Abstract
Throughout most of their lives, women are at greater risk for depression than men. Hormones and neurotransmitters share common pathways and receptor sites in areas of the brain linked to mood, particularly through the hypothalamic-pituitary-gonadal axis. It has been hypothesized that women presenting with episodes of depression associated with reproductive events (i.e., premenstrual, postpartum, menopausal transition) may be particularly prone to experiencing depression, in part because of a heightened sensitivity to intense hormonal fluctuations. The menopausal transition, for example, appears to represent a window during which some women might be more vulnerable to the development of first onset or recurrent depressive symptoms and major depressive episodes. In this review, we examine the association between hormone changes and increased risk of developing depression. Some of the underlying mechanisms that may contribute to such an increased risk are discussed critically, with a special emphasis on the events occurring during the menopausal transition. Last, we explore some of the clinical and therapeutic implications of hormone-modulated depression in women.
Medical subject headings: depression, estrogens, hormone replacement therapy, menopause, premenstrual syndrome, pregnancy.
Abstract
Durant la majeure partie de leur vie, les femmes sont plus vulnérables à la dépression que les hommes. Les hormones et les neurotransmetteurs ont des voies et des récepteurs communs dans des régions du cerveau reliées à l'humeur, en particulier l'axe hypothalamus-hypophyse-gonades. On a posé en hypothèse que les femmes qui vivent des épisodes de dépression associés à des événements génésiques (c.-à-d. prémenstruels, postnataux, transition vers la ménopause) peuvent être particulièrement vulnérables à la dépression, en partie parce qu'elles sont plus sensibles aux fluctuations hormonales intenses. La transition vers la ménopause, p. ex., semble représenter une période pendant laquelle des femmes peuvent être plus vulnérables à l'apparition de symptômes dépressifs nouveaux ou répétitifs et à des épisodes dépressifs majeurs. Dans cette synthèse, nous analysons le lien entre les changements hormonaux et le risque accru d'apparition de la dépression. Nous analysons d'un œil critique certains des mécanismes sous-jacents qui peuvent contribuer à l'augmentation de ce risque et nous accordons une attention spéciale aux événements qui se produisent pendant la transition vers la ménopause. Enfin, nous explorons certaines des répercussions cliniques et thérapeutiques de la dépression d'origine hormonale chez les femmes.
Introduction
Epidemiologic studies suggest that an increased risk for major depressive disorder (MDD) exists in women, compared with men1,2; such increased risk appears to become more evident after puberty and continues throughout the reproductive life cycle. The National Comorbidity Survey (NCS) found that, between the ages of 15 and 54 years, the lifetime prevalence of MDD is 12.7% for men and 21.3% for women.3 More recent NCS data corroborated these earlier findings, showing a nearly 2-fold greater lifetime risk of developing MDD in women (odds ratio [OR] 1.7, 95% confidence interval [CI] 1.5–2.0).1 It has been postulated that this increased prevalence is somewhat associated with female-specific reproductive events such as perimenstrual changes, pregnancy, the postpartum period and menopause.4–6
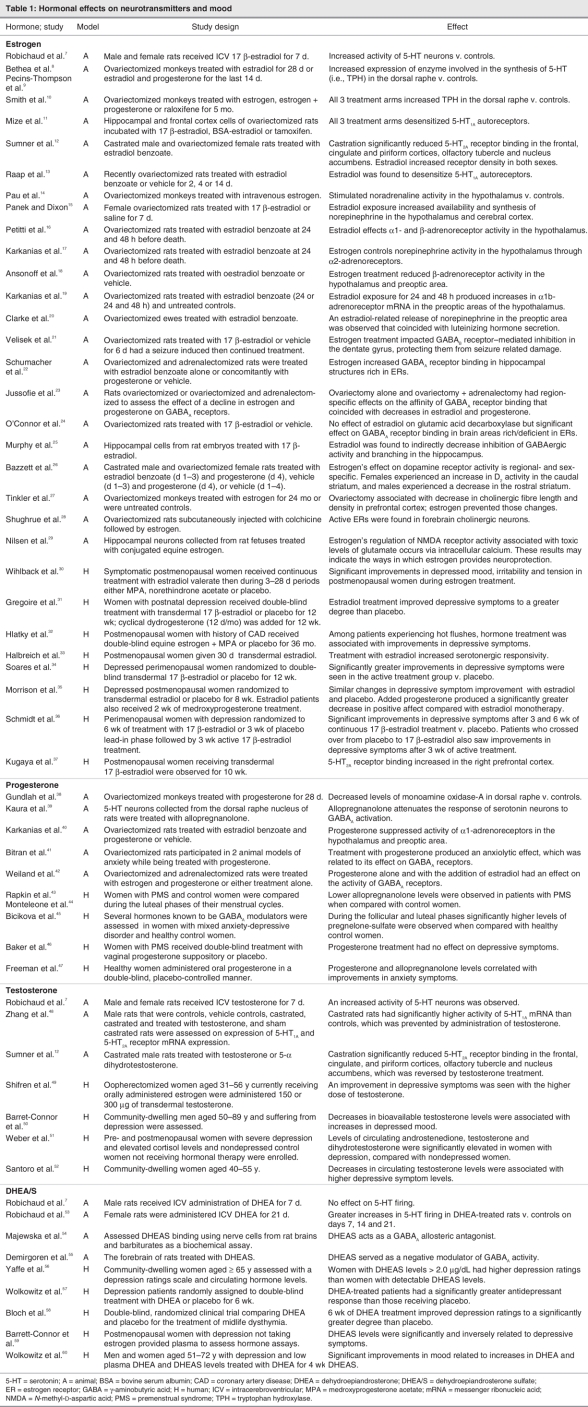
The hypothesis that fluctuations in sex hormones marking female reproductive events could influence neurochemical pathways linked to depression is extensively supported by animal and human studies and existing clinical data (Table 1).61,62 The gonadal steroids estrogen and progesterone have been shown to affect brain regions known to be involved in the modulation of mood and behaviour. Receptor sites for these hormones have been identified in the prefrontal cortex, hippocampus, thalamus and brain stem.63,64
Table 1
Other data support the association between changes in the hormone milieu and the occurrence of depressive symptoms, suggesting a potential window of vulnerability during times of hormone instability. In a community-based study, rapidly increasing levels of follicle-stimulating hormone (FSH) were associated with an increased risk of experiencing depressive symptoms among menopausal women.65 The effects of hormonal interventions, however, indicate a potential window of opportunity to modulate the risk for mood symptoms by stabilizing the hormone environment. In a clinical trial of midlife (45–65 y) men and women with minor and major depression, significant improvements (p < 0.01 v. placebo) on the Hamilton Depression Rating Scale and Center for Epidemiologic Studies Depression Scale (CES-D) were observed in patients who received 6 weeks of dehydroepiandrosterone (DHEA) treatment (weeks 1–3, 90 mg/d; weeks 3–6, 450 mg/d).66 The antidepressant effect associated with DHEA treatment was not the result of ameliorating a deficiency of DHEA because no correlation between DHEA levels and therapeutic response was observed.66
Hormonal changes may also play a role in the development of somatic symptoms and may contribute to the exacerbation of medical disorders. For example, the occurrence of vasomotor symptoms (e.g., hot flashes and night sweats) during the menopausal transition and early postmenopausal years is thought to be directly associated with the hormonal fluctuations that occur during this period. These hormonal changes not only adversely affect the hypothalamic thermoregulatory centre67,68 but also contribute to dysregulation of the serotonergic and noradrenergic systems involved in mood and behaviour.5,69 Studies of premenstrual asthma70 and perimenstrual migraines71 also suggest that progesterone-and estrogen-driven modulation, respectively, play a role in the occurrence of other clinical conditions.
