Abstract
Articular cartilage function depends on the molecular composition and structure of its extracellular matrix (ECM). The collagen network (CN) provides cartilage with tensile integrity, but must also remodel during growth. Such remodeling may depend on matrix molecules interacting with the CN to modulate the tensile behavior of cartilage. The objective of this study was to determine the effects of increasingly selective matrix depletion on tensile properties of immature and mature articular cartilage, and thereby establish a framework for identifying molecules involved in CN remodeling. Depletion of immature cartilage with guanidine, chondroitinase ABC, chondroitinase AC, and Streptomyces hyaluronidase markedly increased tensile integrity, while the integrity of mature cartilage remained unaltered after depletion with guanidine. The enhanced tensile integrity after matrix depletion suggests that certain ECM components of immature matrix serve to inhibit CN interactions and may act as modulators of physiological alterations of cartilage geometry and tensile properties during growth/maturation.
Keywords: Articular Cartilage, Tensile Properties, Maturation, Remodeling, Cartilage Matrix, Glycosaminoglycan, Collagen Crosslinks
Introduction
Articular cartilage is a layer of connective tissue located on the ends of long bones [1] that normally functions as a load-bearing, low friction, wear-resistant material, facilitating joint motion [2, 3]. The ability of cartilage to withstand compressive, tensile, and shear forces depends on the composition and structure of the extracellular matrix (ECM) [2-4]. The proteoglycan constituent of the extracellular matrix provides the tissue with a fixed negative charge that increases the tissue's propensity to swell and to resist compressive loading [5, 6]. The crosslinked collagen network (CN) resists the swelling tendency of the proteoglycan molecules and provides the tissue with tensile and shear stiffness and strength [3, 7, 8]. In adult cartilage, the tensile modulus and strength have been attributed primarily to the CN [3, 9]. An indirect, but dramatic contribution of the proteoglycan component to the tensile properties or cartilage has also been demonstrated. Chondroitinase ABC (C-ABC) treatment, which specifically depolymerizes chondroitin and dermatan sulfate GAG chains [10], resulted in a reduction of the initial slope of the stress-strain curve [11, 12] and an increase in the overall tensile modulus of cartilage [13-15] and tensile strength in tendon [16]. In addition, collagen to proteoglycan ratio correlated strongly with equilibrium tensile modulus of cartilage [17]. Thus, the load-bearing function of cartilage may depend on the ECM components, as well as their ability to transfer stress through intermolecular interactions.
The nature of the interactions among collagen fibrils and between fibrils and other members of the extracellular matrix to form a functional CN remains to be established. Sites for covalent pyridinoline crosslinks, catalyzed by lysyl oxidase, are located both within and between fibrils, linking collagens type II and IX [18]. Such sites on collagen type IX are a source of covalent interfibrillar bonds [19, 20]. Other type of bonds may exist between collagen type IX and proteoglycans, providing a link between the CN and the extrafibrillar matrix components of cartilage [21, 22]. Collagen-binding molecules, including small proteoglycans, such as decorin and fibromodulin, associate with distinct and specific bands at the surface of the collagen fibrils through noncovalent binding of their core proteins [23] and therefore can be considered as constituents of the CN. These molecules have been proposed as functional non-covalent linkages within the ECM [23-26]. Aggrecan, the major proteoglycan in cartilage, while distinct from the CN, may also form interactions with the CN through hyaluronic acid, or its keratan sulfate- and chondroitin sulfate-rich regions [27-30]. Together, extrafibrillar matrix components may mediate the adherence and sliding between collagen fibrils to modulate cartilage tissue shape [31-33].
The regulation of collagen fibril synthesis, assembly, and remodeling suggests that the presence of certain extrafibrillar matrix molecules may alter the transfer of forces between collagen fibrils and the overall tissue integrity. Various studies suggest that small proteoglycans that bind to collagen fibrils may have a role in regulation of collagen fibril length and diameter [34-37] and, consequently, of tensile integrity of connective tissues [38]. Removal of such components appears to enhance the association of collagen fibrils from tendon in vitro and in vivo [37, 39] and enhance cartilage repair in vivo [40]. These findings suggest that a decrease in certain extrafibrillar molecules is necessary to permit the association of collagen segments. In corollary, binding of collagen-binding molecules to the collagen fibrils competitively inhibits the accretion of additional collagen segments and, in this way, regulates the growth of collagen fibrils during development [39, 41, 42]. A similar mechanism may be responsible for controlling the restraining function of the collagen network during cartilage growth and maturation.
Articular cartilage has distinct needs for remodeling at different stages of maturation. During prenatal or early postnatal growth periods, expansion of articular cartilage tissue occurs to accommodate the rapidly increasing size of the underlying bones and to meet the loading demands ex utero [43, 44]. At skeletal maturity, after cessation of growth, articular cartilage homeostasis in terms of geometry, as well as composition, and function is desirable. It is possible that different interactions among matrix components may be preferred at various stages of cartilage tissue maturity to accommodate the needs of the tissue.
While the exact nature of interactions between collagen fibrils and extrafibrillar matrix components to form a functional collagen network remains to be established, cartilage growth and maturation may depend critically on the modulation of the CN and cartilage tensile properties. A detailed understanding of the tensile properties of cartilage may provide insight into molecular mechanisms that regulate interactions within the CN and the associated changes in the CN function during growth as well as in cartilage homeostasis and disease. Thus, the objective of this study was to determine the effects of increasingly selective matrix depletion on tensile mechanical properties of immature and mature articular cartilage, and thereby establish a framework for identifying molecules that regulate remodeling of the CN.
Materials and Methods
Sample Preparation
Articular cartilage blocks, 9 × 3 mm2 in area, were harvested from the patellofemoral groove of bovines that were immature (1−3 week calf, bCALF, n=3) or were young adults (1−2 years, bYOUNG, n=3) and of humans that were teen aged (15−16 years, hTEEN, n=2) or were skeletally mature adults (37−39 years, hADULT, n=3). Bovine joints were obtained from an abattoir and harvested as described previously [45]. Human donor tissue was obtained from San Diego Life Sharing (San Diego, CA) within 72 hours after death and stored at −70°C. Only normal human articular cartilage (grade 1A [46]) was used in the study. Cartilage blocks were prepared using a sledge microtome to either include the intact articular surface (superficial layer, ∼0.45 mm thick, S) or to include the middle zone, starting at a distance of ∼0.6 mm from the articular surface (middle layer, 0.25 mm thick, M). The long axes of the blocks were in the anterior-posterior direction [45] and, thus, approximately perpendicular to the split line direction.
Experimental Design
Blocks were weighed wet (WWi), treated, weighed wet again (WWf), rinsed, tested in tension, and analyzed for biochemical composition as described below. All solutions included protease inhibitors (2 mM Na2-EDTA, 1 mM PMSF, 5 mM Benz-HCl, 10 mM NEM). Two series of experiments were carried out.
