Abstract
Peripheral facial nerve palsy (FNP) may (secondary FNP) or may not have a detectable cause (Bell’s palsy). Three quarters of peripheral FNP are primary and one quarter secondary. The most prevalent causes of secondary FNP are systemic viral infections, trauma, surgery, diabetes, local infections, tumor, immunological disorders, or drugs. The diagnosis of FNP relies upon the presence of typical symptoms and signs, blood chemical investigations, cerebro-spinal-fluid-investigations, X-ray of the scull and mastoid, cerebral MRI, or nerve conduction studies. Bell’s palsy may be diagnosed after exclusion of all secondary causes, but causes of secondary FNP and Bell’s palsy may coexist. Treatment of secondary FNP is based on the therapy of the underlying disorder. Treatment of Bell’s palsy is controversial due to the lack of large, randomized, controlled, prospective studies. There are indications that steroids or antiviral agents are beneficial but also studies, which show no beneficial effect. Additional measures include eye protection, physiotherapy, acupuncture, botulinum toxin, or possibly surgery. Prognosis of Bell’s palsy is fair with complete recovery in about 80% of the cases, 15% experience some kind of permanent nerve damage and 5% remain with severe sequelae.
Keywords: Cranial nerves, Neuropathy, Diabetes, Idiopathic nerve palsy, Therapy, Corticosteroids, Antiretroviral therapy
Introduction
Unilateral peripheral facial nerve palsy may have a detectable cause (secondary facial nerve palsy) or may be idiopathic (primary) without an obvious cause (Bell’s palsy) [1–3]. Secondary facial nerve palsy is due to various causes (Table 1) and is generally less prevalent than Bell’s palsy (25 vs. 75%) [2], first described by NA Friedreich in 1797 [4]. Bell’s palsy is thus a diagnosis of exclusion [5]. Due to the lack of sufficiently powered studies, therapy of primary and secondary facial nerve palsies is controversially discussed, particularly if causes for a secondary facial nerve palsy coexist with Bell’s palsy or if multiple causes of a secondary facial nerve palsy coexist in case of a secondary facial nerve palsy. This mini-review wants to give an overview on the current knowledge about the prevalence, causes, pathogenesis, diagnosis, treatment, and prognosis of primary and secondary facial nerve palsies.
Table 1.
Causes of secondary, unilateral facial nerve palsy
| Cause | Reference |
|---|---|
| Metabolic disease | |
| Diabetes | [16, 82] |
| Preeclampsia | [11] |
| Stroke | |
| Ipsilateral pontine infarction | [83] |
| Pontine tegmental hemorrhage | [82] |
| Infection | |
| Hansen’s disease (leprosy) | [82] |
| Otitis media | [6] |
| Mastoiditis | [6] |
| Herpes simplex infection | [6] |
| Varicella zoster infection | [6] |
| Ramsey–Hunt syndrome | [9] |
| Influenza viruses | [8] |
| Borreliosis | [6] |
| Cryptococcosis | [82] |
| Neurocysticercosis | [16] |
| Toxocarosis | [84] |
| Tuberculous meningitis | [82, 85] |
| Parotitis, parotid abscess | [52] |
| Malignant external otitis | [86] |
| Syphillis | [82] |
| Surgery | |
| Removal of cerebellopontine angle tumours | [71] |
| Trauma | |
| Head trauma (crush injury) | [6, 82, 87] |
| Birth injury | [88] |
| Tumour | |
| Facial nerve neurinoma | [1] |
| Cerebello-pontine angle tumours (neurinoma) | [1] |
| Pons tumour | [1] |
| Tumours of the petrosal bone | [1] |
| Tumours of the middle ear | [1] |
| Leucemia | [1] |
| Tumours of the parotid gland | [1] |
| Lymphoma | [6] |
| Immune system disorder | |
| Guillain–Barre syndrome | [6, 82] |
| Miller–Fisher syndrome | [82] |
| Systemic lupus erythematodes | [82] |
| Myasthenia gravis | [6] |
| Drugs | |
| Interferon | [89] |
| Linezolid | [90] |
| Varia | |
| Moebius syndrome (impaired sixth’s and seventh cranial nerve) | [82] |
| Melkersson–Rosenthal syndromea | [91] |
| Sarkoidosis | [82] |
| Histiocytosis X | [92] |
| Autism | [8] |
| Asperger’s syndrome (hypo/hypersensitivity to sounds) | [8] |
| Parkinson syndrome | [8] |
aMelkersson–Rosenthal syndrome is characterized by recurrent attacks of Bell’s palsy, painless edema of the face, cheilitis granulomatosa (lingua plicata), and fissured tongue
Presentation
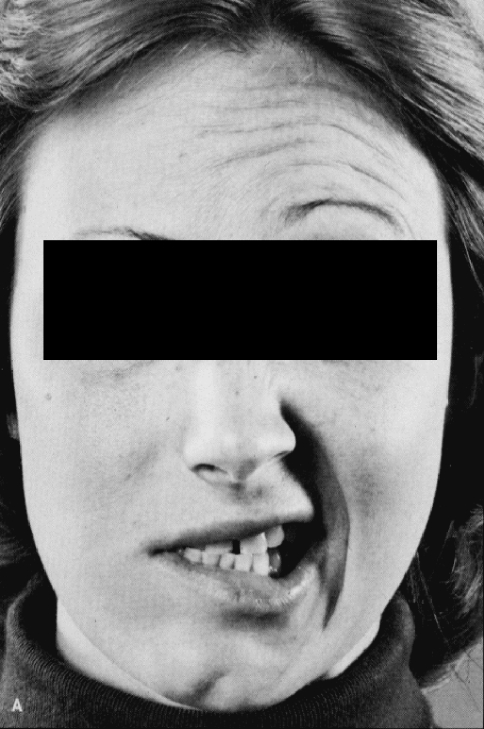
Bell’s palsy is an acute peripheral facial nerve affection, usually affecting only one side of the face. The clinical picture varies, depending on the location of the lesion of the facial nerve along its course to the muscles. Symptoms and signs result from the fact that the facial nerve not only carries motor fibers including fibers to the stapedius muscle but also supplies autonomic innervation of the lacrimal gland, submandibular gland, sensation to part of the ear, and taste to the anterior two thirds of the tongue via the chorda tympani [6]. Thus, Bell’s palsy is diagnosed upon abrupt onset of impaired facial expression due to unilateral facial weakness of all facial nerve branches, dry eye, if saliva runs out of the mouth, the inability to close or wink the eye or close the mouth, to droop the brow or the corner of the mouth, numbness or pain around the ear, temple, mastoid, or angle of the mandible, an altered sense of taste, hypersensitivity to sounds, or decreased tearing (Fig. 1) [6, 7]. If the patient wants to smile he appears unilaterally expressionless [8]. Patients may also mention otalgia, aural fullness, or mild retroauricular pain, which may even precede the palsy [9]. Speech and eating may be also disturbed. Severe pain suggests herpes simplex or zoster infection and may precede a vesicular eruption and progression to Ramsey–Hunt syndrome, characterized by typical cutaneous vesicles and vesicles in the conchal bowl, soft palate, or tongue and by vestibulo-cochlear dysfunction [9]. In half of the herpes zoster infections, vesiculation not necessarily appears or may be delayed (zoster sine herpete). Sometimes the only clinical indication for a herpes zoster is dysesthesia before vesiculation (preherpetic neuralgia). Considering zoster sine herpete is important since it is thought to be the cause of Bell’s palsy in quite a number of cases [9].