The hypothesis that mood changes are related to a heightened vulnerability to hormonal fluctuations experienced during reproductive life events does not, however, preclude the role of psychosocial stressors. In fact, several psychosocial factors have been associated with mood changes during puberty (e.g., sexual maturity, increased social pressures and obligations),6 premenstrual dysphoria (e.g., past trauma, lower education)72,73 and childbirth (e.g., lower income, stressful life events, medical complications).74,75 Similarly, the menopausal transition and postmenopausal years may be accompanied by health problems, inadequate social support, marital/sexual issues and other stressful events (e.g., divorce, death of a spouse, children leaving home) that could increase the risk for depression.76
In this review, we mainly focus on the available evidence linking female reproductive life events and the occurrence of depressive symptoms and disorders. A special emphasis is given to the events occurring during the menopausal transition. Last, we review some common clinical scenarios that could eventually help physicians to improve their ability both to identify patients at risk for developing mood disturbances during reproductive events and to tailor their treatment strategies for these conditions.
Depression across the reproductive life cycle
Premenstrual period
Different hypotheses involving the ovarian steroid hormones have been postulated to explain the occurrence of severe premenstrual symptoms. Hormone fluctuations are particularly intense in the late luteal phase of the menstrual cycle and early days of menses.5,77 Although differences in the concentrations of the ovarian steroids may underlie the occurrence of mood symptoms in this disorder, the evidence that support this hypothesis remains limited.
Within the patient population that frequently reports physical and psychological complaints during the premenstrual period, a subgroup appears to be particularly affected by dysphoric symptoms of mood lability, depressed mood, increased irritability and tension. These symptoms characterize the core of premenstrual dysphoric disorder (PMDD).78 These symptoms generally begin to occur during the late luteal phase of the menstrual cycle (i.e., the last 7–10 d) and continue until the first few days of the subsequent follicular phase. Although restricted to a short period of time, symptoms of PMDD adversely affect psychosocial functioning and quality of life.78
PMDD occurs during periods of progesterone and estrogen instability.43 More recently, some investigators examined the concentration of ovarian steroid hormones across the menstrual cycle in women with and without PMDD, using a cross-sectional and prospective experimental design.
Across the menstrual cycle, the overall percentage of free estradiol was significantly lower, while the percentage of sex hormone binding globulin (SHBG) was significantly greater in the PMDD group when compared with control subjects. Moreover, during the luteal phase, free estradiol was significantly lower in the PMDD group when compared with control subjects. The authors speculate that high concentrations of SHBG in subjects with PMDD could limit the bioavailability of estradiol and ultimately affect mood in these individuals.79
The use of hormonal interventions for the treatment of PMDD seems intuitive; however, data supporting the efficacy of hormone-based treatments have been inconsistent,80–82 and only recently have a few controlled clinical trials demonstrated that improvements in hormonal stability (e.g., with the use of continuous oral contraceptives) could prevent or alleviate the occurrence and severity of premenstrual symptoms.83–85 Contrary to the scarce data on hormone-based interventions for PMDD, abundant evidence does support the role of changes in serotonin-mediated systems in the occurrence of premenstrual syndrome (PMS)86,87 and PMDD.88 Moreover, serotonergic antidepressants have been shown to effectively treat PMDD.89–91
Prevalence of perimenstrual-related depressive symptoms: PMDD and premenstrual exacerbation
Community-based studies indicate a 3%–9% prevalence of PMDD in the general population.92,93 A study of 1488 women who were prospectively followed for 48 months found a 12-month prevalence of 5.8% and a cumulative lifetime incidence of 7.4%. This same study found that women with PMDD were likely to develop psychiatric comorbidities. Rates of comorbid anxiety, affective and somatoform disorders were 47%, 30% and 28%, respectively. Only 27% of participants with PMDD had no other psychiatric diagnosis.94 In a survey of 206 women seeking treatment for PMS, 39% screened positively for a psychiatric disorder. Dysthymia and depression were most commonly comorbid with PMDD.95 Thus a most probable clinical scenario is the occurrence of premenstrual exacerbation of an underlying (sometimes undiagnosed) mood or anxiety disorder. Its recognition is a fundamental step toward the formulation of a more accurate diagnosis and effective treatment strategies.96
Risk factors
Prior psychiatric disorders, especially anxiety disorders, have been found to be significant risk factors for PMDD.73 In addition, a history of depression72 and posttraumatic stress disorder,94 smoking72 and nicotine dependence94 and lower educational status were also more common in patients with PMDD.72
Pregnancy
Estrogen and progesterone levels steadily increase throughout pregnancy until just before birth, and serotonergic activity follows a corresponding pattern. Results from animal models suggest that a lower baseline level of serotonergic activity may lead to a greater risk for developing depressive symptoms during times when these neuroendocrine networks are challenged.97
It was once believed that for most women pregnancy could provide protection against psychiatric disorders. Unfortunately, accumulating evidence has demonstrated the contrary. In fact, for some women, pregnancy can be a time of elevated risk for psychiatric morbidity, particularly depressive and anxiety disorders.98,99
Prevalence of depression
In a wide-ranging review of the published literature, rates of depression during the first, second and third trimesters were found to be 7.4%, 12.8% and 12.0%, respectively, suggesting a substantial risk for pregnant women, especially in the second and third trimesters.98 Notably, a recent naturalistic study conducted in patients with a history of recurrent depression who were followed throughout pregnancy found that 51% of the women who experienced a return of their MDD did so during the first trimester. The overall rate of relapse for this population was 43%.99
Risk factors
Women who experience a major depressive episode during pregnancy are more likely to be younger, in a lower socioeconomic stratum,74,98 not married74 and to have less education than women who do not experience such an episode.74 Cohen and colleagues99 conducted a naturalistic study in pregnant women who had been euthymic since at least 3 months before their last menstrual period but who had a history of MDD and were currently receiving or had recently received antidepressant treatment. When followed throughout pregnancy, women who discontinued their antidepressant treatment were 5 times more likely to experience a depressive relapse than those who maintained treatment (hazard ratio 5.0, 95% CI 2.8–9.1).
