Abstract
Purpose
Several studies have compared conventional open ileocolic resection with a laparoscopic-assisted approach. However, long-term outcome after laparoscopic-assisted ileocolic resection remains to be determined. This study was designed to compare long-term results of surgical recurrence, quality of life, body image, and cosmesis in patients who underwent laparoscopic-assisted or open ileocolic resection for Crohn’s disease.
Methods
Seventy-eight consecutive patients who underwent ileocolic resection during the period 1995 to 1998 were analyzed; 48 underwent a conventional open approach in the Academic Medical Centre (Amsterdam, The Netherlands) and 30 underwent a laparoscopic-assisted approach in the Leiden University Medical Centre (Leiden, The Netherlands). Primary outcome parameters were reoperation and readmission rate. Secondary outcome parameters were quality of life, body image, and cosmesis.
Results
The two groups were comparable for characteristics of sex, age, and immunosuppressive therapy. Seventy-one patients had a complete follow-up of median 8.5 years. Resection for recurrent Crohn’s disease was performed in 6 of 27 (22 percent) and 10 of 44 (23 percent) patients in the laparoscopic and open groups, respectively. Reoperations for incisional hernia were only performed after conventional open ileocolic resection (3/44 = 6.8 percent). Quality of life and body image were comparable, but cosmesis scores were significantly higher in the laparoscopic group.
Conclusions
Despite small numbers, we found that surgical recurrence and quality of life after laparoscopic-assisted and open ileocolic resection were comparable. Incisional hernias occurred only after open ileocolic resection, and laparoscopic-assisted ileocolic resection resulted in a significantly better cosmesis.
Key words: Crohn’s disease, Laparoscopy, Ileocolic resection, Laparoscopic, CD, Long-term morbidity, Body image, Quality of life
Crohn’s disease is a chronic inflammatory bowel disease that can involve the entire digestive tract. In approximately 50 percent of cases, the disease is confined to the terminal ileum. Despite medical therapy, which is the first choice of treatment, 70 to 90 percent of patients with ileocolic Crohn’s disease will need a surgical intervention once during their lifetime. If a strictureplasty is considered inappropriate, resection of the diseased segment, most often the terminal ileum, is necessary.1,2
Ileocolic resection can be performed both by an open and a laparoscopic-assisted approach. In the past decades, several studies have been conducted comparing the conventional open approach with a laparoscopic approach.3–9 A systematic review of these studies concluded that laparoscopic surgery for ileocolic Crohn’s disease is feasible and safe, and a good alternative for open surgery with the advantage of a shorter hospital stay.10 Although the applicability of laparoscopic ileocolic resection increases, only two randomized, controlled trials comparing open and laparoscopic ileocolic resection have been conducted at present.6,7 Most studies, including the two available randomized, controlled trials, have been focusing on short-term results. It is known that Crohn’s disease is characterized by relapses and frequent surgical reinterventions despite improvements made in medical management. Little data are available with respect to long-term outcome after open vs. laparoscopic ileocolic resection, such as the incidence of surgical reintervention and the frequency of readmissions caused by small-bowel obstruction.11
It can be hypothesized that laparoscopic-assisted ileocolic resection, in addition to the demonstrated short-term benefits, has benefits over open ileocolic resection on long-term follow-up. Attenuated adhesion formation, reducing the number of patients requiring reoperation for small-bowel obstructions, and a reduced incidence of incisional hernias because of the less invasive approach have been proposed.10 Currently, no good data are available addressing this issue.
The most obvious benefit of the laparoscopic technique seems to be the cosmetic result. Compared with open surgery, a laparoscopic-assisted ileocolic resection results in a better cosmetic outcome.12 The initial presentation of the disease can be at any age but often is early in life. Therefore, the advantage of a superior cosmesis might be of particular interest in these relatively young patients. Data with respect to both short-term and long-term quality of life (QOL) in patients with Crohn’s disease who underwent laparoscopic ileocolic resection are scarce. Although a few studies have described short-term results with regard to QOL, indicating that there was no difference between laparoscopic and open resection,6,12 long-term QOL remains to be determined; only one study addressing long-term QOL has been published.13
The present study was designed to compare long-term results in terms of both clinical outcome and QOL after open compared with laparoscopic-assisted ileocolic resection for Crohn’s disease.
Patients and Methods
A consecutive series of 78 patients who underwent ileocolic resection for Crohn’s disease from 1995 until 1998 were included in this study. Of those, 48 patients underwent open ileocolic resection at the Academic Medical Centre (AMC; Amsterdam, The Netherlands) and 30 patients underwent laparoscopic-assisted ileocolic resection in the Leiden University Medical Centre (LUMC; Leiden, The Netherlands). Short-term outcome of the same patient population has been described in a previous publication. Full methodologic and operative details of the 78 patients can be found in the original article.3
The medical files of all patients were retrieved. To rule out potential treatments in other hospitals, a questionnaire with detailed questions about (number of) surgical reinterventions, relapses, and readmissions was sent in addition to the medical file search. QOL, body image, and cosmesis were assessed by using a postal survey. After retrieval of the medical files and completion of the questionnaires, all patients were invited to the outpatient department to verify the findings from the medical files. Physical examination also was performed during this visit to determine the presence of incisional hernia. If patients were unable to visit our department, they were contacted by telephone. Detailed questions about potential symptoms or presence of an incisional hernia were asked by a trained interviewer from our department (EJE).
