Abstract
In this study we investigated, using intravital microscopy, how neutrophil extravasation across mouse mesenteric postcapillary venules is inhibited by the glucocorticoid-regulated protein lipocortin (LC; also termed annexin) 1. Intraperitoneal injection of 1 mg of zymosan into mice induced neutrophil rolling on the activated mesenteric endothelium followed by adhesion (maximal at 2 hr: 5–6 cells per 100-μm of vessel length) and emigration (maximal at 4 hr: 8–10 cells per high-powered field). Treatment of mice with human recombinant LC1 (2 mg/kg s.c.) or its mimetic peptide Ac2–26 (13 mg/kg s.c.) did not modify cell rolling but markedly reduced (≥50%) the degree of neutrophil adhesion and emigration (P < 0.05). Intravenous treatment with peptide Ac2–26 (13 mg/kg) or recombinant human LC1 (0.7–2 mg/kg) promoted detachment of neutrophils adherent to the endothelium 2 hr after zymosan administration, with adherent cells detaching within 4.12 ± 0.75 min and 2.36 ± 0.31 min, respectively (n = 20–25 cells). Recruitment of newly adherent cells to the endothelium was unaffected. The structurally related protein LC5 was inactive in this assay, whereas a chimeric molecule constructed from the N terminus of LC1 (49 aa) attached to the core region of LC5 produced cell detachment with kinetics similar to LC1. Removal of adherent neutrophils from activated postcapillary endothelium is a novel pharmacological action, and it is at this site where LC1 and its mimetics operate to down-regulate this aspect of the host inflammatory response.
The events that regulate the initial phases of the interaction between the circulating neutrophil and the endothelium of postcapillary venules have been elucidated recently by using intravital microscopy techniques (1). Upon application of an inflammatory stimulus, neutrophils roll on the endothelium of inflamed postcapillary venules, an action mediated by selectins CD62P, CD62E, or CD62L (2). During this phase the neutrophil can be activated, causing it to firmly adhere. Examples of such activators include chemoattractants such as leukotriene B4 or complement factor C5a (3, 4), chemokines such as interleukin-8 (IL-8) or monocyte chemoattractant protein 1 (5, 6), members of the S100 family such as CP-10 and the complex MRP8/14 (7, 8), and, finally, nonclassical activators such as substance P (9). Firm adhesion is associated with integrin activation and increased avidity for the counter-ligands (10). In- and outward signaling may also occur (11), and the neutrophil undergoes an intense cytoskeletal reorganization to begin the actual emigration process (diapedesis).
In previous studies on the antimigratory action of lipocortin 1 (LC1; also termed annexin I), we and others have reported that LC1 and LC1-mimetics (such as the N-terminal peptide Ac2–26) inhibit recruitment of neutrophils in several models of experimental inflammation (12–14). In addition, passive immunization of mice with specific anti-LC1 neutralizing antisera abrogated the antimigratory action of systemic dexamethasone (12). This suggests that the steroid acted to inhibit neutrophil migration by promoting endogenous LC1 synthesis/release. Recent studies have substantiated this assumption, as we demonstrated that (i) immunoreactive LC1 levels were elevated in mouse polymorphonuclear leukocytes and monocytes after i.v. dexamethasone (15); (ii) the glucocorticoid affected the actual emigration process in an LC1-dependent way (16); and, finally, (iii) there was extensive externalization of LC1 on the cell surface of neutrophils that were adherent to endothelial cell monolayers in vitro (17).
In this study we sought to investigate the cellular mechanism(s) by which LC1 and its mimetics affect neutrophil emigration in vivo. To do so, we have set up (i) a technique of intravital microscopy for the mouse mesenteric vascular bed and (ii) a method of observation that visualizes the fate of neutrophils adherent to the endothelium of inflamed postcapillary venules.
METHODS
Intravital Microscopic Studies.
Male Swiss Albino mice (10–15 g) were purchased from Banton and Kingsman (Hull, U.K.) and maintained on a standard chow pellet diet with tap water ad libitum. Animals were housed in groups of eight per cage in a room with controlled lighting (lights on 8:00–20:00) in which the temperature was maintained at 21–23°C, and the animals were used 2–3 days after their arrival. Animal work was performed according to Home Office regulations (guidance on the operation on animals was from the Scientific Procedures Act 1986). Experiments began at 9:00 with i.p. injections of 1 mg of zymosan (in 0.5 ml of sterile saline). Control animals received sterile saline alone. Either 2 or 4 hr later the vascular mesenteric bed was prepared for microscopic observation.