Clinicians and patients frequently raise concerns regarding the putative teratogenic risks of antidepressant use during pregnancy. Other concerns include the risk of the newborn developing withdrawal symptoms attributed to prenatal exposure to antidepressants and the relative increased risk for persistent pulmonary hypertension in the neonate.100,101 Conversely, evidence suggests that untreated depression has negative consequences for both mother and child. Depression in late pregnancy has been associated with poorer obstetric and neonatal outcomes, including increased risk for cesarean sections and instrumental vaginal deliveries, growth retardation and premature delivery.102,103 The impact of untreated depression during pregnancy is not limited to immediate adverse outcomes. A prospective study of depressed and nondepressed mother–child pairs found that a mother's depression during pregnancy can adversely affect a child's development of language skills and overall cognitive development. This negative impact was not associated with antidepressant use.104
Overall, most physicians and patients remain extremely reluctant to use antidepressants during pregnancy. Such reluctance could be explained, at least in part, by the existing confusion and complexity of evaluating absolute and relative risks and by the difficulty in balancing these risks for both the infant and the mother.105 As a general rule, a risk–benefit consideration must be made concerning pharmacologic treatments during pregnancy (for more details, see106–108). The severity of maternal depressive symptoms is a key factor in the decision-making process.
Postpartum
The postpartum period is a time of abrupt decreases in the amounts of circulating estrogen and progesterone. The relative contribution of these hormonal changes to the occurrence of depressive episodes during the postpartum period is a subject of controversy. For example, there have been some attempts to link the vulnerability of specific subgroups (e.g., patients with a diagnosis of bipolar disorder) to susceptibility genes that could predispose women to puerperal psychosis.109 Nonetheless, clinical experiments support the hypothesis that the abrupt changes in the hormonal milieu associated with the immediate postpartum period may exert a key role in the heightened risk for depression during this period.62 Bloch and colleagues62 induced a hypogonadal state in women with and without a history of postpartum depression. First, they used the gonadotropin-releasing hormone agonist leuprolide acetate followed by 8 weeks of estrogen and progesterone treatment. At the end of this 8-week add-back period, both hormones were withdrawn in a double-blind manner. Sixty-three percent of the women with a history of postpartum depression and none from the comparison group developed significant depressive symptoms, suggesting that hormonal changes associated with the postpartum period may trigger a depressive episode in at-risk women. A potential window of opportunity for hormonal interventions in postpartum depression was further explored in studies demonstrating the efficacy of estrogen-based treatments for severe postpartum depression.31,110
Prevalence and risk factors
A longitudinal study of 1558 pregnant women conducted in Sweden found a prevalence of 13% to 18% for postpartum depressive symptoms during the 6 months after delivery.111 Worldwide rates of postpartum major depression have been found to range from 4.4% to 9%.112–115 It appears that depressive symptoms before and concurrent with pregnancy are reliable predictors of postpartum mood disturbances. In a large study (n = 1622) of midpregnancy and 6-month postpartum women, the postpartum women who experienced depressive symptoms before becoming pregnant (OR 3.82, 95% CI 2.31–6.31) and during pregnancy (OR 6.78, 95% CI 4.07–11.31) had an increased risk for postpartum depressive symptoms.114 These results are supported by a longitudinal study that followed pregnant women until they were 8 months postpartum. The results showed a significant association between antenatal anxiety symptoms and postpartum depressive episodes, even after controlling for prior depressive episodes (OR 3.22, 95% CI 2.28–4.55; p < 0.001).116 Additionally, early postpartum depressive symptoms, a history of PMDD and depression related to the use of oral contraceptives have been identified as risk factors for postpartum depressive symptoms and episodes.115,117
The menopausal transition
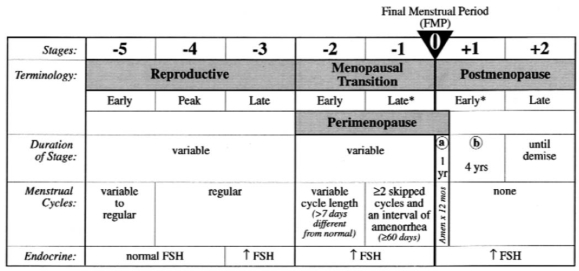
The onset of the perimenopause or the menopausal transition is marked by the end of menstrual cycle regularity and is associated with decreases in the production of ovarian inhibiting hormones, which leads to increases in FSH and estrogen and increased activity of the hypothalamus and pituitary.63,118,119 Initially, menstrual cycles become slightly irregular in their length and/or flow. As women continue to advance toward the late perimenopausal period, menstrual cycles become increasingly irregular (i.e., occurrence of 2 or more skipped menstrual periods with gaps of more than 60 days between menstrual periods).
From a hormonal perspective, in the perimenopausal years slightly elevated but highly fluctuating FSH levels may be observed during the early follicular phase of the menstrual cycle. These levels gradually become consistently elevated into the late perimenopause and postmenopausal years, while estrogen and progesterone levels decrease and luteinizing hormone levels increase as the woman approaches menopause.119
Recent studies have examined the patterns of change in FSH and estradiol levels in different subpopulations across the menopausal transition and early postmenopausal years. Data from the Study of Women's Health Across the Nation revealed that serum concentrations of estradiol and FSH may vary by race or ethnicity. For example, Chinese and Japanese women had consistently lower (20%) estradiol concentrations when compared with women of European ethnicity, with no comparable differences seen in serum FSH concentrations. This finding suggests either a difference in the feedback regulation of the pituitary or an ethnospecific difference in pituitary sensitivity to gonadal-negative feedback.120 The same investigators revealed that FSH measures may be a better independent predictor of vasomotor symptom prevalence than serum estradiol levels: higher FSH concentrations were associated with a greater risk of reporting more frequent symptoms even among regularly cycling women, whereas estradiol concentrations did not show an independent effect. They therefore proposed that an annual serum FSH concentration of 40 IU/L could be incorporated, in conjunction with bleeding markers, into the Stages of Reproductive Aging Workshop (STRAW) paradigm for markers of the late menopausal transition.121
Figure 1 illustrates the stages of menopause based on a consensus statement developed by 27 invited professionals to the STRAW. The final menstrual period (FMP) serves as the anchor point of this staging system, which describes the components of each menopausal phase (i.e., menstrual cycle regularity, endocrine and biochemical changes, fertility, other organ system changes and uterine/ovarian anatomy).119
Fig. 1: Stages and nomenclature of normal reproductive aging in women. *Stages most likely to be characterized by vasomotor symptoms. FSH = follicle-stimulating hormone. Reproduced with permission from Soules et al.119
Clinically, symptoms such as breast tenderness, insomnia, migraines, vasomotor symptoms and dysphoria may be reported with the onset of the perimenopause. In the late perimenopausal years, women also experience significant increases in the intensity of vasomotor symptoms as estrogen levels fluctuate widely and unpredictably and menstrual cycles become increasingly irregular.119,122 Recent evidence also suggests an increased risk for developing depressive symptoms during this period even in the absence of a history of depression.76,99,123 Thus, for some women, the perimenopause and early postmenopausal years may constitute a window of vulnerability wherein the occurrence of challenging physical and emotional discomforts could result in significantly impaired functioning and poorer quality of life.124,125
Postmenopause
Menopause begins following the FMP and is confirmed only after 12 months of amenorrhea. The postmenopausal period is divided into an early (i.e., 5 years since the FMP) and late phase and is marked by significant decreases in estrogen production, an overall state of hypogonadism, stability in the hypothalamic-pituitary-gonadal axis and elevated FSH levels.119 The decreased circulating androgen levels associated with menopause have been linked to loss of libido, fatigue and an increase in depressive symptoms.126,127 Some data suggest that the significant hormone changes and the related physical and emotional symptoms associated with menopause may differ depending on whether a woman experiences menopause naturally or through surgical means (i.e., resulting from bilateral oophorectomy).126,128 In a cross-sectional study of postmenopausal women, those experiencing menopause naturally were found to have fewer hot flashes than those with surgically induced menopausal status.129
