Abstract
Receptors for the Fc region of immunoglobulin G (FcγRs) are expressed on a broad range of haematopoietic cell types and are responsible for regulating antibody production and linking the humoral and effector responses. In response to a number of stimuli, such as cytokine signals or inflammation, FcγR expression at the cell surface is dynamically regulated. On B cells, we observed what appeared to be a correlation between CD22 expression and FcγRIIb expression when the latter was varied in a number of models. Further investigation revealed that this was specific to a particular anti-CD22 monoclonal antibody, which appeared to require stabilization by interaction with FcγRIIb for optimal binding to CD22. Since alterations in the regulation of FcγR expression are important in controlling immune responses and have been associated with a number of immune-mediated disease states, we suggest that it might be prudent to confirm the expression of cell surface markers by two independent methods. Furthermore, because the efficacy of therapeutic antibodies may depend upon their interaction with FcγRs, our results are relevant to their design and assessment.
Keywords: B cell, CD22, FcγRIIb, interleukin-4, therapeutic antibody
Introduction
Receptors for the Fc portion of immunoglobulin G (IgG), the Fc gamma receptors (FcγRs), are cell surface glycoproteins of the immunoglobulin superfamily. These receptors bind IgG through a conserved extracellular structural motif, comprising two or three immunoglobulin-like domains.1 FcγRs then transmit signals to the cell via highly divergent transmembrane and cytoplasmic regions, to control antibody production and link the humoral response to effector mechanisms.2
In mice, four classes of FcγR have so far been identified, which vary with regard to their cellular expression patterns and effector functions.3 FcγRI (CD64), FcγRIII (CD16), and the recently identified Fcγ, are activatory receptors and mediate signalling through a common immunoreceptor tyrosine-based activation motif-containing γ chain. FcγRIIb (CD32b), on the other hand, is an inhibitory receptor with an immunoreceptor tyrosine-based inhibitory motif in its cytoplasmic tail. In addition to their divergent signalling potential, the family of FcγRs vary with regard to their affinity for IgG and their preference for the different IgG subclasses. FcγRI is a high-affinity receptor with a dissociation constant of 10−8–10−9 m for IgG that is capable of binding monomeric IgG.2 FcγRIIb and FcγRIII on the other hand are low-affinity IgG receptors (Kd ∼ 10−6–10−7 m) and bind immunoglobulin of the IgG1, IgG2a and IgG2b isotypes.2 FcγRIV has an intermediate affinity for immunoglobulin (Kd ∼ 10−8 m), but only binds the IgG2a and IgG2b isotypes.4 These lower-affinity receptors interact only with multivalent IgG, presented as immune complexes. The functions of the FcγRs in vivo therefore reflect both their signalling properties and their varying affinities for the different IgG subclasses presented in different contexts.
FcγRs are expressed on a broad range of haematopoietic cell types, including macrophages, eosinophils, neutrophils, dendritic cells, natural killer cells and lymphocytes.2,4 Expression of FcγRs on these different cell types varies between individuals and is dynamically regulated in response to various stimuli and the effects of inflammation.5–9 For example, murine B cells exhibit an approximately threefold increase in FcγRIIb expression upon activation, which is abrogated if the cells are cultured in the presence of interleukin-4 (IL-4), or possess an FcγRIIb promoter polymorphism.5,10,11 Expression of FcγRIIb, and indeed other Fc receptors, is therefore subject to multiple levels of control and is dynamically regulated in response to many stimuli, both in vitro and in vivo.
In this study, we have identified an apparent correlation of CD22 expression with FcγRIIb expression on murine B cells using flow cytometry. Further investigation has, however, revealed that this is specific to a particular anti-CD22 monoclonal antibody (mAb), which apparently requires stabilization through its interaction with FcγRIIb for optimal binding to CD22. Since FcγR levels on cells are frequently altered in disease states,12 and artificially when studying the mechanisms involved in disease,13 this result has important implications for the study of other cell surface molecules in these contexts. In addition, the potential for surface-bound mAbs to interact with FcγRs expressed in cis on the cell surface has implications for the design and assessment of therapeutic antibodies.