Fig. 1.
Right-sided peripheral facial nerve palsy with inability to wrinkle the forehead and nose, unequal lid fissures, and inability to lift the corner of the mouth
Pathogenesis
The etiology of Bell’s palsy is unknown but viral infection, vascular ischemia, or autoimmune disease has been postulated as possible pathomechanisms [6]. Bell’s palsy disproportionally attacks pregnant women, patients who have diabetes, influenza, a cold, some other respiratory alignment, or have undergone tooth root extraction [8]. Some patients report exposure to an air-condition outlet, or an open window before the attack [8]. Also familial occurrence has been reported [10–12]. Increasing evidence implies that Bell’s palsy is caused by latent herpes viruses (herpes simplex, herpes zoster) [9], being reactivated from cranial nerve ganglia [13]. Reactivation of these viruses presumably causes inflammation of the facial nerve [6, 14]. Initially, inflammation of the nerve results in reversible neurapraxia but ultimately Wallerian degeneration [9]. Virus infection with herpes simplex type 1 or herpes zoster may predominantly occur if the immune system is simultaneously compromised [5]. Herpes viruses have been detected by PCR within the facial nerve [15]. There are conflicting results concerning the role of Borrelia burgdorferi in the occurrence of Bell’s palsy. Some studies found an increased prevalence of Borrelia antibodies among patients with Bell’s palsy, whereas others could not confirm these results.
Secondary facial nerve palsy is due to a number of different causes (Table 1). Though it is often difficult to decide if any of these causes is responsible for the clinical picture, it is important to differentiate between the primary and the secondary forms since this may influence therapy and prognosis significantly [16]. Among 180 patients Bell’s palsy was associated with arterial hypertension in 12%, with diabetes in 11%, with pregnancy or puerperium in 4% and neurocysticercosis in 1% [16]. In another study, facial nerve palsy was most frequently associated with viral infections, borreliosis, or diabetes [17]. However, if Bell’s palsy occurs together with a disorder, which also may cause secondary facial nerve palsy, this does not necessarily imply a causal relation.
Frequency
The incidence of Bell’s palsy is estimated to be 20–25 cases per 100,000 population annually [3, 9, 18]. The peak incidence occurs between the second and fourth decade (15–45 years) [2, 19]. Depending on the study, there is either a slight female preponderance [19] or women and men are equally affected [9]. Definitively, the prevalence is increased in pregnant women (43 cases per 100,000) [9]. Facial nerve palsy is uncommon in children under age 2 years. It occurs with equal frequency on the right and left side of the face [19]. Simultaneous, bilateral facial palsy is extremely rare with a prevalence of 0.3–2% of the facial palsies (Table 2) [20]. Bell’s palsy arises more frequently in the spring and fall than any other time of the year [8]. In the US, the annual incidence of newly diagnosed cases is 40,000–50,000 [8].
Table 2.
Causes of bilateral facial nerve palsy [93]
| Diabetes |
| Bilateral Bell’s palsy |
| Borreliosis |
| Mycoplasma pneumoniae infection |
| Guillain–Barre syndrome, Miller–Fisher syndrome |
| Sarcoidosis |
| Moebius syndrome |
| Leukemia |
| Viral infections (Herpes simplex) |
| Syphilis |
| Basal skull fractures |
| Pontine gliomas |
| Linezolid therapy |
| Leprosy |
| Mononucleosis |
| Pregnancy |
| Brainstem encephalitis |
| Hansen’s disease |
| Cryptococcal meningitis |
| Pontine tegmental hemorrhaghe |
| Systemic lupus erythematodes |
| Bulbospinal muscular atrophy |
Assessment of severity
To clinically assess the severity of peripheral facial nerve palsy various scoring systems are available. The most widely applied is the House–Brackmann facial nerve grading system (HBS) (Table 3). Degree of facial nerve palsy can also be assessed by means of the Yanagihara grading system [21], the Sunnybrook scales, the Jadad score of methodological quality, scales on computed systems, and various other systems [22]. Most grading systems rely on the evaluation of resting symmetry, degree of voluntary excursion of the facial muscles, and the degree of synkinesis (involuntary movement accompanying a voluntary movement) triggered by specific voluntary movements [23]. Facial nerve palsy can be categorized as complete if there is inability to voluntarily contract the facial muscles, hyperacusis, or loss of taste [24] or incomplete (partial). The progression of weakness may be additionally assessed by reviewing old photos and comparing them with the actual status. The degree of nerve damage can also be assessed by nerve conduction studies of the facial nerve. Reduction of the compound muscle action potential suggests axonal degeneration whereas increase in latency suggests demyelination of the nerve [25].
Table 3.
House–Brackmann score (HBS) and Yanagihara grading system (Y-system) to grade severity of facial nerve palsy [21] by assessing motility of forehead, eye, nose, and mouth as 1–6
| Grade | HBS | Y-system |
|---|---|---|
| Normal, symmetrical function in all areas | I | 40 |
| Slight weakness on close inspection, complete eye closure with minimal effort, slight asymmetry of smile with maximal effort, slight synkinesis, absent contracture or spasm | II | 32–38 |
| Obvious weakness but not disfiguring, unable to lift eyebrow, complete and strong eye closure, asymmetrical mouth movement with maximal effort, obvious but not disfiguring synkinesis, mass movement or spasm | III | 24–30 |
| Obvious disfiguring weakness, inability to lift brow, incomplete eye closure, and asymmetry of mouth with maximal effort, severe synkinesis, mass movement, spasms | IV | 16–22 |
| Motion barely perceptible, incomplete eye closure, slight movement corner mouth, synkinesis, contracture, spasm usually absent | V | 8–14 |
| No movement, loss of tone, no synkinesis, contracture, spasm | VI | 0–6 |
Diabetes and Bell’s palsy
There are indications that the facial nerve is subclinically involved in 6% of the patients with diabetes [26]. Facial nerve affection, however, is less frequent than limb nerve affection. In a series of 126 patients with Bell’s palsy chemical or overt diabetes was found in 39% of the cases [27]. In this study, impairment of taste was found in 83% of the patients without diabetes as compared to only 14% in diabetic patients [27]. These findings suggest that the lesion in diabetic facial nerve palsy is distal to the chorda tympany but proximal to it and more severe in non-diabetic patients with Bell’s palsy that some cases of Bell’s palsy with normal taste may in fact represent diabetic mononeuropathy [27]. In another study the rate of diabetes was 10% among 38 outpatients with Bell’s palsy. This figure did not differ from the anticipated frequency of diabetes in the general population [28], why it was doubted if a diabetic mononeuropathy of the facial nerve in fact exists. Neither the severity of facial nerve degeneration, as assessed by facial nerve conduction studies, nor the clinical outcome was significantly different between diabetic and non-diabetic patients in a prospective study on 37 patients with Bell’s palsy [29]. In a study on 22 patients with Bell’s palsy, who also suffered from diabetes, auditory brainstem evoked potentials indicated subclinical affection of the eighth cranial nerve [30]. In another study on 21 diabetics with chronic hyperglycemia, the latency of the facial nerve was normal or only slightly prolonged in most of them [31].