Mice were anesthetized with diazepam (60 mg/kg s.c.) and Hypnorm (0.7 mg/kg fentanyl citrate and 20 mg/kg fluanisone i.m.). Cautery incisions were made along the abdominal region, and the mesentery vascular bed was exteriorized and placed on a viewing Plexiglas stage. The preparation then was mounted on a Zeiss Axioskop “FS” with a water-immersion objective lens (magnification of ×40; Zeiss) and an eyepiece (×10 magnification; Zeiss) was used to observe the microcirculation. The preparation was transilluminated with a 12-V, 100-W halogen light source. A Hitachi charge-coupled device color camera (model KPC571; Tokyo) acquired images that were displayed onto a Sony Trinitron color video monitor (model PVM 1440QM) and recorded on a Sony super-VHS video cassette recorder (model SVO-9500 MDP) for subsequent off-line analysis. A video time–date generator (FOR.A video timer, model VTG-33, Tokyo) projected the time, date, and stopwatch function onto the monitor. Mesenteries were superfused with thermostated (37°C) bicarbonate-buffered solution (g/liter: NaCl, 7.71; KCl, 0.25; MgSO4, 0.14; NaHCO3, 1.51; and CaCl2, 0.22, pH 7.4, gassed with 5% CO2/95% N2) at a rate of 2 ml/min. The temperature of the stage was maintained at 37°C. In preliminary experiments, rectal temperature and blood pressure of the animal were monitored for 20 min without showing significant changes.
Red blood cell velocity was measured in venules by using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University). Venular blood flow was calculated from the product of mean red blood cell velocity (Vmean = centerline velocity/1.6) and microvascular cross-sectional area, assuming a cylindrical geometry. Wall shear rate (SR) was calculated by the Newtonian definition: SR = 8,000 × (Vmean/diameter) (18).
In the “fixed-time” protocol, one to three randomly selected postcapillary venules (diameter between 20 and 40 μm; length of at least 100 μm) were observed for each mouse 4 hr after zymosan injection. The extent of the inflammatory response elicited by zymosan was analyzed by measuring white blood cell velocity (VWBC) in μm/sec. Cell adhesion was quantified by counting, for each vessel, the number of adherent neutrophils in a 100-μm length. Leukocyte emigration from the microcirculation into the tissue was quantified by counting the number of cells that had emigrated up to 50 μm away from the wall of the 100-μm vessel segments.
A “real-time” protocol was established to investigate the detachment phenomenon. Mesenteries were exposed 2 hr after zymosan injection as described above, and postcapillary venules with a similar number of adherent neutrophils (at least 4–6 cells per 100-μm vessel) were selected and monitored. Either sterile PBS (100 μl) or one of the different LC preparations was given i.v. into the tail vein, and the fate of the adherent neutrophils within the postcapillary venules was monitored for up to 10 min. Off-line analysis was performed to monitor (i) the fate of adherent cells, (ii) the number of newly adherent neutrophils, and (iii) the cell flux, assessed as the number of cells passing a fixed point in the vessel at 2-min intervals. In the case of the cells detaching from the endothelium, the time lapse between the i.v. injection and the detachment also was recorded. In few animals SR of the postcapillary venule was measured before and 10 min after i.v. injection of LC1.
Drug Treatment.
LC1 (37 kDa), LC5 (32 kDa), and LC1/5 chimera (37 kDa) were prepared as detailed below and stored at high concentration (>100 μg/ml) at 4°C. Peptides Ac2–26 and Ac2–12 were stored as lyophilized powder, and a stock solution (1–2 mg/ml) was prepared in PBS immediately before use. In most cases, LC1 and the other derivatives were given i.v. 2 hr after zymosan and the fate of adherent neutrophils was monitored as described above. In initial experiments, LC1 or peptide Ac2–26 was administered s.c. 15 min before zymosan, and the intensity of neutrophil interaction with the endothelium was assessed at the 4-hr time point.
The doses of peptide Ac2–26, LC1, and LC5 were chosen from previous publications in which at least 70% reduction of neutrophil recruitment was obtained with each of these agents (13, 19, 20).
As a positive control, the effect of the nitric oxide donor sodium nitroprusside (SNP) was determined by using the real-time protocol. To avoid indirect effects secondary to alterations in systemic blood pressure, the drug was applied topically. Buffer superfusion was stopped for 2 min, and 2.62 μg of SNP (10 nmol) in 100-μl volume was added onto the microvascular bed. Superfusion then was reestablished and the vessel was monitored for a further 8 min.
