Abstract
Introduction
Because of its high complication rate, the endovascular treatment (EVT) of anterior communicating artery (ACoA) aneurysms less than 3 mm in maximum diameter remains controversial. We evaluated EVT of tiny ruptured ACoA aneurysms with Guglielmi detachable coils (GDCs).
Methods
We treated 19 ruptured ACoA aneurysms with a maximum diameter of ≤3 mm with GDCs. The pretreatment Hunt and Hess score was grade 1 in four patients, grade 2 in six, grade 3 in six, and grade 4 in three. The patients were clinically assessed before and after treatment and with multiple angiographic follow-up studies.
Results
All EVTs were successful; there were no aneurysm perforations or any other treatment-related complications. In five patients older than 80 years the transfemoral approach was difficult, and the direct carotid approach was used. Complete and near-complete occlusion was achieved in 16 patients (84.2%) and 3 patients (15.8%), respectively. Of the 19 patients, 16 (84.2%) were followed angiographically for a median of 38.5 months (range 16–72 months). None demonstrated recanalization of the aneurysm requiring additional treatment. In 15 patients (78.9%) the final outcome was good (modified Rankin scale, mRS, score 0–2), and 3 patients (15.8%) died or suffered severe disability (mRS score 4–6). None of 18 patients who were followed clinically for a median of 39.5 months (range 17–84 months) experienced rebleeding.
Conclusion
Even tiny ruptured ACoA aneurysms can be safely treated by EVT by expert neurointerventionalists using advanced techniques.
Keywords: Aneurysm size, Anterior communicating artery, Cerebral aneurysm, Endovascular treatment, Subarachnoid hemorrhage
Introduction
Anterior communicating artery (ACoA) aneurysms in the circle of Willis represent up to 39% of treated aneurysms [1, 2]. The standard method of treating ACoA aneurysms has been microsurgical clipping. However, advances in endovascular techniques and the development of new devices have made it possible to manage certain ACoA aneurysms by endovascular treatment (EVT) and the placement of Guglielmi detachable coils (GDCs) has proven feasible and effective [3–6]. In fact, as EVT has been shown to be superior to microsurgical clipping in patients with ruptured aneurysms that could be treated by either method [7], the use of EVT for ACoA aneurysms has increased. However, the treatment of tiny ACoA aneurysms whose maximum diameter is less than 3 mm by EVT remains controversial because of a high complication rate, including intraoperative aneurysm rupture [8].
Although detailed indications for selecting microsurgical clipping or EVT to treat tiny aneurysms have yet to be established, at our institution we tend to treat ruptured aneurysms, including tiny ACoA aneurysms, with EVT. We report here a retrospective review of a consecutive series of patients with tiny ruptured ACoA aneurysms to assess the feasibility, safety, and efficacy of EVT.
Methods
Patient population
Between January 1999 and August 2007, 246 patients with intracranial aneurysms were admitted to our institution for EVT as the primary treatment option. Of these, 19 had ruptured ACoA aneurysms with a maximum diameter of 3 mm or less. Surgery was considered as an alternative to coil placement only after attempting EVT. For each patient we recorded demographic data, clinical presentation (including Hunt and Hess grade), any prior therapeutic intervention, immediate angiographic results, treatment-related complications, immediate outcome, and outcome on follow-up studies. Immediate angiographic results and angiographic follow-up results were recorded retrospectively by two neurosurgeons (M.T. and K.K.).
Aneurysm classification
Two senior neurosurgeons (M.T. and K.K.) analyzed the characteristics of the aneurysms (dome and neck sizes) by precise measurements on three-dimensional (3-D) angiograms. Aneurysms were defined as tiny when the largest diameter of the sac was 3 mm or less.