Children
Peripheral facial nerve palsy not only occurs in adults but also in children. Nevertheless, facial nerve palsy has been much more intensively investigated in adults than in children [32]. Despite a lot of similarities concerning pathogenesis, treatment, and outcome there are also some important differences. Facial nerve palsy appears 2–4 times less frequent in children than in adults [33]. Additionally, facial nerve palsy appears to be more frequently associated with viral infection and with Borreliosis than in adults [34, 35]. Whether corticosteroids are necessary or even more effective than in adults remains controversial [36]. Overall, the outcome of facial nerve palsy appears more favorable in children as compared to adults. Almost all young patients with facial nerve palsy have a complete recovery within 6 months [32, 36]. However, as long as there are no sufficiently powered studies on children available, management of childhood facial palsy should not differ from that in adults.
Diagnosis
Peripheral facial nerve palsy is diagnosed upon the clinical presentation with weakness of all facial nerve branches, drooping of the brow, incomplete lid closure, drooping of the corner of the mouth, impaired closure of the mouth, dry eye, hyperacusis, impaired taste, or pain around the ear. Bell’s phenomenon (upward diversion of the bulb on attempted closure of the lid) occurs if the eye closure is incomplete [9]. Nerve conduction studies (prolonged distal latency, reduced compound muscle action potential) may provide useful information about the severity and nature of the lesion [9], although more prospective studies are required to assess the validity of nerve conduction studies for the prognosis of facial nerve lesions. Transcranial magnetic stimulation seems capable of localizing the site of the lesion within the Fallopian channel [37]. Assessment of the ear should include pneumatic otoscopy, tuning fork tests, otomicroscopy, and audiometry. Additional investigations may include electronystagmography, videonystagmography, and videooculoscopy. The stapedius reflex may be reduced or absent [9]. PCRs are essential to demonstrate the presence of herpes viruses and antibody tests to demonstrate the presence of Borrelia burgdorferi. CSF investigations may show pleocytosis, increased or decreased glucose, increased protein, antibodies against viruses or against Borrelia burgdorferi, or virus DNA or RNA. Appropriate tests are required to exclude other causes of secondary facial nerve palsy as listed in Table 1.
Therapy
Therapy, particularly of Bell’s palsy, is controversial due to the lack of large, prospective, randomized, and controlled trials [38]. Main goals of treatment are to speed recovery, to make recovery more complete, to prevent corneal complications and other sequelae, and to inhibit viral replication [9]. Psychological support is also essential. Patients require regular follow-ups. Therapy of secondary facial nerve palsy aims to omit the particular cause of the palsy. Patients with Bell’s palsy should be referred to a specialist and treatment should start as soon after onset as possible [9]. Treatment may be subdivided into acute measures and measures to treat moderate or severe sequelae.
Acute measures
Eye protection
One of the greatest problems with Bell’s palsy is the involvement of the eye if the lid fissure remains open. In this case, eye care focuses on the protection of the cornea from dehydration, drying, or abrasions due to insufficient lid closure or tearing [9]. Eye ointment is proposed during day and night supported by a watchglas bandage during the day or night.
Mime and physiotherapy
There are only few controlled trials available on the effectiveness of physical therapy for facial palsies [9]. In a randomized trial on 50 patients with Bell’s palsy and a mean HBS of IV, mime therapy, including automassage, relaxation exercises, inhibition of synkinesis, coordination exercises, or emotional expression exercises, resulted in improvement of facial stiffness, lip motility, and the physical and social indices of the facial disability index [39]. Patients with remaining symptoms from Bell’s palsy appear to experience positive effects from physiotherapy [40] and biofeedback training [41]. In a controlled study on 24 patients with Bell’s palsy neuromuscular retraining exercises were effective in improving facial movements [42].
Acupuncture and moxibustion
Though only limited experience has been reported with acupuncture for Bell’s palsy [9], several studies provide increasing evidence for a beneficial effect of acupuncture and moxibustion as an adjunctive treatment of Bell’s palsy [43–45].
Steroids
Although steroids are widely used in Bell’s palsy its efficacy in this indication has not been clearly demonstrated [38]. Most of the studies on steroids in Bell’s palsy have a small sample size, have a retrospective, observational design, rely on chart reviews, lack randomization, a control group, or blinding. On the one hand there are studies, which clearly showed a beneficial effect of steroids in the treatment of Bell’s palsy, on the other hand there are studies, which did not. There is general consensus, however, that steroids are ineffective for Bell’s palsy in children [6], although even in children some studies showed a beneficial effect of steroids [46] contrary to others [47].
