Abstract
We present an agent-based model of the key activities of a troop of chacma baboons (Papio hamadryas ursinus) based on the data collected at De Hoop Nature Reserve in South Africa. We analyse the predictions of the model in terms of how well it is able to duplicate the observed activity patterns of the animals and the relationship between the parameters that control the agent's decision procedure and the model's predictions. At the current stage of model development, we are able to show that across a wide range of decision parameter values, the baboons are able to achieve their energetic and social time requirements. The simulation results also show that decisions concerning movement (group action selection) have the greatest influence on the outcomes. Those cases where the model's predictions fail to agree with the observed activity patterns have highlighted key elements that were missing from the field data, and that would need to be collected in subsequent fieldwork. Based on our experience, we believe group decision making is a fertile field for future research, and agent-based modelling offers considerable scope for understanding group action selection.
Keywords: group decision making, agent-based modelling, voting, democracy, foraging
1. Introduction
Group living is a common strategy among mammals and is key to understanding the success of the primate order in general and early humans in particular. For animals that forage or move in social groups, decisions about when and where to move often depend upon social interactions between group members (Krause & Ruxton 2002; Couzin et al. 2005). Little is actually known, however, about how groups of animals arrive at such collective decisions (Conradt & Roper 2003). We focus on the problem of action selection in groups, i.e. where an individual's action choice is constrained by the choices of other members of the group.
We present an agent-based model of the key activities of a troop of chacma baboons (Papio hamadryas ursinus) based on the data collected at De Hoop Nature Reserve in South Africa. Modern common baboons (Papio sp.) are one of the most widely studied primate species and are ideal for the studies of primate ecology since they often live in open, terrestrial habitats, and can be observed closely for long periods of time (Richard 1985). This means that there is a wealth of data available documenting most aspects of their behaviour in great detail. Many of these studies have managed to quantify the activity patterns of individuals in terms of both durations and also the costs and benefits of the activity. Papio sp. is found across most of sub-Saharan Africa (Jolly 2001), at a range of altitudes, with attendant large changes in average rainfall and temperature. Thus, they can be said to inhabit a wide variety of habitats and ecotypes, and studies have shown that their diet and foraging vary in response to the environmental determinants (Hill & Dunbar 2002).
We analyse the predictions of the model in terms of how well it is able to duplicate the observed activity patterns of the animals and the relationship between the parameters that control the agent's decision procedure and the model's predictions. We use measures such as range size, daily travel distance, energy and time budgets, as these are good candidates for testing agent-based approaches: they have measurable numerical values and so can be tested objectively, and are highly dependent on the activities and choices of the individuals within the population.
Our long-term aim is a robust model of baboon behaviour which is valid across a wide range of habitats and baboon species (including extinct species). Our methodology is to first build a model that can successfully predict the behaviour of a particular group of baboons and then attempt to generalize, conserving the decision procedure while tailoring the decision parameters to a particular species or habitat type. The work reported in this paper is the first step in this process, namely the modelling of a particular group of baboons in a particular habitat.
The rest of the paper is organized as follows. In §2, we review recent theoretical work on decision making in animal groups. In §3, we review the relevant agent-based modelling literature and motivate our modelling approach. In §4, we briefly summarize the field data on which our model is based, and in §5, we outline our agent-based model and the decision procedure which the agents use to choose their activities. In §6, we present the results of a Monte Carlo sensitivity analysis of the parameters used in the agent's decision procedure. In §7, we discuss the results and outline directions for future work.
2. Decision making in animal groups
Animal groups must routinely arrive at collective decisions, such as when and where to feed and the location of nesting sites. Many group decisions will be crucial to the individual fitness of the group members, yet may also involve a conflict of interest between individuals since not all animals will have the same preference for an activity or travel destination. Consensus must nevertheless be reached or the group will split and members will forfeit many of the advantages of group living. The existence of collective decision making in animals that do not communicate verbally is a field of considerable current theoretical interest.
Conradt & Roper (2005) distinguish two conceptually different types of group decisions: combined decisions and consensus decisions. Combined decisions refer to the situations where animals decide individually, but in a manner that is dependent on other group members. These decisions often affect the group as a whole, such as decisions to leave or join groups in fission–fusion species (Deneubourg et al. 2002) or the allocation of tasks in eusocial insects (Beshers & Fewell 2001). Consensus decisions, on the other hand, are made by spatially cohesive groups and concern issues such as movement direction (Couzin et al. 2005), travel destination (Stewart & Harcourt 1994) and activity timing (Conradt & Roper 2003). Consensus decisions thus require mechanisms (such as voting) for groups to arrive at agreements. Although group decision making is an issue of fundamental importance in evolutionary biology, little is known about how animal groups arrive at such consensus decisions (Conradt & Roper 2003).
Two mechanisms which represent the extremes of how groups can reach collective decisions are despotism and democracy (Conradt & Roper 2003). Although democracy seems improbable for the majority of animal species given the perceived implicit cognitive requirements, empirical examples of ‘voting’ behaviours have been documented across a range of animal taxa: honeybees (Apis mellifera; Seeley & Buhrman 1999); whooper swans (Cygnus cygnus; Black 1988); African buffalo (Syncerus caffer; Prins 1996); capuchins (Cebus capucinus; Leca et al. 2003); and gorilla (Gorilla gorilla; Stewart & Harcourt 1994). Furthermore, models of group decision making have shown democratic decisions to be more beneficial than despotic decisions across a wide range of conditions (Conradt & Roper 2003). Indeed, ‘majority rules’ appears to be a robust and highly adaptive form of decision making in groups (Hastie & Kameda 2005). Nevertheless, there is also theoretical support for the emergence of effective leadership in certain contexts (Rands et al. 2003; Couzin et al. 2005). Group decision making is thus a field of considerable current theoretical interest (List 2004; Simons 2004; Conradt & Roper 2005) that provides significant scope for further work. Agent-based modelling offers a valuable technique for exploring how animal groups arrive at consensus decisions.
3. Agent-based modelling
In this section, we briefly review previous work on agent-based modelling of non-human primates and motivate our modelling approach.
Individual-based ecological models have been growing in importance over the last 20 years and it has been predicted that this reductionist approach will provide valuable insight into system-wide properties (Lomnicki 1992). Early work on artificial intelligence has shown that complex group behaviours such as flocking and following can be produced using simple rules applied to individuals (Reynolds 1987). Agent-based modelling is an extension of this approach where each individual retains information about its current and past states, and its behaviour is controlled by an internal decision process. An agent in this context is a software system that perceives its environment and acts in that environment in pursuit of its goals. Agents integrate a range of (often relatively shallow) competences, e.g. goals and reactive behaviour, emotional state, memory and inference. In agent-based modelling, the agents are situated in a simulated environment and are equipped with sensors with differing ranges and directional properties (e.g. smell, hearing and vision) and the ability to perform a range of actions which change the state of the environment or the perceptible characteristics of the agent. The environment may contain passive objects (e.g. topography) and active objects and processes which change spontaneously during the course of the simulation (e.g. weather) and/or in response to the actions of the agents (e.g. food-bearing plants).