Patients were only considered for analysis of the clinical outcome parameters if follow-up was complete. Follow-up was defined as complete if patients’ medical files were up to date (last contact with the hospital between December 1, 2004 and June 1, 2005). The end of the study period was set on the date the questionnaires were sent: June 1, 2005. In case medical files were not up to date, patients were considered lost to follow-up, except for those who completed the questionnaires (including detailed questions about surgical reinterventions, readmissions, and relapses), or those patients who could be contacted by telephone. In case a patient had died, the date of death was defined as the end of the study period. These patients were only considered for analysis if the medical files were up to date at the time of death.
Primary outcome parameters of the present study were the number of patients with intestinal disease recurrence requiring surgery, the number of readmissions for intestinal disease recurrence or other causes: small-bowel obstruction (SBO) caused by adhesions, treatment of perianal abscesses or fistula, and the number of patients requiring a surgical reintervention for incisional hernia. Secondary outcome parameters were results of QOL, body image, and cosmesis.
For intestinal recurrence rates, three groups of patients were distinguished. The first group consisted of patients with intestinal disease activity that could be managed conservatively at the outpatient department. The second group consisted of patients with intestinal disease activity that could be managed conservatively, although a readmission was necessary for treatment of the exacerbation. In these two groups, the diagnosis of exacerbation of Crohn’s disease was based on history, physical examination, laboratory tests, endoscopy, and radiologic findings. The decision to admit or treat patients in the outpatient department was at the discretion of the attending gastroenterologist. The third group consisted of patients who required a re-resection (whether or not in combination with a strictureplasty) of the diseased bowel to manage the recurrence. In these cases, disease activity was confirmed by pathologic examination of the resected bowel. In the present study, perianal disease was not considered an intestinal disease recurrence.
Quality of Life
The Short Form-36 health survey (SF-36) was used to assess general QOL.14 This is a generic, validated QOL questionnaire, consisting of 36 questions combined to form 8 domains. It has 0 to 100 scales in the 8 domains. The results of this questionnaire were compared with QOL data of a healthy control group of the Dutch population matched for age and sex. The Gastrointestinal Quality of life Index (GIQLI) was used to specifically assess bowel-related QOL. This disease-specific questionnaire consists of 36 questions with 5 response categories and is described in detail by Eypasch et al. 15
Body Image and Cosmesis
The body image questionnaire (BIQ) was used to assess body image and cosmesis. The questionnaire consists of eight questions that are combined to form two scales: a body image scale and a cosmesis scale.12 Five questions regarding body image assess patients’ perception of and satisfaction with their own body and evaluates patients’ attitude toward their bodily appearance. The body image scale ranges from 5 (lowest body image score) to 25 (highest body image score). Three questions regarding the cosmetic result after the operation assess the degree of satisfaction with respect to the physical appearance of the incisional scar(s). First patients were asked to give a score for the appearance of their scar(s) on a scale from 1 (lowest score) to 10 (highest score). Then patients were asked to grade the extent to which they were satisfied with their scar on a Likert scale ranging from 1 (very unsatisfied) to 7 (very satisfied). Finally patients were asked to describe their scar on a Likert scale ranging from 1 (very repulsing) to 7 (very beautiful). The combined scores of these three questions resulted in the cosmesis scale ranging from 3 (lowest satisfaction) to 24 (highest satisfaction).
A photoseries questionnaire (PSQ) was used additionally to assess whether the degree of satisfaction with one’s own scar would be affected by seeing the cosmetic result of the alternative procedure.12 Patients were first asked to grade their own scar on a scale from 1 (lowest score) to 10 (highest score). Next, they had to grade the pictures of two other patients (1 male, 1 female) who underwent the operation by the alternative approach. After seeing these pictures, patients were again asked to grade their own incisional scar. Next, patients were asked to state their preference for one of the two surgical approaches (laparoscopic-assisted or open). The patients who chose laparoscopy were asked whether they were willing to spend an extra amount of Euros in a hypothetical situation where the only differences between open and laparoscopic surgery would be the cosmetic result and the costs of surgery (laparoscopy being more expensive).
Statistical Analysis
All data were analyzed according to an intention-to-treat principle. Quantitative data were expressed as mean values accompanied by range unless otherwise specified. Statistical analysis was performed by using SPSS® software version 12.0 (SPSS, Chicago, IL). A power analysis was performed by calculating the odds ratios (OR) and 95 percent confidence interval (CI) of potential confounding effects of patient characteristics on the primary endpoints, regardless of study arms. The nonparametric Mann-Whitney U test was used to compare quantitative variables between two groups. A Student’s t-test was used to compare the results of QOL and the BIQ. The chi-squared test or Fisher’s exact test was used to compare categorical or dichotomous variables between two groups. To test for differences between quantitative variables within a group, the nonparametric Wilcoxon’s signed-rank test was used. In addition to the absolute number of surgical reinterventions, the interval between the primary resection and re-resection was determined by conducting a Kaplan-Meier analysis of re-resection-free survival. To test for a difference in re-resection-free survival, the log rank test was performed. P < 0.05 was accepted as significant for all tests. To correct for multiple testing and to reduce the possibility of Type I error, the Bonferroni correction was adjusted in analysis of QOL, meaning that P < 0.005 was accepted as significant for these tests.