Studies showing a beneficial effect
In a recent randomized, double-blind, placebo-controlled, factorial study on 496 patients with Bell’s palsy 83% recovered facial functions in the corticosteroid group compared to 64% in the placebo group after 3 months [48]. After 9 months of follow-up this proportion increased to 94% for the corticosteroid group and 82% for the placebo group. The authors concluded that treatment with steroids within 3 days after onset significantly improves the chance for complete recovery at 3 or 9 months [48]. In a study on 62 patients, high-dose intravenous prednisone together with vitamins within 72 h after onset resulted in a better outcome as compared to controls, who received only vitamins [49]. In a study on 71 patients with Bell’s palsy administration of intravenous high dose hydrocortisone together with low molecular dextrane resulted in a better outcome of patients in HBS I-II as compared to patients who received prednisone exclusively [50]. The authors also reported that the addition of dextrane was associated with a lower rate of side effects as compared to those not receiving dextrane [50]. In a study on 76 patients, the simultaneous administration of methyl-prednisolone and acyclovir resulted in a reversion of the deficits to HBS I or II in 92% of the cases [51]. All patients in HBS grade I-II recovered completely. For patients in HBS grade IV, V, and VI complete recovery after 1 year was observed in only 94, 86, and 50%, respectively [51]. In a randomized study on 46 patients with Bell’s palsy, of whom 23 received acyclovir and prednisone and 23 prednisone alone, those receiving the combination therapy had a better outcome on the facial nerve function index, as compared to those who were only on steroids alone [51]. If steroids were combined with valacyclovir complete recovery has been observed in 88% of 56 patients with Bell’s palsy, whereas complete recovery was observed in only 68% of the patients who did not receive any therapy at all [5]. Most studies recommend steroids for moderate to severe Bell’s palsy within the first 72 h after onset and for the one-fifth of patients in whom the palsy progresses [9]. A side effect of steroids for Bell’s palsy is the frequent temporary aggravation of a pre-existing diabetes [53]. This is the reason why patients with uncontrolled diabetes should receive corticosteroids only under close monitoring of blood glucose [48].
Studies showing no effect from steroids
According to two recent Cochrane reviews there is no benefit of steroids alone or in combination [54, 55]. However, these reviews admit that the available studies were insufficiently powered to detect a treatment effect [55]. From three randomized trials, one with steroids versus placebo, one with steroids and vitamins versus vitamins, and one with steroids without a placebo group, including a total of 117 patients, the authors concluded that more randomized trials with a larger number of patients are needed to determine if there is harm or benefit from steroids in Bell’s palsy [55]. In a study on 147 patients with peripheral facial palsy 44% received corticosteroids, which did not significantly improve the functional outcome [56]. In a study on 56 patients with Bell’s palsy steroids did not result in a significant improvement of the lesions 3 and 6 weeks after onset [38]. In a study on 29 children, there was no significant difference between the rate of recovery in those treated with a short course of steroids (n = 23) and those not receiving steroids [52]. All patients in HBS I-II recovered completely. In a retrospective study on 879 patients with Bell’s palsy steroids did not significantly influence the outcome [19]. In a study on 221 patients with Bell’s palsy valacyclovir and corticosteroids were significantly better than corticosteroids alone [57].
Antiviral agents
Though application of antiviral agents for Bell’s palsy appears logical, they are only rarely given. In a British study, only 0.6% of the patients with Bell’s palsy received acyclovir [58]. Two recent Cochrane reviews on 246 and 200 patients, including three [54], respectively, two randomized trials [59] with acyclovir and steroids versus steroids alone [60–62], acyclovir versus steroids [63], and valacyclovir with steroids versus steroids [5] concluded that the results of all three trials were inconclusive with regard to a short or long-term benefit and that a large, multicenter, randomized, controlled, and blinded study with a minimum follow-up of 1 year is required before a definite recommendation regarding the effect of acyclovir or valacyclovir can be given [5]. At least there does not seem to be a difference between acyclovir and steroids orally versus acyclovir and steroids intravenously [15]. A recent study on 221 patients with Bell’s palsy, treated with valacyclovir and prednisolone within 7 days after onset, showed a better outcome for patients receiving the combination therapy than corticosteroids alone [57]. In a study on 247 patients receiving acyclovir complete recovery was observed in 71% after 3 months and in 85% after 9 months [48]. The authors found no benefit of acyclovir alone or an additional benefit of acyclovir in combination with corticosteroids [48]. For patients with zoster sine herpete, however, acyclovir appears to be effective [9].
Pentoxifyllin
The efficacy of pentoxifylline on the recovery of Bell’s palsy has been only tested together with other drugs, particularly steroids and low-molecular dextrane [64, 65]. These studies showed a beneficial effect of such a combination therapy, but which of these drugs is the one actually responsible for the beneficial effect, is so far unknown.
Poor outcome measures
Pulsatile electrical current (transcutaneous electrical stimulation)
Particularly, in patients with poor outcome and chronic facial nerve damage long-term electrical stimulation may be beneficial. In a study on 12 patients with chronic Bell’s palsy and 5 patients whose facial nerves had been surgically sacrificed with a mean latency between onset and electrotherapy of 3.7 year stimulation of the most affected muscles at a submotor level for 6 h a day during 6 months significantly reduced facial nerve latencies, the HBS, and collective scores of the 12 clinical impairment measures after 6 months [66]. An improvement by 40, 30, or less than 10% was reported in 5, 4, and 8 patients respectively. The beneficial effect was explained by facilitation of re-innervation through electrical stimulation [66].
Transmastoid decompression
In a study on 58 patients with Bell’s palsy having denervation exceeding 95%, transmastoid decompression of the facial nerve resulted in significant improvement of the HBS and Yanagihara scores 60 days after onset [67]. In a prospective, multi-center trial on patients with a chance of long-term sequelae from Bell’s palsy, as assessed by nerve conduction studies and electromyography, surgical decompression of the facial nerve through a middle cranial fossa exposure, including the tympanic segment, geniculate ganglion, labyrinth segment, and meatal foramen, significantly improved the chances to normal or near-normal return of facial nerve functions if surgery was carried out within 2 weeks after onset of total paralysis [68]. Since middle fossa craniotomy carries the risk of bleeding, infection, seizures, deafness, leakage of cerebrospinal fluid, or facial nerve injury, this surgical approach cannot be routinely recommended to patients with acute Bell’s palsy [9].
Gold weight implant
Implantation of gold into the upper eyelids of 16 patients with lagophthalmus due to Bell’s palsy resulted in significant reduction of lagophthalmus and improved corneal coverage of 100% [69]. Owing to delayed closure time and disrupted tear film, irritation of the cornea and sklera may persist, such that some patients require ongoing topical treatment of the eye, which may compromise visual acuity [69].
Facial nerve cable grafting
In a retrospective study on 27 patients undergoing facial nerve grafting between 1982 and 1997 those who had the nerve grafted to a site distal to the meatal foramen had a better outcome than those with an anastomosis proximal to the meatal foramen [70]. Microneurovascular free muscle transfer and cross-face nerve grafting are other therapeutic options. The latter involves one of the nerves used for biting [8]. In a study on 29 patients who had undergone previous removal of cerebello-pontine angle tumor, hypoglossal-facial nerve anastomosis resulted in significant improvement in all of them [71]. A HBS of III or better was achieved in 65 of the included patients [71].
Subperiostal facial suspension (face lifting)
In an observational study on five patients with a HBS of III–V face lifting resulted in a marked improvement in four of them [72].