Agents are commonly described using anthropomorphic terms, such as beliefs (what the agent believes the state of the environment and other agents to be), desires (those states of the environment it is designed to bring about) and intentions (the state(s) of the environment it is currently engaged in bringing about)—indeed the so-called Belief–Desire–Intention, or BDI, model of agency (Rao & Georgeff 1991) is perhaps the dominant paradigm in agent theory (Georgeff et al. 1999). In some cases, the agent's beliefs and desires are explicitly represented within the software state of the agent. However, not all agents represent beliefs and goals explicitly, even though they act in a goal-directed manner. For example, the behaviour of an agent may be controlled by a collection of decision rules or reactive behaviours which simply respond to the agent's current environment. In such cases, it can still be useful to view the agent as an intentional system, that is we ascribe to it the beliefs and goals it ought to have, given what we know of its environment, sensors and (putative) desires (Dennet 1987, 1996). For example, an agent which has an ‘avoid obstacles’ behaviour, can be said to have a goal of ‘avoiding collisions’ even though this goal is not explicitly represented in the agent. This approach, which Dennett calls ‘adopting the intentional stance’, allows us to ascribe propositional attitudes to agents which do not explicitly represent beliefs and goals, and is licensed on the grounds that viewing an agent as an intentional system is more likely to yield useful insights than would a description couched in terms of the low level details of the agent's implementation.
The outcomes determined by an agent-based model depend on the set of desires and goals within each individual agent, its current internal state (which may include an internal world model) and the sensory information it receives. This reliance on individual choice makes agent-based modelling especially useful when dealing with animals which live in groups, since it is probable that the optimal strategy for an individual depends on the strategies adopted by others in the group (Milinski & Parker 1991). Moreover, while the factors influencing the decisions made by an individual may vary as the environment changes, the decision process itself is likely to be conserved, and an agent with a robust decision procedure will demonstrate reasonable behaviour under a wide range of conditions. Such models can therefore be used to explore the potential effects of situational changes: climate; food distribution; and body size can all be altered and the effects on the agents' behaviour can be observed. If we are confident that the decision procedure is robust, then we can use the behaviour of the agents to predict the behaviour of real populations.
There are a number of different types of ‘agent-based’ model, and different terms are used in the literature for essentially the same kind of model. In some cases, e.g. population ecology models, the emphasis is on tracking the properties of individuals and/or of the population as a whole, with little or no interaction between the individual agents or between the agents and their environment. For example, Robbins & Robbins (2004) have developed a model to simulate the growth rate, age structure and social system of mountain gorillas (Gorilla gorilla beringei) in the Virunga Volcanoes region. The model uses a 1-year time-step and is based on the probabilities of life-history events (birth rates, mortality rates, dispersal patterns, etc.) as determined by the census data from habituated research groups of gorillas. The gorillas do not interact with an environment model (or each other) and the only decisions the gorillas make as individuals is whether to move to a new group.
In other cases, the agents do interact with each other and/or their environment. In these models, individuals base their decisions on their beliefs about the state of the environment and/or the state of other agents, and their actions (changing position and eating food) change the environment perceived by other agents, which may in turn change their state and influence the decisions they make. This focus on interaction through the medium of the environment means that these models are often spatially explicit, i.e. the individuals are associated with a location in geometrical space. Such spatially explicit agent systems are sometimes called situated in the agent literature (Ferber 1999).
Situated models allow consideration of social and spatial interactions between individuals. As a result, they have become a popular technique for modelling interactions in humans and non-human primates. Much of the work on agent-based modelling of non-human primates has been done by Charlotte Hemelrijk and her colleagues. They have developed an agent-based model which has been used to investigate dominance interactions in primates, e.g. chimpanzees (Pau sp.) and macaques (Macaca sp.) (Hemelrijk 1999a,b, 2000, 2002; Hemelrijk et al. 2003; Hemelrijk & Wantia 2005; Hemelrijk et al. 2005). For example, Hemelrijk (2002) presents a model of primate social behaviour in which agents have two tendencies: to group and to perform dominance interactions, and shows how increased levels of aggression can induce female dominance over males. (See Bryson et al. (2007) for an analysis of the Hemelrijk (2002) results.) Bryson & Flack (2002) have used an agent-based model to investigate primate social interactions. The agents are represented as two-dimensional rectangles in a walled enclosure and alternate between two behaviours: grooming neighbours and wandering (feeding in relative isolation). The model investigated the effect of a ‘tolerance behaviour’ on the amount of time spent grooming. These models explicitly take into account the spatial position and orientation of individuals: in the Hemelrijk model, cohesiveness is determined by the ‘SearchAngle’, the angle by which an agent will rotate to locate other agents when there are none in sight; in the Bryson & Flack model, grooming requires being adjacent to and properly aligned with another agent.
Although agent-based models are increasingly finding acceptance, such computer simulations are not without their critics. Maynard-Smith (1995) has famously described these approaches as ‘fact-free science’. To overcome such objections and enable us to use this technique as a tool for exploring primate behavioural ecology, the models produced must be tested by using them to predict behaviours in a given population and comparing the predictions with field observations. For example, the predictions of the model developed by Robbins & Robbins (2004) are directly comparable with future population data from this region, and Hemelrijk et al. (2003) have proposed model-guided studies of female dominance in real animals.
Existing agent-based models have tended to focus on interactions between the agents and their environment, or pairwise interactions (e.g. dominance interactions) between individuals and emergent properties arising from such interactions. There has been relatively little work to date on agent-based modelling of decision making in animal groups. However, work on social insects in particular allows good experimental testing of the role of individuals in group decisions such as the classic work on honeybees (Seeley et al. 1991) as well as more recent agent-based modelling approaches using ants (Pratt et al. 2005). While there is an extensive literature on agent-based models of human behaviour in a wide range of domains, including resource exploitation, economics and politics (see, for example, the article by Laver (2007), this work has not been applied (and in many cases would be difficult to apply) to group decision making in animals. In artificial intelligence, there is a substantial literature on joint actions (Grosz & Sidner 1990; Cohen & Levesque 1991; Tambe 1997). However, this work has tended to view actions by individuals within a group as directed towards the achievement of a joint intention, with each agent committing to performing a (possibly different) action from a shared or team plan, rather than the selection of an action which is performed by all agents but which only serves the interests of a subset. It seems unlikely that non-human primates have explicit joint intentions or the shared plans and models of teamwork necessary to achieve them.
In the rest of this paper, we present a model of group action selection in baboons. Each baboon is modelled as an agent which chooses actions and interacts with its environment (and indirectly with other agents) based on its individual state. A key feature of the model is that an agent's choice of which action to perform is constrained by a group level decision whether the group as a whole will move at the next time-step, which in turn is determined by the actions proposed by the other agents. To the best of our knowledge, this integration of individual and group level action selection (where all the members of the group participate in the selection and execution of a common action) has not been addressed in previous work.
4. Field data
The model is based on data from a study of chacma baboons (P. h. ursinus) at De Hoop Nature Reserve, a coastal reserve in Western Cape Province, South Africa. The baboons ranged in an area surrounding the De Hoop Vlei, a large landlocked body of brackish water lined by cliffs along its eastern edge and fed by several freshwater springs. Owing to its southerly latitude, De Hoop is a highly seasonal environment with significant annual variation in rainfall, temperature and day length that has important implications for the behavioural ecology of this population (Hill et al. 2003, 2004; Hill 2005). Vegetation is dominated by coastal fynbos, a unique and diverse vegetation type comprising Proteacae, Ericaceae, Restionaceae and geophyte species. Six distinct habitat types were classified on the basis of vegetation structure within the home range of the baboons (Hill 2006) (table 1; see R.A. Hill (1999, unpublished) for detailed descriptions and further information on the ecology of the reserve).