Preparation and Purification of Recombinant Lipocortins.
Human LC1 cDNA originally from pUC13 vector (kindly provided by J. L. Browning, Biogen) was subcloned into the expression vector pGEX-4T-3. The human recombinant LC5 cDNA was cloned into the expression vector pGEX-2T-KG as previously reported (21). The coding sequence for the chimera LC1/5 was recovered from the plasmid pGN35 (22, 23) (a gift from R. Dick-Gudenus, Bender Wien, Vienna, Austria) and then subcloned in the restriction sites BamHI-HindIII of the plasmid pGEX-2T-KG. Plasmids were designed for inducible, high-level expression of proteins as fusion with Schistosoma japonicum glutathione S-transferase (GST) (24). Bacterial cultures containing the different expression vectors were treated with 0.1 mM isopropyl β-d-thiogalactoside for 4 hr before lysis processing as described [Mira et al. (21)]. Fusion proteins were purified from bacterial lysates by affinity chromatography using prepacked glutathione-Sepharose 4B, and the GST moiety was removed by using the thrombin cleavage site (25). LC1, LC5, and the chimera LC1/5 were purified further by fast protein liquid chromatography (FPLC, Pharmacia). As analyzed by SDS/PAGE, FPLC profiles, and Western blot analyses, proteins were >95% pure. Endotoxin contamination was less than 20 pg/ml as measured by the Limulus amebocyte chromogenic assay.
Other Materials.
Peptide Ac2–26 (acetyl-AMVSEFLKQAWFIENEEQEYVVQTVK, Mr 3,005) and peptide Ac2–12 (acetyl-AMVSEFLKQAW, Mr 1,424) were prepared by The Advance Biotechnology Centre (The Charing Cross and Westminster Medical School, London) by using solid-phase stepwise synthesis. Purity was more than 90% as assessed by HPLC and capillary electrophoresis (data supplied by the manufacturer).
Zymosan type A, PBS, SNP, and all other chemicals and salts were obtained from Sigma.
Statistics.
Statistical differences were calculated on original values by using ANOVA followed by the Bonferroni test for intergroup comparisons. Nonparametric data were analyzed by Fisher’s exact test (2 × 2 tables) (26). In all cases a threshold value of P < 0.05 was taken as the minimum level of significance.
RESULTS
Mouse Mesenteric Preparation for Intravital Microscopy.
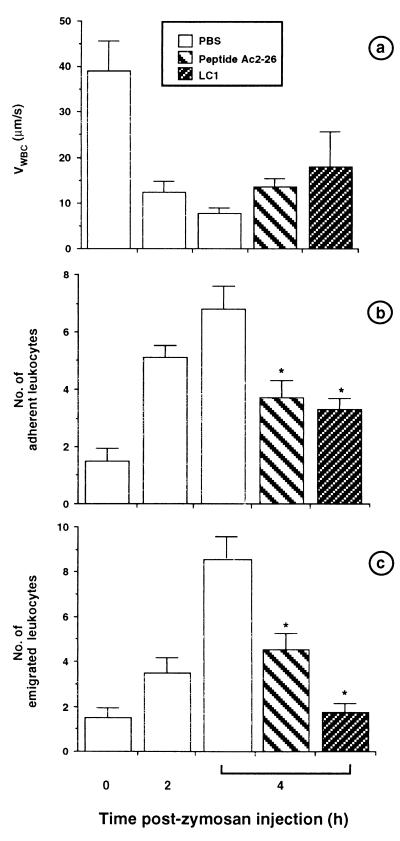
In control animals injected with saline, neutrophils rolled on the endothelium at high speed (VWBC, 39.1 ± 6.6 μm/sec), whereas cells moved more slowly (VWBC, 7.7 ± 1.3 μm/sec) after i.p. administration of zymosan. This phenomenon was time-dependent with a significant reduction in VWBC at 2 hr after zymosan, a further decrease being seen at the 4-hr time point (Fig. 1). This sharp decrease in VWBC was mirrored by a marked increase in cell adhesion, which was almost maximal at 2 hr after zymosan. A similar increase in cell emigration was also seen, but it clearly peaked at 4 hr after zymosan. From these data it appears that most neutrophils emigrate across murine mesenteric postcapillary venules between 2 and 4 hr, and this reflects the recovery of extravasated neutrophils from the peritoneal cavity of mice treated with this inflammogen (27).