Results
Of the total group of 78 potential patients, 7 patients were lost to follow-up because the current address was unknown and/or the medical file was not up-to-date (Fig. 1). Therefore, 71 patients were available for analysis of clinical outcome. Median age of these 71 patients was 37.7 (mean, 42; range, 26.3–85) years at the time of the end of the study period. The median follow-up was 8.6 (mean, 8.5; range, 4.2–10.4) years. There were 17 male patients and 54 female patients.
Figure 1.
Flowchart. SF-36 = Short Form-36 health survey; GIQLI = Gastrointestinal Quality of Life Index; BIQ = body image questionnaire.
For analysis of QOL, body image, and cosmesis, data for 61 patients who completed the questionnaires were available (Fig. 1). The median age of these 61 patients was 37.7 (mean, 41.2; range, 26.3–85) years at the time of the end of the study period. Median follow-up for these 61 patients was 8.8 (mean, 8.6; range, 6.3–10.4) years. Thirteen male patients and 48 female patients completed the questionnaires.
Clinical Outcome
Of the 71 patients available for analysis of clinical outcome parameters, 44 were operated on by an open approach and 27 by a laparoscopic-assisted approach (Fig. 1; Table 1). The open and laparoscopic group were comparable for characteristics of sex, age, body mass index, length of follow-up, and number of patients who were smoking (both at time of surgery and at the end of the study period). Length of resected bowel segment at the time of the initial operation, type of anastomosis, number of patients with postoperative immunomodulating medication, and number of patients with perianal fistulas also were comparable for the two groups. In all patients, complete resection of the macroscopically affected bowel was achieved. More patients from the laparoscopic group than from the open group required additional procedures during their primary ileocolic resection (P = 0.043). In the open group, three patients died during the study period. In all three cases, the cause of death was not related to Crohn’s disease: one patient died at the aged 50 years because of a gastric carcinoma 91 months after ileocolic resection; two other patients died at aged 69 and 77 years, respectively, both as a result of myocardial infarction. Follow-up after ileocolic resection for these patients at the time of death was 50 and 94 months, respectively.
Table 1.
Characteristics of 71 patients available for clinical outcome analysis
| Open group (n = 44) | Lap group (n = 27) | P value | |
|---|---|---|---|
| Male/female ratio | 10/34 | 7/20 | 0.759† |
| Age (yr)* | 44 (26–85) | 40 (26–66) | 0.139‡ |
| BMI (kg/m2) | 23.14 (16.41–34.63) | 23.87 (19.11–34.54) | 0.441‡ |
| Follow-up (yr) | 0.384‡ | ||
| Mean (range) | 8.6 (4.2–10.41) | 8.3 (6.3–10.2) | |
| Median | 8.7 | 8.6 | |
| No. of patients smoking at the end of study period (%) | 18 (41) | 15 (56) | 0.311† |
| No. of patients smoking at time of ileocolic resection (%) | 21 (48) | 14 (52) | 0.736† |
| Length of resected bowel (cm) | 25.27 (5–80) | 23.76 (3–60) | 0.683‡ |
| Type of anastomosis (%) | 0.189† | ||
| S-S | 11 (25) | 10 (37) | |
| E-S/S-E | 29 (66) | 12 (45) | |
| E-E | 4 (9) | 5 (18) | |
| Postoperative immunosuppression prophylaxis (%) | 11 (25) | 4 (15) | 0.307† |
| Azathioprine | 8 (18) | 0 | |
| Budesonide | 2 (5) | 3 (11) | |
| Methotrexate | 1 (2) | 0 | |
| Prednisone, for 2 years | 0 | 1 (4) | |
| Pentasa/salofalk/mesalazine (%) | 13 (30) | 12 (44) | 0.202† |
| Additional procedures at time of ileocolic resection (%) | 7 (16) | 10 (37) | 0.043† |
| No. of patients having perianal fistulas at time of ileocolic resection (%) | 4 (9) | 3 (11) | 1† |
| Mortality | 3 | 0 | 0.283† |
Lap = laparoscopic-assisted; BMI = body mass index; S-S = side-side; E-S/S-E = end-side or side-end; E-E = end-end.
*Age at time of follow-up; age of the patients who died was determined at the date of death.
†Chi-squared test or Fisher’s exact test.
‡Mann-Whitney U test.
Disease Recurrence
Twenty-four patients (18/44 from the open group and 6/27 from the laparoscopic group; P = 0.106) had an uncomplicated long-term course after their initial ileocolic resection (Table 2). Seventeen patients (10 from the open group and 7 from the laparoscopic group) had at least one disease exacerbation, which in all cases could be managed at the outpatient department (P = 0.759). In most cases treatment with oral steroids was started. None of these 17 patients had surgical reintervention.
Table 2.