Materials and methods
Mice
FcγRIIb-deficient mice on a C57BL/6 background were provided by J. Ravetch and S. Bolland (Rockefeller University, New York, NY). CD22-deficient mice on a C57BL/6 background were provided by M. Neuberger (Laboratory of Molecular Biology, Cambridge, UK). B-cell-specific FcγRIIb transgenic mice and non-transgenic littermate controls were on a mixed CBA/C57BL/6 background. ST6Gal I-sialyltransferase knockout splenocytes were a kind gift from L. Nitschke (University of Erlangen, Germany). All other mice were purchased from Charles River Laboratories (Margate, UK).
Cell lines
The A20 cell line was maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich, Poole, UK), supplemented with 10% fetal bovine serum (Invitrogen, Paisley, UK), 1 mm glutamine (Sigma-Aldrich), antibiotics and 1 × 10−5 m 2-mercaptoethanol. The FcγRIIb-deficient cell line IIA1.6, described in ref. 14 was maintained in a similar way.
Cell purification and stimulation
Splenic B cells were selected by magnetic cell purification using anti-CD19 beads (Miltenyi Biotech, Woking, UK), to > 95% purity, according to the manufacturer's instructions. Cells were cultured (4 × 106 cells/ml) in 12-well flat-bottomed plates (Corning Inc., Artington, UK) in RPMI-1640 (Sigma-Aldrich), supplemented with 10% fetal bovine serum, 1 mm glutamine, antibiotics and 1 × 10−5 m 2-mercaptoethanol. Stimulation was with either 10 μg/ml goat anti-mouse IgM μ-chain specific F(ab′)2 (Jackson ImmunoResearch Laboratories, West Grove, PA) or 10 ng/ml lipopolysaccharide (LPS) from Salmonella minnesota (Sigma-Aldrich) for 48 hr, with or without 10 ng/ml IL-4 (Peprotech, London, UK).
Flow cytometry
The fluorescein isothiocyanate-conjugated anti-CD22 mAbs 2D6 (NIM-R6) and Cy34.1 were obtained from Southern Biotech and BDPharmingen (Oxford, UK), respectively, and used at a 1 : 400 dilution for flow cytometry. Other antibodies were purchased from BDPharmingen. Biotinylated Sambucus nigra agglutinin was obtained from Vector Laboratories (Peterborough, UK), and used at 2 μg/ml. Allophycocyanin-conjugated streptavidin was from Molecular Probes (Invitrogen, Paisley, UK) and biotinylated sialoside probes (used at 10 μg/ml) were provided by the Consortium for Functional Glycomics (grant number GM62116; compound numbers PA211 and PA209).15 The probes were: Neu5Gcα2-6Galβ1-4GlcNAcβ-SpNH-PAA and Neu5Gcα2-3Galβ1-4GlcNAcβ-SpNH-PAA, abbreviated to α2,6 NeuGc and α2,3 NeuGc, respectively. Cells were stained with the appropriate antibody/probe combination in phosphate-buffered saline (PBS) containing 0·5 μg/ml 2.4G2 (anti-FγRII/III) and 1% normal rat serum. Cells were counterstained with 7-aminoactinomycin D to exclude dead cells and analysed using a FACSCalibur™ flow cytometer (Becton Dickinson, Plymouth, UK) and FCS Press software (Ray Hicks, University of Cambridge, UK).
Semi-quantitative real-time polymerase chain reaction
RNA was extracted from cells using RNeasy™ columns and reagents (Qiagen, Crawley, UK) and reverse transcribed using Super reverse transcriptase (HT Biotechnology, Cambridge, UK). Levels of CD22 messenger RNA (mRNA) were assessed relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using real-time semi-quantitative polymerase chain reaction (ABI Prism 7700 Sequence Detection System; Applied Biosystems, Foster City, CA). The GAPDH control primers and probe were Taqman rodent control reagents (Applied Biosytems). The CD22 primers and probe were designed using Primer Express software (Applied Biosystems) and manufactured by Sigma-Genosys (Haverhill, UK).
Western blotting
Cells were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (Upstate, Lake Placid, NY), supplemented with 1 × Complete™ Mini, ethylenediaminetetraacetic acid-free protease inhibitors (Roche, Burgess Hill, UK), 2 mm phenylmethylsulphonyl fluoride, 2 mm Na3VO4 and 2 mm NaF. Lysates were clarified by centrifugation (14 000 g for 15 min at 4°), boiled for 5 min in Laemmli buffer containing 0·1 m dithiothreitol and resolved by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. CD22 was detected using the anti-CD22 mAb MB22-1, which was a kind gift from T. Tedder (Duke University, Durham, NC). β-Actin was used as a loading control and was detected with the mouse AC-15 mAb (Abcam, Cambridge, UK). Densitometry was performed using Quantity One software (Bio-Rad Laboratories, Inc., Hemel Hempstead, UK).
