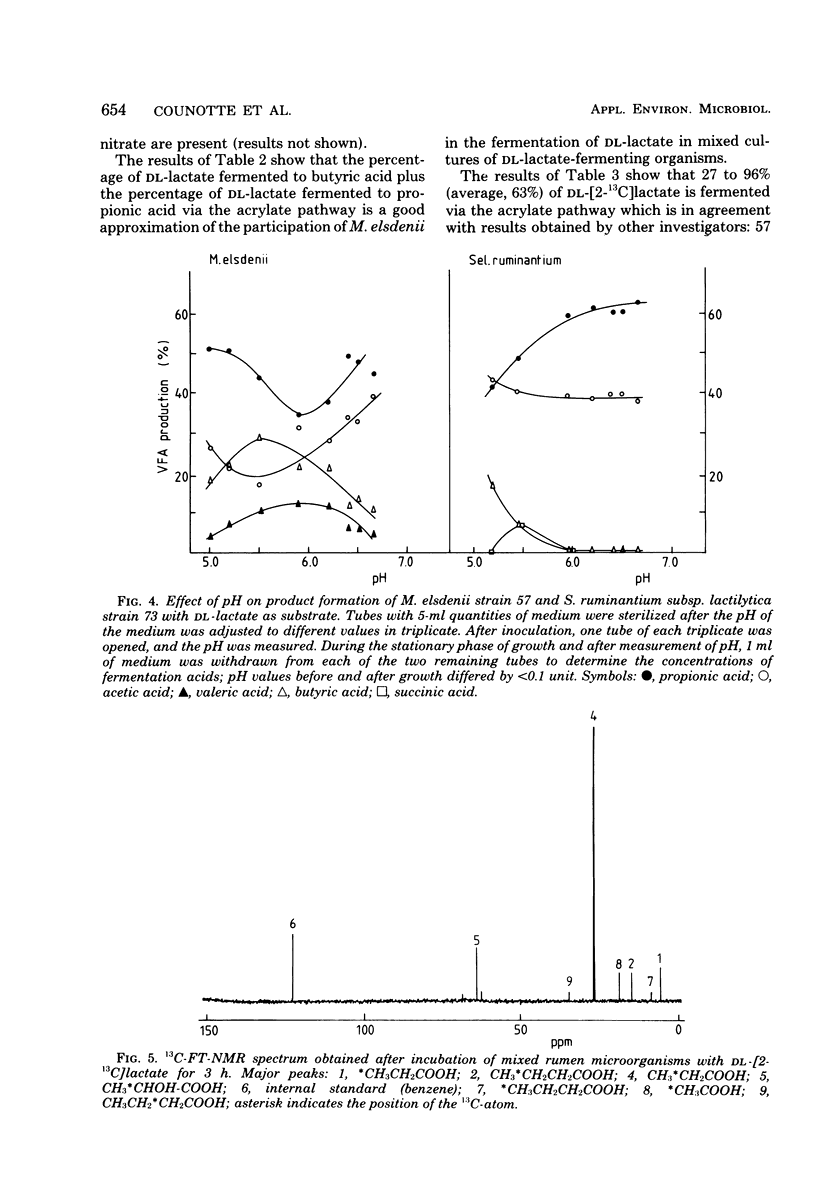
Since Megasphaera elsdenii ferments a variable part of dl-lactate to butyrate, measurement of the percentage of dl-lactate fermented to propionate via the acrylate pathway in rumen contents will underestimate the participation of M. elsdenii in the dl-lactate fermentation. The percentage of dl-[2-13C]lactate fermented via the acrylate pathway and the percentage of dl-lactate fermented to butyrate can be measured with 13C-FT (Fourier transform)-nuclear magnetic resonance. On the average, the contribution of M. elsdenii to dl-lactate fermentation in the rumen of dairy cattle was found to be 74% (standard deviation, 13%), but differed with animal or diet. After feeding a cow readily fermentable carbohydrates, the contribution of M. elsdenii to the fermentation of dl-lactate increased as a consequence of catabolite repression in other dl-lactate-fermenting bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., WOOD W. A., EMERY R. S. Conversion of lactate-C14 to propionate by the rumen microflora. J Bacteriol. 1962 Apr;83:907–913. doi: 10.1128/jb.83.4.907-913.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte G. H., de Groot M., Prins R. A. Kinetic parameters of lactate dehydrogenases of some rumen bacterial species, the anaerobic ciliate Isotricha prostoma and mixed rumen microorganisms. Antonie Van Leeuwenhoek. 1980;46(4):363–381. doi: 10.1007/BF00421983. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W. Nutritional characteristics of Megasphaera elsdenii. Can J Microbiol. 1978 Aug;24(8):981–985. doi: 10.1139/m78-161. [DOI] [PubMed] [Google Scholar]
- Herbeck J. L., Bryant M. P. Nutritional features of the intestinal anaerobe Ruminococcus bromii. Appl Microbiol. 1974 Dec;28(6):1018–1022. doi: 10.1128/am.28.6.1018-1022.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins R. A., Van Der Meer P. On the contribution of the acrylate pathway to the formation of propionate from lactate in the rumen of cattle. Antonie Van Leeuwenhoek. 1976;42(1-2):25–31. doi: 10.1007/BF00399446. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter L. D., Esdale W. J. In vitro lactate metabolism by ruminal ingesta. Appl Microbiol. 1968 May;16(5):680–688. doi: 10.1128/am.16.5.680-688.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallnöfer P., Baldwin R. L., Stagno E. Conversion of C-labeled substrates to volatile Fatty acids by the rumen microbiota. Appl Microbiol. 1966 Nov;14(6):1004–1010. doi: 10.1128/am.14.6.1004-1010.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whanger P. D., Matrone G. Metabolism of lactic, succinic and acrylic acids by rumen microorganisms from sheep fed sulfur-adequate and sulfur-deficient diets. Biochim Biophys Acta. 1967 Feb 7;136(1):27–35. doi: 10.1016/0304-4165(67)90317-0. [DOI] [PubMed] [Google Scholar]



