Abstract
A long-standing but poorly understood observation in experimental cancer therapy is the heterogeneity in cancer susceptibility to energy deprivation. Here we show that the hexose kinase inhibitor, 2-deoxyl glucose (2-dG), preferentially kills cancer cells with defective Laforin expression and significantly increases the survival of mice with aggressive lymphoma due to a genetic defect of the Laforin-encoding Epm2a gene. Normal cells from Epm2a−/− mice also had greatly increased susceptibility to 2-dG. Thus, Laforin is a novel regulator for cellular response to energy-deprivation and its defects in cancer cells may be targeted for cancer therapy.
Introduction
An important hallmark of many poorly differentiated and rapidly growing malignant tumors is higher rates of glucose usage and glycolysis as compared to corresponding normal tissues (1, 2). Increase in glucose uptake is the earliest change observed in cells after malignant transformation (3, 4). The abnormalities in energy metabolism of cancer cells have been targeted for cancer therapy with mixed success (5–8). The heterogeneity of cancer cell response to energy deprivation suggests that additional modifiers exist. As such, understanding the mechanism for cellular energy response may offer critical insights for cancer therapy. It is established that energy deprivation results in activation of AMPK which in conjunction with GSK3β activates the TSC pathway to inhibit mTOR. Genetic defects of this pathway render cancer cells more susceptible to energy deprivation (9, 10).
Laforin is a dual-specific phosphatase encoded by Epm2a, which was initially identified as one of the causative genes for progressive myoclonus epilepsy (11). Its known substrates include GSK3β (12, 13) and carbohydrates (14). Recently, an elegant genetic study showed that Laforin complements starch excess 4 (SEX4) mutations in the Arabidopsis thaliana (15). In immune compromised mice, Epm2a works as a tumor suppressor gene (13). It is unclear, however, whether Laforin is involved in cellular response to energy deprivation. Here we showed that Laforin is a novel regulator for cellular response to energy-deprivation and its defects in cancer cells may be targeted for cancer therapy.
Results and discussion
In analyzing Laforin expression among cancer cell lines, we found great heterogeneity of its expression. For example, among 4 murine tumor cell lines tested, MC38 had a high level of Laforin expression, while the lymphoma cell line TGB had no detectable Laforin, and the remaining cell lines had low to intermediate levels (Fig. 1A). Given the function of Laforin in regulating GSK3β activity and the role for GSK3β in the cellular response to energy, we compared these cell lines for their susceptibility to 2-dG treatment. While the TGB lymphoma cells underwent extensive apoptosis, as judged by the % of cells with less than 2C DNA contents in response to 2-dG treatment, MC38 cells were largely resistant (Fig. 1B upper panel). When a panel of tumor cell lines expressing different levels of Laforin were subject to the same treatment, we observed a strong inverse correlation between the Laforin levels and the % of apoptotic cells (Fig. 1B, lower panel).
Fig. 1. Laforin controls cellular response to energy-deprivation.