Experiment 1
To investigate the effect of non-covalent intermolecular bonds among the extracellular matrix molecules on the tensile integrity of articular cartilage, the specimens were extracted with 4M guanidine HCl (Gnd), a strong chaotrophic agent [47]. The maximum amount of GAG, as measured by hexuronate content, that can be extract with Gnd is ∼85% of total, while the residual ∼15% remains behind with the insoluble matrix [48]. To investigate the effects of Gnd on tensile properties apart from the effect on Gnd-induced matrix depletion alone, Gnd extracted tissue was mechanically tested under associative conditions (in PBS) and under dissociativev conditions (in Gnd). Blocks of bCALF and bYOUNG, as well as hTEEN and hADULT cartilage were treated (600 μl/block, 48 hr, 4°C, pH 7.0), rinsed (200 μl/block, 30 min, 22°C, pH 7.0), and mechanically tested in treat/rinse/test combinations: (1a) PBS/PBS/PBS (sham control), (1b) Gnd/PBS/PBS, or (1c) Gnd/Gnd/Gnd.
Experiment 2
While Gnd is a relatively non-specific depletion agent, more specific enzymatic tissue digestions [49] were used with bCALF specimens. Chondroitinase ABC (C-ABC) depolymerizes chondroitin sulfate (CS) and dermatan sulfate (DS) and, to some degree, hyaluronic acid (HA) [10], thus potentially affecting CS and DS-containing molecules such as aggrecan, decorin, and collagen type IX as well as HA. Depending on the extent of HA cleavage, C-ABC may also affect proteoglycan aggregates, as some of the aggregates which are attached to small HA fragments, may be able to diffuse out of the tissue. Collagen and collagen network arrangement are generally unaffected by C-ABC treatment [50-52]. Chondroitinase AC II (C-AC) is more specific than C-ABC in that it catalytically cleaves CS and HA but not DS [10]. Thus, C-AC may affect similar molecules as C-ABC, except DS-containing molecules like decorin. Streptomyces hyaluronidase (sHAase) splits HA [53] and, thus, may remove some proteoglycan aggregates, similar to C-ABC and C-AC.
Explants from bCALF were subjected to either digestion with protease-free enzyme preparations or the corresponding sham solution (buffer solution that did not contain the enzyme) treatment (0.1 ml/block, 24hr at 37°C): (2a) 0.2 U/ml C-ABC in a buffer solution containing 0.05 M Tris buffer, pH 8.0, 0.01 M sodium acetate, and 0.02% BSA, (2b) 0.1 U/ml C-AC in a buffer solution containing 0.033 M acetic acid/sodium acetate buffer, pH 6.0, and 0.01% BSA, (2c) 10 TRU/ml sHAase in a buffer solution containing 0.02 M acetic acid/sodium acetate buffer, pH 6.0, and 0.15M NaCl. After treatment, the samples were washed and mechanically tested in PBS. C-ABC, C-AC, and sHAase were from Seikagaku America (East Falmouth, MA).
The effects of Gnd on bCALF tissue were also examined further. To investigate whether the effects of PBS wash of Gnd treated blocks (as in 1b) are reversible (i.e. if interactions that form in PBS, under associative conditions, are covalent) the blocks were transferred to Gnd (dissociative conditions) and tested as such: (2d) Gnd/PBS/Gnd. To investigate if the effects of Gnd on tensile properties depend on extraction of matrix components, bCALF tissue was incubated in Gnd saturated with cartilage extract (Gnd+), solution that allows incubation under dissociative conditions without extracting matrix components: (2e) (Gnd+)/PBS/PBS. To prepare Gnd+, articular cartilage from bCALF stifle joints was finely minced (1−3 mm3 pieces) and incubated in Gnd (48 hr, 4°C, pH 7.0) in a ratio of cartilage to Gnd of 2:1. The resulting Gnd+ (i.e. liquid portion) was filtered through gauze and used immediately.
Biochemical Analysis
Residual cartilage and the failed portions of the corresponding tensile strip (see below) were analyzed together to quantify the biochemical composition of the samples. Samples were solubilized with proteinase K [54] and analyzed to quantify the content of sulfated GAG [55] and hydroxyproline [56]. In experiment 1, portions of tissue digest from each animal or donor/layer and certain experimental conditions were pooled for analysis of HA (Corgenix Inc., Denver, CO) and pyridinoline (PYR) [57]. In experiment 2, HA content was determined for individual samples. Hydroxyproline content was converted to COL content by assuming a mass ratio of collagen to hydroxyproline equal to 7.25 for bovine cartilage [58, 59] and 7.1 for human cartilage [60]. The molar ratio of PYR per collagen molecule was calculated, assuming the molecular weight of collagen triple helix of 300,000. Biochemical parameters were normalized to initial wet weight of the tissue (WWi) to represent constituent content.
Biomechanical Analysis
Tapered tensile specimens were analyzed to determine mechanical properties as described previously [54]. From each cartilage block, a tapered strip [61] with a gage region of 4 mm × 0.80 mm was prepared using a punch. The thickness of each tensile strip was measured at three locations in the gage region and the average was used for cross-sectional area calculations. Tapered specimens were then secured in clamps (4.0 mm apart) of a mechanical tester. The test sequence consisted of applying a positive displacement at 0.5 mm/min until a tare load of 0.05 N (equivalent to a stress of ∼0.2 MPa) was attained, then elongating the specimen to 10% strain (relative to the length at the tare load) at a constant strain rate of 0.25%/s, allowing stress relaxation to equilibrium over 900 seconds, elongating the specimen to 20% strain, allowing stress relaxation to equilibrium over 900 seconds, and then elongating the specimen at a constant rate of 5 mm/min until failure.
The measured load and displacement were converted to stress (defined as load normalized to the initial cross-sectional area of the gage region) and strain (defined as the elongation distance normalized to the initial clamp to clamp distance). The equilibrium tensile modulus was calculated as the slope of the equilibrium stress-strain data by linear regression. Tensile ramp modulus was calculated from the last part of the mechanical test (elongation to failure) as the slope of the linear regression of the stress-strain curve between 25% and 75% of the maximum stress. Tensile strength was determined as the maximum stress sustained at failure, and strain at failure was determined as the strain at which maximum stress was attained. Blocks that were tested in Gnd fractured during the tare phase of the test. Tensile ramp modulus was calculated as the slope of the linear regression of the stress-strain curve for tare phase of the test and equilibrium modulus was calculated by multiplying the calculated ramp modulus by the average ratio of equilibrium to ramp modulus for blocks tested in PBS of the same layer. The failed portions of each tensile strip, resulting from the tensile test, were saved for biochemical analysis (described above) in addition to the adjacent cartilage samples obtained during preparation of the tensile strips.