Intestinal disease recurrence for 71 patients with complete follow-up
| Open group (n = 44) | Lap group (n = 27) | P value* | |
|---|---|---|---|
| No intestinal recurrence | 18 (41) | 6 (22) | 0.106 |
| Intestinal recurrence managed at the outpatient department (never readmission) | 10 (23) | 7 (26) | 0.759 |
| Intestinal disease recurrence managed by conservative treatment (never re-resection) | 6 (14) | 8 (30) | 0.1 |
| Medication | 2 | 3 | |
| Combination | 4 | 5 | |
| Med, npo, df | 2 | 4 | |
| Nasogastric tube, npo | 0 | 1 | |
| Med, npo, nasogastric tube | 1 | 0 | |
| Med, npo, nasogastric tube, balloon dilatation | 1 | 0 | |
| Intestinal recurrence requiring re-resection | 10 (23) | 6 (22) | 0.961 |
| Neoterminal ileum and ascending colon | 7 (16) | 6 (22) | |
| Stenosis | 5 | 6 | |
| Fistulizing disease | 2 | 0 | |
| Jejunum | 1 (2) | 0 (0) | |
| Stenosis | 1 | 0 | |
| Fistulizing disease | 0 | 0 | |
| Sigmoid | 2 (5) | 0 (0) | |
| Stenosis | 1 | 0 | |
| Fistulizing disease | 1 | 0 |
Lap = laparoscopic-assisted; med = medication prescribed; npo = nihil per os; df = drip feed.
Data are numbers with percentages in parentheses unless othewise indicated.
*Chi-squared test or Fisher’s exact test.
The remaining 30 patients (16 from the open group vs. 14 from the laparoscopic group) were readmitted at least once as a result of intestinal disease recurrence. All patients, including those with at least one readmission caused by intestinal disease recurrence, are shown in Table 2. Of those 30 readmitted patients (6/16 from the open group vs. 8/14 from the laparoscopic group), the disease recurrence was managed conservatively during their readmission. For the remaining 16 patients (10 from the open group and 6 from the laparoscopic group), surgery was required at least once during one of their readmissions. The average length of resected bowel at the time of the first re-resection was 17 vs. 14 cm in the open and laparoscopic groups, respectively. All six patients from the laparoscopic group who required a re-resection underwent re-resection by a laparoscopic-assisted approach. In Figure 2, re-resection-free survival time in years between the initial ileocolic resection and the first re-resection for both the open and laparoscopic-assisted group is shown. In addition to a comparable number of re-resections between the groups, the time between initial resection and the first re-resection was comparable as well (P = 0.982).
Figure 2.
Analysis of re-resection-free survival (P = 0.982).
The potential confounding effect of patient characteristics on the primary end point (disease recurrence) is shown in Table 3. None of these potential confounding factors had a significant effect on disease recurrence.
Table 3.
Odds ratio, 95 percent CI, and P values of patient characteristics as potential confounding factors regardless of study-arm for all four primary outcome parameters
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| No intestinal disease recurrence | |||
| Gender | 0.9 | 0.3–2.9 | 0.882 |
| Age (dichotomized at median age of 37.7 yr) | 0.8 | 0.3–2.2 | 0.677 |
| BMI (dichotomized at median BMI of 23) | 1.2 | 0.5–3.3 | 0.671 |
| No. of patients smoking at time of ileocolic resection | 0.5 | 0.2–1.3 | 0.155 |
| Length of resected bowel (dichotomized at median length of 20 cm) | 0.7 | 0.3–2 | 0.541 |
| Postoperative immunosuppression prophylaxis | 0.2 | 0.049–1.2 | 0.059 |
| Additional procedures | 0.8 | 0.2–2.5 | 0.661 |
| No. of patients having fistulas at time of ileocolic resection | 0.3 | 0.3–2.6 | 0.41 |
| Intestinal recurrence managed at the outpatient department (never readmission) | |||
| Gender | 1 | 0.3–3.7 | 0.963 |
| Age (dichotomized at median age of 37.7 yr) | 0.9 | 0.3–2.6 | 0.832 |
| BMI (dichotomized at median BMI of 23) | 0.5 | 0.2–1.7 | 0.289 |
| No. of patients smoking at time of ileocolic resection | 0.9 | 0.3–2.6 | 0.832 |
| Length of resected bowel (dichotomized at median length of 20 cm) | 1.4 | 0.4–4.2 | 0.593 |
| Postoperative immunosuppression prophylaxis | 1.2 | 0.3–4.4 | 0.781 |
| Additional procedures | 1 | 0.3–3.5 | 0.963 |
| No. of patients having fistulas at time of ileocolic resection | 2.7 | 0.5–13.4 | 0.217 |
| Intestinal disease recurrence managed by conservative treatment (never re-resection) | |||
| Gender | 1.2 | 0.3–4.9 | 0.806 |
| Age (dichotomized at median age of 37.7 yr) | 3.2 | 0.9–11.4 | 0.065 |
| BMI (dichotomized at median BMI of 23) | 1.7 | 0.5–5.6 | 0.372 |
| No. of patients smoking at time of ileocolic resection | 1.5 | 0.5–4.8 | 0.512 |
| Length of resected bowel (dichotomized at median length of 20 cm) | 1.3 | 0.4–4.4 | 0.663 |
| Postoperative immunosuppression profylaxis | 1 | 0.2–4.3 | 0.975 |
| Additional procedures | 2.1 | 0.6–7.4 | 0.249 |
| No. of patients having fistulas at time of ileocolic resection | 1.7 | 0.3–10 | 0.535 |
| Intestinal recurrence requiring re-resection | |||
| Gender | 0.9 | 0.3–3.4 | 0.91 |
| Age (dichotomized at median age of 37.7 yr) | 0.5 | 0.2–1.7 | 0.284 |
| BMI (dichotomized at median BMI of 23) | 0.9 | 0.3–2.7 | 0.804 |
| No. of patients smoking at time of ileocolic resection | 2 | 0.6–6.3 | 0.23 |
| Length of resected bowel (dichotomized at median length of 20 cm) | 0.9 | 0.3–2.6 | 0.788 |
| Postoperative immunosuppression profylaxis | 3.1 | 0.9–10.6 | 0.068 |
| Additional procedures | 0.7 | 0.2–2.7 | 0.58 |
| No. of patients having fistulas at time of ileocolic resection | 0.5 | 0.1–4.9 | 0.582 |
CI = confidence interval; BMI = body mass index.
