Abstract
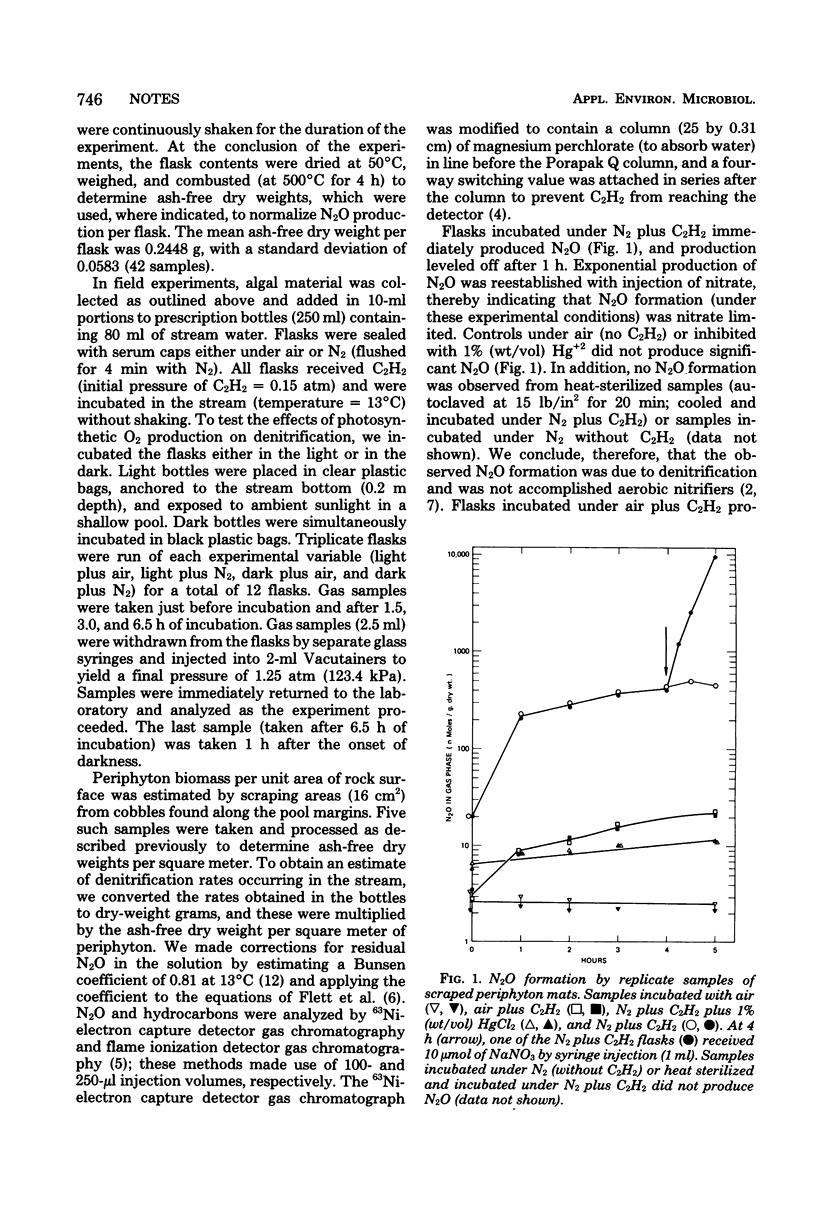
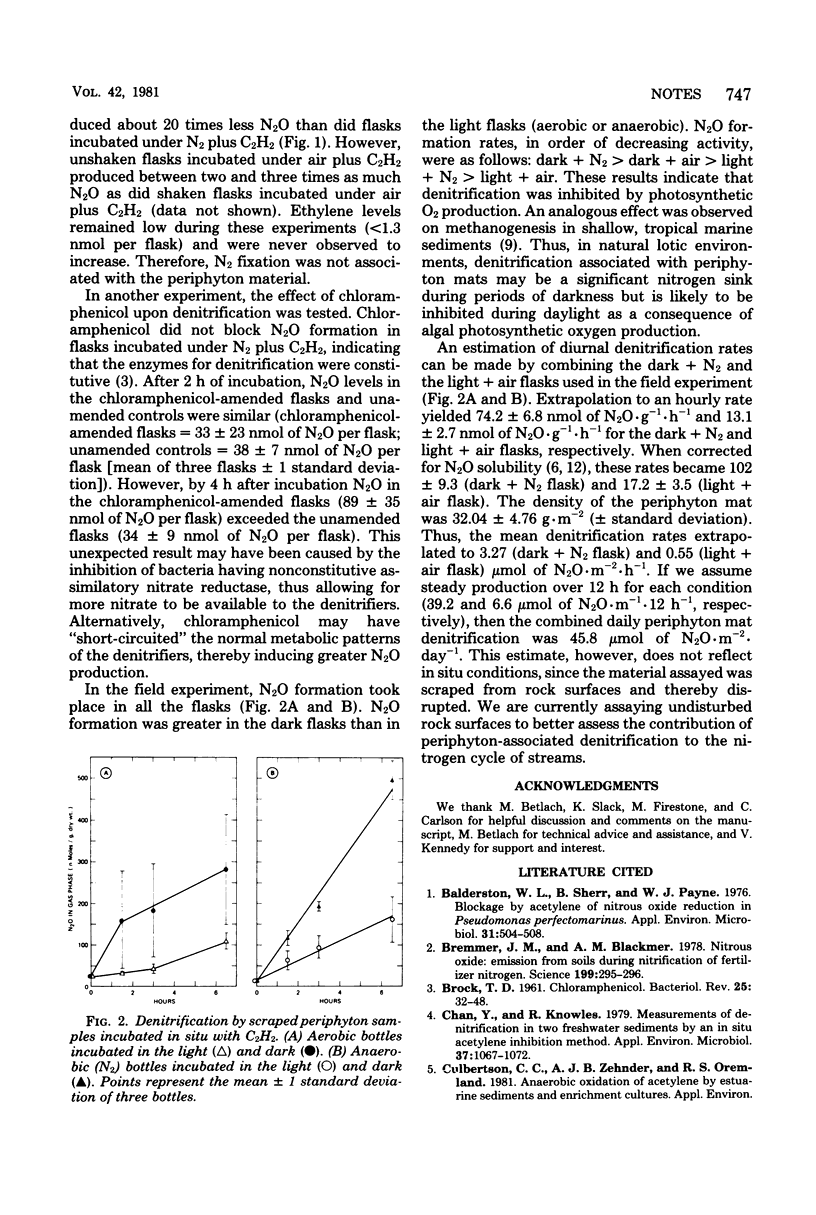
Scrapings of decomposing Cladophora sp. mats (periphyton) covering stream bed rocks produced N2O when incubated under N2 plus 15% C2H2. Denitrification (N2O formation) was enhanced by NO3− and was inhibited by autoclaving, Hg2+, and O2. No N2O was formed in the absence of C2H2 (air or N2 atmosphere). Chloramphenicol did not block N2O formation, indicating that the enzymes were constitutive. In field experiments, incubation of periphyton scrapings in the light inhibited denitrification because of algal photosynthetic O2 production. The diurnal periphyton-associated denitrification rate was estimated to be 45.8 μmol of N2O·m−2·day−1, as determined by averaging light, aerobic plus dark, and anaerobic rates over a 24-h period.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. M., Blackmer A. M. Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen. Science. 1978 Jan 20;199(4326):295–296. doi: 10.1126/science.199.4326.295. [DOI] [PubMed] [Google Scholar]
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. K., Knowles R. Measurement of denitrification in two freshwater sediments by an in situ acetylene inhibition method. Appl Environ Microbiol. 1979 Jun;37(6):1067–1072. doi: 10.1128/aem.37.6.1067-1072.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Methane production in shallow-water, tropical marine sediments. Appl Microbiol. 1975 Oct;30(4):602–608. doi: 10.1128/am.30.4.602-608.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