Statistical Analysis
For each maturation stage/species (bCALF, bYOUNG, hTEEN, and hADULT) and each layer (S and M), the effects of experimental conditions were assessed by analysis of variance (ANOVA) with experimental condition as fixed factor and animal/human donor as a random factor. For the content of HA, which was determined from a pool of samples, the effects of experimental conditions were assessed by ANOVA for each maturation stage/species with experimental conditions and layer as fixed factors and donor animal as a random factor. Tukey post-hoc testing was performed to compare groups. Data for equilibrium modulus of bCALF blocks in Figures 1 and 3 were log-transformed to improve normality. To analyze the effect of experimental conditions on wet weight, repeated measures ANOVA was performed for each layer and experimental condition with wet weight (WWi, WWf) as a repeated factor. Data are expressed as mean ± SEM or mean ±5% CI, where applicable, and significance level was set to 0.05. Statistical analysis was performed using Systat 10.2 (Systat Software, Inc., Richmond, CA).
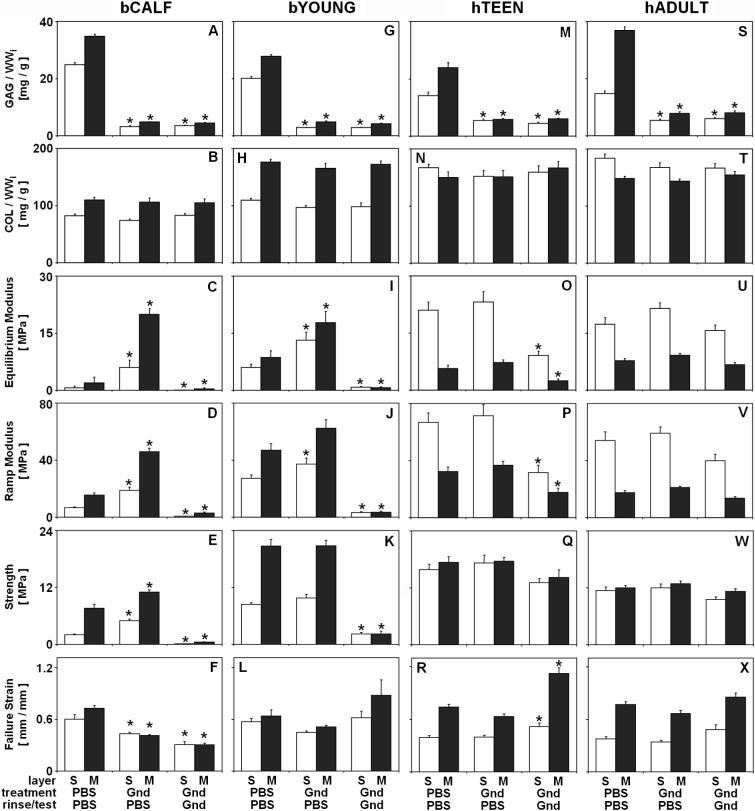
Figure 1.
Effect of experimental conditions on composition and tensile mechanical properties of bCALF (A-F), bYOUNG (G-L), hTEEN (M-R), and hADULT (S-X) articular cartilage explants from S and M layers. Blocks were incubated (48hr at 4°C), rinsed and mechanically tested in combinations of PBS and Gnd. * indicates p<0.05 vs. PBS/PBS/PBS control for a corresponding layer. Data are mean ± SEM or ± 5% CI, n = 12−16 blocks from 3 bCALF animals, n=12−15 block from 3 bYOUNG animals, n=10−12 blocks from 2 hTEEN donors, and n=14−18 blocks from 3 hADULT donors. from 3 hADULT donors.
Figure 3.
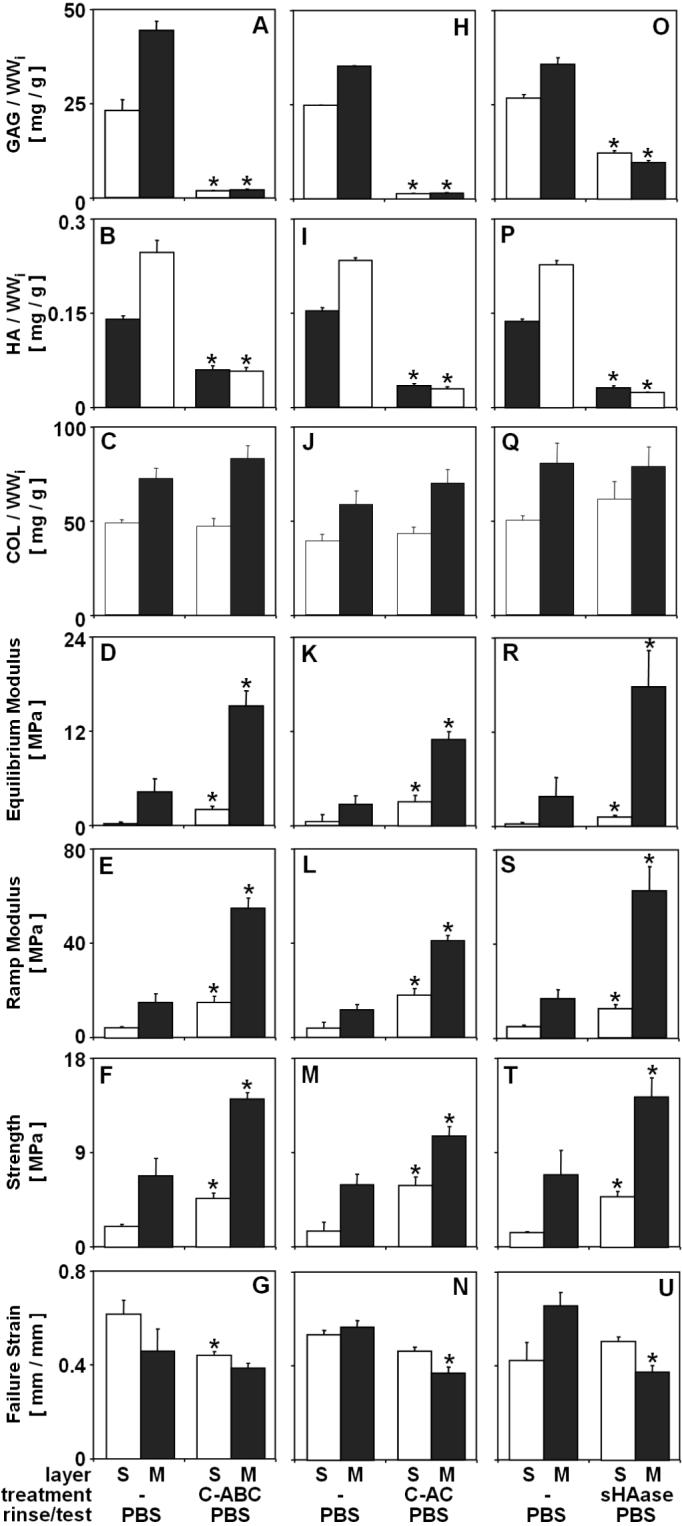
Effect of experimental conditions on composition and tensile mechanical properties of bCALF articular cartilage explants from S and M layers. Blocks were incubated (24hr at 37°C) in either C-ABC (A-G), C-AC (H-N), sHAase (O-U) or a corresponding sham buffer (-) and mechanically tested in PBS. * indicates p<0.05 vs. sham control for a corresponding layer. Data are mean ± SEM, n = 4−6 blocks.