Perianal Disease
In 11 patients, surgical reintervention was required for perianal fistulas: 5 patients (11 percent) were from the open group and 6 (22 percent) were from the laparoscopic group (P = 0.312). Treatment consisted of fistulotomy, seton placement, or drainage of a perianal abscess.
Incisional Hernia and SBO
Reoperation for incisional hernia was performed in three patients, all from the open group (7 percent). In all three patients the incisional hernia was symptomatic.
It was not possible to quantify the number of adhesions and to differentiate between patients who were readmitted for SBO or disease recurrence in case of nonsurgical readmission and conservative treatment. In only one patient from the laparoscopic group, adhesiolysis was the indication for relaparoscopy. At relaparoscopy, neither adhesions nor a disease recurrence was found. After the operation, this patient remained symptomatic for unknown reasons.
Quality of Life
Sixty-one of the potential 75 patients who were alive at the time of the postal survey completed the questionnaires (81 percent response rate). Of those, 38 were from the open group and 23 from the laparoscopic group (Fig. 1). In the open group, five patients could not be contacted and two patients refused participation. In the laparoscopic group, six patients could not be contacted and one patient refused participation. Patient characteristics of the 61 patients who completed the questionnaires were comparable for both groups (data not shown).
General and Bowel-Related QOL
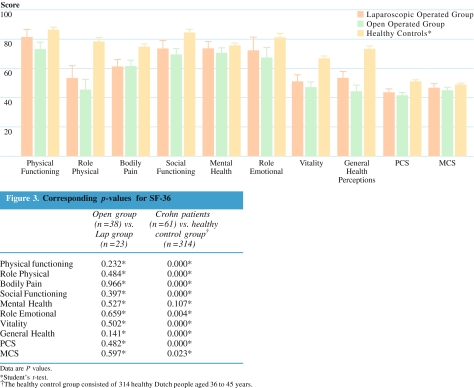
Although scale scores in seven of the eight subscales and the physical and mental component scores (PCS and MCS) of the SF-36 were higher in the laparoscopic compared with the open group, the differences were not statistically significant (Fig. 3). Compared with a healthy control group matched for age and sex, patients from both groups had statistically significant lower scores on seven of the eight subscales from the SF-36. If corrected for multiple testing, the PCS of the SF-36 was significantly lower for the patients with Crohn’s disease compared with the healthy control group (Fig. 3); however, the MCS was not accepted as statistically significant. No differences were found between the two groups with the GIQLI on any of the subscales and the total score (data not shown).
Figure 3.
Analysis of SF-36 scores. SF-36 = Short Form-36 health survey; Lap = laparoscopic-assisted; PCS = physical component score; MCS = mental component score.
Body Image and Cosmesis
There was no statistically significant difference in the body image scale scores between the open and the laparoscopic group (Table 4); however, the cosmesis scale score was significantly higher in the laparoscopic group.
Table 4.
Results of the BIQ after open compared with laparoscopic ileocolic resection for 61 patients who completed the questionnaires
| Open group (n = 38) | Lap group (n = 23) | P value* | |
|---|---|---|---|
| Body image scale | 15.63 (6–20) | 16.3 (7–20) | 0.512 |
| Cosmetic scale | 13.33 (3–24) | 19.61 (7–24) | 0 |
Lap = laparoscopic-assisted; BIQ = body image questionnaire.
Data are means with ranges in parentheses unless otherwise indicated.
* Student’s t-test.
The results of the PSQ are presented in Table 5. Although we have not measured the exact length, typically, the length of the minilaparotomy of laparoscopic-assisted ileocolic resection varies from 4 to 5 cm, mostly hidden in the shallow of the umbilicus, compared with a 10 to 15 cm full exposed midline incision after open ileocolic resection. Patients of the laparoscopic group rated their scars significantly higher than patients from the open group. In both groups, patients rated pictures of the alternative approach comparable to the rates of their own scars in each group. Only one patient from the laparoscopic group preferred an open approach rather than a laparoscopic operation if she had the choice. Two-thirds of the patients from the open group preferred the laparoscopic approach. Thirty-nine percent (32 percent in the open group and 52 percent in the laparoscopic group) were willing to spend an extra amount of Euros to have the laparoscopic approach.