Figure 1.
Time dependency of zymosan-induced neutrophil rolling, adhesion, and emigration and the inhibitory action of LC1 and peptide Ac2–26. Mice were treated with zymosan (1 mg i.p.) at time 0, and mesenteries were exposed at the reported time points to quantify neutrophil rolling (expressed as VWBC, a), adhesion (number of cells per 100 μm of vessel length, b), and emigration (number of cells per 100 × 50 μm2, c). Other animals were treated s.c. with 100 μl of PBS/2 mg/kg LC1 or 13 mg/kg peptide Ac2–26 15 min before zymosan injection. The extent of neutrophil–endothelium interaction was quantified 4 hr later. Data are mean ± SE of n = 5–7 mice per group. ∗, P < 0.05 vs. PBS group.
Treatment with animals with LC1 (2 mg/kg s.c., 810 pmol per mouse) or with its pharmacophore, peptide Ac2–26 (13 mg/kg s.c., 65 nmol per mouse) (13) did not affect the changes in VWBC produced by zymosan, but significantly reduced the extent of cell adhesion and emigration as assessed at the 4-hr time point (Fig. 1).
LC1 Alters the Fate of Adherent Neutrophils.
To discover whether this effect could be explained by reduced cell adhesion or prevention of cell emigration, the real-time protocol was developed to enable us to observe the inflamed mesenteric microcirculation after i.v. administration of different drugs.
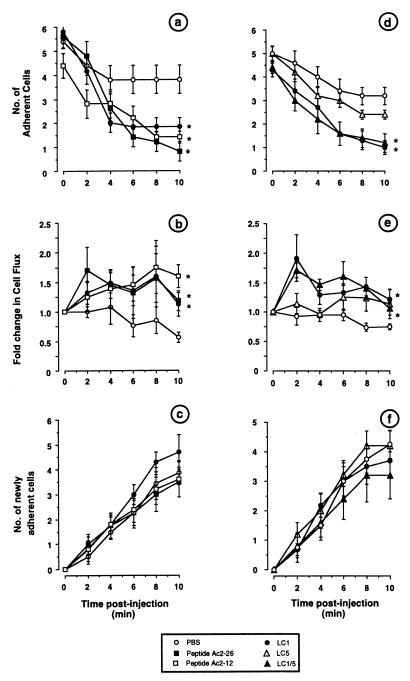
Fig. 2a shows that i.v. treatment with 100 μl of PBS caused a small and not significant detachment of adherent cells from the endothelium of the postcapillary venule under observation. Most (≈70%, as shown in Table 1) of the adherent cells monitored had remained in place or migrated within the 10 min of recording. In contrast, administration of 2 mg/kg LC1 or 13 mg/kg peptide Ac2–26 produced a marked and significant detachment of adherent cells (71% in both cases; see Table 1). Leukocytes detached and rejoined the bloodstream 2.36 ± 0.31 min after LC1 treatment and 4.12 ± 0.75 min after peptide Ac2–26 treatment. The incidence of this phenomenon in the entire microvascular bed was assessed by measuring cell flux after the i.v. treatments: although there was some degree of variability between animals, a significant increase in cells passing through the postcapillary venule was seen after administration of LC1 and peptide Ac2–26 when compared with the PBS group (Fig. 2b). No significant alterations in the number of newly recruited adherent cells was seen among the different groups within the 10-min monitoring period (Fig. 2c). A shorter N-terminal fragment, peptide Ac2–12 (6.7 mg/kg, ≈70 nmol per mouse), was found to mimic the actions of LC1 and Ac2–26 (Fig. 2 a–c).
Figure 2.
Alteration of the fate of adherent neutrophils by LC1 and its mimetics. Mice were treated with zymosan (1 mg i.p.) at time 0, and mesenteries were exposed 2 hr later. PBS (100 μl), LC1 (2 mg/kg in a–c; 0.7 mg/kg in d–f), peptide Ac2–26 (13 mg/kg), peptide Ac2–12 (6.7 mg/kg), LC5 (0.7 mg/kg), or LC1/5 (0.7 mg/kg) then was injected i.v. (time 0 in a–f). The detachment of adherent neutrophils (a and d), cell flux (b and e), and the recruitment of newly adherent cells (c and f) were monitored up to 10 min after injection. Data are mean ± SE of n = 6–8 mice per group. ∗, P < 0.05 vs. area under the curves of the PBS group.