Endovascular treatment
The timing of EVT relative to the date of subarachnoid hemorrhage (SAH) was recorded in all patients. All aneurysms were embolized with GDCs (Boston Scientific, Natick, MA) with the patient under general anesthesia. Systemic heparinization was not performed; rather, heparinized saline was continuously infused from a guiding catheter. In the transfemoral approach, a 6F guiding catheter (Guider Softip; Boston Scientific) was inserted into the petrous portion of the internal carotid artery (ICA). When severe arteriosclerotic changes in the aortic arch and carotid artery made it impossible to insert a guiding catheter into the petrous portion of the ICA, the direct carotid approach was employed. In this approach, we percutaneously inserted a 5F sheath introducer into the common carotid artery. A 5F guiding catheter (Tracker-38; Boston Scientific) was then placed in the distal segment of the ICA petrous portion.
Prior to EVT we obtained rotational angiograms and performed 3-D reconstruction of the native projections. Then we identified the working projections that provided the surgeon with the best-achievable view of the aneurysm neck and its relationships to the parent vessel and adjacent branches. Using the road-mapping technique we performed superselective catheterization of the aneurysm with an Excel 14 or SL10 microcatheter (Boston Scientific). In all procedures we used steam to form a microcatheter of the same length as the horizontal portion (A1 segment) of the anterior cerebral artery (ACA) to be used as an access route (Fig. 1b). The tip of the microcatheter was shaped according to the direction of the aneurysm.
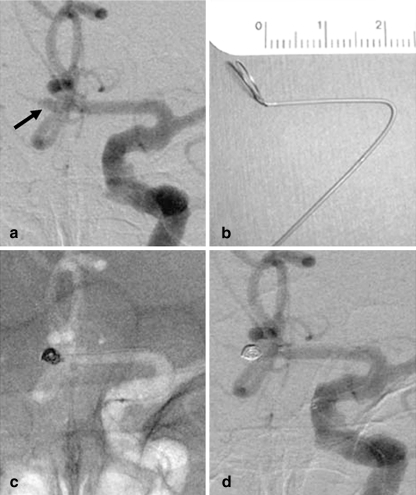
Fig. 1.
Patient 4. a Angiogram of the left ICA shows a tiny ACoA aneurysm with a maximum diameter of 2.5 mm (arrow). b Photograph of a steam-shaped microcatheter. The shaping mandrel is bent to conform to the shape of the horizontal portion of the anterior cerebral artery. c Unsubtracted image of the skull obtained with the road-mapping technique during treatment. Embolization was with a GDC-10 Soft coil measuring 2×60 mm. d Angiogram of the left ICA obtained at the end of the procedure shows complete occlusion of the aneurysm. The diameter of the coil mass was larger than that of the aneurysm sac before embolization, indicating aneurysm distention
The aneurysms were embolized as densely as possible using a detachable coil system; the coil diameter was 0.010 inch (GDC-10 Soft, GDC-10 Ultrasoft; Boston Scientific). At the end of the procedure we obtained angiograms (frontal, lateral, and working projection) to rule out occlusion of the parent artery and branches.
Complications, clinical outcomes, and follow-up
Using medical records, we retrospectively recorded procedure-related complications and clinical outcomes. The patients were clinically assessed before and after the procedure and followed up every 3–6 months after discharge from the hospital. The clinical outcome was determined at the last follow-up visit. Patients who were not followed at our institution were interviewed by telephone. All outcomes were graded on the modified Rankin scale (mRS) [9], where 0 = no symptoms, 1 = no marked disability despite symptoms, 2 = ability to carry out all usual duties and activities, 3 = moderate disability requiring some help and ability to walk without assistance, 4 = moderately severe disability, inability to walk without assistance and to attend bodily needs without assistance, 5 = severe disability, bedridden, incontinent and need for constant nursing care, and 6 = death.