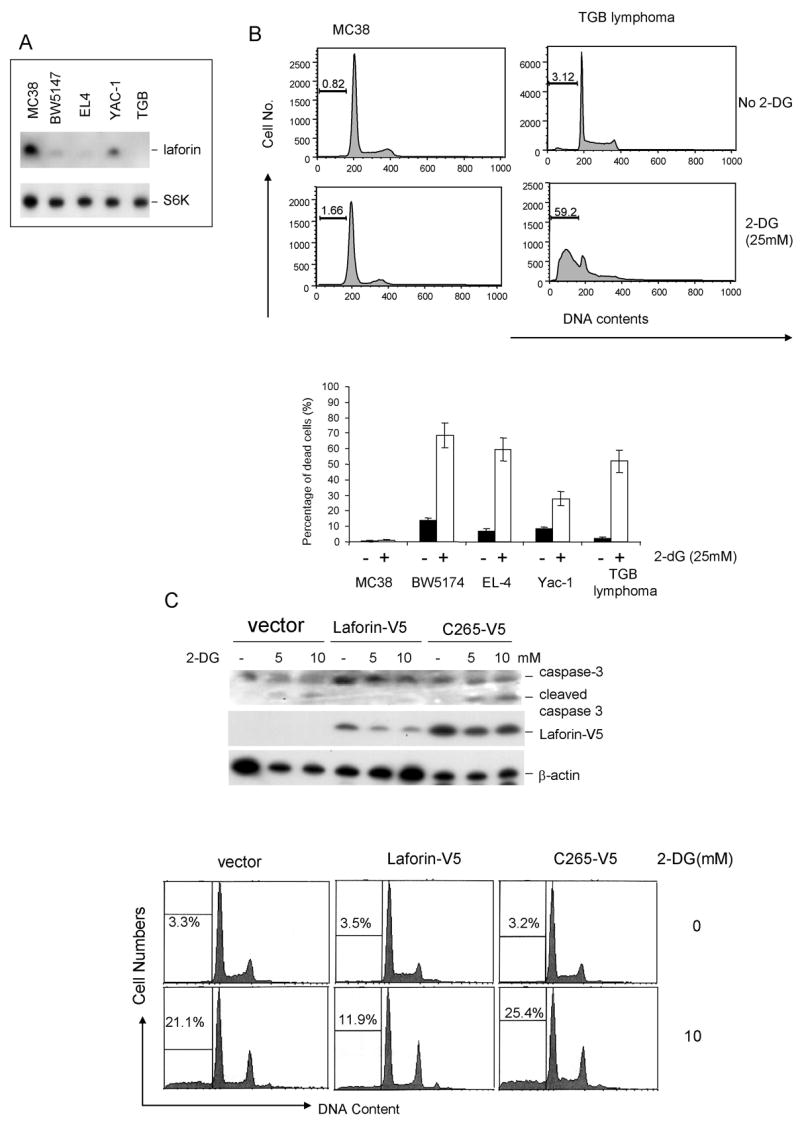
A &B. Inverse correlation between Laforin levels and cancer cell susceptibility to energy deprivation. A. Levels of Laforin in the colorectal cancer cell line MC38, thymoma cell lines BW5147 and EL4, T lymphoma YAC-1 and a cell line derived from TGB lymphoma, as determined by Western blot with anti-Laforin antibody. B. Correlation between Laforin levels and susceptibility to energy-deprivation. Upper panel shows the representative FACS profiles of Laforinhi MC38 and Laforin− TGB lymphoma, depicting DNA contents. The numbers in the panels are % of cells with < 2C DNA contents. The lower panel depict summary of data from 2 independent experiments.
C. Inducible expression of Laforin confers resistance to energy deprivation. EL4 cells were transfected with Tet-off vector with Laforin-V5 cDNA in the presence or absence of tetracyclin for 36 hours and treated with given doses of 2-dG for 24 hours. The harvested cells were analyzed for caspase 3 activation (top panel) or DNA content (lower panel). The % of cells with less than 2C DNA contents is listed in the panels. Data shown have been repeated 3 times.
To directly demonstrate the protective effect of Laforin to energy-deprivation-induced apoptosis, we generated a Tet-off system in the EL4, a mouse T lymphoma cell line with low levels of endogenous Laforin (13). As shown in Fig. 1C, removal of deoxycycline resulted in a significant induction of the transfected Laforin, regardless of the 2-dG in the medium. With the induction of Laforin expression, the EL4 cells gained resistance to 2-dG treatment, as revealed both by activation of caspase 3 and the % of cells with sub-2C DNA contents (Fig. 1C). Thus, expression of Laforin conveyed resistance to 2-dG..