Table 1.
Cumulative data for the detachment phenomenon observed in mouse mesenteric postcapillary venules
| Treatment | Fate of adherent neutrophils
|
||||
|---|---|---|---|---|---|
| Detached
|
Stationary or migrated
|
Total no. | |||
| No. | % | No. | % | ||
| PBS (n = 8) | 13 | 32 | 28 | 68 | 41 |
| Peptide Ac2–26 (n = 6) | 20 | 71* | 8 | 29 | 28 |
| Peptide Ac2–12 (n = 6) | 15 | 68* | 7 | 32 | 22 |
| LC1#(n = 5) | 25 | 71* | 10 | 29 | 35 |
| LC1§(n = 6) | 23 | 77* | 7 | 23 | 30 |
| LC5 (n = 6) | 13 | 52 | 12 | 48 | 25 |
| LC1/5 (n = 6) | 16 | 73* | 6 | 27 | 22 |
Cumulative data are shown, with the total number of adherent cells in the postcapillary venules before i.v. challenge with the different treatments and their fate 10 min after drug administration. Injected doses (in 100 μl of PBS) were as follows: peptide Ac2–26, 13 mg/kg; peptide Ac2–12, 6.7 mg/kg; LC1#, 2 mg/kg; LC1
, LC5, and the chimera LC1/5, 0.7 mg/kg. ∗, P < 0.05 vs. PBS group (Fisher’s exact test).
The latter data were controlled for and extended by the next series of experiments. Intravenous injection of 0.7 mg/kg LC1 (≈270 pmol per mouse) promoted detachment to the same extent as the 2 mg/kg dose (Fig. 2d), with >75% adherent cells reentering the blood stream within 10 min (Table 1). This is in contrast to the situation observed after i.v. administration of LC5 (0.7 mg/kg; ≈310 pmol), where ≈50% of the cells detached (not significant vs. the value, 32%, for PBS group; Table 1). In addition, whereas a marked increase in cell flux was seen after 0.7 mg/kg LC1 (providing an indication of a sustained neutrophil detachment from within the entire vascular bed), this effect was clearly absent after treatment of mice with 0.7 mg/kg LC5 i.v. (Fig. 2e). Finally, the chimera LC1/5 was equiactive with the full LC1 protein (Fig. 2 d and e), promoting 72 ± 9% cell detachment (Table 1). Neither of these treatments modified the degree of cell adhesion observed during the 10-min period that was monitored (Fig. 2f).
In vessels exposed 2 hr after zymosan injection, an SR value of 262 ± 22 sec−1 was calculated. This figure was not modified after i.v. injection with 0.7 mg/kg LC1, calculating at 10 min an SR of 253 ± 32 sec−1 (n = 5, not significant).
Finally, topical application of SNP promoted cell detachment from the activated mesenteric endothelia, such that only 2.75 ± 0.45 cells were found at the end of the 10-min period compared with 5.0 ± 0.58 adherent cells initially counted (n = 4). No changes were seen after topical application of PBS (data not shown).
DISCUSSION
LC1 and LC1 mimetics, such as N-terminal fragments of the protein, inhibit recruitment of neutrophils in acute and chronic inflammation (12, 13, 28, 29). In several of these cases, the protein appears to be the key endogenous mediator by which glucocorticoid hormones inhibit the host cellular response (12, 28, 29). We recently have suggested a model to account for this finding. Human (30, 31) and murine neutrophils (15) contain abundant cytosolic LC1, and upon adhesion to endothelial monolayers in vitro, the protein is recruited to the neutrophil plasma membrane, where it acts negatively to regulate the extent of cellular activation and emigration (17). An LC1-dependent effect of dexamethasone on cell emigration also was seen in vivo, when the hamster cheek pouch microcirculation inflamed with fMet-Leu-Phe or substance P was used (16).
Since many experimental data have been produced in murine models, we decided to develop a mouse mesenteric preparation to visualize the antimigratory effect of LC1 and LC1 mimetics in this species. Zymosan was used to inflame the murine mesenteric postcapillary venules since LC1 and peptide Ac2–26 have been shown to inhibit neutrophil (early phase of cell accumulation) and monocyte (>8 hr cell accumulation) recruitment into the peritoneal cavity in response to administration of this inflammogen (13, 19). Intraperitoneal injection of zymosan produced a time-dependent activation of the vascular mesenteric bed, and the well described phenomena of neutrophil rolling, adhesion, and emigration (1) could be visualized and quantified. At 4 hr, zymosan produced a sharp reduction in VWBC compared with saline-treated mice, and this was mirrored by a significant increase in cell adhesion and emigration. The latter two processes were dynamic, such that neutrophil adhesion to the endothelium essentially was maximal at the 2-hr time point, whereas neutrophil emigration appeared to occur mainly between 2 and 4 hr postinjection. A similar time course of neutrophil accumulation into the mouse peritoneal cavity is seen (27).