Table 1.
Home range composition, vegetation food availability and predation risk of the major habitat types at De Hoop.
| habitat type | proportion of range (%) | bush cover (%) | tree cover (%) | food availability | predation risk |
|---|---|---|---|---|---|
| acacia woodland | 15.8 | 55.8 | 34.4 | high | high |
| burnt acacia woodland | 1.2 | 3.2 | 0.4 | low | intermediate |
| burnt fynbos | 27.6 | 3.6 | 0.0 | low | intermediate |
| climax fynbos | 25.7 | 54.0 | 3.4 | low | high |
| grassland | 11.0 | 1.6 | 1.2 | intermediate | low |
| vlei | 18.7 | 0.0 | 0.0 | high | low |
The data presented here are for a seven-month period (June to December 1997) from a single troop of chacma baboons that ranged in size from 40 to 44 individuals over the course of the study. Data were collected by means of instantaneous scan samples (Altmann 1974) at 30 min intervals, with 2–4 adult males and 12 adult females sampled for a minimum of 5 full days each month. At each sample point, information was recorded on the identity, habitat type and activity state (feeding, moving, socializing or resting) of all visible individuals. Each scan lasted a maximum of 5 min. A more detailed description of the data collection methods is given in R. A. Hill (1999, unpublished), with further information on patterns of habitat use in Hill & Weingrill (2006).
5. The model
Based on the field data, we developed and implemented an agent-based model of baboon behaviour and used this to simulate a troop of 50 baboons over a seven month period (June to December). The model determined the activity chosen by each baboon at each time-step and the resulting energy balance, time between drinking, time spent socializing and time spent resting.
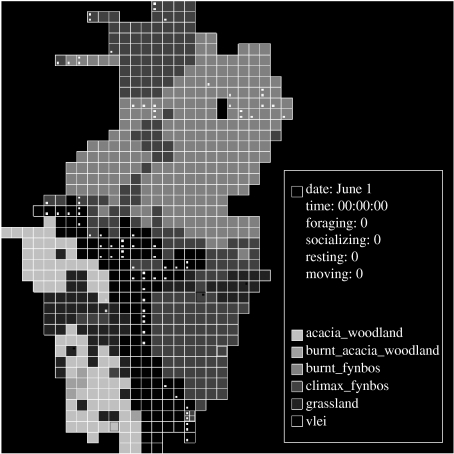
The simulation model consists of two components: the environment model and the baboon model. (The electronic supplementary material contains the code necessary to run the model and the environment data used for the runs in the paper.) The environment model is based on the 200 m×200 m map grid used for field data recording, and consists of 660 cells within an area 5.4×8.4 km. Each cell contains a mixture of the six primary habitat types found at De Hoop (acacia woodland, burnt acacia woodland, climax fynbos, burnt fynbos, grassland and vlei) and may also include one or more ‘special features’: water sources; sleeping sites; and refuges (primarily cliffs). When more than one habitat type occurs in a cell, it is assumed that they are present in equal proportions. Each habitat type is characterized by maximum food availability, food intake rate when foraging and travel–foraging and replenishment rate for each month of the study period. The energy value of the food available was estimated at 13.98 kJ g−1 (Stacey 1986) for all habitat types. The habitat types and distributions are illustrated in the graphical output of the simulator, as shown in figure 1.
Figure 1.
Graphical output from the simulator showing the habitat types and distributions. Cells with dots at top right indicate water sources; dots at top left indicate sleeping sites and dots at bottom left and bottom right indicate refuges.
The second component of the simulation is the baboon model. Each baboon is modelled as an agent with physical parameters based on well-known baboon physiology. In addition, each agent maintains an individual score for water, energy and social time which function as ‘drives’ in biasing the agent's choice of preferred activity. At each time-step, an agent can perform one out of the four actions corresponding to the activities recorded for the baboons at De Hoop: foraging, moving (travel foraging), socializing or resting. In addition, an agent can perform an instantaneous drinking action which can be combined with any of the other four actions (assuming the agent is in a cell which contains a water source). Foraging, moving, socializing and resting actions have an associated energy cost which decreases the agent's energy score. Energy costs were calculated using the formulae given in Tucker (1970) for an average adult female baboon with a body mass of 16.1 kg (with the heavier males offset by the lighter infants and juveniles) and assuming that the baboons moved relatively slowly (0.5 ms−1) since they customarily foraged while moving. Thus, foraging requires 36.71 W, moving (travel–foraging) 50.59 W, socializing 64.04 W and resting 34.63 W. These values are not directly based on the field data from De Hoop and hence must be viewed as approximate. The agents rest at night and the energy required for this is also subtracted from their energy score. In addition to its energy cost, each action also updates the energy, water and social time scores. Thus, foraging increases the agent's energy score depending on the type of food consumed (i.e. the habitat type(s) of the current cell). The agents also forage while moving, which increases the agent's energy score at a lower travel–foraging rate. Drinking adds 1 to the agent's water score. Socializing increases the agent's social time score by the length of the time-step. Not drinking causes the agent's water score to decrease by the reciprocal of the time-step, and any action other than socializing causes the social score to decrease by the length of the time-step.
The baboon model simulates the activities of each baboon during daylight hours at a 5 min time-step. At the beginning of each time-step, the agents execute a two-stage decision procedure which determines the action performed by each agent at this time-step. In the first phase, each agent chooses a preferred action and whether it would prefer to move, allowing it to perform the action more effectively. In the second phase, a group decision is taken to determine whether the agents actually move to another cell. This may force some agents to choose an alternative, less preferred, action, as explained below.
In the first phase of the decision procedure, each agent's preferred action is determined by a combination of individual constraints and the agent's goals. The agents have a single individual constraint: they must return to a sleeping site to rest each night. In addition, they have three goals: maintaining their energy balance (i.e. to eat sufficient food to make up for the energy expended each day), drinking (i.e. visit a grid cell constraining a water source) at least once every 2 days and spending 2 h a day in social activities. The requirement that the agents must return to a sleeping site each night constrains the choice of the preferred action (and ultimately the preferred cell) so that the agent can always reach a sleeping site in the time remaining before nightfall. If the individual constraint does not force the agent to move at this time-step, the agent's preferred action is determined using a weighted random function with weights proportional to the current desire to forage, drink and socialize. Desires are linear functions of the corresponding scores with gradients proportional to relative importance values for each action: WF (the relative importance of foraging); WD (the relative importance of drinking); and WS (the relative importance of socializing). These desire functions fall to zero when the target amount has been reached and when they are all zero, the agent will opt to rest. By aiming to keep all desires at zero, the agents will eat enough food to balance their energy expenditure, drink on average once per day and socialize on average 2 h per day.