Botulinum toxin
Synkinesis and facial spasms, common features of partially recovered facial nerve palsies, can be effectively managed by subcutaneous or intramuscular injections of botulinum toxin [9]. In a study on ten patients with synkinesis during a period of 7 years on the average, periorbital injections of Botulinum toxin A resulted in marked subjective and objective improvement in nine [73].
Prognosis
Facial nerve palsy can improve up to 1 year later [6]. Patients with incomplete palsy have a better prognosis than patients with complete palsy [74] and the younger the patient the better the prognosis [2, 75, 76]. In patients with incomplete palsy up to 94% make a full recovery [9]. For elderly patients and those with severe weakness the outcome is less favorable [77]. Without treatment the prognosis of complete Bell’s palsy is generally fair, but about 20–30% of the cases are left with varying degrees of permanent disability [3, 5, 8]. About 80–85% of the patients recover spontaneously and completely within 3 months, whereas 15–20% experience some kind of permanent nerve damage [8]. About 5% may remain with severe sequelae [2, 5]. In a study on 496 patients with Bell’s palsy full recovery after 9 months was achieved in 94% of the patients receiving corticosteroids either alone or in combination with acyclovir [48]. In a retrospective study on 334 patients with Bell’s palsy treated with 250 mg prednisolone in addition to dextrane and pentoxyfyllin, the functional outcome was independent of age, arterial hypertension, or diabetes [64]. In this study, the outcome was better if therapy had started within 3 days after onset of symptoms. The general outcome was regarded superior to patients without receiving any therapy at all [64]. Prognosis of Bell’s palsy may be assessed clinically, by nerve conduction studies [25, 78], transcranial magnetic stimulation [79], or quantitative analysis of MRI [80]. About 10% of the patients with Bell’s palsy experience one or more recurrences after a mean latency of 10 years [81].
Long-term sequelae of facial nerve palsy may be persisting weakness, contractures, facial spasms, synkinesis, decreased tearing, crocodile tears, or psychosocial effects [2]. In patients who recover without treatment, major improvement occurs within 3 weeks. A new wave of recovery of function starts 3 months after onset. If it does not occur within this time then it is unlikely to be seen by 6 months. By 6 months it becomes clear who will have moderate or severe sequelae. Indicators for poor prognosis are listed in Table 4. In case of incomplete recovery facial nerve palsy may go along with facial synkinesis.
Table 4.
Indicators for poor prognosis of Bell’s palsy
| Complete palsy |
| Absent recovery by 3 weeks |
| Age >60 years |
| Severe pain |
| Ramsey Hunt syndrome |
| Presence of conditions causing secondary facial nerve palsy |
| Reduction of the compound muscle action potential >50% |
Conclusion
Patients developing Bell’s palsy should be seen by a neurologist, oto-rhino-laryngologist, and ophthalmologist with the least possible latency after onset of the palsy. All patients in whom secondary facial nerve palsy is suspected a diagnostic work-up for the presence or absence of possible causes should be promptly initiated. If any of these causes is detected, it should be assessed if there is a causal relation between the palsy and the detected cause or not. Though a final decision on the optimal therapy of acutely developing Bell’s palsy cannot be actually proposed, patients should be provided with all measures to avoid secondary affection of the eyes if the lid closure is insufficient or in case of impaired tearing. According to a recent double-blind, placebo-controlled trial on 496 patients early administration of corticosteroids resulted in a significantly better outcome than placebo [48]. In case steroids are used in diabetic patients, serum glucose should be frequently followed.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kawiak W, Dudkowska A, Adach B. Diagnostic difficulties in etiology of the lesion of peripheral neuron of the facial nerve during the growth of sialoma. Ann Univ Mariae Curie Sklodowska [Med] 1993;48:125–128. [PubMed] [Google Scholar]
- 2.Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;549:4–30. [PubMed] [Google Scholar]
- 3.Shaw M, Nazir F, Bone I. Bell’s palsy: a study of the treatment advice given by neurologists. J Neurol Neurosurg Psychiatry. 2005;76:293–294. doi: 10.1136/jnnp.2004.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf SR. Idiopathic facial paralysis. HNO. 1998;46:786–798. doi: 10.1007/s001060050314. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson S, Lindberg S, Stjernquist-Desatnik A. Outcome of treatment with valacyclovir and prednisone in patients with Bell’s palsy. Ann Otol Rhinol Laryngol. 2003;112:197–201. doi: 10.1177/000348940311200301. [DOI] [PubMed] [Google Scholar]
- 6.Atzema C, Goldman RD. Should we use steroids to treat children with Bell’s palsy? Can Family Physician. 2006;52:313–314. [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed A. When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med. 2005;72:398–401. doi: 10.3949/ccjm.72.5.398. [DOI] [PubMed] [Google Scholar]
- 8.Slavkin HC. The significance of a human smile: observations on Bell’s palsy. JADA. 1999;130:269–72. doi: 10.14219/jada.archive.1999.0178. [DOI] [PubMed] [Google Scholar]
- 9.Holland NJ, Weiner GM. Recent developments in Bell’s palsy. Br Med J. 2004;329:553–557. doi: 10.1136/bmj.329.7465.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole J. About face. Cambridge: MIT Press; 1998. [Google Scholar]
- 11.Mylonas I, Kastner R, Sattler C, Kainer F, Friese K. Idiopathic facial paralysis (Bell’s palsy) in the immediate puerperium in a patient with mild preeclampsia: a case report. Arch Gynecol Obstet. 2005;272:241–243. doi: 10.1007/s00404-005-0742-2. [DOI] [PubMed] [Google Scholar]