Prevalence of depression
Notably, most women have a positive attitude toward approaching menopause and proceed through the menopausal transition without experiencing significant depressive symptoms or a major depressive episode.130 However, recent epidemiologic studies suggest an increased risk for the development of psychiatric morbidity even among those who never experienced depression during the premenopausal years. In an 8-year longitudinal study of women with no history of depression (n = 224), Freeman and colleagues123 found that 50% experienced an increase in depressive symptoms (CES-D ≥ 16) during the perimenopausal transition and 26% met formal MDD diagnostic criteria as assessed with the Primary Care Evaluation of Mental Disorders measure. Perimenopausal women were more than 4 times more likely (OR 4.29, 95% CI 2.39–7.72) to experience clinically significant depressive symptoms and 2.5 times more likely (OR 2.50, 95% CI 1.25–5.02) to be diagnosed with MDD than when they were premenopausal.123 Another community-based, prospective study, the Harvard Study of Moods and Cycles, also identified a 2-fold greater risk of developing significant depressive symptoms during the perimenopause in women who had never reported prior depression. Interestingly, this increased risk remained significant after adjusting for common confounding factors (e.g., cigarette smoking, socioeconomic status).72 Moreover, the risk for a new onset of depression was accentuated by the presence of significant vasomotor symptoms.99 In subjects with a lifetime history of depression, the risk of developing a depressive episode during the late perimenopause is 14 times greater than during the 31-year period preceding perimenopause, compared with a 3-fold increase in risk associated with the early perimenopause.131
Risk factors
As in the other reproductive life events discussed, a history of previous depressive symptoms or episodes is a significant risk factor for developing menopausal depression.65,132 Freeman and colleagues65 reported a nearly 5-fold increase in the risk of experiencing a major depressive episode during the menopausal transition in women with a history of depression. A similar increased risk was observed in data derived from the Moods and Cycles Study (Dr. Bernard Harlow, University of Minnesota, Division of Epidemiology, personal communication, December 2006). Additional risk factors included a history of severe PMS or PMDD,133 poor sleep patterns65 and hot flashes.134
Accumulating evidence suggests a link between the presence of vasomotor symptoms and the occurrence of menopause-related depression. For example, in a study of 476 women aged 40 to 60 seeking primary care treatment, perimenopausal women with depression were significantly more likely to report experiencing vasomotor symptoms than those who were perimenopausal and not suffering from depression. Perimenopausal women with vasomotor symptoms were more than 4 times more likely to meet criteria for depression (CES-D ≥ 25) than those who did not have vasomotor symptoms (4.39 95% CI 1.40–13.83).135 No significant increase in the risk for depression was noted for older premenopausal and postmenopausal women who were also experiencing hot flashes, reinforcing the concept that the perimenopausal period may represent a time of unique vulnerability.135
Androgen levels may also play some role in menopause-related depression. Barret-Connor and colleagues59 found a significant relation between decreased dehydroepiandrosterone sulfate (DHEAS) levels and depressed mood assessed with the Beck Depression Inventory. Another group of investigators found a weak inverse relation between depressive symptoms (according to CES-D scores) and DHEAS levels in women aged 42–52 years. Even after controlling for factors such as waist circumference, ethnicity and age, levels of testosterone and free androgen index levels remained inversely associated with depressed mood.52
Reproductive-related depressive disorders: diagnosis and clinical considerations
The occurrence of a similar symptom profile (e.g., increased irritability, disturbed sleep, depressed mood, cognitive dysfunction) associated with the various reproductive life events already discussed has led some to speculate that a continuum of reproductive depressive disorders exists. It has been postulated, for instance, that the hypoestrogenism associated with each of these phases in a woman's life could lead to an increased risk for depression.4 In addition, some suggest the hypothesis of a kindling effect; women who experience a depressive episode during one reproductive phase (e.g., during the premenstrual period) could be at a greater risk for developing future depressive episodes when facing hormonally challenging situations (e.g., during the postpartum period).117,136 A preliminary study of 72 women with depression found significant correlations between premenstrual (r = 0.41; p = 0.04) and postpartum (r = 0.64; p = 0.001) depression ratings and the presence of perimenopausal depression.137 Other studies also point to the higher occurrence of lifetime depressive events related to the reproductive cycle.138 Nonetheless, larger prospective studies confirming these associations are lacking.
The major caveat of the hypoestrogenic state theory is that the hormonal milieu differs with each reproductive event, so the relation between depressed mood and reproductive hormones may not be direct. The occurrence of mood disturbances during separate reproductive events and the wide variations in hormone levels observed during such events suggest that absolute hormone levels do not cause reproductive-related mood disorders. Rather, it is the dramatic — sometimes chaotic — hormone fluctuations that contribute to the increased risk for depression. As discussed above, premenstrual depressive symptoms occur during the luteal phase and continue through the first few days of the follicular phase, when dramatic fluctuations of progesterone and estrogen levels are most prevalent.5,77 Similarly, estrogen levels in perimenopausal women fluctuate widely as menstrual cycles become increasingly erratic.119 Conversely, the late postmenopausal years, clearly marked by a constant hypoestrogenic state, are not generally characterized by an increase in depressive episodes (either first onset or reemergent).
The complexity of the interactions between hormone changes and mood is corroborated by the different responses to hormonal fluctuations observed in women going through reproductive events and by different therapeutic responses obtained after the administration of exogenous hormones. For example, the antidepressant properties of estrogen therapy appear to vary according to the population treated. Most clinical trials have shown the positive effects of estrogen treatment for depression in perimenopausal but not postmenopausal women.34,35,139 Moreover, the formulation and pathways used (e.g., transdermal estradiol as opposed to oral conjugated estrogen) may also affect estrogen's antidepressant effect, with more robust response observed with the use of transdermal estradiol34,36 and limited antidepressant response with oral preparations.140
The response to antidepressants for the management of reproductive-related mood symptoms has also been a point of some controversy. Serotonergic antidepressants are quite efficacious for the treatment of PMDD.141–143 However, the consistent efficacy of these agents in treating depression in women has been questioned, particularly with regard to women who have entered the postmenopausal years.144–146
From the therapeutic point of view, it is plausible that the use of hormonal interventions could have an impact on the presence and severity of mood symptoms when used either alone or in combination with antidepressants. Bloch and colleagues117 found a significant association between subjects' mood symptoms while taking oral contraceptives and the experiencing of postpartum depressive symptoms and episodes (p = 0.007). In the Harvard Study of Moods and Cycles, women receiving hormone therapy to ease the symptoms associated with the perimenopause were less likely to experience a severe depressive episode, compared with those who entered the perimenopause without any hormonal intervention.134 Last, some but not all depression studies suggest that estrogen-based therapies may constitute a valid treatment strategy (either as monotherapy or an augmenting tool) for the improvement of depression during menopause or for premenstrual exacerbation of depression.34–36,140,147
When the treatment of mood disorders in women is being considered, assessment of reproductive history and inquiry about menstrual history or somatic symptoms are important to the decision-making process. Incorporating appropriate questions into psychiatric assessments may improve the ability to identify women vulnerable to experiencing depressive episodes during reproductive events and to effectively manage depression in these women. For example, the decision to use long-term maintenance treatment could be affected by the recognition that a woman at midlife with a history of MDD who is currently in remission could be at an increased risk for recurrence when approaching menopause.