The agent then determines whether it could perform its preferred action more effectively in another cell. The agent will vote to move if the best grid cell within a search radius, S, is more than an action-specific threshold better than the current cell for its preferred action. (If the search radius is greater than the maximum distance, the baboon can travel and still reach the closest sleeping site by dusk, only cells within the maximum travel distance are considered.) In the case of foraging, the threshold is denoted by TF and depends on the food availability; however, in the case of socializing and resting, the thresholds (denoted by TS, TR) are a measure of the predation risk. For example, if the agent would prefer to forage, it will vote to move if the best cell within the search radius has more than TF times as much food as the current cell. It is assumed that the agent has prefect information about food availability and predation risk for all cells within the search radius of its current position, and that the agent knows where the nearest water source is, irrespective of the search radius.
In the second phase, the votes for all the agents are counted, and a group decision is taken on whether all the agents will move. If the number of agents which voted to move is higher than a user specified threshold, V, then the whole group moves in the most commonly preferred direction (i.e. each agent performs a move action in the specified direction at this time-step). If fewer than V agents opt to move, then the agents which voted to move choose their most preferred action for the current cell at this time-step. This is because the group decision not to move may invalidate an individual's initial choice of preferred action: for example, it is impossible to drink if there is no water in the current cell, or if the current cell's predation risk is greater than TK, then the agent will not want to socialize or rest.
The interaction between individual and group level decisions is summarized in table 2. Note that the adoption of the group-level decision by all agents may involve an element of coercion for some agents. If the group decision is not to move, then all the agents that did not vote to move get to perform their preferred action and those which did vote to move are forced to choose another action to execute in the current cell. This could be the same action as their first choice (but in this case, they are forced to perform it in the current cell which is at least TX worse than their preferred cell for this action). In other cases, it may be impossible or too risky to perform their preferred action in the current cell and they must choose another action. If, on the other hand, more than V agents voted to move, then those that preferred to stay in the current cell are coerced to move (travel–forage) in the most commonly preferred direction. Moreover, since the group decision is to move in the most preferred direction, it is possible that some of the agents which voted to move will ultimately not be able to perform their preferred action in their preferred cell. For example, an agent which wants to move west to a water source may be forced to move east towards a cell which is better for foraging or resting/socializing.
Table 2.
Interaction between individual and group level decisions.
| group decides to move | individual votes to move | |
|---|---|---|
| yes | no | |
| yes | travel–forage in the most preferred direction | |
| no | choose best available action for the current cell | perform preferred action in the current cell |
The agents then spend the next 5 min either moving in the chosen direction or performing their chosen action, and their scores in terms of energy, water and social time are adjusted accordingly. If the agent's preferred action is to forage, socialize or rest, then the agent will opportunistically drink if it finds itself in a grid cell containing a water source.
Critically, the actions of the agents also affect the environment, which in turn affects the action chosen by the agents at the next time-step. For example, foraging and travel–foraging cause the food available in the grid cell containing a baboon to be depleted at the appropriate food intake rate for each habitat type occurring in the cell. While food consumed is replaced at the replenishment rate for the current simulation month for each of the habitat type(s) occurring in the grid cell, this is lower than the corresponding food intake rate. Foraging therefore reduces the availability of food at the next time-step (relative to other cells within the agents' perceptual range), making it more probable that an agent will choose to forage in another cell (or perform some other action) at the next time-step.
6. Sensitivity analysis
We do not currently have values for the parameters used in the agents' decision procedure. We may be able to estimate some empirically with more detailed field observations, but others are essentially unknowable. To overcome this, we choose plausible ranges for each decision parameter and performed a Monte Carlo sensitivity analysis (Campolongo et al. 2000) where the simulation was repeated a large number of times and the values of the parameters randomly sampled from the appropriate ranges for each run. This allows us both to estimate the importance of a particular parameter on the outcome and to calculate the range of possible outcomes. We chose to perform a sensitivity analysis as our objectives in this initial study were to see whether the range of possible outcomes predicted by the model were able to bracket those observed in the field and to highlight gaps in the field data, rather than to find the parameter values that produced the best fit to the field data (although this will be a goal of future work). The parameter ranges used in the analysis are shown in table 3.
Table 3.
Key parameters in the decision procedures showing the ranges used in the Monte-Carlo sensitivity analysis.
| parameter | min | max |
|---|---|---|
| V | 0.1 | 0.9 |
| S | 200 | 2200 |
| WF | 1 | 10 |
| WS | 1 | 10 |
| WD | 1 | 10 |
| TF | 1 | 3 |
| TS | 1 | 3 |
| TR | 1 | 3 |
| TK | 0 | 0.25 |
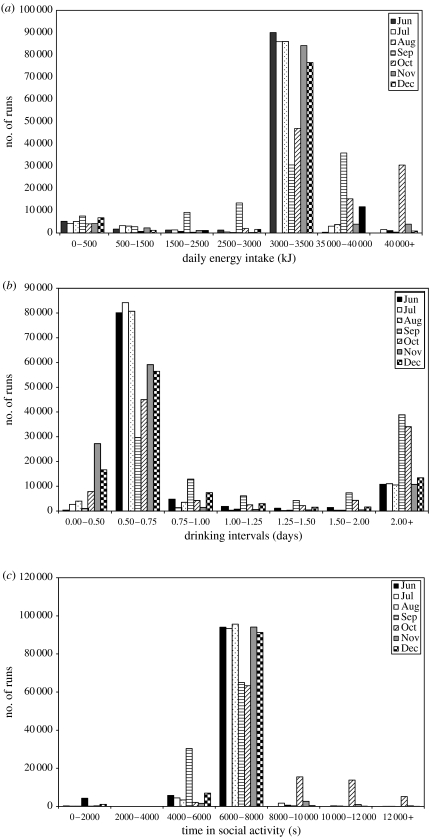
We analysed the predictions of the model in terms of how well it was able to duplicate the observed activity patterns of animals found in field data. The model was run 100 000 times sampling the decision parameters from table 3 each time. Figure 2 shows the distribution of outcomes for each of the agent's goals: daily energy intake, time between drinking and daily social time. The values appear highly consistent between runs and are clustered around the target value for each goal. Agents aim for an energy intake of approximately 3500 kJ (depending on the activity pattern), an interval between drinking of less than 2 days and a target of 2 h (7200 s) of social activity. This suggests that, for single goals considered in isolation, the model may be relatively robust to variations in the decision parameters and that the agents are able to satisfy each of their goals under a range of input conditions.
Figure 2.
Frequency of the primary outcomes for 100 000 repeats of the simulation with the input parameters randomly sampled from the ranges in table 3. (a) Daily energy intake (target approx. 3500 kJ depending on activity pattern); (b) Interval between drinking; (c) Time spent socializing (target 7200 s).
However, the agents find it harder to achieve all three of their goals simultaneously. We say a run is successful if all of the agents simultaneously achieve their energetic, water and social requirements over the whole seven month simulation period. Out of the 100 000 simulations, only 39 400 were successful in this sense. For the 60 600 failures, the agents failed to achieve their foraging requirements in 37 585 runs, failed in their social requirements in 18 223 runs and did not obtain their water requirements in 60 531 simulations. As a consequence, 71.0% of failures (43 045 runs) were as a result of the agents failing to achieve multiple requirements over the simulation period, with only 29.0% (17 555 runs) resulting from a single failure (energy failure only—64 runs; social failure only—5 runs; water failure only—17 486). Thus, while the agents successfully achieve their requirements in a large proportion of cases, certain combinations of input criteria clearly lead to a significant level of failure, particularly in relation to the agents' water requirements.