Results
Experiment 1
Treatment of bovine and human cartilage with Gnd did not affect thickness of cartilage blocks (p>0.16) and, consistent with this, changes in tissue wet weight were minimal (data not shown). In bCALF and bYOUNG cartilage, incubation in PBS did not change tissue wet weight (p>0.12), but during incubation in Gnd tissue wet weight decreased slightly (−7.4−10.2%, p<0.001). In hTEEN and hADULT cartilage, both incubation in PBS and Gnd decreased tissue wet weight (−1.3 −3.2% in PBS and −2.8 −8.2% in Gnd; p<0.01), but such decrease was generally larger after incubation with Gnd than in PBS.
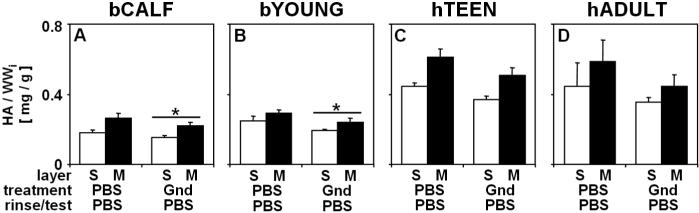
Gnd treatment affected biochemical composition of cartilage blocks from bCALF, bYOUNG, hTEEN, and hADULT. Gnd treatment released 70−85% (p<0.001) of GAG (Fig. 1A, G, M, S), with little effect on collagen content (<5% release, p>0.13) (Fig. 1B, H, N, T). Gnd treatment also reduced the content of HA in bCALF and bYOUNG tissue (17−20%, p<0.05) (Fig. 2A-B) and slightly in hTEEN and hADULT tissue (16−22%, p>0.16) (Fig. 2C-D).
Figure 2.
Effect of Gnd treatment on the content of hyaluronic acid (HA) of bCALF (A), bYOUNG (B), hTEEN (C), and hADULT (D) articular cartilage explants from S and M layers. Blocks were incubated (48hr at 4°C), rinsed and mechanically tested in PBS or Gnd. __*__ indicates p<0.05 vs. PBS/PBS/PBS control for both layer collectively. Data are mean ± SEM, n = 12−16 blocks from 3 bCALF animals, n=12−15 block from 3 bYOUNG animals, n=10−12 blocks from 2 hTEEN donors, and n=14−18 blocks from 3 hADULT donors.
Experimental conditions affected the tensile biomechanical properties of cartilage explants from bCALF, bYOUNG, and hTEEN, but not hADULT. Depletion of matrix with Gnd and then rinse/test in PBS markedly affected bCALF cartilage, with increased equilibrium modulus (+775−1176%, p<0.001) (Fig. 1C), ramp modulus (+189%−199%, p<0.001) (Fig. 1D) and strength (+44−148%, p<0.001) (Fig. 1E) and a decrease in strain at failure (−28 −43%, p<0.05) (Fig. 1F), and moderately affected bYOUNG cartilage with a slightly increased equilibrium modulus (+105−120%, p<0.05) (Fig. 1I) and ramp modulus (+37% in S, p<0.05; +33% in M, p=0.18) (Fig. 1J), but not strength (p>0.14) (Fig. 1K) and strain at failure (p>0.28) (Fig. 1L). In contrast, such treatment did not affect tensile integrity of hTEEN (p>0.25) (Fig. 1O-R) or hADULT (p>0.16) (Fig. 1U-X) cartilage.
Depletion of matrix with Gnd and then rinse/test in Gnd markedly affected bCALF cartilage, with decreased equilibrium modulus (−77−93%, p<0.001) (Fig. 1C), ramp modulus (−82−92%, p<0.001) (Fig. 1D), strength (∼ −94 %, p<0.001) (Fig. 1E), and strain at failure (−49−58%, p<0.05) (Fig. 1F), as well as bYOUNG cartilage with decreased equilibrium modulus (−86−92%, p<0.05) (Fig. 1I), ramp modulus (−88−93%, p<0.001) (Fig. 1J), and strength (−74%−89%, p<0.001) (Fig. 1K) but not strain at failure (p>0.30) (Fig. 1L). Such treatment affected hTEEN cartilage, but only moderately, with decreased equilibrium modulus (∼ −57%, p<0.01) (Fig. 1O), ramp modulus (−45−53%, p<0.05) (Fig. 1P), but not strength (p>0.28) (Fig. 1Q) and a slight increase in strain at failure (+32−52%, p<0.05) (Fig. 1R). In contrast, such treatment did not affect tensile integrity of hADULT cartilage (p>0.13) (Fig. 1U-X).
The maturity of the source tissue, as indicated by tissue age, agreed with measures of content of collagen, PYR crosslinks, and PYR/COL ratio, parameters that are sensitive to the degree of tissue maturity [45]. The content of collagen and PYR crosslinks, and PYR/COL ratio were low in bCALF tissue and increased with growth to bYOUNG tissue (Fig. 1B, H; Fig. 5). In hTEEN and hADULT cartilage, the contents of collagen, PYR crosslinks and PYR/COL ratio were higher than those of bCALF and bYOUNG cartilage (Fig. 1N, T; Fig. 5).
Figure 5.
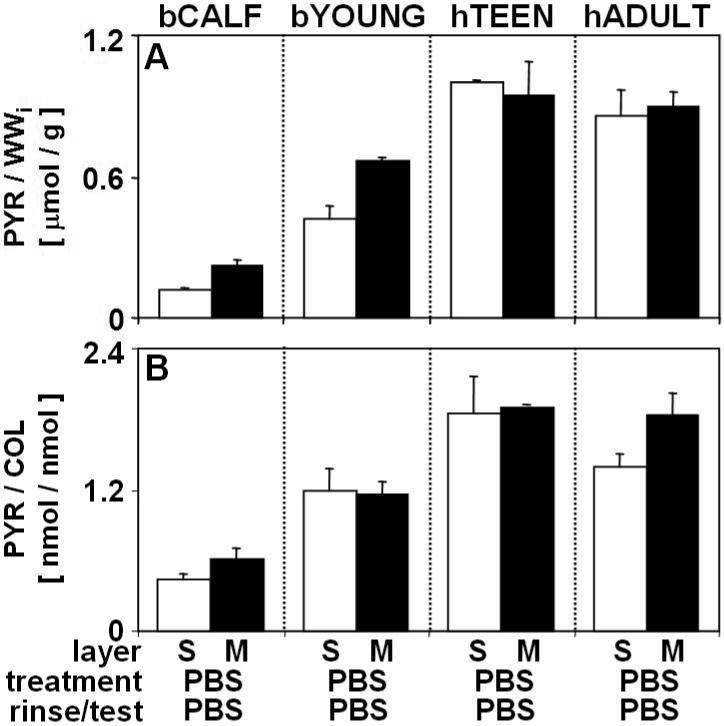
The content of pyridinoline crosslinks (PYR) and molar ratio of PYR to collagen (PYR/COL) in of bCALF, bYOUNG, hTEEN, and hADULT articular cartilage explants from S and M layers. Blocks were incubated (48hr at 4°C), rinsed and mechanically tested in PBS. Data are mean ± SEM, n = 12−16 blocks from 3 bCALF animals, n=12−15 block from 3 bYOUNG animals, n=10−12 blocks from 2 hTEEN donors, and n=14−18 blocks from 3 hADULT donors.