An important issue is whether Laforin is required for resistance of normal cells to 2-dG. In order to address this issue, we compared the sensitivity of T cells from WT and Laforin-deficient mice for their sensitivity to 2-dG. As shown in Fig. 2, targeted mutation of the Epm2a gene drastically increased the sensitivity of the splenic T cells to 2-dG., as revealed by cellular viability (MTT assay, Fig. 2A) and DNA content (Fig. 2B). Taken together, our data demonstrated that Laforin plays a critical role in protecting cells from apopotosis induced by energy deprivation.
Fig. 2. Targeted mutation of the Epm2a gene dramatically increases susceptibility of normal T cells.
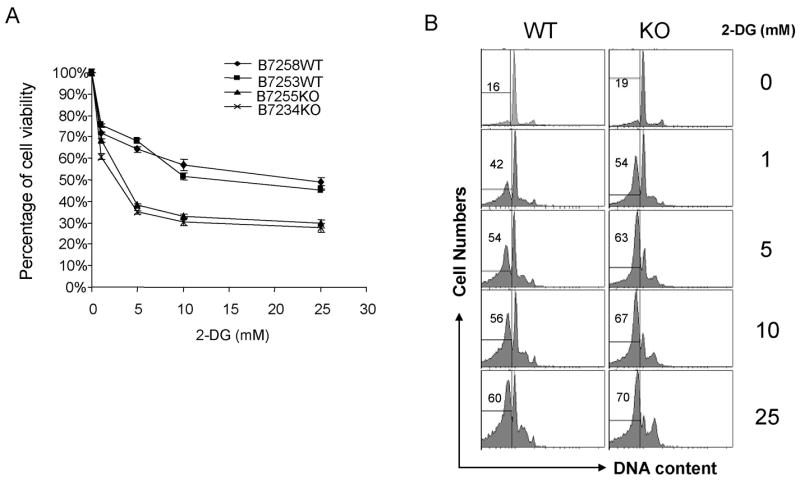
Spleen cells from the WT and Epm2−/− mice were stimulated with anti-CD3 (400ng/ml) for 48 h. After washing with culture medium, the cells were cultured in the presence of given concentration of 2-dG for 24h. The survival of T cells was determined by either MTT assay (A) using the O.D. of the untreated group as 100% (data shown were means and S.D. of triplicates, T cells from 2 mice per group are shown), or by DNA contents (B). These experiments have been repeated twice.
The AMPK-GSK3β-TSC-mTOR pathway plays an important role in regulating cellular energy response (9, 10). As a genetic test, we compared the effect of Laforin knockdown in TSC2-deficient and TSC2-reconstituted EEF8 cells (9, 10) (Fig. 3A). SiRNA silencing of Epm2a significantly increased GSK3β phosphorylation, with the notable exception of 50 mM 2-dG treatment when the cell viability was poor. This, however, did not appreciably affect mTOR activation as revealed by comparable levels of S6K P70 phosphorylation at the T389 site. As expected, activation of AMPK was also unaffected (Fig. 3B). Nevertheless, Laforin knockdown did increase cellular susceptibility to 2-dG regardless of TSC2 expression (Fig. 3C &D). Thus, the Laforin works independently of the TSC-mTOR pathway in regulating cellular response to energy deprivation.
Fig. 3. Laforin confers cellular resistance to energy-deprivation by a TSC-mTOR independent mechanism.

A. Efficacy of siRNA on Laforin levels in TSC2+ and TSC2− cell lines. B. In the TSC2− cell line, Epm2a silencing increases GSK3β phosphorylation without affecting activation of AMPK and S6K. The TSC2−-vector and TSC2−siRNA transfectants were treated with given concentration of 2-dG for 0.5 hours. The cell lysates were harvested and probed with antibodies specific for phosphorylated AMPK (T172), S6K70 (T389), and GSK3β (S9) or total S6K and Laforin. Data shown have been repeated 4 times. C. Cell viability after treatment with given concentration of 2-dG (upper panel) and apoptosis as revealed by caspase 3 activation (lower panel). D. Impact of Epm2a SiRNA on 2-dG-induced apoptosis of TSC2+ and TSC2− cells. The culture media used were: ( C ): DMEM medium+ 4.5g/L glucose+10% dialyzed fetal bovine serum (FBS); G(−):MEM medium+ 0 g/L glucose+10% dialyzed FBS; "−": DMEM medium+1g/L glucose (no FBS); 2-DG: DMEM medium+1g/L glucose+ 2DG (no FBS). Data have been repeated 3 times.