Antibody deglycosylation
Fluorescein isothiocyanate-conjugated 2D6 mAb was deglycosylated by treatment with peptide N-glycosidase F, purified from Flavobacterium meningosepticum (Roche Applied Biosciences, Burgess Hill, UK), for 24 hr at 37°. One unit of enzyme was used per microgram of antibody.
Results
Binding of mAb 2D6 to CD22 correlates with FcγRIIb expression
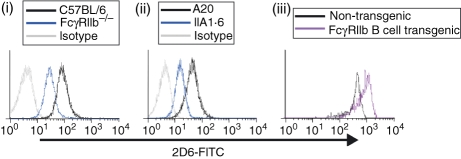
Previous studies in our laboratory, and others, have demonstrated that when murine B cells are activated in the presence of IL-4, surface expression of FcγRIIb, CD22 and CD72 is reduced compared to in cells activated in the absence of cytokine.10 It appeared that expression of these receptors was co-ordinately regulated, so we sought to establish whether deficiency in one receptor, namely FcγRIIb, could affect the expression of other receptors. B cells were isolated from the spleens of FcγRIIb-deficient mice and C57BL/6 control mice and the expression of CD22 (Fig. 1) and CD72 (data not shown) was examined by flow cytometry. Whether the cells remained untreated or were cultured for 48 hr in the presence of LPS or anti-μ F(ab′)2 (data not shown), staining of cells by the rat anti-CD22 IgG1κ mAb 2D6 was reduced in FcγRIIb-deficient cells compared to control cells. This effect was also observed in the FcγRIIb-deficient cell line IIA1.6, which demonstrated reduced 2D6 staining compared to the FcγRIIb-sufficient A20 parental cell line (Fig. 1).
Figure 1.
Expression of CD22 as assessed by monoclonal antibody (mAb) 2D6 is affected by expression of FcγRIIb. Binding of the anti-CD22 mAb 2D6 to B220+ splenocytes (i and iii), or B-cell lines (ii), was examined by flow cytometry. Histograms are representative of at least four independent experiments in each case.
Since FcγRIIb deficiency could reduce binding of the mAb 2D6, we wondered whether overexpression of this receptor might serve to enhance antibody binding. This was indeed the case, as demonstrated by the staining of B cells from an FcγRIIb-overexpressing transgenic mouse (RJ Brownlie, KE Lawlor, HA Niederer, AJ Cutler, Z Xiang, MR Clatworthy, RA Floto, DR Greaves, PA Lyons, KGC Smith, unpublished data). Compared to B cells from non-transgenic littermates, FcγRIIb-overexpressing B cells bound considerably more 2D6 mAb (Fig. 1).
Expression of CD22 is unaffected by FcγRIIb deficiency
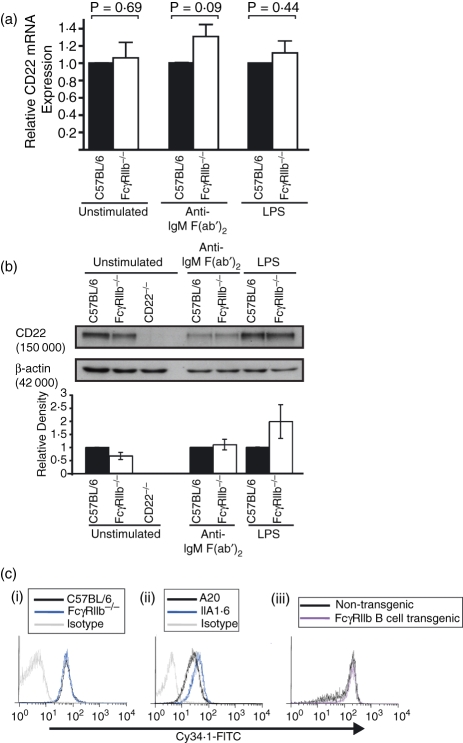
Given that binding of mAb 2D6 to murine B cells correlated with the expression of FcγRIIb, we investigated whether CD22 expression was reduced in the FcγRIIb-deficient mouse. CD19+ B cells were purified from the spleens of FcγRIIb-deficient and control mice by magnetic cell sorting. Cells were either left untreated, or were cultured for 48 hr in the presence of anti-μ F(ab′)2, or LPS. Expression of CD22 mRNA was examined by semi- quantitative real-time polymerase chain reaction and normalized to GAPDH. Compared to control B cells, FcγRIIb-deficient cells expressed similar levels of CD22 mRNA (Fig. 2a). Similarly, the abundance of CD22 protein in FcγRIIb-deficient B cells was comparable to controls, as demonstrated by Western blotting and subsequent densitometry (Fig. 2b). Upon stimulation with anti-μ F(ab′)2 or LPS, no significant difference in CD22 expression was observed at either the mRNA or protein level.