Angiographic results and follow-up
Aneurysm occlusion at the end of the procedure and at follow-up was considered complete when there was angiographic evidence that the aneurysm sac and neck were packed and there was no filling of the aneurysm sac by contrast material; near-complete when the sac was occluded but a neck remnant was suspected or obviously present; and incomplete when there was persistent opacification of a sac remnant. Aneurysm distention was evaluated at the end of the procedure. Aneurysm distention after coiling was recorded when the diameter of the coil mass was larger than the aneurysm sac before coiling. Our angiographic follow-up protocol for patients with tiny ruptured ACoA aneurysms was the same as that typically used for other aneurysms; the first follow-up study was carried out 3–6 months after treatment, the second 12–18 months, the third 24–36 months, and the fourth 48 months or later after EVT. For angiographic follow-up we obtained frontal, lateral, and working projections defined during the endovascular procedure.
Aneurysm recanalization was recorded when a previously completely occluded aneurysm exhibited partial or small neck recanalization during follow-up or when the size of the neck remnant of a previously nearly completely occluded aneurysm was found to have increased in size during follow-up.
Results
Patient demographics
Of the 19 patients, 8 (42.1%) were men and 11 (57.9%) were women; their average age at presentation was 65.2 years (range 44–90 years). The Hunt and Hess grade at the time of EVT was grade 1 in four patients (21.1%), grade 2 in six (31.6%), grade 3 in six (31.6%), and grade 4 in three (15.8%); none was grade 5 (Table 1).
Table 1.
Summary of 19 tiny ruptured ACoA aneurysm treated with endovascular treatment
| Patient | Aneurysm | Coils (no. × mm)a | Angiographic occlusion | Complication | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (years) | Sex | Hunt & Hess grade | Size (mm) | Dome/neck ratio | Angiographic | Clinical | |||||
| Period (months) | Recanalization | Period (months) | Outcome (mRS score) | |||||||||
| 1 | 56 | M | 2 | 3×3×2.5 | 1.3 | 3×40, 2×30 | Complete | None | 72 | − | 84 | 0 |
| 2 | 58 | F | 2 | 3×3×3 | 1.2 | 3×60, 2×20 | Near-complete | None | 64 | + | 80 | 0 |
| 3 | 51 | M | 1 | 3×2.5×2.5 | 1.4 | 2×60, 2×30, 2×20 | Complete | None | 60 | − | 60 | 0 |
| 4 | 89 | F | 3 | 2.5×2×2 | 1.5 | 2×60 | Complete | None | NA | − | 36 | 4 |
| 5 | 82 | F | 2 | 2.5×2.5×2 | 1.7 | 2×60 | Complete | None | 48 | + | 75 | 2 |
| 6 | 50 | M | 4 | 3×3×3 | 1.1 | 3×40, 2×40, 2×20 | Complete | None | 52 | − | 70 | 1 |
| 7 | 44 | F | 1 | 3×2.5×3 | 1.6 | 3×60, 2×40 | Complete | None | 48 | − | 64 | 0 |
| 8 | 58 | F | 3 | 3×3×3 | 1.8 | 3×60, 2×40, 2×10 | Complete | None | 40 | − | 40 | 2 |
| 9 | 66 | M | 3 | 3×2.5×2.5 | 1.9 | 2.5×60, 2×30, 2×10 | Near-complete | None | 36 | − | 58 | 1 |
| 10 | 79 | F | 2 | 2.5×2×2 | 1.1 | 2×60 | Complete | None | 48 | − | 48 | 1 |
| 11 | 78 | M | 1 | 3×2.5×2.5 | 1.5 | 3×60, 2×60 | Complete | None | 36 | − | 39 | 0 |
| 12 | 53 | M | 2 | 2.5×2.5×2 | 1.2 | 2.5×60, 2×30 | Complete | None | 37 | − | 37 | 0 |
| 13 | 84 | F | 3 | 3×2.5×2.5 | 1.8 | 3×60, 2×20, 2×10 | Complete | None | NA | − | 24 | 5 |
| 14 | 53 | M | 1 | 3×2×2 | 2.1 | 2×60, 2×40 | Complete | None | 36 | − | 36 | 0 |
| 15 | 52 | F | 3 | 2×2×2 | 1.4 | 2×40, 2×30 | Complete | None | 27 | − | 30 | 0 |
| 16 | 66 | M | 3 | 3×2.5×2.5 | 2.0 | 3×40, 2×20, 2×10 | Complete | None | 25 | − | 30 | 1 |
| 17 | 83 | F | 4 | 3×3×3 | 1.7 | 3×40, 2×40 | Complete | None | 24 | − | 27 | 3 |
| 18 | 47 | F | 4 | 3×2.5×3 | 1.9 | 3×80 | Complete | None | NA | − | NA | 6 |
| 19 | 90 | F | 2 | 2.5×2×2 | 1.7 | 2×40, 2×20 | Near-complete | None | 16 | − | 17 | 2 |
NA not avairable
aAll coils used were GDC-10 Soft or GDC-10 Ultrasoft.