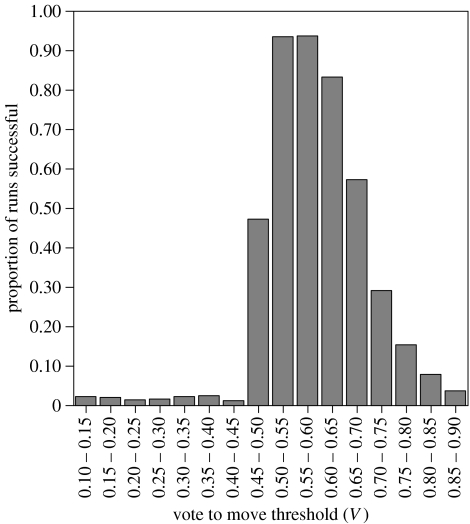
Table 4 presents the results of a forward logistic regression analysis to determine which of the nine parameters in the decision procedure of the model have significant effects on the success and failure of each run. Since the relationship between success or failure of the model and the voting threshold was nonlinear (see figure 3), the vote to move threshold was recoded as a categorical variable for analysis, with each category representing a proportional increase in threshold of 0.05. Although seven parameters are significant determinants of the success or failure of the model, it is clear that V, the proportion of agents required to vote in order to move, has the greatest effect. Figure 3 illustrates that only intermediate vote to move threshold values produce successful outcomes, with thresholds between 0.5 and 0.65 most consistently leading to successful runs of the model. In contrast, simulations with low or very high voting thresholds rarely result in the agents achieving their requirements, indicating that majority decision making is the key to a successful foraging strategy within the model.
Table 4.
Results of a logistic regression analysis to determine which factors within the model have the greatest effect on whether the agents succeed or fail in achieving their goals. (V, vote to move threshold; WF, relative importance of foraging; S, search radius; WD, relative importance of drinking; TK, predation risk threshold; WS, relative importance of social activity; TS, move to socialize threshold; TF, move to forage threshold; TR, move to rest threshold.)
| model | Nagelkerke r2 | −2 log L | Χ2 | d.f. | p |
|---|---|---|---|---|---|
| 0.732 | 56 404.30 | 77 696.449 | 21 | <0.0001 | |
| variables included | r2 change | B | Wald | d.f. | p |
| V | 0.658 | 26 600.07 | 15 | <0.0001 | |
| WF | 0.027 | 0.367 | 3903.97 | 1 | <0.0001 |
| S | 0.023 | 0.002 | 3415.58 | 1 | <0.0001 |
| WD | 0.019 | −0.309 | 2855.75 | 1 | <0.0001 |
| TK | 0.003 | −4.071 | 425.20 | 1 | <0.0001 |
| WS | 0.001 | 0.075 | 187.70 | 1 | <0.0001 |
| TS | 0.001 | −0.158 | 41.18 | 1 | <0.0001 |
| variables excluded | score | d.f. | p |
|---|---|---|---|
| TF | 1.96 | 1 | 0.161 |
| TR | 1.65 | 1 | 0.199 |
Figure 3.
Proportion of runs resulting in the agents successfully achieving their goals against proportion of agents required in order to vote to move V.
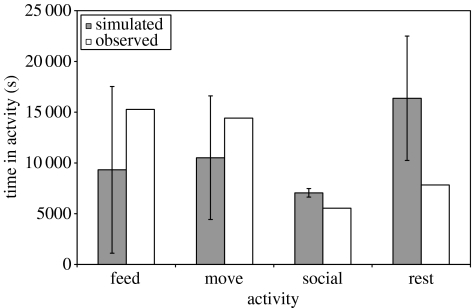
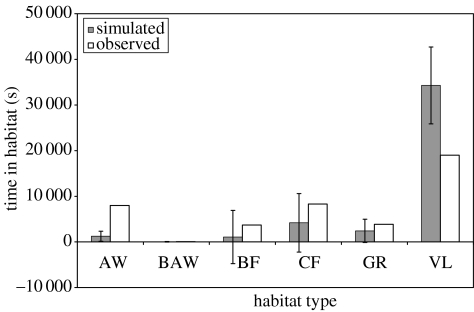
However, while the model successfully meets its requirements in terms of goal achievement in a large proportion of cases, it is less successful in predicting energy budgets and habitat utilization. Figure 4 shows the average amount of time spent in each activity by the agents over all runs. Although the observed field data fall within one standard deviation of the mean of the simulated values for both feeding and moving, it is clear that the agents spend significantly less time foraging to satisfy their nutritional requirements than the natural population. Conversely, the agents spend significantly more time resting. A similar pattern is seen if we consider the habitat types used by the agents. Figure 5 illustrates that in the simulations the agents use the vlei habitat significantly more than observed within the field data, with the acacia woodland habitat significantly underused. Since the vlei habitat is both high in food availability and close to the sleeping sites (see table 1 and figure 1), these results suggest that on average that the agents are able to satisfy their foraging requirements without needing to move to the more distant acacia woodland. This is almost certainly a reflection of the coarseness of our environmental model where groups are foraging in 200×200 m patches that they can deplete evenly without additional search costs. In reality, of course, baboons will cause local resource depletion on a much finer scale with more rapidly diminishing foraging returns. As a consequence, the animals are likely to make frequent movements over small distances between discrete food patches within a cell and these elements are currently not captured in our model. A significant improvement may therefore be achieved if we were to simulate baboon foraging at an appropriate temporal and spatial scale (such as 1 m2 at sub-minute intervals). Although our current ecological data do not permit such an approach, a number of studies have recently started to incorporate landscape dynamics and geographical information systems (GIS) data into individual-oriented models (Gimblett 2002; Topping et al. 2003). In future, coupling multi-agent simulation tools with GIS mapping data will offer opportunities for the production of highly realistic multi-agent simulations of individual behaviour and population processes within precise spatial contexts (Schüle et al. 2004).
Figure 4.
The mean (±s.d.) duration of time spent by the agents in each of the four activities over all runs compared with the values from the field data.
Figure 5.
The mean (±s.d.) duration of time spent by the agents in each of the six habitat types over all runs compared with the values from the field data. AW, acacia woodland; BAW, burnt acacia woodland; BF, burnt fynbos; CF, climax fynbos; GR, grassland; VL, vlei.
