Abstract
We have studied the temporal variation in viral abundances and community assemblage in the eutrophic Lake Loosdrecht through epifluorescence microscopy and pulsed field gel electrophoresis (PFGE). The virioplankton community was a dynamic component of the aquatic community, with abundances ranging between 5.5 × 107 and 1.3 × 108 virus-like particles ml−1 and viral genome sizes ranging between 30 and 200 kb. Both viral abundances and community composition followed a distinct seasonal cycle, with high viral abundances observed during spring and summer. Due to the selective and parasitic nature of viral infection, it was expected that viral and host community dynamics would covary both in abundances and community composition. The temporal dynamics of the bacterial and cyanobacterial communities, as potential viral hosts, were studied in addition to a range of environmental parameters to relate these to viral community dynamics. Cyanobacterial and bacterial communities were studied applying epifluorescence microscopy, flow cytometry, and denaturing gradient gel electrophoresis (DGGE). Both bacterial and cyanobacterial communities followed a clear seasonal cycle. Contrary to expectations, viral abundances were neither correlated to abundances of the most dominant plankton groups in Lake Loosdrecht, the bacteria and the filamentous cyanobacteria, nor could we detect a correlation between the assemblage of viral and bacterial or cyanobacterial communities during the overall period. Only during short periods of strong fluctuations in microbial communities could we detect viral community assemblages to covary with cyanobacterial and bacterial communities. Methods with a higher specificity and resolution are probably needed to detect the more subtle virus–host interactions. Viral abundances did however relate to cyanobacterial community assemblage and showed a significant positive correlation to Chl-a as well as prochlorophytes, suggesting that a significant proportion of the viruses in Lake Loosdrecht may be phytoplankton and more specific cyanobacterial viruses. Temporal changes in bacterial abundances were significantly related to viral community assemblage, and vice versa, suggesting an interaction between viral and bacterial communities in Lake Loosdrecht.
Introduction
Traditionally, nutrient availability, sedimentation, and grazing were considered the major driving forces of microbial and algal communities in aquatic environments. Since the discovery of high viral abundances almost two decades ago [3], awareness of the virioplankton community as major player in the aquatic food web has grown [59]. Reports of viral lysis contributing up to 70% of cyanobacterial mortality in marine systems [38] and up to 90–100% of bacterial mortality in freshwater systems [14, 58] led to the conclusion that viral lysis can be a major mortality cause, comparable to grazing-induced mortality. Several studies employing molecular methods such as pulsed field gel electrophoresis (PFGE) and denaturing gradient gel electrophoresis (DGGE) revealed the viral community as a very diverse and dynamic component of the aquatic community [13, 16, 40, 60]. Metagenome analysis of the viral community in coastal waters indicated that viral diversity is probably even up to an order of a magnitude higher than bacterial richness [7].
Due to the selective and parasitic nature of viruses, viral and host abundances are expected to covary [17]. One would therefore also expect a close linkage over time between host and virus community composition [24, 42]. Viral population dynamics have indeed been reported to be closely linked to microbial and algal population dynamics in aquatic environments [9, 18, 23, 26, 33, 48]. Changes in viral community structure have also been associated with environmental factors such as seasonality, location, water depth, degree of stratification, tide height, salinity, and Chl-a concentration [13, 16, 40, 60]. In freshwater environments, there is still limited knowledge to what extent viral community composition reflects changes in environmental conditions and host community composition [2, 13].
The present study examined temporal changes in viral abundances and community composition in Lake Loosdrecht, employing epifluorescence microscopy and PFGE. Lake Loosdrecht is a highly eutrophic (Chl-a annual average of ca 60 mg m−3), shallow (mean depth 1.9 m), and turbid (Secchi depth approximately 0.5 m) peat lake in the Netherlands. The lake is dominated by a group of related filamentous cyanobacteria belonging to the Limnothrix/Pseudoanabaena group [54, 63]. The virioplankton community in this lake is particularly interesting since earlier research repeatedly showed a dramatic collapse of the cyanobacterial community associated with viral activity during lake water enclosure experiments [20, 41, 53]. Furthermore, it is known from previous research that the zooplankton community largely depends on eukaryotic algae for growth and that grazing only accounts for part of the cyanobacterial mortality [22, 37]. Therefore, the viral community is thought to play a significant role in cyanobacterial mortality in this lake. Heterotrophic bacteria are generally also considered to be an important viral host in freshwater environments [14, 58]. During the present study, the community dynamics of these potential viral hosts were examined employing flow cytometry and DGGE. If cyanobacteria and heterotrophic bacteria are indeed important viral hosts in Lake Loosdrecht, a close linkage with viral community dynamics would be expected. We thus studied the temporal dynamics of the total viral community in Lake Loosdrecht, in relation to environmental parameters and in particular to potential viral host community dynamics, aiming to improve our knowledge of the virioplankton community in eutrophic lakes.