Aneurysm characteristics
All 19 were tiny saccular aneurysms; their maximum diameter ranged from 2 to 3 mm (mean 2.8 mm). The dome/neck ratio ranged from 1.1 to 2.1 (mean 1.57). None of the aneurysms had been treated previously.
Results of EVT, treatment-related complications, and angiographic results
All 19 aneurysms were successfully treated by EVT within 3 days of SAH onset (mean 1.4 days); there were no aneurysm perforations or any other procedure-related complications. The direct carotid approach was used in five patients (26.3%) older than 80 years because of systemic arteriosclerosis (patients 4, 5, 13, 17 and 19). Neither the balloon-assisted (remodeling technique) nor the stent-assisted technique was used to treat any of the 19 aneurysms. Complete occlusion was achieved in 16 aneurysms (84.2%; Figs. 1 and 2), and near-complete occlusion in 3 aneurysms (15.8%). Ten aneurysms (52.6%) showed distention (Figs. 1 and 3).
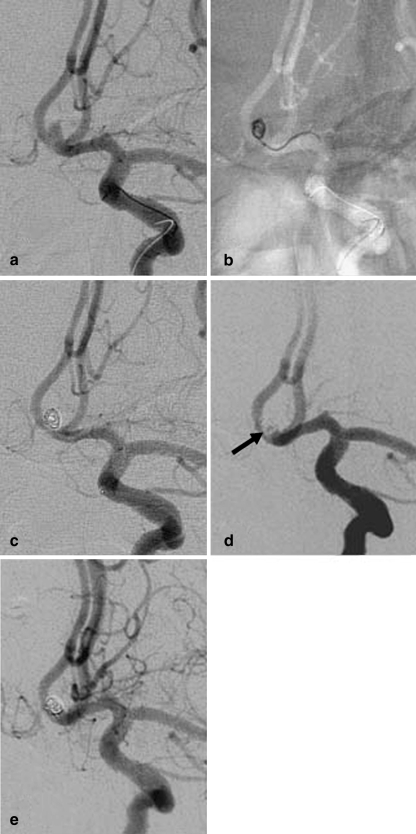
Fig. 2.
Patient 5. a Angiogram of the left ICA shows a tiny ACoA aneurysm with a maximum diameter of 2.5 mm. b Unsubtracted image of the skull obtained with the road-mapping technique during treatment. Embolization was with a GDC-10 Soft coil measuring 2×60 mm. c Angiogram of the left ICA obtained at the end of the procedure shows complete occlusion of the aneurysm. d Angiogram of the left ICA obtained at the 3-month follow-up shows minor recanalization of the neck (arrow). e The minor neck filling was stable at the 48-month follow-up
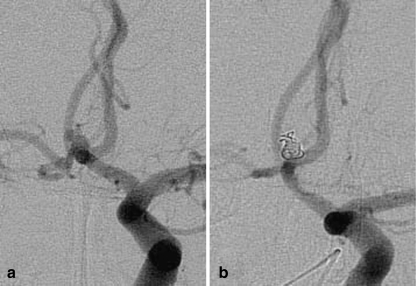
Fig. 3.