The fact that a large proportion of both mouse and human lymphomas have depressed Laforin expression (13) and that cells with defective Laforin have increased susceptibility to energy deprivation suggest that 2-dG may be effective in treating this type of cancer. We reported that insertional mutation of one allele of the Epm2a gene in conjunction with epigenetic silencing caused lymphoma with rapid onset and 100% lethality within one year (13). This model allowed us to determine if energy deprivation can be therapeutic for lymphoma. Thus, we treated the TgB mice with 2-dG and followed the survival of the mice. As shown in Fig. 4A, regardless of the age of treatment, at either 8–12 weeks when some untreated mice had begun to become moribund, or at 15–20 weeks of age, when 25 to 50% of untreated mice had succumbed to cancer, 2-dG significantly prolonged mouse survival.
Fig. 4. Laforin expression and cancer susceptibility to energy-deprivation.
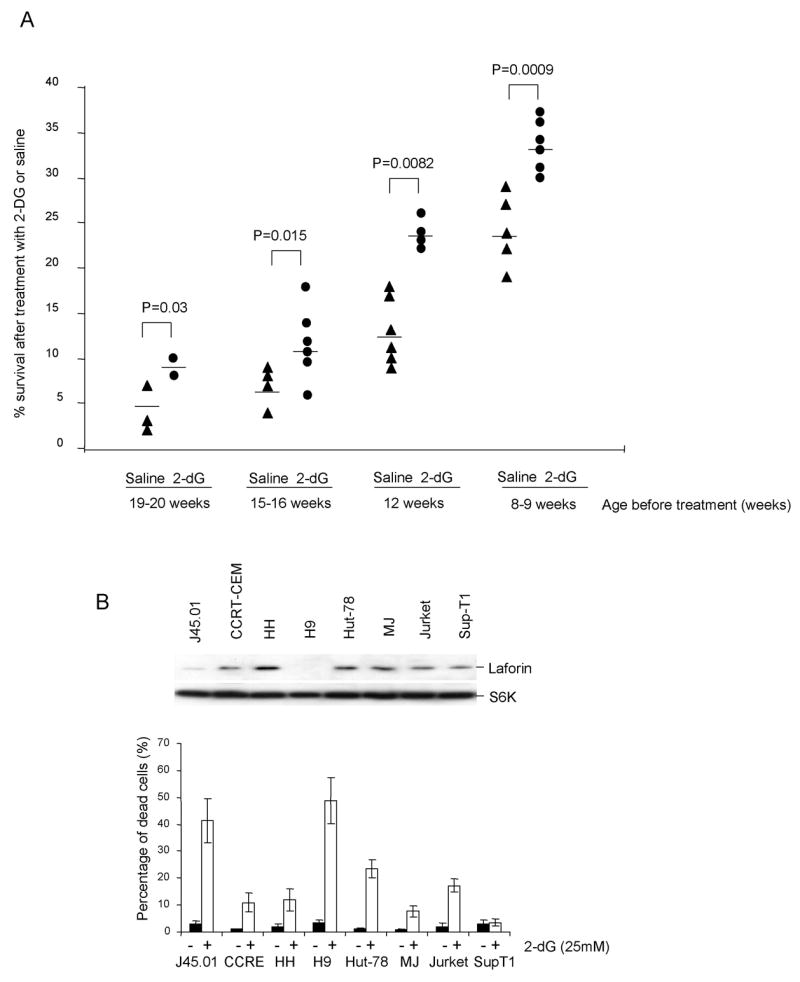
A. 2-dG treatment significantly increases the life span of the TGB mice that develop lymphoma due to genetic and epigenetic defects of the Epm2a gene. TGB mice were grouped and paired according to age and treated with either PBS or 2-dG at the dose of 20mg/mouse/3day at the beginning week, and then 20mg/mouse/week in the following 1month until moribund or death. Data shown indicate the life span after initiation of treatment. B. Inverse correlation between LAFORIN levels and cellular response to 2-dG-treatment. The upper panels show the levels of LAFORIN as determined by Western blot, using total S6K as a loading control. The lower panel shows % of cells with less than 2C DNA contents, as determined by flow cytometry. Data shown are means and S.D. and have been repeated twice.