Table 5.
Results of the PSQ after open compared with laparoscopic ileocolic resection for 61 patients who completed the questionnaires
| Open group (n = 38) | Lap group (n = 23) | P value | |
|---|---|---|---|
| Rating own scars before seeing pictures | 5.5 | 8 | 0† |
| Rating pictures of other approach | 8.3 | 5.5 | 0† |
| Rating of own scars after seeing pictures | 4.8 | 8.4 | 0† |
| Difference (before – after) | −0.7 (P = 0.001*) | +0.4 (P = 0.08*) | |
| Preference for specific approach | 0.082‡ | ||
| Open | 4 (10.5) | 1 (4.3) | |
| Laparoscopic | 25 (65.8) | 21 (91.3) | |
| No preference | 8 (21.1) | 1 (4.3) | |
| No answer | 1 (2.6) | 0 | |
| Personal additional fee to undergo the laparoscopic approach (in Euros) | 0.039‡ | ||
| Nothing | 25 (65.8) | 10 (43.5) | |
| 0–499 | 6 (15.8) | 1 (4.3) | |
| 499–999 | 1 (2.6) | 3 (13) | |
| >1,000 | 5 (13.2) | 8 (34.8) | |
| No answer | 1 (2.6) | 1 (4.3) |
Lap = laparoscopic-assisted; PSQ = photo series questionnaire.
Data are numbers with percentages in parentheses unless othewise indicated.
*Wilcoxon’s signed-rank test.
†Mann Whitney U test.
‡Chi-squared test or Fisher’s exact test.
Within the open and laparoscopic group, there were no differences between male and female patients with regard to body image, cosmesis, and the rating of incisional scars.
Discussion
With 71 patients being evaluated at a median postoperative follow-up of more than eight years, the present study investigated the long-term results comparing two cohorts of patients who underwent an open and a laparoscopic-assisted ileocolic resection for Crohn’s disease. The study demonstrated that there was no difference in surgical recurrence between open and laparoscopic-assisted ileocolic resection for Crohn’s disease. Supposed attenuated depression of the immunologic system after a laparoscopic resection did not lead to a higher surgical recurrence. QOL at long-term follow-up was significantly reduced compared with the general population. Although QOL was lower in the open group in most subscales, the reduction was not statistically significant. Cosmesis after the laparoscopic approach was significantly better than after the open approach.
Although patients were treated in two different university hospitals, which might have resulted in a hospital bias, the possibility of patient selection for one of both approaches was excluded in this way because each hospital only used one surgical approach (i.e., open or laparoscopic). An overall response rate of 81 percent to the questionnaires is acceptable, in particular because follow-up was more than eight years. Moreover, 90 percent of the patients of the laparoscopic group and 92 percent of the patients of the open group were available for analysis of clinical outcome, which indicates that the current study is representative.
There were more readmissions in the laparoscopic group, but the incidence of surgical recurrences was similar. Re-resection of a diseased segment of the bowel, confirmed by pathologic examination, is a more objective measurement for disease recurrence than is a readmission, as indications might differ between the two hospitals. It is unlikely that the difference in readmission rate, in combination with a similar number of re-resections, can be explained by a true difference in disease recurrence rate; a hospital bias in terms of a different treatment protocol is a more likely explanation for this difference.
A comparable study with assessment of long-term clinical outcome has been performed previously by Lowney et al. 11 In contrast to that study, the present study did not find a higher recurrence rate in the open group. This can be explained by several confounding factors that might have influenced outcome in that study. In the study by Lowney et al. the open and laparoscopic groups were not comparable for several important parameters: length of follow-up in the open group was significantly longer than in the laparoscopic group (81.2 vs. 60.4 months), resulting in a higher probability for the development of disease recurrence. In that study, a selection bias probably has confounded the results, selecting the best patients for the laparoscopic approach. This can be deducted from the higher incidence of additional procedures in the open group. In the present study, length of follow-up of the open and laparoscopic groups was similar (means, 8.3 vs. 8.6 years) and the incidence of preexistent fistulas was comparable. In contrast to the study by Lowney et al., in which more additional procedures were performed in the open group, indicating a potentially more severe disease phenotype, in the present study patients from the laparoscopic group underwent more additional procedures. Other studies available in literature have reported outcome at a medium long-term follow-up of 20 to 60 months,7,8,16–19 of which only one study had long-term follow-up as its primary goal.17 Three studies found similar surgical recurrence rates in the open and laparoscopic groups, in accordance with the results of the present study.16,17,19 Although the three other studies found no recurrences in both groups, these results must be interpreted cautiously because follow-up was limited to only 19 months.7,8,18
The three operations for incisional hernias in the present study occurred in the open group vs. none in the laparoscopic group. Although not statistically significant, a tendency of a higher incidence of incisional hernias after open surgery seems to exist. The authors have no explanation for the higher proportion of patients in the laparoscopic group requiring surgical treatment for perianal fistulas. Although previous studies found a reduction in SBO after a laparoscopic approach, the present study could not confirm these results.16,17 None of the patients from the laparoscopic or the open group required reoperation for small-bowel obstruction caused by adhesions, which is remarkable. In the present series a laparoscopic-assisted procedure was performed. Compared with the open approach, the procedure not only differs with respect to the place and extent of the scar, but also with respect to the mobilization of the right colon, which is performed laparoscopically. Although the operation can be performed completely laparoscopically, most surgeons prefer to use a laparoscopic-assisted procedure because it is faster, safer, and less expensive. Vascular ligation, transection, and fashioning of the anastomosis are done extracorporeally. In this way, additional procedures, such as proximal strictureplasty or segmental small-bowel resection, can be performed easily and safely. It is unclear whether a complete laparoscopic compared with a laparoscopic-assisted procedure would have advantages with respect to SBO and incisional hernia.