Materials and Methods
Sampling
Samples were collected from Lake Loosdrecht (The Netherlands) approximately every 2 weeks from the 12th of February to the 25th of November 2003. Upon sampling Secchi-disk depth, pH and water temperature were determined. Surface water was sampled in high-density polyethylene containers and immediately transported to the laboratory. All samples were processed or fixed within 3 h after sampling. Data on chemical and environmental variables were obtained from the Service for Inland Water Management and Wastewater Treatment Amstel, Gooi en Vecht (DWR).
Phytoplankton Enumeration
Different cyanobacterial groups and eukaryotic algae were distinguished using an Epics Elite flow cytometer (Coulter, Miami, USA) equipped with an ion argon laser (excitation 30 mW at 488 nm) and a cell sorter. Fresh samples were analyzed for 5 min at a flow rate of 43 μl min−1. The fluorescence of Chl-a was recorded at 675 nm with full width-half maximum (FWHM) of 40 nm. Phycocyanin fluorescence was recorded at 635 nm (FWHM 15 nm). Side scatter was used to trigger count events. A small volume of a known concentration of 1-μm diameter fluorescent beads (Polyscience, Warrington, USA; no. 15702) was added for exact volume determination.
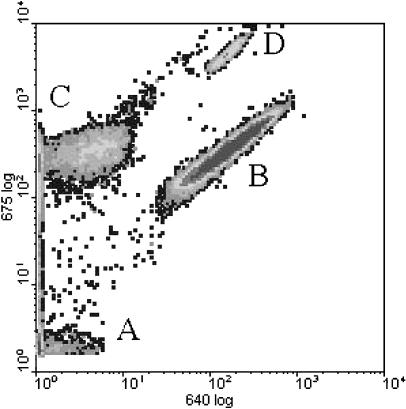
Typically, three major cell clusters of filamentous cyanobacteria, prochlorophytes [49], and eukaryotic algae, respectively, were detected (Fig. 1). This classification was confirmed by light microscopy examination and fatty acids analysis after cell sorting [36].
Figure 1.
Phytoplankton clusters in typical Lake Loosdrecht sample as detected by flow cytometry. (A) bacteria/detritus cluster (B) cyanobacterial cluster (C) prochlorophyte cluster (D) algal cluster
Nanoflagellate Enumeration
Samples were fixed with 0.5% glutaraldehyde and stored at 4°C in the dark. All samples were counted within a week after sampling applying Utermöhl chambers [50] and an inverted Leica, Fluovert microscope, counting at least 200 nanoflagellates. Flagellates <10 μm were counted as nanoflagellates.
Heterotrophic Bacteria and Virus Enumeration
Samples were fixed with 0.02-μm filtered formalin (1% final volume), frozen in liquid nitrogen and stored at −80°C until analysis. Triplicate bacteria and virus counts were obtained after staining of samples with SYBR Green I (Molecular Probes, Europe), as described by Noble and Fuhrman [35]. In brief, samples were 100× diluted in 0.02 μm filtered MQ to facilitate counting and 1 ml of this dilution was filtered through a 0.02-μm Anodisc filter (Whatman, Brentford, England). The filters were then placed on 100 μl of a solution of SYBR Green I (1:2500 dilution of original stock) and incubated for 15 min in the dark. After incubation, the backside of the filters were dried gently on a tissue and mounted onto glass slides with 20 μl of antifading solution [35]. At least 200 virus-like particles (vlp) and bacteria were counted using a Zeiss Axiophot epifluorescence microscope at blue light excitation.
Bacterial and Cyanobacterial Community Composition
The composition of bacterial and cyanobacterial communities was obtained on a monthly basis, by denaturing gradient gel electrophoresis (DGGE) [15] after PCR-amplification applying general bacteria specific as well as cyanobacteria specific primers targeting the small subunit ribosomal RNA gene (SSU rDNA), using the exact protocol as described in [61, 63]. We amplified the cyanobacterial SSU rDNA sequences using first a nested procedure with primers Cya-b-F371 and Cya-R783. After this cyanobacteria-selective preamplification, a second amplification procedure was performed employing the general bacterial primers F357GC and R518.
The product of both the bacteria and cyanobacteria-specific PCR procedure is a GC clamp containing PCR product of approximately 200-bp long. This product was subjected to DGGE using a clone ladder composed of 23 previously sequenced and described Lake Loosdrecht clones. Of these clones, 11 are of cyanobacterial origin [61, 62]. This procedure enables to directly compare the cyanobacterial community profiles with those of the total bacterial community and was optimized using Lake Loosdrecht water [63].