Experiment 2
Enzymatic treatment of bCALF cartilage did not affect thickness of cartilage blocks (p>0.26) and, consistent with this, changes in tissue wet weight were minimal (data not shown). Incubation in sham solutions did not change tissue wet weight in the S layer (p>0.13) and increased wet weight slightly in the M layer (up to +3%, p<0.001), while during incubation in solutions containing C-ABC, C-AC and sHAase tissue wet weight decreased (−7.5−10.5%, p<0.001). Similar to effects of Gnd on tissue wet weight in experiment 1, incubation in Gnd decreased tissue wet weight slightly (−7.4−10.2%, p<0.001), while during incubation in PBS and Gnd+, tissue wet weight remained unchanged (p>0.08) (data not shown).
The effects of treatments with C-ABC, C-AC and sHAase of bCALF blocks were similar to the effect of Gnd, in terms of both biochemical and biomechanical parameters. C-ABC, C-AC and sHAase treatments released ∼95%, ∼95% and ∼63% of GAG (p<0.001) (Fig. 3A, H, O), and ∼64%, ∼85% and ∼87% of HA (p<0.001) (Fig. 3B, I, P), respectively, with little effect on collagen content (<5% release, p>0.17) (Fig. 3C, J, Q). Depletions of tissue matrix with C-ABC, C-AC and sHAase and subsequent rinse/test in PBS resulted in a marked increase in equilibrium modulus (+246−782%, p<0.01) (Fig. 3D, K, R), ramp modulus (+149−373%, p<0.01) (Fig. 3E, L, S) and strength (+80−300%, p<0.05) (Fig. 3F, M, T). The strain at failure did not vary or decreased after enzymatic treatment (up to −45%, p>0.05) (Fig. 3G, N, U).
Further investigation of the effect of Gnd on bCALF tissue agreed with findings of experiment 1 on both biochemical and biomechanical parameters and yielded more information about the nature of the effects of ECM depletion. Gnd treatment released ∼85% (p<0.001) of GAG (Fig. 4A), with little effect on collagen content (<5% release, p>0.30) (Fig. 4B). In contrast, incubation in Gnd+ did not affect biochemical parameters, with GAG and COL similar to those in tissue incubated in PBS (p>0.99). Similar to effects of Gnd on biomechanical properties in experiment 1, depletion of matrix with Gnd and then rinse/test in PBS resulted in a marked increase in equilibrium modulus (+530−736%, p<0.001) (Fig. 4C), ramp modulus (+246−295, p<0.001) (Fig. 4D) and strength (+63−178%, p<0.05) (Fig. 4E) and a decrease in strain at failure (−3 8% in S, p=0.12; −48 % in M, p<0.001) (Fig. 4F), and depletion of matrix with Gnd and then rinse/test in Gnd resulted in a decrease in equilibrium modulus (−77−90%, p<0.05) (Fig. 4C), ramp modulus (−78 −90%, p<0.05) (Fig. 4D), strength (∼ −95 %, p<0.05) (Fig. 4E), and strain at failure (∼ −60 %, p<0.05) (Fig. 4F). Incubation in Gnd, with a PBS rinse, and subsequent test in Gnd, resulted in a decreased equilibrium modulus (−59 −86%, p<0.05) (Fig. 4C), ramp modulus (−68−86%, p<0.05) (Fig. 4D), strength (∼ −93 % p<0.05) (Fig. 4E), and strain at failure (−48 % in S, p=0.13; −60% in p<0.001) M,(Fig. 4F), and these mechanical properties approximated those of Gnd-treated tissue tested in Gnd but not exposed to PBS (p>0.39). Finally, incubation in Gnd+ restored mechanical properties to native levels, with equilibrium and ramp moduli, strength, and strain at failure similar to those of tissue incubated and tested in PBS (p>0.93) (Fig. 4C-F).
Figure 4.
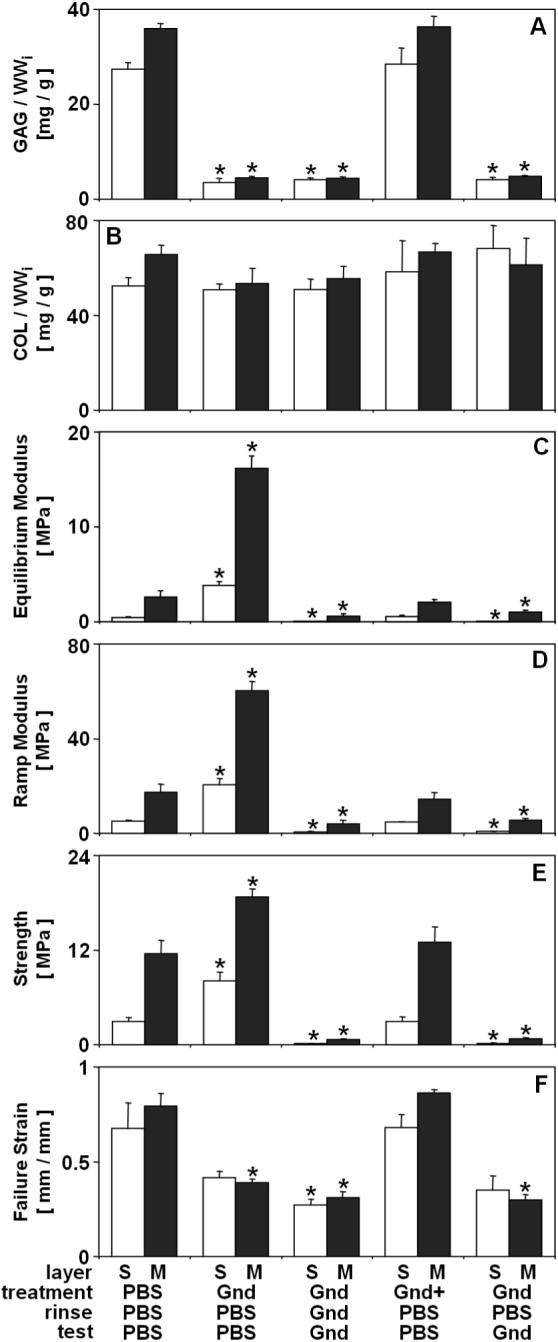
Effect of experimental conditions on composition and tensile mechanical properties of bCALF articular cartilage explants from S and M layers. Blocks were incubated (48hr at 4°C), rinsed and mechanically tested in combinations of PBS, Gnd, and Gnd saturated with cartilage extract (Gnd+). * indicates p<0.05 vs. PBS/PBS/PBS control for a corresponding layer. Data are mean ± SEM or ± 5% CI, n = 4−6 blocks.