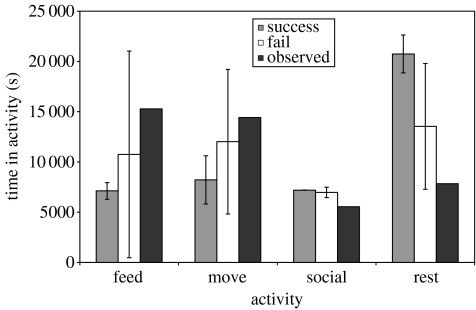
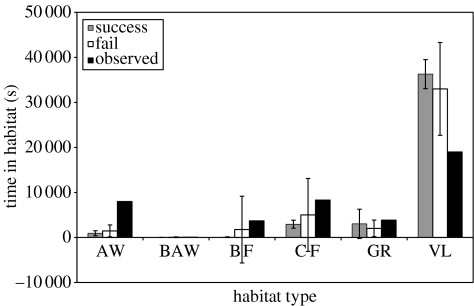
Although the coarseness of the foraging environment is a possible factor explaining the lack of congruence between the simulated and the field data, the initial analysis considered both successful and unsuccessful simulations. It is possible therefore, that the differences between the observed data and the simulation output may result from our inclusion of runs where agents failed to achieve all their goals simultaneously. The reverse in fact appears to be the case. Figure 6 shows the time spent in each of the four activities in successful simulations where the agents were able to achieve their goals, failed simulations where the goals were not achieved and the observed field data. It is clear that much of the variation in the output of the model is produced by the failed simulations. The successful runs show far less variability, with the agent's easily achieving their energetic requirements with levels of foraging far below the observation data. A similar result is seen with the patterns of habitat choice (see figure 7) where successful simulations are characterized by an even greater dependence on the vlei habitat. These findings reinforce the notion that future developments of the model must be made on more refined temporal and spatial scales.
Figure 6.
The mean (±s.d.) duration of time spent in each of the four activities by agents in successful and failed runs of the model compared with the values from the field data.
Figure 7.
The mean (±s.d.) duration of time spent in each of the six habitat types by agents in successful and failed runs of the model compared with the values from the field data. AW, acacia woodland; BAW, burnt acacia woodland; BF, burnt fynbos; CF, climax fynbos; GR, Grassland; VL, vlei.
7. Discussion
The data show that although the model is based upon detailed ecological data, the coarseness of the environment and the way in which resources are depleted by the agents mean that it is relatively easy for them to satisfy their energetic requirements over a broad range of decision parameter values. It is important to remember though that we would never expect a model to precisely match the observed activity patterns at even a monthly level, let alone daily or hourly time-scale, although the results presented here do suggest that this should be possible. Some of the disparities between the model's predictions and the field data may be attributable to the fact that the field data actually represent a subset (scan samples at 30 min intervals on 5 days per month) of the complete monthly behavioural profile of the baboons. In contrast, the model simulates the behaviour of the baboons every 5 min on every day each month. As a consequence, the field data are more susceptible to stochastic sampling variation where ‘atypical’ behaviour patterns could produce misleading monthly averages. Nevertheless, it is clear from our results that future developments of the model will need to define the environment on a more precise scale so that local resource depletion is more realistically incorporated.
Given the ease with which agents appear to be able to forage within our model, it is extremely interesting that the agents fail to achieve all of their goals simultaneously in over 60% of simulations. The results of the Monte-Carlo sensitivity analysis indicate that while the agents are able to achieve their minimum requirements across a range of decision parameter values, unsuccessful runs are most likely to arise from individuals gaining insufficient access to water. The primary determinant of the success of the model, however, is determined not by WD, the relative importance of drinking, but instead by the proportion of individuals voting to move. Only in situations where a majority is required for movement (and thus changes in activity and location) does the model consistently result in the agents achieving their minimum requirements. In fact, the voting threshold is also the primary variable underlying variation in the time spent in various behaviours and in the different habitat types (Sellers et al. 2005). This is almost certainly a result of democratic decision making tending to produce less extreme decisions (Conradt & Roper 2003) and it is certainly true that the unsuccessful runs produce far more variable output. While all grid cells in the model contain at least some food, and many habitats are suitable for socializing and resting, water is restricted to just a few localities. As a consequence, while it is probably still possible for individuals to adequately forage, socialize and rest under less democratic voting thresholds, because suitable habitats are still likely to be encountered, low (and to a lesser degree very high) voting thresholds can lead to more extreme variation in drinking intervals resulting in the agents being unable to obtain their basic requirements in many cases.
It might be thought that the reverse should be the case: with a lower value of V, the group will move more often, making it easier for individuals to obtain water. However, we believe the observed results are due to the small number of water sources and the consequent relatively long journey times required to obtain water. Reaching a water source therefore requires consensus within the group (since the decision whether and in what direction to move is repeated at every time-step). While the group moves more often with lower V, they tend to oscillate back and forward towards different resources since there is very little consensus within the group. This is particularly ineffective when trying to access water resources since these are rare and the group needs to make a concerted effort to get there. With a higher V, once a decision is made, there is sufficient consensus that the group will maintain the decision long enough to reach the water source. Another possibility is that with a low V the group moves much further. This allows the group to get itself into positions that are too far from water resources and cannot therefore get to a water resource in time when it becomes critical.
Although the voting procedure employed in this paper might be considered unrealistic, anecdotal observations of wild baboons have reported voting behaviours where a simple majority determines changes in group activity based on movement (Byrne 2000) or where the majority of adults decide on the direction of travel through body orientation (Norton 1986). While constraining a social group to remain together, the voting mechanism in our model may have greater similarities to natural systems than might be supposed. Nevertheless, while this study adds to the growing body of evidence that democracy and majority decision making should be widespread across a range of animal taxa, it is clear that the questions of how animal groups coordinate movement and reach decision are a fertile field for future research.
While the need for more detailed environmental models is clear, there are a number of other areas where additional detail could also be beneficially incorporated into the agent-based model. First, the incorporation of a full diet model may be essential. While the agents' preference for foraging within the vlei habitat may be explained in terms of its proximity to sleeping sites and the nature of resource depletion ensuring that it always offers high energetic returns, it may be equally true that in nature the baboons move on in order to seek a more diverse diet. In reality, the vlei has only limited diversity in terms of food types (R. A. Hill 1999, unpublished), but since the model only examines energy intake this may explain why the more food species diverse habitats such as acacia woodland and burnt climax fynbos are underused in the simulations. A full diet model would be easy in modelling terms but difficult in terms of validation, since it would require much more detailed chemical and calorific analysis of what the baboons actually eat in different areas. While this is not possible with our existing ecological data, it does serve to highlight the value of agent-based modelling in identifying areas of empirical data that are important for future field studies.
A second area that could usefully be integrated in greater detail is predation risk. At present, we only consider risk in relation to resting and social activity, but predation risk is known to be an important factor shaping patterns of habitat choice by the baboons when foraging at De Hoop (Hill & Weingrill 2006). Fortunately, this is precisely where agent-based modelling reveals its power and generality, since the predators can be modelled as agents themselves. The difficulty here is that we know considerably less about the behaviours of any of the predator species than we do the prey animals, so that validation may be extremely difficult. Nevertheless, since leopards (Panthera pardus), the primary predator of baboons (Cowlishaw 1994), are solitary predators, this objective should be easier to achieve than for many other predator–prey systems.
Finally, it seems likely that primates, and in particular baboons owing to their larger than normal brains (for equivalent sized mammals: Jerison 1973), do have some sort of a mental map of their home range and do plan their daily activities to some extent. It is obviously almost impossible to know how a baboon might view the world but an agent-based model is an ideal way of investigating possible approaches and can certainly quantify the costs and benefits associated with various levels of planning. It would also be interesting to extend the model to explore the relationship between individual- and group-level action selection in more detail. For example, it would be straightforward to incorporate a weighted voting scheme in which the votes of some individuals have a greater effect on action choice (and in the limit some subset of individuals determines group actions). It would be more interesting, however, to try to model the emergence of group-level action selection from the sum of interactions between individual agent's action choices (i.e. without an explicit voting scheme). This would require a much finer grained model of baboon sensing and behaviour, and a greater time resolution of the model. Nevertheless, agent-based modelling offers the potential to address these issues, and the current model should provide a valuable springboard for examining the relationship between individual and group level action selection.