Figure 2.
CD22 expression as assessed by semi-quantitative real-time polymerase chain reaction (SQRT-PCR), Western blot and monoclonal antibody (mAb) Cy34.1 is not affected by expression of FcγRIIb. To determine CD22 expression at the messenger RNA (mRNA) and protein levels, B cells were purified by magnetic cell sorting and cultured with anti-μ F(ab′)2 or lipopolysaccharide (LPS) for 48 hr. Levels of CD22 mRNA were quantified (relative to GAPDH) using SQRT-PCR, and expressed relative to controls for each stimulation condition (a). CD22 protein was detected by Western blotting and normalized to a β-actin loading control by densitometry (b). Binding of the anti-CD22 mAb Cy34.1, to B220+ splenocytes (i and iii), or B-cell lines (ii), was examined by flow cytometry (c). Results for SQRT-PCR and protein analysis are expressed as mean + SD for three independent experiments. P-values refer to a Student's t-test analysis.
Since expression of CD22 in resting cells is not altered by FcγRIIb deficiency, we examined the CD22 surface expression by flow cytometry using an alternative anti-CD22 antibody; the mouse anti-CD22 IgG1κ mAb, Cy34.1. In agreement with our expression data, this antibody bound equally well to both FcγRIIb-deficient and control B cells. Furthermore, Cy34.1 showed no significant difference in its ability to stain A20 and IIA1.6 cells, nor could it differentiate between the FcγRIIb transgenic and control B cells (Fig. 2c). Therefore, we conclude that surface expression of CD22 is not affected by FcγRIIb deficiency but that binding of mAb 2D6 to CD22 depends upon the abundance of FcγRIIb at the cell surface.
Alterations in 2D6 binding do not reflect changes in CD22 accessibility
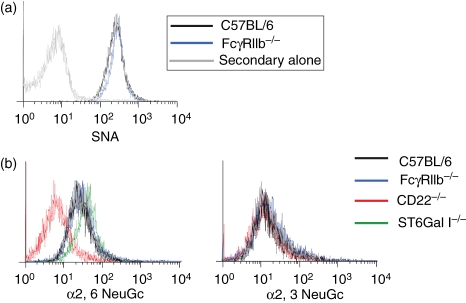
On the B-cell surface, CD22 exists predominantly as homomultimeric clusters, located in clathrin-rich membrane microdomains.16 Maintenance of this distribution is critically dependent upon cis interactions between CD22 and its ligand; α2,6-linked sialic acid (α2,6Sia).17 In the absence of α2,6Sia, CD22 becomes more dispersed throughout the cell membrane and its binding site becomes ‘unmasked’ such that it becomes more available to bind exogenous ligands, such as synthetic sialoside probes.18 The mAb 2D6 binds to a more membrane-proximal epitope than Cy34.1,19,20 so one might envisage that accessibility to this epitope would be more sensitive to CD22 clustering than that of Cy34.1. We therefore sought to establish whether clustering of CD22 was altered in the absence of FcγRIIb by examining two parameters: the abundance of CD22 ligand (α2,6Sia) at the cell surface, and the ability of CD22 to bind exogenous sialoside probes. Using Sambucus nigra agglutinin, a lectin that binds specifically to α2,6Sia, we observed that ligand abundance at the B-cell surface was unaffected by the absence of FcγRIIb (Fig. 3a). We next examined the ability of an α2,6-linked synthetic sialoside (α2,6 NeuGc) to bind to CD22 on FcγRIIb-deficient and control B cells (Fig. 3b). Binding of α2,6 NeuGc to B cells was demonstrated to be specific for CD22, because only background levels of staining were achieved with CD22-deficient cells, and because binding of a negative control (α2,3 NeuGc) was equivalent for cells of all genotypes tested. In accordance with published results,21 B cells from ST6Gal I knockout mice, which lack the enzyme required to produce the CD22 ligand, stained very strongly with the α2,6-linked probe, indicating that CD22 on these cells is unmasked and available to interact with ligands delivered in trans. Less staining was observed in control C57BL/6 B cells, as has been described previously, and critically this was equivalent to the level of staining achieved in FcγRIIb-deficient cells. We can therefore conclude that masking of the CD22 ligand binding site is not affected by FcγRIIb and, as a result, it seems unlikely that changes in CD22 membrane localization are responsible for the differential binding of the two anti-CD22 mAbs.