Discussion
The data presented here demonstrate that tensile mechanical properties of articular cartilage can be modulated through depletion of certain extracellular matrix components; however the extent of this modulation is dependent on the maturity of the source tissue. In immature bovine cartilage (bCALF), matrix depletion with Gnd resulted in a marked enhancement of tensile integrity when mechanically tested under associative conditions in PBS and a marked decrease in tensile integrity when mechanically tested under dissociative conditions in Gnd (Fig. 1C-E). The enhanced tensile integrity of immature cartilage after matrix depletion and testing in PBS suggests that certain ECM components of immature matrix serve to inhibit CN interactions that promote tensile integrity, while reduction of tensile integrity to very low levels after depletion and testing in Gnd indicates that certain endogenous linkages within the CN can be disrupted by Gnd. As the tissue maturity increases (from bCALF to bYOUNG to hTEEN to hADULT), a progressively more moderate enhancement of tensile integrity after matrix depletion in Gnd and testing under associative conditions in PBS and less marked diminution of tensile integrity after matrix depletion in Gnd and testing under dissociative conditions in Gnd is observed, until hADULT tissue that is not affected by matrix depletion with Gnd (Fig. 1). The lack of effect of matrix depletion with Gnd on tensile integrity in hADULT cartilage suggests that in mature human cartilage tissue, the CN attains a state in which further tensile integrity-promoting interactions can not be induced by removing matrix molecules and that the endogenous linkages within the CN can not be disrupted by Gnd.
The effects of increasingly selective enzymatic treatments of bCALF tissue, along with further examination of the effects of Gnd, provide insight into the nature of the modulated CN interactions that promote tensile integrity after matrix depletion. For bCALF, the effects of treatments with C-ABC, C-AC and sHAase were similar to the effects of Gnd both in terms of biochemical and biomechanical parameters (Fig. 1A-F, Fig. 3). This result, along with substrate specificity of C-ABC, C-AC and sHAase, may allow the identification of ECM molecules that serve to inhibit CN interactions that form as a result of matrix depletion. The decrease in tensile integrity in Gnd-treated and PBS-rinsed bCALF tissue tested in Gnd to levels similar to those observed in tissue tested in Gnd but not exposed to PBS (Fig. 4C-E) indicates that the effects of PBS rinse on the tensile integrity were reversible, suggesting that CN interactions involved in the remodeling of the CN in response to matrix depletion are not covalent. Therefore, the enhancement of tensile integrity after matrix depletion does not involve the formation of collagen crosslinks. In addition, the lack of effects on biochemical composition and tensile integrity of incubation in Gnd+ confirms that the formation of modulated, tensile integrity-promoting CN interactions is dependent on the depletion of certain matrix components.
The use of bovine and human cartilage explants for studying maturation-dependent modulation of CN and cartilage tensile properties required consideration of a number of issues. Both bovine and human cartilage was examined to represent different states of maturity of cartilage tissue. While examining a range of maturation states from one species would be desirable it could not be implemented for practical issues. Bovine tissue older than 1−2 years old could not be examined because of difficulty finding joints that were macroscopically normal, while human tissue from donors younger than 15 years old could not be examined because of supply limitations. The tissue was harvested in layers, using the top ∼1mm of articular cartilage. Because the various zones of normal cartilage exhibit differences in biochemical composition and mechanical properties, both superficial and middle layer were analyzed. The patellofemoral groove of bovines was used as the source of tissue, similar to tissue used in previous studies [45, 54, 62]. Consequently, the biochemical and biomechanical properties of bCALF and bYOUNG tissue samples examined after incubation and testing in sham conditions were similar to those reported previously [45, 54]. The tensile specimens of human cartilage were also taken from patellofemoral groove, based on preliminary studies that showed little variation among specimens from this location for an individual joint. Biochemical and biomechanical parameters of hTEEN and hADULT tissue examined after incubation and testing in PBS were generally similar to those reported previously for human cartilage [17, 63].
Immature cartilage can be rendered stiff or soft experimentally by alterations in the tissue matrix through enzymatic or chemical treatments, suggesting that the restraining function of the CN can be regulated through interactions of components of the extracellular matrix. Studies of collagen molecules in vitro as well of tendon in vitro and in vivo indicate that additional interactions or interactions of a different type may occur among collagen fibrils after depletion of certain matrix molecules. Enzymatic treatment with trypsin, which may remove at least some of the collagen-coating small proteoglycans, appears to enhance the association of collagen fibrils from tendon in vitro [39], while treatment of tendon explants with C-ABC results in reduced propensity to swell and enhanced tensile strength [16]. In addition, during embryonic tendon growth, a dramatic decrease in fibril-associated decorin occurs contemporary with lateral collagen fibril fusion to form more continuous fibrils [37]. The reduced tensile integrity of bCALF tissue after matrix depletion with Gnd, similar to the reduction in tensile strength observed after 1M Gnd treatment in sea urchin ligament [64], indicates the importance of the non-covalent endogenous interaction among matrix components in cartilaginous tissues.
Certain components of the extracellular matrix may be responsible for controlling the stiffness and the restraining function of the collagen network through mediating the adherence and sliding between collagen fibrils. It has been proposed that the collagen fibrils, or groups of fibrils, slide past one another mediating the response of cartilaginous tissues to mechanical loading and modulating tissue length/shape. The deflection patterns of lines photobleached or fluorescently stained into the extracellular matrix suggest that collagen sliding occurs in rat tendon and ligament and bovine annulus fibrosis [32, 33, 65]. The presence of gaps within transversely sectioned fibril bundles in electron micrographs of experimentally elongated sea urchin ligament, is also indicative of fibril sliding [64]. It is possible that if molecules that mediate sliding are removed, collagen fibrils can associate linearly (end-to-end) or laterally (side-to-side) to increase the fibril diameter and consequently increase tensile stiffness and strength of the tissue overall [38, 39, 66]. Conversely, if the endogenous linkages within the CN are disrupted, sliding can be enhanced resulting in a decrease in tensile integrity.
While the enhanced tensile integrity of immature cartilage after matrix depletion suggests the involvement of different interactions between collagen fibrils within the collagen network, some of the increase in the overall tensile integrity after matrix depletion demonstrated in this study and observed by others [13, 14] can be explained by direct individual contributions of GAG and the collagen network components to the tensile properties of cartilage. In normal cartilage, proteoglycans act to inflate the collagen network and establish a level of pre-stress [67], while in matrix-depleted cartilage, in the absence of pre-stress, the collagen network would be expected to relax and straighten. Although matrix depletion did not affect thickness and had little effects on tissue wet weight, the small decrease in wet weight due to treatment may indicate a contraction of the collagen network. The absence of pre-stress in the collagen network of treated cartilage may contribute to a higher strength, while collagen network straightening can account for some of the decrease in failure strain and, consequently, an increase in the ramp stiffness/modulus. Thus, although the tensile behavior of cartilage in the high strain region is generally attributed to the tensile response of the collagen network [11, 45], these results demonstrate an indirect, but dramatic, contribution of the proteoglycan component.