Acknowledgments
R.A.H. gratefully acknowledges the financial support of the Leverhulme Trust in contributing to this project.
Footnotes
One contribution of 15 to a Theme Issue ‘Modelling natural action selection’.
Supplementary Material
The ESM folder contains the code necessary to run the model, and the environment data used for the runs in the paper. The field data is contained in the file EnvironmentData.xml and the ranges chosen for the Monte Carlo analysis are in MonteCarloData.xml. Both these files are extensively annotated. The code to run the model is contained in the src subdirectory. The simulations is written in C++ with dependencies on libxml2, libglut, libgl and libglui all of which are available on the web. This code has been tested under Linux and MacOSX
References
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Beshers S.N, Fewell J.H. Models of division of labor in social insects. Annu. Rev. Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. doi:10.1146/annurev.ento.46.1.413 [DOI] [PubMed] [Google Scholar]
- Black J.M. Preflight signalling in swans—a mechanism for group cohesion and flock formation. Ethology. 1988:143–157. [Google Scholar]
- Bryson, J. J. & Flack, J. C. 2002 Action selection for an artificial life model of social behavior in non-human primates. In Proc. Int. Workshop on Self-Organization and Evolution of Social Behaviour (ed. C. Hemelrijk), pp. 42–45. Switzerland: Monte Verita.
- Bryson J.J, Yasushi A, Lehmann H. Agent-based modelling as a scientific methodology: a case study analyzing primate social behaviour. Phil. Trans. R. Soc. B. 2007;362:1685–1698. doi: 10.1098/rstb.2007.2061. doi:10.1098/rstb.2007.2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R.W. How monkeys find their way: leadership, coordination, and cognitive maps of African baboons. In: Boinski S, Garber P, editors. On the move. University of Chicago Press; Chicago, IL: 2000. pp. 491–518. [Google Scholar]
- Campolongo F, Saltelli A, Sorensen T, Taratola S. Hitchhikers' guide to sensitivity analysis. In: Saltelli A, Chan K, Scott E.M, editors. Sensitivity analysis. Wiley; Chichester, UK: 2000. pp. 15–47. [Google Scholar]
- Cohen P.R, Levesque H.J. Teamwork. Noûs. 1991;25:487–512. [Google Scholar]
- Conradt L, Roper T.J. Group decision-making in animals. Nature. 2003;421:155–158. doi: 10.1038/nature01294. doi:10.1038/nature01294 [DOI] [PubMed] [Google Scholar]
- Conradt L, Roper T.J. Consensus decision making in animals. Trends Ecol. Evol. 2005;20:449–456. doi: 10.1016/j.tree.2005.05.008. doi:10.1016/j.tree.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Couzin I.D, Krause J, Franks N.R, Levin S.A. Effective leadership and decision-making in animal groups on the move. Nature. 2005;433:513–516. doi: 10.1038/nature03236. doi:10.1038/nature03236 [DOI] [PubMed] [Google Scholar]
- Cowlishaw G.C. Vulnerability to predation in baboon populations. Behaviour. 1994;131:293–304. [Google Scholar]
- Deneubourg J.L, Lioni A, Detrain C. Dynamics of aggregation and emergence of cooperation. Biol. Bull. 2002;202:262–267. doi: 10.2307/1543477. doi:10.2307/1543477 [DOI] [PubMed] [Google Scholar]
- Dennett D.C. MIT Press; Cambridge, MA: 1987. The intentional stance. [Google Scholar]
- Dennett D. Basic Books; New York, NY: 1996. Kinds of minds: towards an understanding of consciousness. [Google Scholar]
- Ferber J. Addison Wesley Longman; Boston, MA: 1999. Multi-agent systems. [Google Scholar]
- Georgeff M.P, Pell B, Pollack M.E, Tambe M, Wooldridge M. The belief-desire-intention model of agency. In: Müller J.P, Singh M.P, Rao A.S, editors. Intelligent agents v, agent theories, architectures, and languages, 5th Int. Workshop, (ATAL'98), Paris, France, July 4–7, 1998, Proc. vol. 1555. Springer; Berlin, Germany: 1999. pp. 1–10. [Google Scholar]
- Gimblett H.R. Oxford University Press; New York, NY: 2002. Integrating geographic information systems and agent-based modelling techniques for simulating social and ecological processes. [Google Scholar]
- Grosz B.J, Sidner C.L. Sidner plans for discourse. In: Cohen P.R, Morgan J, Pollack M, editors. Intentions in communication. MIT Press; Cambridge, MA: 1990. pp. 417–445. [Google Scholar]
- Hastie R, Kameda T. The robust beauty of majority rules in group decisions. Psychol. Rev. 2005;112:494–508. doi: 10.1037/0033-295X.112.2.494. doi:10.1037/0033-295X.112.2.494 [DOI] [PubMed] [Google Scholar]
- Hemelrijk C.J. Effects of cohesiveness on intersexual dominance relationships and spatial structure among group-living virtual entities. In: Floreano D, Nicoud J.-D, Mondada F, editors. Proc. Fifth Eur. Conf. on Artificial Life (ECAL99) Springer; Berlin, Germany: 1999a. pp. 524–534. [Google Scholar]
- Hemelrijk C.J. An individual-oriented model of the emergence of despotic and egalitarian societies. Proc. R. Soc. B. 1999b;266:361–369. doi:10.1098/rspb.1999.0646 [Google Scholar]
- Hemelrijk C.J. Towards the integration of social dominance and spatial structure. Anim. Behav. 2000;59:1035–1048. doi: 10.1006/anbe.2000.1400. doi:10.1006/anbe.2000.1400 [DOI] [PubMed] [Google Scholar]
- Hemelrijk C.J. Self-organization and natural selection in the evolution of complex despotic societies. Biol. Bull. 2002;202:283–288. doi: 10.2307/1543480. doi:10.2307/1543480 [DOI] [PubMed] [Google Scholar]
- Hemelrijk C.J, Wantia J. Individual variation by self-organisation. Neurosci. Biobehav. Rev. 2005;29:125–136. doi: 10.1016/j.neubiorev.2004.07.003. doi:10.1016/j.neubiorev.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Hemelrijk C.J, Wantia J, Datwyler M. Female co-dominance in a virtual world: ecological, cognitive, social and sexual causes. Behaviour. 2003;140:1247–1273. doi:10.1163/156853903771980585 [Google Scholar]
- Hemelrijk C.J, Wantia J, Gygax L. The construction of dominance order: comparing performance of five methods using an individual-based model. Behaviour. 2005;142:1043–1064. doi:10.1163/156853905774405290 [Google Scholar]
- Hill R.A. Day length seasonality and the thermal environment. In: Brockman D.K, van Schaik C.P, editors. Primate seasonality: implications for human evolution. Cambridge University Press; Cambridge, UK: 2005. pp. 197–212. [Google Scholar]
- Hill R.A. Thermal constraints on activity scheduling and habitat choice in baboons. Am. J. Phys. Anthropol. 2006;129:242–249. doi: 10.1002/ajpa.20264. doi:10.1002/ajpa.20264 [DOI] [PubMed] [Google Scholar]
- Hill R.A, Dunbar R.I.M. Climatic determinants of diet and foraging behaviour in baboons. Evol. Ecol. 2002;16:579–593. doi:10.1023/A:1021625003597 [Google Scholar]
- Hill, R. A. & Weingrill, T. 2006 Predation risk and habitat use in chacma baboons (Papio hamadryas ursinus) In Primates and their predators (eds S. Gursky & K. A. I. Nekaris), pp. 339–354. New York, NY: Kluwer Academic Publishers.