As we have reported previously (13), human lymphoma cell lines were heterogenous in LAFORIN levels. This feature allowed us to determine whether LAFORIN levels predict cancer susceptibility to 2-dG treatment. As shown in Fig. 4B, when a panel of 8 lymphomas were tested, those with no or the lowest levels of Laforin were significantly more susceptible 2-dG treatment compared to cells displaying high levels of LAFORIN. Notably, among the 6 lines with high levels of LAFORIN and relatively more resistant to 2-dG, there is no strict correlation between LAFORIN levels and susceptibility to 2-dG. These data are best explained by involvement of other factors may regulate cellular energy response (9, 10).
Taken together, we have demonstrated Laforin as a critical checkpoint for cellular susceptibility to energy deprivation-induced apoptosis and that Laforin regulate cell survival under energy-deprivation by a TSC independent mechanism. Since down-regulation of Laforin is quite wide-spread in human lymphoma (13), and since the human chromosome 6q24, where EPM2A resides, is often deleted in human cancer (16), targeting this defect may have a significant impact for cancer therapy.
Experimental Procedure
Antibodies, reagents
Antibodies against the following proteins were used in this study: Anti-Akt, phospho-Akt (Ser 473), phospho-GSK-3β (Ser 9), phospho-AMPK, phospho-S6K70 (T389), phospho-mTor, and caspase-3 (Cell Signaling, Beverly, MA). Anti-V5 and anti-Myc (Invitrogen Corp., Carlsbad, CA), Anti-β-actin (I-19), TSC-2, Hexokinase, S6K, VDAC (Santa Cruz Biotechnology, Inc. Santa Cruz, CA). 2-Deoxy-D-Glucose, and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)(Sigma, St. Louis, MO). Anti-Laforin polyclonal antibody was produced by Genemed Synthesis, Inc. (San Francisco, CA). Rabbits were immunized with the synthetic peptide of 16 amino acid residues (YKFLQREPGGELHWEG, residues 85–100 in Laforin protein, Accession No. AAD26336) coupled with keyhole limpet hemocyanin (KLH) in complete Freund’s adjuvant. The antiserum was purified using peptide conjugated Affigel column.
Plasmids and Transfection
Full length cDNA of Epm2a were amplified by reverse-transcription PCR using high fidelity Taq enzyme (Invitogen) from mouse spleen and cloned into vectors of pcDNA4-V5/His (Invitrogen) at restriction enzyme sites of HindIII/BamHI to get fusion proteins with Myc or V5 tag fused in the C terminus of Laforin. Human embryonic kidney HEK293 cells were transiently transfected or cotransfected with different expression plasmids for 24 hours under a culture of O-PTEM medium containing 10% fetal bovine serum and the pre-mixed mixture of the plasmids with lipofectman-2000 that was done according to the vendor protocol (Invitrogen, San Diego, CA).
EPM2A siRNA constructs
Oligonucleotides encoding siRNA directed against EPM2a at C terminal region of 934 to 954 nucleotides (5’-AAGGTGCAGTACTTCATCATG-3’) (C) inserted into a modified pLenti6/V5-D-TOPO vector (Invitrogen). Lentivirus stocks were produced in 293FT cells according to manufacturer’s protocol. Viral transduced cells were selected with blasticidin.