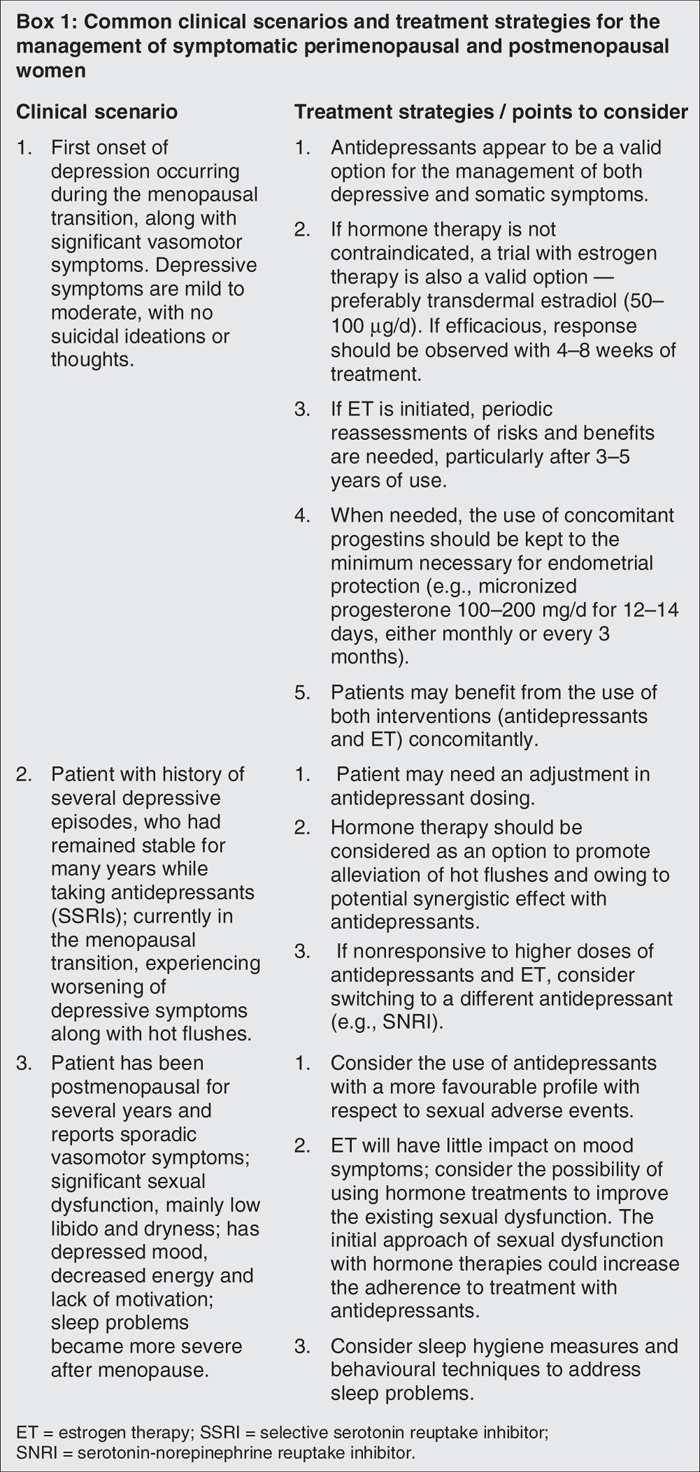
Given the complex overlap between menopause-related symptoms and some of the somatic and psychological complaints commonly seen in depression, the diagnostic process becomes more difficult and requires different strategies. The domino theory suggests that the sheer increase in the number of symptoms is responsible for more menopausal women meeting the criteria for MDD.148 However, depression may occur even in the absence of one or more of these symptoms. Use of more specific instruments or scales may help physicians make this delineation. For example, the Edinburgh Postnatal Depression Scale149 has been successfully used to screen for and monitor the occurrence and severity of MDD during pregnancy and the postpartum period. Similarly, instruments such as the Greene Climacteric Scale,150 the Menopause-Specific Quality of Life Questionnaire151 and the Utian Menopause Quality of Life Score152 could be used to assess and monitor menopause-related physical and psychological symptoms, as well as to assess the impact of these symptoms on functioning and overall well-being. Clinical assessments of menopausal women presenting with symptoms of depression should determine the presence of comorbidities, explore the course of illness (i.e., chronic or recurrent) and identify triggers (i.e., reproductive events, life events, seasonal patterns) and psychosocial contributors to depression.153 Box 1 highlights some of the most common clinical scenarios seen when depression is diagnosed during the menopausal transition and also includes practical guidelines for addressing them.
Box 1.
Conclusion
Despite the mounting evidence of an association between reproductive life events and the development of depressive episodes, further research is needed to more clearly identify the driving factors of this association. A better understanding of both systemic and brain-specific biological changes experienced during different reproductive phases may provide the keys to developing better treatment options for these cases and effectively reducing the disease burden and risk for recurrence. Future research should also assess the efficacy and safety of hormonal and nonhormonal strategies to modulate the occurrence of these disturbances or to alleviate their symptoms. With regard to managing menopause-related symptoms, a particular emphasis has been put on identifying safer and more effective treatments such as selective estrogen receptor modulators and serotonergic, noradrenergic and γ-aminobutyric acid–ergic psychoactive agents. In addition, accurate and effective screening strategies and updated treatment guidelines are necessary to provide physicians and patients with the necessary tools to meet the needs of this population.
Acknowledgments
The authors thank Dennis Stancavish, MA, and Jennifer Hutcheson for their writing and editing assistance on this manuscript.
Footnotes
Contributors: Drs. Soares and Zitek conducted the literature review, wrote and reviewed the article and gave final approval for its publication.
Competing interests: Dr. Soares is a recipient of grant/research support from Eli Lilly, AstraZeneca, NARSAD and Physicians Services Incorporated. He is also a research consultant for GlaxoSmithKline, Wyeth, Forest, Lundbeck, Sepracor Inc. and Neurocrine. Dr. Soares is a part of the Speakers' Bureau of GlaxoSmithKline, AstraZeneca, Wyeth, Forest, Berlex and Lundbeck. Dr. Soares did not receive any direct financial compensation for writing this manuscript. Dr. Zitek is an employee of Wyeth Research.