Patient 8. a Angiogram of the left ICA shows a tiny ACoA aneurysm with blebs. b Angiogram of the left ICA obtained at the end of the procedure. The aneurysm sac and blebs are completely occluded with coils. The coil mass is larger than the aneurysm sac before embolization (indicating aneurysm distention)
Treatment outcomes
Of the 19 patients, 1 (5.3%) died as a consequence of vasospasm 9 days after EVT. The other 18 patients were followed up for 17–84 months (median 39.5 months). None of the patients rebled during this period. The final mRS score was 0 in eight patients (42.1%), 1 in four (21.1%), 2 in three (15.8%), and 3, 4, 5, and 6 in one patient (5.3%) each.
Angiographic follow-up
Of the 19 embolized aneurysms, 16 (84.2%) were followed up angiographically for a median of 38.5 months (range 16–72 months). Three patients were not available for follow-up; one died (patient 18, Table 1) and in two (patients 4 and 13) the clinical outcome was poor. Only 2 (12.5%) of the 16 aneurysms (patients 2 and 5) followed for a median of 38.5 months showed recanalization (Table 1). One completely occluded aneurysm showed a minor neck filling after EVT (patient 5, Fig. 2), and one with near-complete occlusion (patient 2) showed an increase in the size of the neck remnant on angiograms obtained 3 months after the procedure. However, these findings remained stable on angiograms taken 48 and 64 months after EVT, respectively. None of the 16 aneurysms showed recanalization requiring additional treatment.
Discussion
Their deep location, complex arterial relationships, and frequent association with anomalies of the ACoA render microsurgical clipping of ACoA aneurysms difficult. The efficacy and favorable outcome of EVT for ACoA aneurysms have been reported [3–6]. EVT is particularly advantageous when the fundus projects posteriorly because microsurgical clipping tends to result in vessel occlusion that can produce a sizable stroke [6]. Some patients exhibited significant cognitive deficits after treatment of their ruptured ACoA aneurysms [10]. The incidence of cognitive impairment is lower in patients treated with EVT than in those treated with surgical clipping because the former method does not require direct manipulation of surrounding brain tissue and perforator occlusion is rare in patients undergoing EVT [11, 12]. A characteristic of ACoA aneurysms is their development into tiny aneurysms with a diameter of less than 3 mm [3]. We performed the current study because although EVT of ACoA aneurysms offers several advantages, no evaluations of EVT for tiny ruptured ACoA aneurysms have appeared in the literature. We successfully used this method to treat 19 tiny ruptured ACoA aneurysms and encountered no intraoperative rupture or any other treatment-related complications. Moreover, none of the aneurysms rebled during a median clinical follow-up period of 39.5 months.
Intraoperative rupture is one of the most serious complications of EVT for cerebral aneurysms and a small aneurysm size is considered a risk factor [6, 8]. Thus, the delicate control of therapeutic devices such as microcatheters and coils is necessary to prevent intraoperative rupture and tiny aneurysms require particular control because the space in the aneurysm sac is extremely limited. On the other hand, the ability to control and track therapeutic devices is restricted by the tortuosity and acute angle of ACoA aneurysms [5, 6]. Moret et al. [5] found that the presence of a looped cervical artery and intracranial vessels rendered catheterization into the aneurysm impossible in 9% of patients with ACoA aneurysms. Especially in patients undergoing treatment for ACoA aneurysms, the guiding catheter should be inserted as far as possible into the distal portion of the ICA to ensure the control and tracking of therapeutic devices. We consider this key for successful EVT. Therefore, we chose the direct carotid approach in cases where it was impossible to insert the guiding catheter sufficiently into the ICA by the transfemoral approach. In the direct carotid approach we place a 5F guiding catheter into the distal segment of the petrous portion of the ICA [13]. This improves control and tracking of therapeutic devices even in patients with severe arteriosclerosis. The tortuosity of the access route to the aneurysm may result in unexpected microcatheter movement, e.g. a jump into the aneurysm sac, which in turn may lead to aneurysm rupture. Taking into account the length of the A1 segment, we usually place a curve in the microcatheter; this serves as an anchor to prevent jumping of the microcatheter into the aneurysm sac. This made it possible to perform EVT in all 19 patients without encountering intraoperative rupture or any other treatment-related complications.