MTT cell viability assay
Plate out, in triplicate, 100 μl of 1×103 cells per ml into wells of a 96-well microplate, including three control wells of medium alone to provide the blanks for absorbance readings. Incubate the cells under conditions appropriate for the cell line for 12 hrs, the cells were treated with different concentration of 2-DG. 10 μl of 5 mg/ml MTT in PBS were added to each well, including controls. Return plate to cell culture incubator for 2 to 4 hrs. When the purple precipitate is clearly visible under the microscope, remove cultures from incubator and dissolve the resulting MTT formazan crystals by adding 150μl of DMSO. Spectrophotometrically measure absorbance at a wavelength of 490 nm.
PI staining
2-DG treated or control cells were stain by Propidium Iodide (PI) using “PI/RNase Staining Buffer” from BD Biosciences (San Jose, CA) according to product instruction. Briefly, cells were frozen in 70% ethanol overnight, wash with PBS 3 times, and 0.5 ml staining buffer was added to each sample cells. The content of DNA in stained cells was determined by flow cytometry after 15 minutes room temperature incubation.
Acknowledgments
We thank Dr. Kunliang Guan for valuable discussion, for providing us with TSC2+ and TSC2− EEF8 cell lines and for critical reading of the manuscript, Lynde Shaw and Todd Brown for secretarial assistance. This study is supported by grants from the American Cancer Society, the Department of Defense and the National Institutes of Health.
Footnotes
Yin Wang and Yan Liu are equal contributing first authors of the manuscript
References
- 1.Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- 4.Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972;32(10):2007–16. [PubMed] [Google Scholar]
- 5.Cay O, Radnell M, Jeppsson B, Ahren B, Bengmark S. Inhibitory effect of 2-deoxy-D-glucose on liver tumor growth in rats. Cancer Res. 1992;52(20):5794–6. [PubMed] [Google Scholar]
- 6.Kaplan O, Navon G, Lyon RC, Faustino PJ, Straka EJ, Cohen JS. Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 1990;50(3):544–51. [PubMed] [Google Scholar]
- 7.Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64(1):31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 8.Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE 2007. 2007;(381):pe14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- 9.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 11.Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, Dunham I, Gardner R, Fong CY, Carpenter S, Jardim L, Satishchandra P, Andermann E, Snead OC, 3rd, Lopes-Cendes I, Tsui LC, Delgado-Escueta AV, Rouleau GA, Scherer SW. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet. 1998;20(2):171–4. doi: 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- 12.Lohi H, Ianzano L, Zhao XC, Chan EM, Turnbull J, Scherer SW, Ackerley CA, Minassian BA. Novel glycogen synthase kinase 3 and ubiquitination pathways in progressive myoclonus epilepsy. Hum Mol Genet. 2005;14(18):2727–36. doi: 10.1093/hmg/ddi306. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu Y, Wu C, Zhang H, Zheng X, Zheng Z, Geiger TL, Nuovo GJ, Liu Y, Zheng P. Epm2a suppresses tumor growth in an immunocompromised host by inhibiting Wnt signaling. Cancer Cell. 2006;10(3):179–90. doi: 10.1016/j.ccr.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Worby CA, Gentry MS, Dixon JE. Laforin: A dual specificity phosphatase that dephosphorylates complex carbohydrates. J Biol Chem. 2006;281:30412–8. doi: 10.1074/jbc.M606117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry MS, Dowen RH, 3rd, Worby CA, Mattoo S, Ecker JR, Dixon JE. The phosphatase laforin crosses evolutionary boundaries and links carbohydrate metabolism to neuronal disease. J Cell Biol. 2007;178(3):477–88. doi: 10.1083/jcb.200704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noviello C, Courjal F, Theillet C. Loss of heterozygosity on the long arm of chromosome 6 in breast cancer: possibly four regions of deletion. Clin Cancer Res. 1996;2(9):1601–6. [PubMed] [Google Scholar]