Several studies have evaluated QOL after ileocolic resection. Most studies concluded that QOL is good.20,21 In contrast to these studies, in the present study QOL was found to be reduced compared with a healthy control group. Only a study by Thaler et al. 13 found results similar to the present study. A possible explanation for the unsuspected reduction in QOL is that in the present study the patient group was compared with a healthy control group that was matched for age; in the other studies it was not clearly documented whether data were stratified for age and sex. The reduction in QOL might be explained by the chronicity of Crohn’s disease, which can recur any time. This is in contrast to patients with ulcerative colitis who have a QOL comparable to the normal population after restorative colectomy—probably because these patients are cured after their operation.22–24 Fear of recurrence can have a negative impact on QOL as well. In the present study, the difference in QOL between the open and laparoscopic group was not significant. Nonetheless, there was a tendency for better QOL in the laparoscopic group. Because both bowel-related QOL, as measured by the GIQLI, and clinical outcome were comparable between both groups, it is unlikely that a difference in disease activity contributed to the difference in general QOL.
Body image was not affected by the surgical approach, but the laparoscopic group was more satisfied with the physical appearance of the scar, resulting in a better cosmesis score. Besides factors, such as age, BMI, or sex, cosmesis also influences body image. Therefore, it can be hypothesized that with a better cosmetic result, body image increases. This was not the case, however. A response shift might have caused the smaller than expected difference between the open and laparoscopic groups; because surgery has taken place a long time ago, patients may have learned to cope with a larger scar.
The fact that patients from both groups rated the pictures of the PSQ comparable to each other indicates that the pictures were representative. Satisfaction with the own scar decreased significantly in the open group after seeing the superior result of the alternative approach. Conversely, satisfaction did not increase in the laparoscopic group after seeing the inferior cosmetic result of an open operation. This might be explained by a ceiling-effect, which means that the superior cosmetic result of the laparoscopic approach was the reference in these patients beforehand. A majority of patients preferred a laparoscopic approach if the same operation was necessary. Most of them were willing to spend an extra amount of Euros if required.
With 71 patients being evaluated, the present study provided long-term data of a relatively small number of persons. Larger numbers of patients are needed to draw valid conclusions on the incidence of SBO. Still the present study is one of only a few studies that assessed long-term results comparing an open and a laparoscopic-assisted ileocolic resection, and it is therefore of importance. More studies with larger sample sizes should be performed to empower conclusions deduced in the present study.
Conclusions
For the present study, long-term surgical recurrence and QOL after open and laparoscopic ileocolic resection were not different. There is no evidence to suspect a higher incidence of procedure-related morbidity in terms of an increase in the incidence of SBO, although incisional hernias only occurred after open resection. QOL of patients with Crohn’s disease who underwent an open or laparoscopic ileocolic resection is significantly reduced compared with QOL of the general population. For the long-term, the advantage of a superior cosmesis remains important for these generally young patients.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Reprints are not available.