- 12.Zaidi FH, Gregory-Evans K, Acheson JF, Ferguson V. Familial Bell’s palsy in females: a phenotype with a predilection for eyelids and lacrimal gland. Orbit. 2005;24:121–124. doi: 10.1080/01676830490916082. [DOI] [PubMed] [Google Scholar]
- 13.Devriese PP. Bell’s palsy in children. Acta Otorhinolaryngol Belg. 1984;38:261–267. [PubMed] [Google Scholar]
- 14.de Ru JA, van Benthem PP, Hordijk GJ. Arguments favouring the pharmacotherapy of Bells’ palsy. Ned Tijdschr Geneeskd. 2005;149:1454. [PubMed] [Google Scholar]
- 15.Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124:27–30. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Valenca MM, Valenca LP, Lima MC. Idiopathic facial paralysis (Bell’s palsy): a study of 180 patients. Arq Neuropsiquiatr. 2001;59:733–739. [PubMed] [Google Scholar]
- 17.Roob G, Fazekas F, Hartung HP. Peripheral facial palsy: etiology, diagnosis and treatment. Eur Neurol. 1999;41:3–9. doi: 10.1159/000007990. [DOI] [PubMed] [Google Scholar]
- 18.Marson AG, Salinas R. Bell’s palsy. West J Med. 2000;173:266–268. doi: 10.1136/ewjm.173.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott CA. Idiopathic facial nerve palsy (the effect of treatment with steroids) J Laryngol Otol. 1988;102:403–407. doi: 10.1017/s0022215100105201. [DOI] [PubMed] [Google Scholar]
- 20.Stahl N, Ferit T. Recurrent bilateral peripheral facial palsy. J Laryngol Otol. 1989;103:117–119. doi: 10.1017/S0022215100108199. [DOI] [PubMed] [Google Scholar]
- 21.Satoh Y, Kanzaki J, Yoshihara S. A comparison and conversion table of ‘the House-Brackmann facial nerve grading system’ and ‘the Yanagihara grading system’. Auris Nasus Larynx. 2000;27:207–212. doi: 10.1016/S0385-8146(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 22.Ahrens A, Skarada D, Wallace M, Cheung JY, Neely JG. Rapid simultaneous comparison system for subjective grading scales grading scales for facial paralysis. Am J Otol. 1999;20:667–671. [PubMed] [Google Scholar]
- 23.Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg. 1996;114:380–386. doi: 10.1016/S0194-5998(96)70206-1. [DOI] [PubMed] [Google Scholar]
- 24.Ochoa-Sepulveda JJ, Ochoa-Amor JJ. Ondine’s curse during pregnancy. J Neurol Neurosurg Psychiatry. 2005;76:294. doi: 10.1136/jnnp.2004.048025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura J. Electrodiagnosis of the cranial nerves. Acta Neurol Taiwan. 2006;15:2–12. [PubMed] [Google Scholar]
- 26.Urban PP, Forst T, Lenfers M, Koehler J, Connemann BJ, Beyer J. Incidence of subclinical trigeminal and facial nerve involvement in diabetes mellitus. Electromyogr Clin Neurophysiol. 1999;39:267–272. [PubMed] [Google Scholar]
- 27.Pecket P, Schattner A. Concurrent Bell’s palsy and diabetes mellitus: a diabetic mononeuropathy? J Neurol Neurosurg Psychiatry. 1982;45:652–655. doi: 10.1136/jnnp.45.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamboulis E, Vassilopoulos D, Kalfakis N. Symptomatic focal mononeuropathies in diabetic patients: increased or not? J Neurol. 2005;252:448–452. doi: 10.1007/s00415-005-0672-8. [DOI] [PubMed] [Google Scholar]
- 29.Prakash KM, Raymond AA. The use of nerve conduction studies in determining the short-term outcome of Bell’s palsy. Med J Malaysia. 2003;58:69–78. [PubMed] [Google Scholar]
- 30.Ben-David J, Gertner R, Podoshin L, Fradis M, Pratt H, Rabina A. Auditory brain stem evoked potentials in patients suffering from peripheral facial nerve palsy and diabetes mellitus. J Laryngol Otol. 1986;100:629–633. doi: 10.1017/S0022215100099801. [DOI] [PubMed] [Google Scholar]
- 31.Neau JP, Gil R, Marechaud R, Gouet D, Sudre Y, Lefevre JP. Blink reflex and stimulus detection by the facial nerve in 21 diabetics. Testing before and after precise blood sugar normalization by the artificial pancreas. Acta Neurol Belg. 1985;85:310–317. [PubMed] [Google Scholar]
- 32.Ashtekar CS, Joishy M, Joshi R. Best evidence topic report. Do we need to give steroids in children with Bell’s palsy? Emerg Med J. 2005;22:505–507. doi: 10.1136/emj.2005.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Hawrani AS, Eng CY, Ahmed SK, Clarke J, Dhiwakar M. General practitioners’ referral pattern for children with acute facial paralysis. J Laryngol Otol. 2005;119:540–542. doi: 10.1258/0022215054352135. [DOI] [PubMed] [Google Scholar]
- 34.Furuta Y, Ohtani F, Aizawa H, Fukuda S, Kawabata H, Bergstrom T. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr Infect Dis J. 2005;24:97–101. doi: 10.1097/01.inf.0000151032.16639.9c. [DOI] [PubMed] [Google Scholar]
- 35.Ogita S, Terada K, Niizuma T, Kosaka Y, Kataoka N. Characteristics of facial nerve palsy during childhood in Japan: frequency of varicella-zoster virus association. Pediatr Int. 2006;48:245–249. doi: 10.1111/j.1442-200X.2006.02197.x. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka M, Mochizuki M, Sugiyama N, Hamano S. Bell’s palsy in children: analysis of clinical findings and course. No To Hattatsu. 2004;36:461–465. [PubMed] [Google Scholar]
- 37.Nowak DA, Linder S, Topka H. Diagnostic relevance of transcranial magnetic and electric stimulation of the facial nerve in the management of facial palsy. Clin Neurophysiol. 2005;116:2051–2057. doi: 10.1016/j.clinph.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Turk-Boru U, Kocer A, Bilge C. The efficacy of steroids in idiopathic facial nerve paralysis: an open, randomized, prospective controlled study. Kulak Burun Bogaz Ihtis Derg. 2005;14:62–66. [PubMed] [Google Scholar]
- 39.Beurskens CH, Heymans PG. Positive effects of mime therapy on sequelae of facial paralysis: stiffness, lip mobility, and social and physical aspects of facial disability. Otol Neurotol. 2003;24:677–681. doi: 10.1097/00129492-200307000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Cederwall E, Olsen MF, Hanner P, Fogdestam I. Evaluation of a physiotherapeutic treatment intervention in “Bell’s” facial palsy. Physiother Theory Pract. 2006;22:43–52. doi: 10.1080/09593980500422529. [DOI] [PubMed] [Google Scholar]