Figure 3.
CD22 ligand abundance and masking of the CD22 binding site are unaffected by FcγRIIb expression. Splenocytes from FcγRIIb−, CD22− and ST6Gal I-deficient mice were incubated with biotinylated Sambucus nigra agglutinin (SNA) lectin (a) or sialoside probes (b), followed by allophycocyanin-conjugated streptavidin. Staining of B220+ cells was compared to C57BL/6 controls and traces are representative of cells from three mice in each case.
The mAb 2D6 requires stabilization by FcγRIIb for optimal binding to CD22
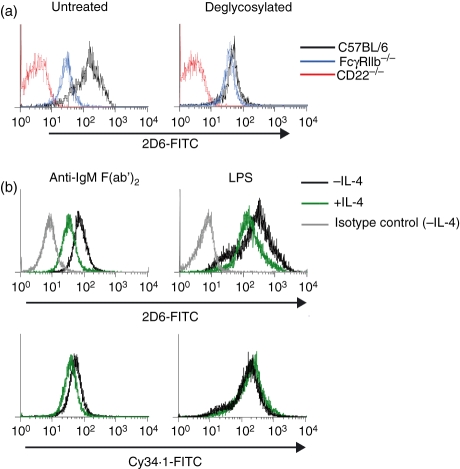
Since optimal binding of 2D6, but not Cy34.1, to B cells is dependent upon the presence of FcγRIIb, we wondered whether the Fc portion of mAb 2D6 might interact with FcγRIIb. This hypothesis appeared unlikely, because all staining was conducted in the presence of the FcγRII/III-blocking antibody 2.4G2, and 1% normal rat serum. Furthermore, staining of B cells with mAb 2D6 was clearly dependent upon the presence of its cognate antigen, because no staining was observed on CD22-deficient B cells (Fig. 4a, ‘untreated’). However, the possibility remained that binding of 2D6 to CD22 could be stabilized through concomitant interaction of its Fc portion with FcγRIIb. Since rat IgG1 antibodies are notoriously difficult to fragment to high purity,22 we chose an alternative method to abrogate binding of this mAb to FcγRs. Removal of the glycan attached at Asn-297 of the Fc portion of antibodies completely prevents binding of IgG to Fc receptors.23,24 We therefore treated mAb 2D6 with protein-N-glycosidase F (PNGase F) to remove all the N-linked glycans, and compared its ability to bind FcγRIIb-deficient and control B cells with that of the mock-treated antibody. While binding of native, and PNGase F-treated, antibody was similar in FcγRIIb-deficient B cells, enzymatic treatment reduced staining of control B cells to a level that was approximately equivalent to that in FcγRIIb-deficient cells (Fig. 4a). Therefore, binding of the Fc portion of mAb 2D6 to FcγRIIb accounts entirely for the apparent correlation of staining with FcγRIIb expression, and this FcγRIIb-dependent binding persisted when higher concentrations of the mAb 2D6 were used (data not shown).
Figure 4.
The monoclonal antibody (mAb) 2D6 interacts with FcγRIIb on B cells and gives rise to artefactual changes in CD22 expression upon interleukin-4 (IL-4) treatment. Binding of protein-N-glycosidase F-treated, or mock-treated, mAb 2D6 to FcγRIIb-deficient and control B220+ splenocytes was examined by flow cytometry (a). (b) Purified splenic B cells were stimulated with anti-μ F(ab′)2 or lipopolysaccharide (LPS) for 48 hr, in the presence or absence of 10 ng/ml IL-4 as indicated. Binding of mAbs 2D6 and Cy34.1 was assessed by flow cytometry. Traces are representative of cells from three mice in each case.