Correspondence to: Dr. C.N. Soares, Department of Psychiatry and Behavioral Neurosciences, McMaster University, 301 James St. S, FB#638, Hamilton ON L8P 3B6; fax 905 521-6098; csoares@mcmaster.ca
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105. [DOI] [PubMed]
- 2.Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996;276:293-9. [PubMed]
- 3.Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord 1993;29:85-96. [DOI] [PubMed]
- 4.Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med 1996;41:633-9. [PubMed]
- 5.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry 1998;44:839-50. [DOI] [PubMed]
- 6.Cyranowski JM, Frank E, Young E, et al. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry 2000;57:21-7. [DOI] [PubMed]
- 7.Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol 2005;17:179-85. [DOI] [PubMed]
- 8.Bethea CL, Mirkes SJ, Shively CA, et al. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry 2000;47:562-76. [DOI] [PubMed]
- 9.Pecins-Thompson M, Brown NA, Kohama SG, et al. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci 1996;16:7021-9. [DOI] [PMC free article] [PubMed]
- 10.Smith LJ, Henderson JA, Abell CW, et al. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology 2004;29:2035-45. [DOI] [PubMed]
- 11.Mize AL, Young LJ, Alper RH. Uncoupling of 5-HT1A receptors in the brain by estrogens: regional variations in antagonism by ICI 182,780. Neuropharmacology 2003;44:584-91. [DOI] [PubMed]
- 12.Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res 1998;59:205-14. [DOI] [PubMed]
- 13.Raap DK, DonCarlos LL, Garcia F, et al. Ovariectomy-induced increases in hypothalamic serotonin-1A receptor function in rats are prevented by estradiol. Neuroendocrinology 2002;76:348-56. [DOI] [PubMed]
- 14.Pau KY, Hess DL, Kohama S, et al. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol 2000;12:899-909. [DOI] [PubMed]
- 15.Panek DU, Dixon WR. Effect of continuous intraventricular estrogen or catechol estrogen treatment on catecholamine turnover in various brain regions. J Pharmacol Exp Ther 1986;236:646-52. [PubMed]
- 16.Petitti N, Etgen AM. Alpha 1-adrenoceptor augmentation of beta-stimulated cAMP formation is enhanced by estrogen and reduced by progesterone in rat hypothalamic slices. J Neurosci 1990;10:2842-9. [DOI] [PMC free article] [PubMed]
- 17.Karkanias GB, Etgen AM. Estradiol attenuates alpha 2-adrenoceptor-mediated inhibition of hypothalamic norepinephrine release. J Neurosci 1993;13:3448-55. [DOI] [PMC free article] [PubMed]
- 18.Ansonoff MA, Etgen AM. Evidence that oestradiol attenuates beta-adrenoceptor function in the hypothalamus of female rats by altering receptor phosphorylation and sequestration. J Neuroendocrinol 2000;12:1060-6. [DOI] [PubMed]
- 19.Karkanias GB, Ansonoff MA, Etgen AM. Estradiol regulation of alpha 1b-adrenoceptor mRNA in female rat hypothalamus-preoptic area. J Neuroendocrinol 1996;8:449-55. [DOI] [PubMed]
- 20.Clarke IJ, Scott CJ, Pereira A, et al. Levels of dopamine beta hydroxylase immunoreactivity in the preoptic hypothalamus of the ovariectomised ewe following injection of oestrogen: evidence for increased noradrenaline release around the time of the oestrogen-induced surge in luteinizing hormone. J Neuroendocrinol 1999;11:503-12. [DOI] [PubMed]
- 21.Velisek L, Veliskova J. Estrogen treatment protects GABA(B) inhibition in the dentate gyrus of female rats after kainic acid-induced status epilepticus. Epilepsia 2002;43(Suppl 5):146-51. [DOI] [PubMed]
- 22.Schumacher M, Coirini H, McEwen BS. Regulation of high-affinity GABAA receptors in the dorsal hippocampus by estradiol and progesterone. Brain Res 1989;487:178-83. [DOI] [PubMed]
- 23.Jussofie A, Korner I, Schell C, et al. Time course of the effects of steroid hormone deprivation elicited by ovariectomy or ovariectomy plus adrenalectomy on the affinity and density of GABA binding sites in distinct rat brain areas. Exp Clin Endocrinol Diabetes 1995;103:196-204. [DOI] [PubMed]
- 24.O'Connor LH, Nock B, McEwen BS. Regional specificity of gamma-aminobutyric acid receptor regulation by estradiol. Neuroendocrinology 1988;47:473-81. [DOI] [PubMed]
- 25.Murphy DD, Cole NB, Greenberger V, et al. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 1998;18:2550-9. [DOI] [PMC free article] [PubMed]
- 26.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res 1994;637:163-72. [DOI] [PubMed]
- 27.Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol 2004;469:507-21. [DOI] [PubMed]
- 28.Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience 2000;96:41-9. [DOI] [PubMed]
- 29.Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res 2002;930:216-34. [DOI] [PubMed]
- 30.Wihlback AC, Sundstrom-Poromaa I, Allard P, et al. Influence of postmenopausal hormone replacement therapy on platelet serotonin uptake site and serotonin 2A receptor binding. Obstet Gynecol 2001;98:450-7. [DOI] [PubMed]
- 31.Gregoire AJ, Kumar R, Everitt B, et al. Transdermal oestrogen for treatment of severe postnatal depression. Lancet 1996;347:930-3. [DOI] [PubMed]
- 32.Hlatky MA, Boothroyd D, Vittinghoff E, et al. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial. JAMA 2002;287:591-7. [DOI] [PubMed]
- 33.Halbreich U, Rojansky N, Palter S, et al. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry 1995;37:434-41. [DOI] [PubMed]
- 34.Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34. [DOI] [PubMed]
- 35.Morrison MF, Kallan MJ, Ten Have T, et al. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biol Psychiatry 2004;55:406-12. [DOI] [PubMed]
- 36.Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20. [DOI] [PubMed]
- 37.Kugaya A, Epperson CN, Zoghbi S, et al. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry 2003;160:1522-4. [DOI] [PubMed]
- 38.Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl) 2002;160:271-82. [DOI] [PubMed]
- 39.Kaura V, Ingram CD, Gartside SE, et al. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol 2007;17:108-15. [DOI] [PubMed]
- 40.Karkanias GB, Petitti N, Etgen AM. Progesterone attenuation of alpha 1-adrenergic receptor stimulation of phosphoinositol hydrolysis in hypothalamus of estrogen-primed female rats. Endocrinology 1995;136:1993-9. [DOI] [PubMed]
- 41.Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol 1995;7:171-7. [DOI] [PubMed]
- 42.Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res Mol Brain Res 1995;32:271-8. [DOI] [PubMed]
- 43.Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol 1997;90:709-14. [DOI] [PubMed]
- 44.Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol 2000;142:269-73. [DOI] [PubMed]
- 45.Bicikova M, Tallova J, Hill M, et al. Serum concentrations of some neuroactive steroids in women suffering from mixed anxiety-depressive disorder. Neurochem Res 2000;25:1623-7. [DOI] [PubMed]
- 46.Baker ER, Best RG, Manfredi RL, et al. Efficacy of progesterone vaginal suppositories in alleviation of nervous symptoms in patients with premenstrual syndrome. J Assist Reprod Genet 1995;12:205-9. [DOI] [PubMed]
- 47.Freeman EW, Purdy RH, Coutifaris C, et al. Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendocrinology 1993;58:478-84. [DOI] [PubMed]
- 48.Zhang L, Ma W, Barker JL, et al. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience 1999;94:251-9. [DOI] [PubMed]