- 41.Shafshak TF. The treatment of facial palsy from the point of view of physical and rehabilitation medicine. Eura Medicophys. 2006;42:41–47. [PubMed] [Google Scholar]
- 42.Cronin GW, Steenerson RL. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngol Head Neck Surg. 2003;128:534–538. doi: 10.1016/S0194-5998(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 43.Liang F, Li Y, Yu S, Li C, Hu L, Zhou D, Yuan X, Li Y. A multicentral randomized control study on clinical acupuncture treatment of Bell’s palsy. J Tradit Chin Med. 2006;26:3–7. [PubMed] [Google Scholar]
- 44.Qu Y. Clinical observation on acupuncture by stages combined with exercise therapy for treatment of Bell palsy at acute stage. Zhongguo Zhen Jiu. 2005;25:545–547. [PubMed] [Google Scholar]
- 45.Li Y, Liang FR, Yu SG, Li CD, Hu LX, Zhou D, Yuan XL, Li Y, Xia XH. Efficacy of acupuncture and moxibustion in treating Bell’s palsy: a multicenter randomized controlled trial in China. Chin Med J (Engl) 2004;117:1502–1506. [PubMed] [Google Scholar]
- 46.Williamson IG, Whelan TR. The clinical problem of Bell’s palsy: is treatment with steroids effective? Br J Gen Pract. 1996;46:743–747. [PMC free article] [PubMed] [Google Scholar]
- 47.Micheli R, Telesca C, Gitti F, Giordano L, Perini A. Bell’s palsy: diagnostic and therapeutical trial in childhood. Minerva Pediatr. 1996;48:245–250. [PubMed] [Google Scholar]
- 48.Sullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B, Davenport RJ, Vale LD, Clarkson JE, Hammersley V, Hayavi S, McAteer A, Stewart K, Daly F. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med. 2007;357:1598–1607. doi: 10.1056/NEJMoa072006. [DOI] [PubMed] [Google Scholar]
- 49.Lagalla G, Logullo F, Di Bella P, Provinciali L, Ceravolo MG. Influence of early high-dose steroid treatment on Bell’s palsy evolution. Neurol Sci. 2002;23:107–112. doi: 10.1007/s100720200035. [DOI] [PubMed] [Google Scholar]
- 50.Tian JF, Li BX, Liu DG, Zhou CY, Wang LL, Han H. Effects of modified SD therapy on Bell’s palsy. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2000;14:551–553. [PubMed] [Google Scholar]
- 51.Lejeune D, Bernat I, Vitte E, Lamas G, Willer JC, Soudant J, Tankere F. Treatment of Bell’s palsy with acyclovir and methylprednisolone. Ann Otolaryngol Chir Cervicofac. 2002;119:209–215. [PubMed] [Google Scholar]
- 52.Chen WL, Yang ZH, Huang ZQ. Outcome of treatment 46 patients with Bell’s palsy with aciclovir and prednisone. Shanghai Kou Qiang Yi Xue. 2005;14:590–592. [PubMed] [Google Scholar]
- 53.Koriyama T, Inafuku S, Kimata K, Banno T, Ishigami H. Recent-onset bell palsy complicated by diabetes: comparison of steroid and lipoprostaglandin E(1) therapy. Arch Otolaryngol Head Neck Surg. 2001;127:1338–1340. doi: 10.1001/archotol.127.11.1338. [DOI] [PubMed] [Google Scholar]
- 54.Allen D, Dunn L. Aciclovir or valaciclovir for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2004;3:CD001869. doi: 10.1002/14651858.CD001869.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2004;4:CD001942. doi: 10.1002/14651858.CD001942.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Hyden D, Roberg M, Forsberg P, Fridell E, Fryden A, Linde A, Odkvist L. Acute “idiopathic” peripheral facial palsy: clinical, serological, and cerebrospinal fluid findings and effects of corticosteroids. Am J Otolaryngol. 1993;14:179–186. doi: 10.1016/0196-0709(93)90027-5. [DOI] [PubMed] [Google Scholar]
- 57.Hato N, Yamada H, Kohno H, Matsumoto S, Honda N, Gyo K, Fukuda S, Furuta Y, Ohtani F, Aizawa H, Aoyagi M, Inamura H, Nakashima T, Nakata S, Murakami S, Kiguchi J, Yamano K, Takeda T, Hamada M, Yamakawa K. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol. 2007;28:408–413. doi: 10.1097/01.mao.0000265190.29969.12. [DOI] [PubMed] [Google Scholar]
- 58.Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell’s palsy in the UK. Eur J Neurol. 2002;9:63–67. doi: 10.1046/j.1468-1331.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 59.Sipe J, Dunn L. Aciclovir for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2001;4:CD001869. Review. Update in: Cochrane Database Syst Rev 2004;3:CD001869 [DOI] [PubMed]
- 60.Adour K, Wingerd J, Doty HE. Prevalence of concurrent diabetes mellitus and idiopathic facial paralysis (Bell’s palsy) Diabetes. 1975;24:449–451. doi: 10.2337/diabetes.24.5.449. [DOI] [PubMed] [Google Scholar]
- 61.Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:830–836. doi: 10.1212/wnl.56.7.830. [DOI] [PubMed] [Google Scholar]
- 62.Hato N, Matsumoto S, Kisaki H, Takahashi H, Wakisaka H, Honda N, Gyo K, Murakami S, Yanagihara N. Efficacy of early treatment of Bell’s palsy with oral acyclovir and prednisolone. Otol Neurotol. 2003;24:948–951. doi: 10.1097/00129492-200311000-00022. [DOI] [PubMed] [Google Scholar]
- 63.De Diego JI, Prim MP, De Sarria MJ, Madero R, Gavilan J. Idiopathic facial paralysis: a randomized, prospective, and controlled study using single-dose prednisone versus acyclovir three times daily. Laryngoscope. 1998;108:573–575. doi: 10.1097/00005537-199804000-00020. [DOI] [PubMed] [Google Scholar]
- 64.Sittel C, Sittel A, Guntinas-Lichius O, Eckel HE, Stennert E. Bell’s palsy: a 10-year experience with antiphlogistic-rheologic infusion therapy. Am J Otol. 2000;21:425–432. doi: 10.1016/S0196-0709(00)80055-1. [DOI] [PubMed] [Google Scholar]
- 65.Kinishi M, Amatsu M, Hosomi H. Conservative treatment of Bell’s palsy with steroids and dextran-pentoxiphylline combined therapy. Eur Arch Otorhinolaryngol. 1991;248:147–149. doi: 10.1007/BF00178925. [DOI] [PubMed] [Google Scholar]
- 66.Targan RS, Alon G, Kay SL. Effect of long-term electrical stimulation on motor recovery and improvement of clinical residuals in patients with unresolved facial nerve palsy. Otolaryngol Head Neck Surg. 2000;122:246–252. doi: 10.1016/S0194-5998(00)70248-8. [DOI] [PubMed] [Google Scholar]
- 67.Yanagihara N, Hato N, Murakami S, Honda N. Transmastoid decompression as a treatment of Bell palsy. Otolaryngol Head Neck Surg. 2001;124:282–286. doi: 10.1067/mhn.2001.112309. [DOI] [PubMed] [Google Scholar]
