Abstract
A patient with obstructive sleep apnea was monitored five times during three years while his weight fluctuated within a range of 26 kg. The number of apneas per hour of sleep varied from 59.6 at 111 kg of weight to 3.1 at 85 kg. The relation between apneas per hour of sleep and body weight was a logarithmic function. A modest decrease in weight was thus associated with a disproportionally larger decrease in the rate of apneas. Typical SaO2 levels during the apneic episodes also had a logarithmic relation with body weight. Apnea-related sinus bradycardia and sinus tachycardia were only present at the highest weight. The results suggested that dieting and weight loss lead to an improvement in sleep apnea and related sequelae.
Obstructive sleep apnea syndrome is characterized by a repetitive and periodic occlusion of the upper airway during sleep. The mechanisms involved in this disorder are not well understood at present.1 Obesity is seen in the majority of patients with sleep apnea. Redundant tissue in the oropharyngeal airway associated with obesity is thought to be a contributing factor. Surgical intervention by tracheostomy or uvulo-palato-pharyngoplasty is the treatment of choice, especially in the presence of apneic-induced cardiac arrhythmias, but case reports have suggested improvement in the obstructive sleep apnea and related sequelae following weight loss.2-4 The exact relation between this syndrome and body weight remains ambiguous, however, as previous reports are based on single observations following weight reduction. We have monitored the sleep of a patient with obstructive sleep apnea five times during the course of nearly three years while his weight fluctuated from 85 kg to 111 kg. A logarithmic relation between the rate of apneas during sleep and body weight is demonstrated by this patient.
Case Report
A 52-year-old white man was referred for evaluation of uncontrolled daytime sleepiness. The patient reported a 19-year history of hypersomnia and falling asleep at inappropriate times. Symptoms were reported as relatively stable over the past ten years. The patient noted loud snoring and excessive sweating during sleep. Although he denied cataplexy, hypnagogic hallucinations and sleep paralysis, narcolepsy was diagnosed two years prior to our evaluation based solely on the reported hypersomnia.
Physical examination revealed an obese man (173 cm tall, weighing 111 kg), extremely drowsy, but otherwise in no distress. Pertinent medical disorders included essential systemic hypertension and a history consistent with chronic bronchitis. His blood pressure was 140/90 mm Hg and there was evidence of 1+ peripheral edema. HEENT examination was within normal limits. The remainder of the examination was unremarkable.
Results of laboratory tests included the following: Na, 141; K, 3.6; Cl, 93; glucose, 116; BUN, 23; Ca, 9.6; SGPT, 12; SGOT, 10; T3, 115.0 (normal range: 58-185); T4, 6.1 (normal range: 3.9-13.0), Hgb 15.2, and Hct 43. Pulmonary function was conducted in the seated position. These values were: TLC, 129 percent; VC, 133 percent; RV, 121 percent; ERV, 30 percent; FRC, 86 percent and Dco, 141 percent predicted; RV/TLC was 32 percent; and FEV1 was 63 percent VC (postbronchodilator, 70 percent VC). Arterial blood taken at this time showed PaO2, 62 mm Hg; O2 saturation, 91 percent; PaCO2, 32 mm Hg; and pH, 7.41. Holter monitoring disclosed sinus bradycardia as low as 30 bpm during the night. Otolaryngologic examination and psychiatric evaluation were unremarkable. Medications were methylphenidate 20 mg po tid, 50 mg chlorthalidone plus 0.25 mg reserpine in the form of Regroton tablets, 1 po qd; digoxin, 0.25 mg po qd; and potassium chloride, 40 mEq po qam.
A diagnostic polysomnographic evaluation was conducted.1 Severe obstructive sleep apnea was present at a weight of 111 kg. His sleep apnea index—average number of obstructive apneas per hour of sleep—was 59.6. In the absence of apnea-related ECG abnormalities other than sinus bradycardia (50 bpm) and sinus tachycardia (120 bpm), the patient started a 1,000 cal per day diet. Daily intake was 100 g carbohydrate, 65 g protein, and 40 g fat. The weight-reduction program was successful for 0.7 yr during which time the patient lost 26 kg. The sleep apnea was essentially obviated at 85 kg of body weight with 3.1 obstructive events per hour of sleep. The severe excessive daytime sleepiness had completely resolved and methylphenidate was discontinued. Unfortunately, the patient was unable to maintain his lower weight. He gained and then lost some weight through the following 2.2 years. His sleep apnea index showed a remarkable relation to weight throughout this time (Fig 1). The relation between body weight and the log transformation of sleep apnea index was significant (r = .960).