Administration of an antiinflammatory dose of peptide Ac2–26 (19) or LC1 (12) did not modify zymosan-dependent VWBC but reduced cell adhesion and emigration to similar extents. An effect on function and/or expression of selectins therefore was ruled out as the mechanism responsible for the antimigratory action of the LC1-derived peptide. This observation confirms that peptide Ac2–26 has a different mechanism of action on neutrophil trafficking to selectin-modifying agents, such as sialidase and fucoidin (32). Because of the causal and time-related link between the three events of cell rolling, adhesion, and emigration (33, 34), the inhibitory effect of LC1 and peptide Ac2–26 on neutrophil emigration could be because of a specific interference with the emigration process or a reduction in the extent of cell adhesion. This question could not be answered by using the simple fixed-time protocol at 4 hr after zymosan injection, and, therefore, we developed the real-time protocol for intravital microscopy.
Since cell adhesion to zymosan-inflamed postcapillary venules was almost 80% of maximum at 2 hr, the acute effect of LC1 and related drugs was investigated at this time point. Intravenous administration of a high dose (2 mg/kg) of LC1 produced a fast detachment of neutrophils that were adherent on the endothelium before the beginning of the complex process of emigration. This detachment phenomenon was completed within 8–10 min of i.v. challenge and was not secondary to detectable changes in blood flow (e.g., without alteration in SR values). The detachment effected by LC1 was mirrored by i.v. administration of the pharmacophore, peptide Ac2–26 (13). Furthermore, by using the real-time protocol we monitored the potential of these agents to alter neutrophil adhesion. Here, no difference was seen between i.v. injection of PBS, LC1, or peptide Ac2–26 on the recruitment to the postcapillary venule of newly adherent neutrophils. The fact that blood-derived neutrophils could adhere within the 10-min observation period with no difference among the experimental groups also acts as an internal control for the absence of potential drug-related alterations in blood flow.
Cell fluxes were also measured as an indication of a generalized neutrophil detachment in the mesenteric vascular bed upstream from the vessel under observation. Although variable, treatment with LC1 or LC1-derived peptides always increased the number of neutrophils passing through the vessel by more than 50% with respect to PBS-injected mice.
The efficacy of the shorter peptide, Ac2–12, may shed light on the potential molecular mechanism(s) underlying the neutrophil-detachment phenomenon. Since this unique portion of the protein is directly involved in the binding to proteins of the S100 family (35), we may postulate that an interaction may be occurring during experimental inflammation. The S100 family of proteins includes some chemotactic peptides, and, interestingly, two members of this family are abundant in neutrophils (36) and may be important in regulating neutrophil adhesion and signaling (8). Future studies are required to address this point.
LC1-induced detachment and the pivotal role played by its N terminus region were confirmed in the experiments in which LC5 and the chimera LC1/5 were tested. LC5 is a protein structurally related to LC1, with a highly homologous 316-aa-long core and a short (5-aa) N terminus (37). Experimentally, LC5 has been shown to reduce neutrophil accumulation in vivo (20, 38). However, in this set of experiments, i.v. injection of LC5 did not promote a significant cell detachment from the postcapillary endothelium when compared with the modest effect because of vehicle injection alone (PBS group). In contrast, 0.7 mg/kg LC1 produced rapid detachment of adherent cells without alteration in the number of newly adherent cells. An identical effect was seen after i.v. administration of the chimera LC1/5. Construction of this chimera, which comprises the LC1 N terminus plus the LC5 core, has been used previously to confirm the specificity of LC1 action and to investigate the role played by the N terminus in governing the interaction between these proteins and membrane preparations in vitro (22, 23).
The cell-detaching effect of LC1 and its N terminus-derived peptides correlates well with the ability of these agents to produce systemic neutrophilia after i.v. administration (32). It is likely that the latter effect is the “macroscopic” consequence of a systemic effect of releasing neutrophils that spontaneously interact with the endothelium of vascular beds.