Some patients show recanalization of the embolized aneurysm in the chronic stage after EVT. This is less common with small aneurysms than it is with large/giant aneurysms in the long term [14]. The reported recanalization rate of large aneurysms (diameter more than 10 mm) is as high as 50.6% while that of small aneurysms (diameter less than 10 mm) is 21.3% [15]. However, the long-term outcome of EVT for tiny aneurysms has not been reported. Although 2 of 16 (12.5%) aneurysms in our series recanalized, angiographic follow-up for a median of 38.5 months indicated that no patients with recanalized aneurysms required additional treatment. Our findings suggest that stable embolization of tiny aneurysms can be obtained over the long term. Because GDCs retain their circular memory shape on delivery into the aneurysm sac, they do not completely pack the aneurysm volume and space remains between coils in the sac. The more coils are introduced, the more dead space develops and this may lead to coil compaction and recanalization of the aneurysm [16]. To treat large aneurysms with a diameter exceeding 10 mm, 0.018-inch coils are frequently used. As they are thicker and stiffer than 0.010-inch coils, they tend to pile up, raising the likelihood of developing dead space in larger aneurysms [16]. In contrast, the amount of dead space between coils is thought to be small in tiny compared to large aneurysms. In the presence of less dead space, clots are easily formed in the dead space and the complex of coils and clots fills the aneurysm sac at an earlier stage. In addition, as the neck of tiny aneurysms tends to be small, there tends to be less blood flow at that site, reducing the risk of coil compaction [17]. However, dense packing is considered necessary even for tiny aneurysms since insufficient packing can result in coil compaction [18].
The optimal volume embolization ratio (VER) is recommended to maintain long-term stability in embolized aneurysms [17, 19]. With respect to tiny aneurysms, Suzuki et al. [18] reported that the technique of coiling with one coil by calculating the appropriate coil length on preoperative angiograms can achieve optimal VER. However, some ruptured aneurysms harbor an intraaneurysmal thrombus whose presence may result in an underestimation of the aneurysm volume on preoperative angiograms [20]. In fact, we encountered postembolization aneurysm distention suggestive of an intraaneurysmal thrombus in 52.6% of our patients. Since the presence of an intraaneurysmal thrombus prevents the accurate calculation of VER, we did not determine the VER in this study.
Although we were able to achieve complete or near-complete occlusion with the single-catheter technique in all of our patients, some tiny ACoA aneurysms cannot be easily treated by EVT. In patients with tiny aneurysms, the remodeling technique reportedly increases the risk of intraoperative rupture [8]. Microsurgical clipping may be appropriate in these patients who require application of the remodeling technique. This procedure may also be necessary to treat aneurysms with a maximum diameter less than 2 mm for which no appropriate coils are available at present.
Conclusion
EVT can be used by expert neurointerventionalists to treat tiny ruptured ACoA aneurysms if placement of the guiding catheter is performed carefully and the microcatheter is shaped so as to prevent its jumping during the procedure. Under favorable embolization conditions, rebleeding can be prevented and recanalization over the long term occurs infrequently.