Figure 1.
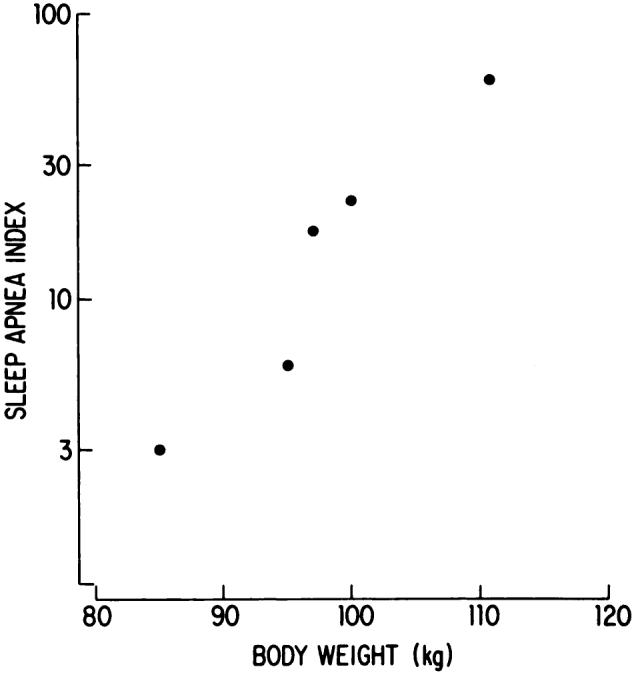
Body weight and obstructive sleep apnea index (apneas per hour of sleep) during five recordings from a single patient. Note the logarithmic scale of the ordinate.
Apnea-related SaO2 levels measured by ear oximetry provided an index of the severity of the upper-airway obstructive events. The lowest O2 desaturation of 20 percent during sleep was observed at a body weight of 111 kg. The typical SaO2 range represented O2 desaturation associated with 80 percent of all apneas. This range also showed a logarithmic relation with body weight. Typical apnea-related SaO2 level by body weight was 40 to 80 percent at 111 kg, 85 to 95 percent at 100 kg, 80 to 90 percent at 97 kg, 70 to 95 percent at 95 kg, and 88 to 95 percent at 85 kg. Sinus bradycardia-tachycardia related to the apnea essentially occurred with each obstructive apneic episode during the first recording. This was not present beyond the initial sleep study.
Sleep structure was responsive to the rate of obstructive apneas during the night (Table 1). The overall distribution of sleep stages is within the normal limits at a body weight of 85 kg when the apnea index is subclinical. Stages 3 and 4 are reestablished and the patient is able to sustain his sleep period. Similarly, there is no clinical evidence of excessive daytime sleepiness at this time. The patient did note at the last visit, however, that he used variations in his hypersomnia as an indicant of changes in body weight.
Table 1.
Sleep Structure in Relation to Obstructive Apnea Index
| Apnea Index | Sleep Latency (min) | Total Sleep (min) | Sleep Stage (%) |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | REM | |||
| 59.6 | 1.5 | 299 | 36 | 56 | 0 | 0 | 8 |
| 22.1 | 4.0 | 415 | 14 | 61 | 0 | 0 | 25 |
| 17.5 | 3.4 | 385 | 10 | 76 | 2 | 0 | 12 |
| 5.9 | 2.0 | 367 | 13 | 69 | 1 | 0 | 17 |
| 3.1 | 5.0 | 383 | 8 | 64 | 7 | 1 | 20 |
Comments
Diet manipulation and a subsequent reduction in weight is associated with a decrease in the number of obstructive apneas during sleep for the patient. The findings suggest the relation between apnea and body weight is best described as a logarithmic function. Beyond some critical weight, there is a rapid increase in the rate of apneas. The initial loss of weight thus provides the greatest reversal of this syndrome. Apparently ideal body weight need not be achieved to resolve the sleep apnea. The patient’s sleep apnea is not a major clinical concern at 85 kg (116 percent of ideal).