Immunophenotyping with mAb 2D6 is affected by FcγRIIb expression
The effect of FcγRIIb on the binding of this mAb, and potentially others, has important implications for immunophenotyping. Changes in FcγR expression, in response to various physiological and experimental stimuli, may give rise to differential staining by certain antibodies, which could be incorrectly interpreted as changes in expression of the corresponding cell surface molecules. In light of this finding, we reconsidered the effect of IL-4 on CD22 expression on activated B cells. We have previously reported that IL-4 reduces CD22 expression, but that changes in CD22 mRNA were significantly less pronounced than those observed for other inhibitory receptors.10 We therefore re-examined expression of CD22 on activated B cells in the presence or absence of IL-4 using Cy34.1 mAb, which is unaffected by FcγRIIb expression (Fig 4b). We discovered that while CD22 measured by mAb 2D6 was apparently reduced in IL-4-treated cells, this was not the case when detected by mAb Cy34.1. Although a small ‘true’ difference in CD22 expression was observed in cells stimulated with anti-μ F(ab′)2, this was of a much smaller magnitude than that previously determined using mAb 2D6 (Fig. 4b and ref. 10). It is interesting to note, therefore, that while IL-4 has little impact on CD22 expression, it completely abolishes its function.10 This suggests another level at which the inhibitory activity of CD22 might be regulated, and is the focus of further investigation.
Discussion
In this study, we demonstrate that binding of the anti-CD22 mAb 2D6 to murine B cells correlates directly with FcγRIIb expression. This appears to be a consequence of stabilization of the mAb through interaction with FcγRIIb, because the effect is completely abrogated upon deglycosylation of the antibody. Nevertheless, we cannot completely rule out the possibility that stabilization of mAb may also involve a third-party molecule, whose expression or accessibility is affected by FcγRIIb. This result has important implications for the study of other cell surface molecules, because FcγRIIb expression is highly variable and may give rise to changes in mAb binding that could be incorrectly interpreted as changes in expression or conformation of the corresponding cell surface molecules. Unlike common staining artefacts, where antibody binds directly to FcγRs, the effect that we observe is antigen specific, and absolutely dependent on the presence of CD22. It would therefore appear that coexpression of FcγRIIb on the cell surface stabilizes the interaction of 2D6 with CD22, shifting the binding equilibrium to favour a greater degree of staining. Even at higher concentrations of 2D6, maximal staining cannot be achieved in the absence of FcγRIIb. It is not entirely clear why this should be so, and why FcγRIIb expression does not affect Cy34.1 binding in the same way. One possibility is that mAb 2D6 has a particularly low affinity for CD22, and is therefore highly susceptible to the increase in avidity afforded by binding to the Fc receptor. However, the affinity of this antibody for CD22 has been estimated at 1·2 × 108 m−1,25 well within the normal range for mAbs. Nevertheless, FcγRIIb does appear to be a limiting factor for 2D6 binding and this effect appears to be ‘dose-dependent’. Remarkably, over a range of FcγRIIb densities, from zero to the 10-fold excess (relative to controls) expressed by FcγRIIb transgenic B cells, we do not appear to reach a level at which the effects of FcγRIIb become saturating. This would indicate that CD22 is present in substantial excess on the cell surface. Consistent with this prediction, the stoichiometry has been estimated at 10 : 1 CD22 molecules to FcγRIIb, though this is likely to be an underestimate given that it was obtained using mAb 2D6 (which from the data presented in this paper would be expected to bind only a fraction of the total CD22 molecules present on the cell surface).25–27
This example of FcγRIIb-dependent mAb binding is not an isolated one. A similar binding pattern was observed with an anti-CD72 mAb,28 and this was interpreted to be the result of the creation of a ‘neo-epitope’ that is influenced by FcγRIIb. It seems likely, however, that this was in fact the result of FcγRIIb-dependent binding of the anti-CD72 mAb, because the effect occurred on resting cells (in the absence of CD72 : FcγRIIb colocalization) and because binding of a F(ab′)2 fragment to B cells showed no dependence on FcγRIIb expression. While these examples relate to murine B cells, and the role of FcγRIIb, it is likely that this phenomenon is more widespread, extending to multiple cell types and involving other members of the Fc receptor family. It would seem prudent that immunophenotyping of surface antigens be verified using an alternative reagent, or independent method, where possible.