The extent of modulation of tensile integrity by matrix depletion was dependent on the stage of maturity of the source tissue and may reflect the intrinsic tissue function at a certain maturation stage. In the bCALF tissue, tensile integrity could be markedly increased or decreased resulting in a fairly large range of modulated tissue state. As the tissue maturity increases, from bCALF to bYOUNG to hTEEN to hADULT, a progressively smaller range of modulated tissue states was observed, until hADULT tissue that was not affected by matrix depletion. It is possible that a relatively lax tissue state that allows modulation of the CN integrity is associated with an immature and growing tissue which needs to remodel as it rapidly increases in size during growth. As the tissue matures, the need to grow decreases and the need to bear load increases to reach a more rigid tissue state desired in adulthood. The transformation of cartilage tissue from the lax immature to a rigid mature state may involve regulation of CN integrity through alterable interactions of matrix components. For example, the increase in tensile integrity that occurs during growth of bovine cartilage in vivo from bCALF to bYOUNG (Fig. 1C-E, I-K), can be attained in bCALF tissue with matrix depletion alone. The residual variations between the modulated integrity of bCALF tissue and native integrity of bYOUNG tissue may be due to changes in the content of other ECM components, such as collagen and PYR crosslinks (Fig 1 B, H; Fig. 5).
Increasingly selective matrix depletion of bCALF cartilage with Gnd, C-ABC, C-AC, and sHAase may provide insights into the identity of molecules that may serve to inhibit the tensile integrity-promoting interactions in the immature tissue. Considering extrafibrillar matrix molecules such as aggrecan, collagen IX, decorin and fibromodulin as candidate “inhibitory” molecules, and the finding that tensile integrity-promoting interactions form when matrix molecules are removed, molecules that are unaltered by a certain treatment are excluded from the list of potential candidates. Treatment with Gnd extracts matrix molecules that are not linked to the CN covalently [48], thereby ruling out collagen IX as it is covalently attached to collagen II fibrils [18]. The involvement of fibromodulin is also excluded, as treatment with C-ABC does not affect keratan sulfate content of cartilage [50]. While aggrecan contains most of tissue's CABC-susceptible material, depletion of GAG with C-ABC may alter or displace other chondroitin sulfate- or dermatan sulfate-containing molecules such as collagen type IX and decorin. The involvement of decorin, however, is excluded as treatment with C-AC does not remove this molecule [10], but resulted in enhancement of tensile integrity similar to that produced by C-ABC treatment. Treatment with sHAase was the most selective as sHAase susceptible material includes HA [53] and, consequently, may also involve proteoglycan aggregates, suggesting that these molecules are the primary candidates as the “inhibitory” components in the immature tissue. While aggrecan is the primary candidate to serve as such “inhibitory” component, other matrix molecules that are not considered here may also be involved. To further confirm aggrecan as the inhibitory molecule, it can be tested as a monomer or with other molecules, such as HA, through depletion/repletion studies.
While the exact nature of the interactions of matrix components within cartilage tissue remains to be established, these findings may have a practical utility for tissue engineering of cartilage tissue grafts for the purposes of repairing articular cartilage defects. Since the tensile integrity-promoting interactions in immature tissue are non-covalent, it may be possible to deconstruct and reconstruct the constituents to modulate CN function. Identification of methods to reconstruct constituents, may allow the assembly of desired components that would enhance functional properties of cartilage tissue, thus producing a mature tissue construct in a short time period.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute, Musculoskeletal Transplant Foundation, National Institute of Health, and National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage. Part I: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg Am. 1997;79-A:600–611. [PubMed] [Google Scholar]
- 2.Maroudas A. Physico-chemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Pitman Medical, Tunbridge Wells; England: 1979. pp. 215–290. [Google Scholar]
- 3.Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Raven Press; New York: 1997. pp. 113–178. [Google Scholar]
- 4.Grodzinsky AJ. Electromechanical and physicochemical properties of connective tissue. CRC Crit Rev Bioeng. 1983;9:133–199. [PubMed] [Google Scholar]
- 5.Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 6.Basser PJ, Schneiderman R, Bank RA, Wachtel E, Maroudas A. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch Biochem Biophys. 1998;351:207–219. doi: 10.1006/abbi.1997.0507. [DOI] [PubMed] [Google Scholar]
- 7.Woo SL-Y, Akeson WH, Jemmott GF. Measurements of nonhomogeneous directional mechanical properties of articular cartilage in tension. J Biomech. 1976;9:785–791. doi: 10.1016/0021-9290(76)90186-x. [DOI] [PubMed] [Google Scholar]
- 8.Venn MF, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempson GE. Mechanical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Pitman Medical, Tunbridge Wells; England: 1979. pp. 333–414. [Google Scholar]
- 10.Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968;243:1523–1535. [PubMed] [Google Scholar]
- 11.Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta. 1973;297:456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 12.Kempson GE, Tuke MA, Dingle JT, Barrett AJ, Horsfield PH. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976;428:741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- 13.Li JT, Mow VC, Koob TJ, Eyre DR. Effect of chondroitinase ABC treatment on the tensile behavior of bovine articular cartilage. Trans Orthop Res Soc. 1984;9 [Google Scholar]
- 14.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 15.Asanbaeva A, Masuda K, Thonar EJ-MA, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2006 doi: 10.1002/art.22298. In Press. [DOI] [PubMed] [Google Scholar]
- 16.Screen HR, Chhaya VH, Greenwald SE, Bader DL, Lee DA, Shelton JC. The influence of swelling and matrix degradation on the microstructural integrity of tendon. Acta Biomater. 2006;2:505–513. doi: 10.1016/j.actbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4:379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 18.Eyre DR, Dickson IR, van Ness K. The collagens of articular cartilage. Sem Arthritis Rheum. 1991;21(S2):2–11. doi: 10.1016/0049-0172(91)90035-x. [DOI] [PubMed] [Google Scholar]
- 19.Eyre DR. Collagens and cartilage matrix homeostasis. Clin Orthop Rel Res Relat Res. 2004:S118–122. doi: 10.1097/01.blo.0000144855.48640.b9. [DOI] [PubMed] [Google Scholar]
- 20.Eyre DR, Pietka T, Weis MA, Wu JJ. Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J Biol Chem. 2004;279:2568–2574. doi: 10.1074/jbc.M311653200. [DOI] [PubMed] [Google Scholar]