- 49.Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000;343:682-8. [DOI] [PubMed]
- 50.Barrett-Connor E, von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab 1999;84:573-7. [DOI] [PubMed]
- 51.Weber B, Lewicka S, Deuschle M, et al. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology 2000;25:765-71. [DOI] [PubMed]
- 52.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the study of women's health across the nation. J Clin Endocrinol Metab 2005;90:4836-45. [DOI] [PubMed]
- 53.Robichaud M, Debonnel G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J Endocrinol 2004;182:11-21. [DOI] [PubMed]
- 54.Majewska MD, Demirgoren S, Spivak CE, et al. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res 1990;526:143-6. [DOI] [PubMed]
- 55.Demirgoren S, Majewska MD, Spivak CE, et al. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience 1991;45:127-35. [DOI] [PubMed]
- 56.Yaffe K, Ettinger B, Pressman A, et al. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biol Psychiatry 1998;43:694-700. [DOI] [PubMed]
- 57.Wolkowitz OM, Reus VI, Keebler A, et al. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry 1999;156:646-9. [DOI] [PubMed]
- 58.Bloch M, Schmidt PJ, Danaceau MA, et al. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry 1999;45:1533-41. [DOI] [PubMed]
- 59.Barrett-Connor E, von Muhlen D, Laughlin GA, et al. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc 1999;47:685-91. [DOI] [PubMed]
- 60.Wolkowitz OM, Reus VI, Roberts E, et al. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry 1997;41:311-8. [DOI] [PubMed]
- 61.Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998;338:209-16. [DOI] [PubMed]
- 62.Bloch M, Schmidt PJ, Danaceau M, et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157:924-30. [DOI] [PubMed]
- 63.Morrison JH, Brinton RD, Schmidt PJ, et al. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci 2006;26:10332-48. [DOI] [PMC free article] [PubMed]
- 64.McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev 2007;55:343-55. [DOI] [PMC free article] [PubMed]
- 65.Freeman EW, Sammel MD, Liu L, et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry 2004;61:62-70. [DOI] [PubMed]
- 66.Schmidt PJ, Daly RC, Bloch M, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry 2005;62:154-62. [DOI] [PubMed]
- 67.Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol 1994;29:319-36. [DOI] [PubMed]
- 68.Barton D, Loprinzi C, Wahner-Roedler D. Hot flashes: aetiology and management. Drugs Aging 2001;18:597-606. [DOI] [PubMed]
- 69.Berendsen HHG. The role of serotonin in hot flushes. Maturitas 2000;36:155-64. [DOI] [PubMed]
- 70.Tan KS, McFarlane LC, Lipworth BJ. Paradoxical down-regulation and desensitization of beta2-adrenoceptors by exogenous progesterone in female asthmatics. Chest 1997;111:847-51. [DOI] [PubMed]
- 71.Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA 2006;295:1824-30. [DOI] [PubMed]
- 72.Cohen LS, Soares CN, Otto MW, et al. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. J Affect Disord 2002;70:125-32. [DOI] [PubMed]
- 73.Perkonigg A, Yonkers KA, Pfister H, et al. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J Clin Psychiatry 2004;65:1314-22. [DOI] [PubMed]
- 74.Field T, Hernandez-Reif M, Diego M. Risk factors and stress variables that differentiate depressed from nondepressed pregnant women. Infant Behav Dev 2006;29:169-74. [DOI] [PubMed]
- 75.Rubertsson C, Wickberg B, Gustavsson P, et al. Depressive symptoms in early pregnancy, two months and one year postpartum-prevalence and psychosocial risk factors in a national Swedish sample. Arch Womens Ment Health 2005;8:97-104. [DOI] [PubMed]
- 76.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN). J Affect Disord 2007;103:267-72. [DOI] [PMC free article] [PubMed]
- 77.Rapkin AJ, Mikacich JA, Moatakef-Imani B, et al. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep 2002;4:419-28. [DOI] [PubMed]
- 78.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 79.Thys-Jacobs S, McMahon D, Bilezikian JP. Differences in free estradiol and sex hormone binding globulin in women with and without premenstrual dysphoric disorder. J Clin Endocrinol Metab 2007;93:96-102 [DOI] [PMC free article] [PubMed]
- 80.Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol 2006;195:1311-9. [DOI] [PubMed]
- 81.Kroll R, Rapkin AJ. Treatment of premenstrual disorders. J Reprod Med 2006;51(Suppl):359-70. [PubMed]
- 82.Joffe H, Cohen LS, Harlow BL. Impact of oral contraceptive pill use on premenstrual mood: predictors of improvement and deterioration. Am J Obstet Gynecol 2003;189:1523-30. [DOI] [PubMed]
- 83.Freeman EW, Kroll R, Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med 2001;10:561-9. [DOI] [PubMed]
- 84.Pearlstein TB, Bachmann GA, Zacur HA, et al. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception 2005;72:414-21. [DOI] [PubMed]
- 85.Yonkers KA, Brown C, Pearlstein TB, et al. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol 2005;106:492-501. [DOI] [PubMed]
- 86.Ashby CR Jr, Carr LA, Cook CL, et al. Alteration of platelet serotonergic mechanisms and monoamine oxidase activity in premenstrual syndrome. Biol Psychiatry 1988;24:225-33. [DOI] [PubMed]
- 87.Rapkin AJ, Edelmuth E, Chang LC, et al. Whole-blood serotonin in premenstrual syndrome. Obstet Gynecol 1987;70:533-7. [PubMed]
- 88.Jovanovic H, Cerin A, Karlsson P, et al. PET study of 5-HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res 2006;148:185-93. [DOI] [PubMed]
- 89.Steiner M. Premenstrual syndrome and premenstrual dysphoric disorder: guidelines for management. J Psychiatry Neurosci 2000;25:459-68. [PMC free article] [PubMed]
- 90.Steiner M, Hirschberg AL, Bergeron R, et al. Luteal phase dosing with paroxetine controlled release (CR) in the treatment of premenstrual dysphoric disorder. Am J Obstet Gynecol 2005;193:352-60. [DOI] [PubMed]
- 91.Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry 2005;66:769-73. [DOI] [PubMed]
- 92.Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. I. Prevalence, natural history and help-seeking behavior. J Reprod Med 1988;33:340-6. [PubMed]
- 93.Rivera-Tovar AD, Frank E. Late luteal phase dysphoric disorder in young women. Am J Psychiatry 1990;147:1634-6. [DOI] [PubMed]
- 94.Wittchen HU, Becker E, Lieb R, et al. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med 2002;32:119-32. [DOI] [PubMed]
- 95.Bailey JW, Cohen LS. Prevalence of mood and anxiety disorders in women who seek treatment for premenstrual syndrome. J Womens Health Gend Based Med 1999;8:1181-4. [DOI] [PubMed]
- 96.Joffe H, Petrillo L, Viguera A, et al. Treatment of premenstrual worsening of depression with adjunctive oral contraceptive pills: A preliminary report. J Clin Psychiatry 2007;1954-62. [DOI] [PubMed]
- 97.Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacology 2002;43:1119-28. [DOI] [PubMed]
- 98.Bennett HA, Einarson A, Taddio A, et al. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol 2004;103:698-709. [DOI] [PubMed]
- 99.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295:499-507. [DOI] [PubMed]
- 100.Hallberg P, Sjoblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73. [DOI] [PubMed]