Our results are also relevant to the design of therapeutic mAbs, because interaction of these agents with Fc receptors is critical for their efficacy.29 Many therapeutic mAbs recruit FcγR-expressing effector cells to facilitate the elimination of target cells through mechanisms such as phagocytosis and antibody-dependent cell-mediated cytotoxicity. Consistent with these functions, the effectiveness of anti-tumour mAb therapy and of B-cell depletion by rituximab (anti-CD20) in the treatment of systemic lupus erythematosus correlates with FcγR genotype.30–33 Numerous attempts have therefore been made to develop mAbs with enhanced FcγR affinity, and these modifications have been accompanied by improvements in the efficacy of these therapies.34–37 Our results show that the degree of binding of mAbs to cell surface antigens may vary up to 10-fold depending on the level of expression of FcγRIIb. This would be expected to impact on the therapeutic efficacy of such antibodies, especially when binding to circulating cells, and may also influence the effect of relevant autoantibodies (e.g. anti-lymphocyte antibodies in systemic lupus erythematosus and anti-neutrophil antibodies in immune-mediated neutropenia38,39). This should be borne in mind when developing and assessing therapeutic mAbs.
Acknowledgments
We would like to thank E. Walker and R. Brownlie for experimental assistance and advice, and G. Häcker, D. Fearon and P. Crocker for helpful discussion. J.A.W. was supported by a Wellcome Trust 4-year PhD studentship. K.G.C.S. is supported by a Wellcome Trust Clinician Research Leave Award and is a Lister Prize Fellow. [Correction added after online publication, 21st February 2008, where the following statement was omitted from the Acknowledgements section]. We wish to acknowledge the Consortium for Functional Glycomics Grant number GM62116 for the provision of sialoside probes.
Conflict of interest
The authors declare that they have no competing financial interests.
Glossary
Abbreviations:
- Anti-μ
anti-immunoglobulin M (μ-chain)
- FcγR
Fc γ receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IgG
immunoglobulin G
- IL-4
interleukin-4
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- α2,6Sia
α2,6-linked sialic acid
References
- 1.Hulett MD, Osman N, McKenzie IFC, Hogarth PM. Chimeric Fc receptors identify functional domains of the murine high affinity receptor for IgG. J Immunol. 1991;147:1863–8. [PubMed] [Google Scholar]
- 2.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Pritchard NR, Cutler AJ, Uribe S, Chadban SJ, Morley BJ, Smith KGC. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–30. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 6.Su K, Yang H, Li X, Gibson AW, Cafardi JM, Zhou T, Edberg JC, Kimberly RP. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–80. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarmay G, Rozsnyay Z, Szabo I, Biro A, Gergely J. Modulation of type II Fc gamma receptor expression on activated human B lymphocytes. Eur J Immunol. 1991;21:541–9. doi: 10.1002/eji.1830210303. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Gao X, Masuda E, Redecha PB, Blank MC, Pricop L. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen–antibody complexes. J Immunol. 2006;177:8440–7. doi: 10.4049/jimmunol.177.12.8440. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Masuda E, Blank MC, Kirou KA, Gao X, Park MS, Pricop L. Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes. J Leukoc Biol. 2005;77:767–76. doi: 10.1189/jlb.0904532. [DOI] [PubMed] [Google Scholar]
- 10.Rudge EU, Cutler AJ, Pritchard NR, Smith KGC. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and Fc gamma RII-mediated B cell suppression. J Exp Med. 2002;195:1079–85. doi: 10.1084/jem.20011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y, Hirose S, Sanokawa-Akakura R, et al. Genetically determined aberrant down-regulation of FcgammaRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int Immunol. 1999;11:1685–91. doi: 10.1093/intimm/11.10.1685. [DOI] [PubMed] [Google Scholar]
- 12.Stefanescu RN, Olferiev M, Liu Y, Pricop L. Inhibitory Fc gamma receptors: from gene to disease. J Clin Immunol. 2004;24:315–26. doi: 10.1023/B:JOCI.0000029105.47772.04. [DOI] [PubMed] [Google Scholar]