Observations produced with techniques of intravital microscopy have indicated that at least two distinct steps operate within the host to control cell recruitment in inflammation: (i) leukocytes rolling on the endothelium can reenter into the circulation without subsequent adhesion to the endothelium, and (ii) a fraction of the adherent leukocytes fail to initiate diapedesis and eventually detach from the postcapillary venule, rejoining the blood flow. Whereas the latter is a well recognized phenomenon (16, 39, 40), no efforts have been made to address the molecular mechanism(s) responsible for the effect. Based on the data presented here using exogenously administered LC1 and LC1 mimetics, together with a previous in vitro study on the externalization of intracellular LC1 on the plasma membrane of adherent neutrophils, we propose that LC1 is one of the mediator(s) that prevents adherent neutrophils from entering into diapedesis and eventually detaching from the postcapillary venule. Future studies are required to elucidate the mechanism responsible for this pharmacological effect, although it is likely that LC1 may interfere with the neutrophil activation process that occurs at the leukocyte–endothelium interface on the inflamed postcapillary venule (41).
The search for novel antiinflammatory drugs that inhibit the interaction between the circulating neutrophil and the activated endothelium of postcapillary venules has, so far, focused on the use of specific antibodies or complex carbohydrate moieties to block cell rolling on, or adhesion to, the inflamed postcapillary venule. Here, we have identified a different target for pharmacological intervention and shown that the “adherent neutrophil” is the target for LC1 and associated mimetics. It is conceivable that other mediators endowed with antiinflammatory activity may act in a similar manner (41, 42).
Acknowledgments
We thank Dr. R. Dick-Gudenus (Bender Wien, Vienna, Austria) for providing the cDNA for the chimera LC1/5. This work was supported by the Arthritis and Rheumatism Council and by the Wellcome Trust (U.K.). M.P. is an Arthritis and Rheumatism Council Postdoctoral Fellow, and R.J.F. is a Principal Fellow of the Wellcome Trust. L.H.K.L. is a Ph.D. student partially funded by the Overseas Research Award Scheme.
ABBREVIATIONS
- LC1 and LC5
lipocortins 1 and 5
- LC1/5
chimeric protein with the N terminus of LC1 and the core of LC5
- SNP
sodium nitroprusside
- SR
shear rate
- VWBC
white blood cell velocity
References
- 1.Granger D N, Kubes P. J Leukocyte Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 2.Kansas G S. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 3.Casale T B, Abbas M K. Am J Physiol. 1990;258:C639–C647. doi: 10.1152/ajpcell.1990.258.4.C639. [DOI] [PubMed] [Google Scholar]
- 4.Collins P D, Jose P J, Williams T J. J Immunol. 1991;146:677–684. [PubMed] [Google Scholar]
- 5.Yoshimura T, Matsushima K, Oppenheim J J, Leonard E J. J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]
- 6.Yoshimura T, Yuhki N, Moore S K, Appella E, Lerman M I, Leonard E J. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 7.Iisma S E, Hu S, Kocher M, Lackmann M, Harrison C A, Thliveris S, Geczy C L. DNA Cell Biol. 1994;13:183–192. doi: 10.1089/dna.1994.13.183. [DOI] [PubMed] [Google Scholar]
- 8.Newton R A, Hogg N. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- 9.Haines K A, Kolasinski S L, Cronstein B N, Reibman J, Gold L I, Weissmann G. J Immunol. 1993;151:1491–1499. [PubMed] [Google Scholar]
- 10.Newton R A, Thiel M, Hogg N. J Leukocyte Biol. 1997;61:422–426. doi: 10.1002/jlb.61.4.422. [DOI] [PubMed] [Google Scholar]
- 11.Wahl S M, Feldman G M, McCarthy J B. J Leukocyte Biol. 1996;59:789–796. doi: 10.1002/jlb.59.6.789. [DOI] [PubMed] [Google Scholar]
- 12.Perretti M, Flower R J. J Immunol. 1993;150:992–999. [PubMed] [Google Scholar]
- 13.Perretti M, Ahluwalia A, Harris J G, Goulding N J, Flower R J. J Immunol. 1993;151:4306–4314. [PubMed] [Google Scholar]