Address of correspondence: Willem A. Bemelman, M.D., Ph.D., Academic Medical Center, Department of Surgery, G4-146.1, P.O. Box 22660, 1100 DD Amsterdam, The Netherlands. E-mail: w.a.bemelman@amc.uva.nl
References
- 1.Roy P, Kumar D. Strictureplasty. Br J Surg. 2004;91:1428–1437. doi: 10.1002/bjs.4804. [DOI] [PubMed] [Google Scholar]
- 2.Michelassi F, Hurst RD, Melis M, et al. Side-to-side isoperistaltic strictureplasty in extensive Crohn’s disease: a prospective longitudinal study. Ann Surg. 2000;232:401–408. doi: 10.1097/00000658-200009000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bemelman WA, Slors JF, Dunker MS, et al. Laparoscopic assisted vs. open ileocolic resection for Crohn’s disease. Surg Endosc. 2000;14:721–725. doi: 10.1007/s004640000186. [DOI] [PubMed] [Google Scholar]
- 4.Diamond IR, Langer JC. Laparoscopic-assisted versus open ileocolic resection for adolescent Crohn disease. J Pediatr Gastroenterol Nutr. 2001;33:543–547. doi: 10.1097/00005176-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Duepree HJ, Senagore AJ, Delaney CP, Brady KM, Fazio VW. Advantages of laparoscopic resection for ileocecal Crohn’s disease. Dis Colon Rectum. 2002;45:605–610. doi: 10.1007/s10350-004-6253-6. [DOI] [PubMed] [Google Scholar]
- 6.Maartense S, Dunker MS, Slors JF, et al. Laparoscopic-assisted versus open ileocolic resection for Crohn’s disease: a randomized trial. Ann Surg. 2006;243:143–149. doi: 10.1097/01.sla.0000197318.37459.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milsom JW, Hammerhofer KA, Böhm B, Marcello P, Elson P, Fazio VW. Prospective, randomized trial comparing laparoscopic vs. conventional surgery for refractory ileocolic Crohn’s disease. Dis Colon Rectum. 2001;44:1–8. doi: 10.1007/BF02234810. [DOI] [PubMed] [Google Scholar]
- 8.Shore G, Gonzalez QH, Bondora A, Vickers SM. Laparoscopic vs. conventional iliocolectomy for primary crohn disease. Arch Surg. 2003;138:76–79. doi: 10.1001/archsurg.138.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Young-Fadok TM, HallLong K, McConnell EJ, Gomez Rey G, Cabanela RL. Advantages of laparoscopic resection for ileocolic Crohn’s disease. Improved outcomes and reduced costs. Surg Endosc. 2001;15:450–454. doi: 10.1007/s004640080078. [DOI] [PubMed] [Google Scholar]
- 10.Rosman AS, Melis M, Fichera A. Meta-analysis of trials comparing laparoscopy and open surgery for Crohn’s disease. Surg Endosc. 2005;19:1549–1555. doi: 10.1007/s00464-005-0114-9. [DOI] [PubMed] [Google Scholar]
- 11.Lowney JK, Dietz DW, Birnbaum EH, Kodner IJ, Mutch MG, Fleshman JW. Is there any difference in recurrence rates in laparoscopic ileocolic resection for Crohn’s disease compared with conventional surgery? A long-term, follow-up study. Dis Colon Rectum. 2006;49:58–63. doi: 10.1007/s10350-005-0214-6. [DOI] [PubMed] [Google Scholar]
- 12.Dunker MS, Stiggelbout AM, van Hogezand RA, Ringers J, Griffioen G, Bemelman WA. Cosmesis and body image after laparoscopic-assisted and open ileocolic resection for Crohn’s disease. Surg Endosc. 1998;12:1334–1340. doi: 10.1007/s004649900851. [DOI] [PubMed] [Google Scholar]
- 13.Thaler K, Dinnewitzer A, Oberwalder M, Weiss EG, Nogueras JJ, Wexner SD. Assessment of long-term quality of life after laparoscopic and open surgery for Crohn’s disease. Colorectal Dis. 2005;7:375–381. doi: 10.1111/j.1463-1318.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–1068. doi: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 15.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 16.Alabaz O, Iroatulam AJ, Nessim A, Weiss EG, Nogueras JJ, Wexner SD. Comparison of laparoscopically assisted and conventional ileocolic resection for Crohn’s disease. Eur J Surg. 2000;166:213–217. doi: 10.1080/110241500750009302. [DOI] [PubMed] [Google Scholar]
- 17.Bergamaschi R, Pessaux P, Arnaud JP. Comparison of conventional and laparoscopic ileocolic resection for Crohn’s disease. Dis Colon Rectum. 2003;46:1129–1133. doi: 10.1007/s10350-004-7292-8. [DOI] [PubMed] [Google Scholar]
- 18.Msika S, Iannelli A, Deroide G, et al. Can laparoscopy reduce hospital stay in the treatment of Crohn’s disease? Dis Colon Rectum. 2001;44:1661–1666. doi: 10.1007/BF02234387. [DOI] [PubMed] [Google Scholar]
- 19.Tabet J, Hong D, Kim CW, Wong J, Goodacre R, Anvari M. Laparoscopic versus open bowel resection for Crohn’s disease. Can J Gastroenterol. 2001;15:237–242. doi: 10.1155/2001/814749. [DOI] [PubMed] [Google Scholar]
- 20.Thirlby RC, Sobrino MA, Randall JB. The long-term benefit of surgery on health-related quality of life in patients with inflammatory bowel disease. Arch Surg. 2001;136:521–527. doi: 10.1001/archsurg.136.5.521. [DOI] [PubMed] [Google Scholar]
- 21.Tillinger W, Mittermaier C, Lochs H, Moser G. Health-related quality of life in patients with Crohn’s disease: influence of surgical operation – a prospective trial. Dig Dis Sci. 1999;44:932–938. doi: 10.1023/A:1026600428484. [DOI] [PubMed] [Google Scholar]
- 22.Carmon E, Keidar A, Ravid A, Goldman G, Rabau M. The correlation between quality of life and functional outcome in ulcerative colitis patients after proctocolectomy ileal pouch anal anastomosis. Colorectal Dis. 2003;5:228–232. doi: 10.1046/j.1463-1318.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 23.Delaney CP, Fazio VW, Remzi FH, et al. Prospective, age-related analysis of surgical results, functional outcome, and quality of life after ileal pouch-anal anastomosis. Ann Surg. 2003;238:221–228. doi: 10.1097/01.sla.0000080825.95166.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazio VW, O’Riordain MG, Lavery IC, et al. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg. 1999;230:575–584. doi: 10.1097/00000658-199910000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]