- 68.Gantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell’s palsy. Laryngoscope. 1999;109:1177–1188. doi: 10.1097/00005537-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Pausch N, Sterker I, Hemprich A, Frerich B. Restoration of lid function in peripheral facial palsy by implanting gold weights. Mund Kiefer Gesichtschir. 2006;10:135–140. doi: 10.1007/s10006-006-0683-3. [DOI] [PubMed] [Google Scholar]
- 70.Gidley PW, Gantz BJ, Rubinstein JT. Facial nerve grafts: from cerebellopontine angle and beyond. Am J Otol. 1999;20:781–788. [PubMed] [Google Scholar]
- 71.Sood S, Anthony R, Homer JJ, Van Hille P, Fenwick JD. Hypoglossal-facial nerve anastomosis: assessment of clinical results and patient benefit for facial nerve palsy following acoustic neuroma excision. Clin Otolaryngol Allied Sci. 2000;25:219–226. doi: 10.1046/j.1365-2273.2000.00348.x. [DOI] [PubMed] [Google Scholar]
- 72.Horlock N, Sanders R, Harrison DH. The SOOF lift: its role in correcting midfacial and lower facial asymmetry in patients with partial facial palsy. Plast Reconstr Surg. 2002;109:839–849. doi: 10.1097/00006534-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Badarny S, Giladi N, Honigman S. Botulinum toxin injection effective for post-peripheral facial nerve palsy synkinesis. Harefuah. 1998;135:106–107. [PubMed] [Google Scholar]
- 74.Adour KK, Wingerd J. Idiopathic facial paralysis (Bell’s palsy): factors affecting severity and outcome in 446 patients. Neurology. 1974;24:1112–1116. doi: 10.1212/wnl.24.12.1112. [DOI] [PubMed] [Google Scholar]
- 75.Unuvar E, Oguz F, Sidal M, Kilic A. Corticosteroid treatment of childhood Bell’s palsy. Pediatr Neurol. 1999;21:814–816. doi: 10.1016/S0887-8994(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 76.Devriese PP, Schumacher T, Scheide A, de Jongh RH, Houtkooper JM. Incidence, prognosis and recovery of Bell’s palsy. A survey of about 1000 patients (1974–1983) Clin Otolaryngol Allied Sci. 1990;15:15–27. doi: 10.1111/j.1365-2273.1990.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 77.Kasse CA, Cruz OL, Leonhardt FD, Testa JR, Ferri RG, Viertler EY. The value of prognostic clinical data in Bell’s palsy. Rev Bras Otorrinolaringol (Engl Ed) 2005;71:454–458. doi: 10.1016/S1808-8694(15)31198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Engstrom M, Jonsson L, Grindlund M, Stalberg E. Electroneurographic facial muscle pattern in Bell’s palsy. Otolaryngol Head Neck Surg. 2000;122:290–297. doi: 10.1016/S0194-5998(00)70258-0. [DOI] [PubMed] [Google Scholar]
- 79.Rimpilainen I, Eskola H, Laippala P, Laranne J, Karma P. Prognostication of Bell’s palsy using transcranial magnetic stimulation. Acta Otolaryngol Suppl. 1997;529:111–115. doi: 10.3109/00016489709124098. [DOI] [PubMed] [Google Scholar]
- 80.Kress BP, Griesbeck F, Efinger K, Solbach T, Gottschalk A, Kornhuber AW, Bahren W. Bell’s palsy: what is the prognostic value of measurements of signal intensity increases with contrast enhancement on MRI? Neuroradiology. 2002;44:428–433. doi: 10.1007/s00234-001-0738-y. [DOI] [PubMed] [Google Scholar]
- 81.Pitts DB, Adour KK, Hilsinger RL., Jr Recurrent Bell’s palsy: analysis of 140 patients. Laryngoscope. 1988;98:535–540. doi: 10.1288/00005537-198805000-00012. [DOI] [PubMed] [Google Scholar]
- 82.Keane JR. Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature. Neurology. 1994;44:1198–1202. doi: 10.1212/wnl.44.7.1198. [DOI] [PubMed] [Google Scholar]
- 83.Thomke F, Urban PP, Marx JJ, Mika-Gruttner A, Hopf HC. Seventh nerve palsies may be the only clinical sign of small pontine infarctions in diabetic and hypertensive patients. J Neurol. 2002;249:1556–1562. doi: 10.1007/s00415-002-0894-y. [DOI] [PubMed] [Google Scholar]
- 84.Finsterer J, Auer H (2007) Neurotoxocariasis. Revista do Medical Trop de Sao Paulo (submitted)
- 85.Aderibigbe A, Ologe FE. Cervical spinal tuberculosis with tuberculous otitis media masquerading as otitis externa malignans in an elderly diabetic patient: case report. East Afr Med J. 2004;81:267–270. doi: 10.4314/eamj.v81i5.9172. [DOI] [PubMed] [Google Scholar]
- 86.Zaky DA, Bentley DW, Lowy K, Betts RF, Douglas RG., Jr Malignant external otitis: a severe form of otitis in diabetic patients. Am J Med. 1976;61:298–302. doi: 10.1016/0002-9343(76)90181-9. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez Tortosa J, Martinez-Lage JF, Poza M. Bitemporal head crush injuries: clinical and radiological features of a distinctive type of head injury. J Neurosurg. 2004;100:645–651. doi: 10.3171/jns.2004.100.4.0645. [DOI] [PubMed] [Google Scholar]
- 88.Salonen IS, Uusitalo R. Birth injuries: incidence and predisposing factors. Z Kinderchir. 1990;45:133–135. doi: 10.1055/s-2008-1042565. [DOI] [PubMed] [Google Scholar]
- 89.Ogundipe O, Smith M. Bell’s palsy during interferon therapy for chronic hepatitis C infection in patients with haemorrhagic disorders. Haemophilia. 2000;6:110–112. doi: 10.1046/j.1365-2516.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 90.Thai XC, Bruno-Murtha LA. Bell’s palsy associated with linezolid therapy: case report and review of neuropathic adverse events. Pharmacotherapy. 2006;26:1183–1189. doi: 10.1592/phco.26.8.1183. [DOI] [PubMed] [Google Scholar]
- 91.Ruza Paz-Curbera E, Fernandez Benitez M. Melkersson-Rosenthal syndrome in a diabetic boy. Allergol Immunopathol (Madr) 1998;26:291–293. [PubMed] [Google Scholar]
- 92.Allard P, Kermarec J, Goasguen J, Ferry M, de Muizon H, Girard P, Herning R. Cerebral and pulmonary histiocytosis X. Neurologic manifestations disclosing a pseudotumoral formation on the floor of the 4th ventricle. Rev Pneumol Clin. 1984;40:305–309. [PubMed] [Google Scholar]
- 93.Kamaratos A, Kokkoris S, Protopsaltis J, Agorgianitis D, Koumpoulis H, Lentzas J, Melidonis A, Giannoulis G. Simultaneous bilateral facial palsy in a diabetic patient. Diabetes Care. 2004;27:623–624. doi: 10.2337/diacare.27.2.623. [DOI] [PubMed] [Google Scholar]