- 101.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354:579-87. [DOI] [PubMed]
- 102.Bonari L, Bennett H, Einarson A, et al. Risks of untreated depression during pregnancy. Can Fam Physician 2004;50:37-9. [PMC free article] [PubMed]
- 103.Chung TK, Lau TK, Yip AS, et al. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med 2001;63:830-4. [DOI] [PubMed]
- 104.Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95. [DOI] [PubMed]
- 105.Rubinow DR. Antidepressant treatment during pregnancy: between Scylla and Charybdis. Am J Psychiatry 2006;163:954-6. [DOI] [PubMed]
- 106.Einarson A, Koren G. Prescribing antidepressants to pregnant women: What is a family physician to do? Can Fam Physician 2007;53:1412-5. [PMC free article] [PubMed]
- 107.Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007;356:2684-92. [DOI] [PubMed]
- 108.Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007;356:2675-83. [DOI] [PubMed]
- 109.Jones I, Hamshere M, Nangle JM, et al. Bipolar affective puerperal psychosis: genome-wide significant evidence for linkage to chromosome 16. Am J Psychiatry 2007;164:1099-104. [DOI] [PubMed]
- 110.Ahokas A, Kaukoranta J, Wahlbeck K, et al. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry 2001;62:332-6. [DOI] [PubMed]
- 111.Josefsson A, Berg G, Nordin C, et al. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstet Gynecol Scand 2001;80:251-5. [DOI] [PubMed]
- 112.Kitamura T, Yoshida K, Okano T, et al. Multicentre prospective study of perinatal depression in Japan: incidence and correlates of antenatal and postnatal depression. Arch Womens Ment Health 2006;9:121-30. [DOI] [PubMed]
- 113.Lee DT, Chan SS, Sahota DS, et al. A prevalence study of antenatal depression among Chinese women. J Affect Disord 2004;82:93-9. [DOI] [PubMed]
- 114.Rich-Edwards JW, Kleinman K, Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health 2006;60:221-7. [DOI] [PMC free article] [PubMed]
- 115.Bloch M, Rotenberg N, Koren D, et al. Risk factors for early postpartum depressive symptoms. Gen Hosp Psychiatry 2006;28:3-8. [DOI] [PubMed]
- 116.Heron J, O'Connor TG, Evans J, et al. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord 2004;80:65-73. [DOI] [PubMed]
- 117.Bloch M, Rotenberg N, Koren D, et al. Risk factors associated with the development of postpartum mood disorders. J Affect Disord 2005;88:9-18. [DOI] [PubMed]
- 118.Santoro N. The menopausal transition. Am J Med 2005;118(Suppl 12B):8-13. [DOI] [PubMed]
- 119.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001;76:874-8. [DOI] [PubMed]
- 120.Randolph JF Jr, Sowers M, Bondarenko IV, et al. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 2004;89:1555-61. [DOI] [PubMed]
- 121.Randolph JF Jr, Crawford S, Dennerstein L, et al. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J Clin Endocrinol Metab 2006;91:3034-40. [DOI] [PubMed]
- 122.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003;78:603-12. [DOI] [PubMed]
- 123.Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006;63:375-82. [DOI] [PubMed]
- 124.Soares CN, Prouty J, Born L, et al. Treatment of menopause-related mood disturbances. CNS Spectr 2005;10:489-97. [DOI] [PubMed]
- 125.Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7:1285-93. [DOI] [PubMed]
- 126.Arlt W. Androgen therapy in women. Eur J Endocrinol 2006;154:1-11. [DOI] [PubMed]
- 127.Freedman MA. Quality of life and menopause: the role of estrogen. J Womens Health (Larchmt) 2002;11:703-18. [DOI] [PubMed]
- 128.Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90:3847-53. [DOI] [PubMed]
- 129.Gallicchio L, Whiteman MK, Tomic D, et al. Type of menopause, patterns of hormone therapy use, and hot flashes. Fertil Steril 2006;85:1432-40. [DOI] [PubMed]
- 130.Woods NF, Mariella A, Mitchell ES. Depressed mood symptoms during the menopausal transition: observations from the Seattle Midlife Women's Health Study. Climacteric 2006;9:195-203. [DOI] [PubMed]
- 131.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry 2004;161:2238-44. [DOI] [PubMed]
- 132.Avis NE, Brambilla D, McKinlay SM, et al. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women's Health Study. Ann Epidemiol 1994;4:214-20. [DOI] [PubMed]
- 133.Richards M, Rubinow DR, Daly RC, et al. Premenstrual symptoms and perimenopausal depression. Am J Psychiatry 2006;163:133-7. [DOI] [PubMed]
- 134.Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: The Harvard Study of Moods and Cycles. Arch Gen Psychiatry 2006;63:385-90. [DOI] [PubMed]
- 135.Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9:392-8. [DOI] [PubMed]
- 136.Stewart DE, Boydell KM. Psychologic distress during menopause: associations across the reproductive life cycle. Int J Psychiatry Med 1993;23:157-62. [DOI] [PubMed]
- 137.Gregory RJ, Masand PS, Yohai NH. Depression across the reproductive life cycle: correlations between events. Prim Care Companion J Clin Psychiatry 2000;2:127-9. [DOI] [PMC free article] [PubMed]
- 138.Soares CN, Cohen LS, Otto MW, et al. Characteristics of women with premenstrual dysphoric disorder (PMDD) who did or did not report history of depression: a preliminary report from the Harvard Study of Moods and Cycles. J Womens Health Gend Based Med 2001;10:873-8. [DOI] [PubMed]
- 139.Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry 2003;160:1519-22. [DOI] [PubMed]
- 140.Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri-and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13:780-6. [DOI] [PubMed]
- 141.Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med 1995;332:1529-34. [DOI] [PubMed]
- 142.Yonkers KA, Halbreich U, Freeman E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. A randomized controlled trial. Sertraline Premenstrual Dysphoric Collaborative Study Group. JAMA 1997;278:983-8. [PubMed]
- 143.Cohen LS, Miner C, Brown EW, et al. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: a placebo-controlled, clinical trial using computerized diaries. Obstet Gynecol 2002;100:435-44. [DOI] [PubMed]
- 144.Cassano P, Soares CN, Cusin C, et al. Antidepressant response and well-being in pre-, peri-and postmenopausal women with major depressive disorder treated with fluoxetine. Psychother Psychosom 2005;74:362-5. [DOI] [PubMed]
- 145.Pinto-Meza A, Usall J, Serrano-Blanco A, et al. Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference? J Affect Disord 2006;93:53-60. [DOI] [PubMed]
- 146.Thase ME, Entsuah R, Cantillon M, et al. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt) 2005;14:609-16. [DOI] [PubMed]
- 147.Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64:473-9. [DOI] [PubMed]
- 148.Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10:471-8. [DOI] [PubMed]
- 149.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6. [DOI] [PubMed]
- 150.Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29:25-31. [DOI] [PubMed]
- 151.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996;24:161-75. [DOI] [PubMed]
- 152.Utian WH, Janata JW, Kingsberg SA, et al. The Utian Quality of Life (UQOL) Scale: development and validation of an instrument to quantify quality of life through and beyond menopause. Menopause 2002;9:402-10. [DOI] [PubMed]
- 153.Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry 1997;58(Suppl 15):12-8. [PubMed]