Acknowledgments
Conflict of interest statement
We declare that we have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kassell NF, Torner JC, Haley EC, Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 2.Yasargil MG. Microneurosurgery, vol II. New York: Thieme; 1984. pp. 169–184. [Google Scholar]
- 3.Birknes JK, Hwang SK, Pandey AS, Cockroft K, Dyer AM, Benitez RP, et al. Feasibility and limitations of endovascular coil embolization of anterior communicating artery aneurysms: morphological considerations. Neurosurgery. 2006;59:43–52. doi: 10.1227/01.NEU.0000219220.25721.B9. [DOI] [PubMed] [Google Scholar]
- 4.Kazekawa K, Tsutsumi M, Aikawa H, et al. Endovascular treatment of anterior cerebral artery aneurysms using Guglielmi detachable coils: mid-term clinical evaluation. Radiat Med. 2002;20:291–297. [PubMed] [Google Scholar]
- 5.Moret J, Pierot L, Boulin A, Castaings L, Rey A. Endovascular treatment of anterior communicating artery aneurysms using Guglielmi detachable coils. Neuroradiology. 1996;38:800–805. doi: 10.1007/s002340050352. [DOI] [PubMed] [Google Scholar]
- 6.Proust F, Debono B, Hannequin D, et al. Treatment of anterior communicating artery aneurysms: complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99:3–14. doi: 10.3171/jns.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux A, Kerr R, Stratton I, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group: International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/S0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 8.Sluzewski M, Bosch JA, van Rooij WJ, Nijssen PC, Wijnalda D. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. 2001;94:238–240. doi: 10.3171/jns.2001.94.2.0238. [DOI] [PubMed] [Google Scholar]
- 9.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 10.Tidswell P, Dias PS, Sagar HJ, Mayes AR, Battersby RD. Cognitive outcome after aneurysm rupture: relationship to aneurysm site and perioperative complications. Neurology. 1995;45:875–882. doi: 10.1212/wnl.45.5.876. [DOI] [PubMed] [Google Scholar]
- 11.Chan A, Ho S, Poon WS. Neuropsychological sequelae of patients treated with microsurgical clipping or endovascular embolization for anterior communicating artery aneurysm. Eur Neurol. 2002;47:37–44. doi: 10.1159/000047945. [DOI] [PubMed] [Google Scholar]
- 12.Fontanella M, Perozzo P, Ursone R, Garbossa D, Bergui M. Neuropsychological assessment after microsurgical clipping or endovascular treatment for anterior communicating artery aneurysm. Acta Neurochir (Wien) 2003;145:867–872. doi: 10.1007/s00701-003-0111-5. [DOI] [PubMed] [Google Scholar]
- 13.Nii K, Kazekawa K, Onizuka M, et al. Direct carotid puncture for the endovascular treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol. 2006;27:1502–1504. [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239–249. doi: 10.1097/00006123-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 16.Tamatani S, Ito Y, Abe H, Koike T, Takeuchi S, Tanaka R. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol. 2002;23:762–767. [PMC free article] [PubMed] [Google Scholar]
- 17.Kai Y, Hamada J, Morioka M, Yano S, Kuratsu J. Evaluation of the stability of small ruptured aneurysms with a small neck after embolization with Guglielmi detachable coils: correlation between coil packing ratio and coil compaction. Neurosurgery. 2005;56:785–792. doi: 10.1227/01.NEU.0000156790.28794.EA. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Kurata A, Ohmomo T, et al. Endovascular surgery for very small ruptured intracranial aneurysms. Technical note. J Neurosurg. 2006;105:777–780. doi: 10.3171/jns.2006.105.5.777. [DOI] [PubMed] [Google Scholar]
- 19.Yagi K, Satoh K, Satomi J, Matsubara S, Nagahiro S. Evaluation of aneurysm stability after endovascular embolization with Guglielmi detachable coils: correlation between long-term stability and volume embolization ratio. Neurol Med Chir (Tokyo) 2005;45:561–565. doi: 10.2176/nmc.45.561. [DOI] [PubMed] [Google Scholar]
- 20.Yu SC, Wong WC, Chung AC, Lee KT, Wong GK, Poon WS. Does endoluminal coil embolization cause distension of intracranial aneurysms? Neuroradiology. 2006;48:653–660. doi: 10.1007/s00234-006-0107-y. [DOI] [PubMed] [Google Scholar]