- 13.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–3. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 14.Jones B, Tite JP, Janeway CA., Jr Different phenotypic variants of the mouse B cell tumor A20/2J are selected by antigen- and mitogen-triggered cytotoxicity of L3T4-positive, I-A-restricted T cell clones. J Immunol. 1986;136:348–56. [PubMed] [Google Scholar]
- 15.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci U S A. 2004;101:6104–9. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S, Collins BE, Bengtson P, Paulson JC. Homomultimeric complexes of CD22 in B cells revealed by protein–glycan cross-linking. Nat Chem Biol. 2005;1:93–7. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 17.Grewal PK, Boton M, Ramirez K, et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol Cell Biol. 2006;26:4970–81. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci U S A. 1998;95:7469–74. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath D, van der Merwe PA, Kelm S, Bradfield P, Crocker PR. The amino-terminal immunoglobulin-like domain of sialoadhesin contains the sialic acid binding site. Comparison with CD22. J Biol Chem. 1995;270:26184–91. doi: 10.1074/jbc.270.44.26184. [DOI] [PubMed] [Google Scholar]
- 20.van der Merwe PA, Crocker PR, Vinson M, Barclay AN, Schauer R, Kelm S. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J Biol Chem. 1996;271:9273–80. [PubMed] [Google Scholar]
- 21.Collins BE, Blixt O, Bovin NV, Danzer CP, Chui D, Marth JD, Nitschke L, Paulson JC. Constitutively unmasked CD22 on B cells of ST6Gal I knockout mice: novel sialoside probe for murine CD22. Glycobiology. 2002;12:563–71. doi: 10.1093/glycob/cwf067. [DOI] [PubMed] [Google Scholar]
- 22.Rousseaux J, Rousseaux-Prevost R, Bazin H. Optimal conditions for the preparation of Fab and F(ab′)2 fragments from monoclonal IgG of different rat IgG subclasses. J Immunol Methods. 1983;64:141–6. doi: 10.1016/0022-1759(83)90392-7. [DOI] [PubMed] [Google Scholar]
- 23.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunol Lett. 2002;82:57–65. doi: 10.1016/s0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 24.Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, Jefferis R. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539–47. doi: 10.1074/jbc.M107478200. [DOI] [PubMed] [Google Scholar]
- 25.Illidge TM, Cragg MS, McBride HM, French RR, Glennie MJ. The importance of antibody-specificity in determining successful radioimmunotherapy of B-cell lymphoma. Blood. 1999;94:233–43. [PubMed] [Google Scholar]
- 26.Pure E, Witmer MD, Lum JB, Mellman I, Unkeless JC. Properties of a second epitope of the murine Fc receptor for aggregated IgG. J Immunol. 1987;139:4152–8. [PubMed] [Google Scholar]
- 27.Honeychurch J, Tutt AL, Valerius T, Heijnen IA, Van De Winkel JG, Glennie MJ. Therapeutic efficacy of FcgammaRI/CD64-directed bispecific antibodies in B-cell lymphoma. Blood. 2000;96:3544–52. [PubMed] [Google Scholar]
- 28.Yamashita Y, Phee H, Tudor KS, Rossi MI, Parnes JR, Coggeshall KM, Kincade PW. A unique CD72 epitope suggests a potential interaction with Fc gamma RII/CD32 on B lineage lymphocytes. Hybridoma (Larchmt) 2006;25:107–14. doi: 10.1089/hyb.2006.25.107. [DOI] [PubMed] [Google Scholar]
- 29.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 30.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 31.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration–effect relationship. Cancer Res. 2004;64:4664–9. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 33.Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, Looney RJ. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48:455–9. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 34.Shields RL, Namenuk AK, Hong K, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 35.Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–10. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda Y, Yamada T, Mori K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17:104–18. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- 37.Siberil S, Dutertre CA, Fridman WH, Teillaud JL. FcgammaR: the key to optimize therapeutic antibodies? Crit Rev Oncol Hematol. 2007;62:26–33. doi: 10.1016/j.critrevonc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Winfield JB. Anti-lymphocyte antibodies in systemic lupus erythematosus. Clin Rheum Dis. 1985;11:523–49. [PubMed] [Google Scholar]
- 39.Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Res Ther. 2005;7:208–14. doi: 10.1186/ar1803. [DOI] [PMC free article] [PubMed] [Google Scholar]