- 21.Pihlajamaa T, Lankinen H, Ylostalo J, Valmu L, Jaalinoja J, Zaucke F, Spitznagel L, Gosling S, Puustinen A, Morgelin M, Peranen J, Maurer P, Ala-Kokko L, Kilpelainen I. Characterization of recombinant amino-terminal NC4 domain of human collagen IX: interaction with glycosaminoglycans and cartilage oligomeric matrix protein. J Biol Chem. 2004;279:24265–24273. doi: 10.1074/jbc.M402865200. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan L, Mendler M, Huber S, Bruckner P, Winterhalter KH, Irwin MH, Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988;106:991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedbom E, Heinegard D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- 24.Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons--a computational study from molecular to microstructural level. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 25.Svensson L, Oldberg A, Heinegard D. Collagen binding proteins. Osteoarthritis Cartilage. 2001;9(Suppl A):S23–28. doi: 10.1053/joca.2001.0440. [DOI] [PubMed] [Google Scholar]
- 26.Roughley PJ, Rodriguez E, Lee ER. The interactions of 'non-aggregating' proteoglycans. Osteoarthritis Cartilage. 1995;3:239–248. doi: 10.1016/s1063-4584(05)80015-9. [DOI] [PubMed] [Google Scholar]
- 27.Hedlund H, Hedbom E, Heinegard D, Mengarelli-Widholm S, Reinholt FP, Svensson O. Association of the aggrecan keratan sulfate-rich region with collagen in bovine articular cartilage. J Biol Chem. 1999;274:5777–5781. doi: 10.1074/jbc.274.9.5777. [DOI] [PubMed] [Google Scholar]
- 28.Junqueira LC, Montes GS. Biology of collagen-proteoglycan interaction. Arch Histol Jpn. 1983;46:589–629. doi: 10.1679/aohc.46.589. [DOI] [PubMed] [Google Scholar]
- 29.Oegema TR, Laidlaw J, Hascall VC, Dziewiatkowski DD. The effect of proteoglycans on the formation of fibrils from collagen solutions. Arch Biochem Biophys. 1975;170:698–709. doi: 10.1016/0003-9861(75)90167-8. [DOI] [PubMed] [Google Scholar]
- 30.Poole AR, Pidoux I, Reiner A, Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982;93:921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott JE. Elasticity in extracellular matrix 'shape modules' of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol. 2003;553:335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruehlmann SB, Matyas JR, Duncan NA. ISSLS prize winner: Collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine. 2004;29:2612–2620. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- 33.Wood ML, Lester GE, Dahners LE. Collagen fiber sliding during ligament growth and contracture. J Orthop Res. 1998;16:438–440. doi: 10.1002/jor.1100160407. [DOI] [PubMed] [Google Scholar]
- 34.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel KG, Trotter JA. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Collagen Rel Res. 1987;7:105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 36.Hedbom E, Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- 37.Birk DE, Linsenmayer TF. Collagen fibril assembly, deposition, and organization into tissue-specific matrices. In: Yurchenco PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. Academic Press; San Diego: 1994. pp. 91–128. [Google Scholar]
- 38.Parry DA. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem. 1988;29:195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- 39.Graham HK, Holmes DF, Watson RB, Kadler KE. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J Mol Biol. 2000;295:891–902. doi: 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki Y, Goldberg VM, Caplan AI. Enzymatical digestion for the repair of superficial articular cartilage lesions. Trans Orthop Res Soc. 1993;18:728. [Google Scholar]
- 41.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto R, Kihara I, Otani H. Perinatal development of the rat hip joint with restrained fetal movement. Congenit Anom (Kyoto) 2002;42:135–142. doi: 10.1111/j.1741-4520.2002.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 44.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 45.Williamson AK, Chen AC, Masuda K, Thonar EJ-MA, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 46.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–513. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 47.Heinegard D, Sommarin Y. Isolation and characterization of proteoglycans. Methods Enzymol. 1987;144:305–319. doi: 10.1016/0076-6879(87)44186-4. [DOI] [PubMed] [Google Scholar]
- 48.Sajdera SW, Hascall VC. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969;244:77–87. [PubMed] [Google Scholar]
- 49.Calabro A, Midura R, Wang A, West L, Plaas A, Hascall VC. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthritis Cartilage. 2001;9:S16–22. doi: 10.1053/joca.2001.0439. [DOI] [PubMed] [Google Scholar]
- 50.Chun LE, Koob TJ, Eyre DR. Sequential enzymic dissection of the proteoglycan complex from articular cartilage. Trans Orthop Res Soc. 1986;11:96. [Google Scholar]
- 51.Lyyra T, Arokoski JP, Oksala N, Vihko A, Hyttinen M, Jurvelin JS, Kiviranta I. Experimental validation of arthroscopic cartilage stiffness measurement using enzymatically degraded cartilage samples. Phys Med Biol. 1999;44:525–535. doi: 10.1088/0031-9155/44/2/017. [DOI] [PubMed] [Google Scholar]
- 52.Nahir AM, Shomrat D, Awad M. Chondroitinase ABC affects the activity of intracellular enzymes in rabbit articular cartilage chondrocytes. J Rheumatol. 1995;22:702–707. [PubMed] [Google Scholar]
- 53.Ohya T, Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970;198:607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- 54.Williamson AK, Masuda K, Thonar EJ-MA, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 55.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 56.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 57.Uebelhart D, Thonar EJ-MA, Pietryla DW, Williams JW. Elevation in urinary levels of pyridinium cross-links of collagen following chymopapin-induced degradation of articular cartilage in the rabbit knee provides evidence of metabolic changes in bone. Osteoarthritis Cartilage. 1993;1:185–192. doi: 10.1016/s1063-4584(05)80090-1. [DOI] [PubMed] [Google Scholar]
- 58.Pal S, Tang L-H, Choi H, Habermann E, Rosenberg L, Roughley P, Poole AR. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151–176. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- 59.Herbage D, Bouillet J, Bernengo J-C. Biochemical and physicochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161:303–312. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson DS, Cleary EG. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- 61.Kempson GE. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann Rheum Dis. 1982;41:508–511. doi: 10.1136/ard.41.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 63.Temple MM. Bioengineering, 2005 University of California. La Jolla; San Diego: [Google Scholar]
- 64.Hidaka M, Takahashi K. Fine Structure and Mechanical Properties of the Catch Apparatus of the Sea-Urchin Spine, a Collagenous Connective Tissue with Muscle-Like Holding Capacity. J Exp Biol. 1983;103:1–14. [Google Scholar]
- 65.Wood ML, Luthin WN, Lester GE, Dahners LE. Tendon creep is potentiated by NKISK and relaxin which produce collagen fiber sliding. Iowa Orthop J. 2003;23:75–79. [PMC free article] [PubMed] [Google Scholar]
- 66.Doillon CJ, Dunn MG, Bender E, Silver FH. Collagen fiber formation in repair tissue: development of strength and toughness. Coll Relat Res. 1985;5:481–492. doi: 10.1016/s0174-173x(85)80002-9. [DOI] [PubMed] [Google Scholar]
- 67.Maroudas A. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]