- Hill R.A, Barrett L, Gaynor D, Weingrill T, Dixon P, Payne H, Henzi S.P. Day length, latitude and behavioural (in)flexibility in baboons. Behav. Ecol. Sociobiol. 2003;53:278–286. [Google Scholar]
- Hill R.A, Barrett L, Gaynor D, Weingrill T, Dixon P, Payne H, Henzi S.P. Day length variation and seasonal analyses of behaviour. S. Afr. J. Wildl. Res. 2004;34:39–44. [Google Scholar]
- Jerison H.J. Academic Press; New York, NY: 1973. Evolution of the brain and intelligence. [Google Scholar]
- Jolly C.J. A proper study for mankind: analogies from the papionin monkeys and their implications for human evolution. Yearbook Phys. Anthropol. 2001;44:177–204. doi: 10.1002/ajpa.10021. doi:10.1002/ajpa.10021 [DOI] [PubMed] [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Laver M. Spatial models of political competition with endogenous political parties. Phil. Trans. R. Soc. B. 2007;362:1711–1721. doi: 10.1098/rstb.2007.2062. doi:10.1098/rstb.2007.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leca J.-B, Gunst N, Thierry B, Petit O. Distributed leadership in semifree-ranging white-faced capuchin monkeys. Anim. Behav. 2003;66:1045–1052. doi:10.1006/anbe.2003.2276 [Google Scholar]
- List C. Democracy in animal groups: a political perspective. Trends Ecol. Evol. 2004;19:168–169. doi: 10.1016/j.tree.2004.02.004. doi:10.1016/j.tree.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Lomnicki A. Population ecology from the individual perspective. In: DeAngelis D.L, Gross L.J, editors. Individual-based models and approaches in ecology. Chapman and Hall; New York, NY: 1992. pp. 3–17. [Google Scholar]
- Maynard-Smith J. Life at the edge of chaos. New York Rev. Books, March. 1995;2:28–30. [Google Scholar]
- Milinski M, Parker G.A. Competition for resources. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. Blackwell; Oxford, UK: 1991. pp. 137–168. [Google Scholar]
- Norton G.W. Leadership: decision processes of group movement in yellow baboons. In: Else J, Lee P, editors. Primate ecology and conservation. Cambridge University Press; Cambridge, UK: 1986. pp. 145–156. [Google Scholar]
- Pratt S.C, Sumpter D.J.T, Mallon E.B, Franks N.R. An agent-based model of collective nest choice by the ant Temnothorax albipennis. Anim. Behav. 2005;70:1023–1036. doi:10.1016/j.anbehav.2005.01.022 [Google Scholar]
- Prins H.H.T. Chapman & Hall; London, UK: 1996. Ecology and behaviour of the African buffalo. [Google Scholar]
- Rands S.A, Cowlishaw G, Pettifor R.A, Rowcliffe J.M, Johnstone R.A. The spontaneous emergence of leaders and followers in a foraging pair. Nature. 2003;423:432–434. doi: 10.1038/nature01630. doi:10.1038/nature01630 [DOI] [PubMed] [Google Scholar]
- Rao, A. S. & Georgeff, M. P. 1991 Modeling rational agents within a BDI-architecture. In Proc. Second Int. Conf. on Principles Of Knowledge Representation and Reasoning (kr'91), pp. 473–484.
- Reynolds, C. W. 1987 Flocks, herds and schools: a distributed behavioral model. In Proc. 14th Annual Conf. on Computer Graphics and Interactive Techniques, pp. 25–34. ACM Press.
- Richard A.F. W.H. Freeman and Company; New York, NY: 1985. Primates in nature. [Google Scholar]
- Robbins M.M, Robbins A.M. Simulation of the population dynamics and social structure of the Virunga mountain gorillas. Am. J. Primatol. 2004;63:201–223. doi: 10.1002/ajp.20052. doi:10.1002/ajp.20052 [DOI] [PubMed] [Google Scholar]
- Schüle M, Herrler R, Klüg F. Coupling GIS and multi-agent simulation—towards infrastructure for realistic simulation. In: Lindemann G, Denzinger J, Timm I.J, Unland R, editors. Multiagent system technologies: Proc. Second German Conf. (MATES 2004) vol. 3187. Springer; Berlin, Germany: 2004. pp. 228–242. [Google Scholar]
- Seeley T.D, Buhrman S.C. Group decision making in swarms of honey bees. Behav. Ecol. Sociobiol. 1999;45:19–31. doi:10.1007/s002650050536 [Google Scholar]
- Seeley T.D, Camazine S, Sneyd J. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1991;28:277–290. doi:10.1007/BF00175101 [Google Scholar]
- Sellers W.I, Hill R.A, Logan B. Biorealistic modelling of baboon foraging using agent-based modelling. In: Bryson J.J, Prescott T.J, Seth A.K, editors. Modelling natural action selection: Proc. Int. Workshop. AISB Press; Edinburgh, UK: 2005. pp. 127–134. [Google Scholar]
- Simons A.M. Many wrongs: the advantage of group navigation. Trends Ecol. Evol. 2004;19:453–455. doi: 10.1016/j.tree.2004.07.001. doi:10.1016/j.tree.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Stacey P.B. Group size and foraging efficiency in yellow baboons. Behav. Ecol. Sociobiol. 1986;18:175–187. doi:10.1007/BF00290821 [Google Scholar]
- Stewart K.J, Harcourt A.H. Gorilla vocalisations during rest periods—signals of impending departure. Behaviour. 1994;130:29–40. [Google Scholar]
- Tambe M. Towards flexible teamwork. J. Artif. Intell. Res. 1997;7:83–124. [Google Scholar]
- Topping C.J, Hansen T.S, Jensen T.S, Nikolajsen J.J.F, Odderskaer P. Almass, an agent-based model for animals in temperate European landscapes. Ecol. Model. 2003;167:65–82. doi:10.1016/S0304-3800(03)00173-X [Google Scholar]
- Tucker V. The energetic cost of locomotion in animals. Comp. Biochem. Physiol. 1970;34:841–846. doi: 10.1016/0010-406x(70)91006-6. doi:10.1016/0010-406X(70)91006-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The ESM folder contains the code necessary to run the model, and the environment data used for the runs in the paper. The field data is contained in the file EnvironmentData.xml and the ranges chosen for the Monte Carlo analysis are in MonteCarloData.xml. Both these files are extensively annotated. The code to run the model is contained in the src subdirectory. The simulations is written in C++ with dependencies on libxml2, libglut, libgl and libglui all of which are available on the web. This code has been tested under Linux and MacOSX