- 14.Cuzzocrea S, Tailor A, Zingarelli B, Salzman A L, Flower R J, Szab/c C, Perretti M. J Immunol. 1997;159:5089–5097. [PubMed] [Google Scholar]
- 15.Perretti M, Flower R J. Br J Pharmacol. 1996;118:605–610. doi: 10.1111/j.1476-5381.1996.tb15444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancuso F, Flower R J, Perretti M. J Immunol. 1995;155:377–386. [PubMed] [Google Scholar]
- 17.Perretti M, Croxtall J D, Wheller S K, Goulding N J, Hannon R, Flower R J. Nat Med. 1996;22:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 18.Bienvenu K, Granger D N. Am J Physiol. 1993;264:H1504–H1508. doi: 10.1152/ajpheart.1993.264.5.H1504. [DOI] [PubMed] [Google Scholar]
- 19.Getting S J, Flower R J, Perretti M. Br J Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Errasfa M, Russo-Marie F. Br J Pharmacol. 1989;97:1051–1058. doi: 10.1111/j.1476-5381.1989.tb12561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mira J-P, Dubois T, Oudinet J-P, Lukowski S, Russo-Marie F, Geny B. J Biol Chem. 1997;272:10474–10482. doi: 10.1074/jbc.272.16.10474. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra D, Buist-Arkema R, Klappe K, Reutelingsperger C P M. Biochemistry. 1993;32:14194–14202. doi: 10.1021/bi00214a019. [DOI] [PubMed] [Google Scholar]
- 23.Andree H A M, Willems G M, Hauptmann R, Maurer-Fogy I, Stuart M C A, Hermens W T, Frederick P M, Reutelingsperger C P M. Biochemistry. 1993;32:4634–4640. doi: 10.1021/bi00068a022. [DOI] [PubMed] [Google Scholar]
- 24.Smith D B, Johnson K S. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaelin W G, Pallas D C, DeCaprio J A, Kaye F J, Livingston D M. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 26.Berry D A, Lindgren B W. Statistics: Theory and Methods. Pacific Grove, CA: Brooks/Cole Publishing Company; 1990. , 650 pp. [Google Scholar]
- 27.Ajuebor M N, Flower R J, Hannon R, Christie M, Bowers K, Verity A, Perretti M. J Leukocyte Biol. 1998;63:108–116. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira M M, Das A M, Miotla J M, Perretti M, Hellewell P G. Br J Pharmacol. 1997;123:538–544. doi: 10.1038/sj.bjp.0701625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Leech M, Hutchinson P, Holdsworth S R, Morand E F. Inflammation. 1997;21:583–596. doi: 10.1023/a:1027330021479. [DOI] [PubMed] [Google Scholar]
- 30.Goulding N J, Godolphin J L, Sharland P R, Peers S H, Sampson M, Maddison P J, Flower R J. Lancet. 1990;335:1416–1418. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- 31.Morand E F, Hutchinson P, Hargreaves A, Goulding N J, Boyce N W, Holdsworth S. Clin Immunol Immunopathol. 1995;76:195–202. doi: 10.1006/clin.1995.1115. [DOI] [PubMed] [Google Scholar]
- 32.Harris J G, Flower R J, Perretti M. Eur J Pharmacol. 1995;279:149–157. doi: 10.1016/0014-2999(95)00145-b. [DOI] [PubMed] [Google Scholar]
- 33.Von Andrian U H, Hansell P, Chambers J D, Berger E M, Filho I T, Butcher E C, Arfors K-E. Am J Physiol. 1992;263:H1034–H1044. doi: 10.1152/ajpheart.1992.263.4.H1034. [DOI] [PubMed] [Google Scholar]
- 34.Von Andrian U H, Chambers J D, McEvoy L M, Bargatze R F, Arfors K-E, Butcher E C. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seemann J, Weber K, Gerke V. Biochem J. 1996;319:123–129. doi: 10.1042/bj3190123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessian P A, Edgeworth J, Hogg N. J Leukocyte Biol. 1993;53:197–204. [PubMed] [Google Scholar]
- 37.Raynal P, Pollard H B. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 38.Becherucci C, Perretti M, Solito E, Galeotti C, Parente L. Med Inflamm. 1993;2:109–113. doi: 10.1155/S0962935193000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatanaka K, Katori M. Microcirc Ann. 1992;8:129–130. [Google Scholar]
- 40.Hatanaka K, Katori M. Microcirc Ann. 1993;9:153–154. [Google Scholar]
- 41.Perretti M. Trends Pharmacol Sci. 1997;18:418–425. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- 42.Szabó C, Lim L H K, Cuzzocrea S, Getting S J, Zingarelli B, Flower R J, Salzman A L, Perretti M. J Exp Med. 1997;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]