The striking correlation between body weight and sleep apnea does not preclude the possible effect of dieting independent of weight reduction on obstructive sleep apnea. Dieting alone may improve ventilatory responsiveness to carbon dioxide in the awake patient.5 This relates to the metabolic and other changes associated with dieting. Although ventilatory effort is present while the oropharyngeal airway is occluded with sleep apnea, the influence of dieting may be at the level of the central nervous system. Whether the obstruction of the upper airway during an apneic episode is an active or passive process remains controversial.1 However, patency of the airway is at least partially under neurologic control via maintenance of muscular tone of the pharnygeal muscles. Thus, dieting and weight loss may act independently or interact to improve sleep apnea in the obese patient. If weight reduction per se is a contributory factor, the relevance of the anatomic location of the lost weight and the ratio of fluid to tissue loss need to be elucidated. Similar delineation of the possible role of dieting is also needed.
The apnea-induced sequelae are improved with dieting and weight loss. There is no evidence of apnea-related heart rate abnormalities, for example, beyond the first recording. Thus, sleep apnea may persist at a clinically significant rate while the severity of sequelae are rapidly obviated. However, this patient did develop complete heart block (dropped sinus beat) during sleep which was unrelated to respiratory abnormalities or O2 desaturations. This was only observed during the final recording. While there was no direct causal relation, the previous significant sleep apnea may have been a contributing factor in the development of this cardiovascular disease.6
The findings show that dieting and weight loss can be nearly as efficacious as tracheostomy in the treatment of sleep apnea. However, compliance with the weight-control program is a major issue when considering this form of treatment. The prognosis for maintenance following surgical or behavioral methods of weight reduction is not optimistic.7
Finally, the patient’s diagnosis of narcolepsy was based on the reported hypersomnia. We found no clinical evidence to support this diagnosis. His hypersomnia fluctuated with the rate of apneas during nighttime sleep and there was no history of cataplexy. Since excessive sleepiness is the presenting symptom of numerous disorders and treatment is specific to etiology, the differential diagnosis is extremely important.
Footnotes
From the Sleep Disorders Center, State University of New York at Stony Brook
References
- 1.Guilleminault C. Sleep and breathing. In: Guilleminault C, editor. Sleeping and waking disorders. Addison-Wesley; Menlo Park: 1982. pp. 155–82. [Google Scholar]
- 2.Kerin NZ, Edelstein J, Goldberg LB, Louridas G. Cardiac dysrhythmias associated with obesity and sleep apnea. Cardiovascular Med. 1979;4:1167–74. [Google Scholar]
- 3.Guilleminault C, van den Hoed J, Mitler MM. Clinical overview of the sleep apnea syndromes. In: Guilleminault C, Dement WC, editors. Sleep apnea syndromes. Alan R. Liss; New York: 1978. pp. 1–12. [Google Scholar]
- 4.Walsh JK, Ulett GA, Schweitzer PA, O’Regan JP. Snoring, sleepiness and the obstructive sleep apnea syndrome. Mo Med. 1982;79:21–6. [PubMed] [Google Scholar]
- 5.Fried PI, McClean PA, Phillipson EA, Zamel N, Murray FT, Marliss EB. Effect of ketosis on respiratory sensitivity to carbon dioxide in obesity. N Engl J Med. 1976;294:1081–86. doi: 10.1056/NEJM197605132942003. [DOI] [PubMed] [Google Scholar]
- 6.Partinen M, Putkonen PTS, Kaprio J, Koskenvuo M, Hilakivi I. Sleep disorders in relation to coronary heart disease. Acta Med Scand (Suppl) 1982;660:69–83. doi: 10.1111/j.0954-6820.1982.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 7.Foreyt JP, Goodrick GK, Gotto AM. Limitations of behavioral treatment of obesity: review and analysis. J Behav Med. 1981;4:159–74. doi: 10.1007/BF00844268. [DOI] [PubMed] [Google Scholar]