DGGE gels were stained using ethidium bromide, and gel images were analyzed and documented using the ImaGo imaging system (Isogen life science). As this method is designed to compare the cyanobacterial and total bacterial communities, the latter DGGE pattern also contains bands corresponding to cyanobacteria. Because the same primers have been used to generate the final product for both DGGE patterns, cyanobacterial bands in the total bacterial DGGE pattern migrate to the same position as their corresponding bands in the cyanobacterial DGGE. Such cyanobacterial bands were subtracted from the total bacterial DGGE pattern and omitted from further analysis for comparing the communities of heterotrophic bacteria and cyanobacteria.
Viral Community Composition
The composition of the total viral community was determined on a monthly basis, based on differences in total genome length obtained with pulsed field gel electrophoresis (PFGE) using a modified version of an existing protocol [27]. For PFGE analysis, 2 l of water was prefiltered over a low-protein binding 0.45-μm filter (Durapore, Millipore, Billerica, MA, USA). The viral community was then concentrated to ca. 50 ml using the VivaFlow 200 assembly (VivaFlow, Vivasciences, Hannover, Germany; 30.000 MWCO PES membrane). After adding 10% Tween 80 to separate viral particles from other particles in solution (1:1,350 final concentration), the sample was centrifuged for 25 min at 15,500×g at 4°C to remove bacteria. The supernatant (45 ml) was stored at −80°C until further processing. When thawed quickly at 35°C before further processing, storage at −80°C was not observed to result in a significant loss in viral particles (A.-C. Baudoux, personal communication). This primary concentrate was further concentrated to a volume of 1–5 ml using centrifugal ultrafiltration units (30,000 MWCO, centricon-plus-20, Millipore, Billerica, MA, USA). The final volume was doubled by adding SM buffer (0.1 M NaCl, 10 mM MgS04, 50 mM Tris, 0.005% glycerol, pH 8) and 0.2 μm filtered Na-azide (0.1% final volume) for optimal storage and preservation, overnight at 4°C in the dark. For a final round of concentration of the viral community, the samples were ultra-centrifuged for 2 h at 135,000×g at 8°C (Sorvall Discovery M120 SE; swinging bucket rotor S52-ST). After dissolving the virus pellet overnight at 4°C in the dark in SM buffer, the virus concentration was determined as described above.
Agarose plugs were prepared according to [27], using InCert agarose (1.5% final volume, FMC, Rockland, ME) taking care to load 5 × 109 viruses per plug, determined by epifluorescence microscopy as described above. The plugs were digested overnight at 30°C in the dark with a lysis buffer [250 mM EDTA pH 8.0; 1% SDS; 1 mg/ml Proteinase K (Sigma-Aldrich, Zwijndrecht, the Netherlands)]. After repeated washings with TE buffer the plugs were loaded onto and run on a 1.0% (w/v) Seakem GTG agarose gel (FMC, Rockland, ME, USA) prepared in 1× TBE gel buffer (90 mM Tris–Borate and 1 mM EDTA, pH 8.0). Samples were electrophorized for 21 h at 14°C, with pulse ramp from 1 to 6 s at 6 V cm−1 using the Bio-Rad CHEF-DR II PFGE apparatus. As a molecular weight ladder, 5 kb and λ concatamers (Bio-Rad, Richmond, CA, USA) were used. The used PFGE settings were optimized to allow optimal separation of marker and PFGE bands in the size regions of interest and allowed the detection of viruses with genome sizes between 10 and 200 kb. The gels were stained with Sybr Green I (Molecular Probes, Europe) for 1 h, destained for 30 min in distilled water and documented as is described for the DGGE gels.
Data Analysis
Correlations between different variables were determined using linear correlation (Pearson r) with Statistica (StatSoft, Tulsa, OK, USA).
Both the DGGE and the PFGE gels were analyzed using Phoretix 5.00 (Nonlinear Dynamics, UK). Background noise was subtracted using the rolling ball algorithm with a radius of 50 pixels and peaks smaller than 2% of the maximum peak were discarded. The band matrices obtained with Phoretix representing the community composition were further analyzed using Primer 5.2.0.9 [11] to obtain similarity matrices of the different data sets using presence–absence analysis of bands. Similarity coefficients were calculated using the Bray-Curtis coefficient [6]. Dendrograms were constructed based on these similarity matrices using hierarchical clustering with group-average linking. Dissimilarity matrices of untransformed environmental variables were obtained using Euclidean distances. Mantel tests, applying spearman rank’s correlation coefficient ρ as the test statistic and 999 permutations, were used to test the null hypothesis of ‘no relationship between matrices’ to determine the presence of a seasonal cycle in the community profiles and if the similarity matrix of the viral community or the Euclidean distance matrix of viral abundances was related to the matrices of the heterotrophic bacterial and the cyanobacterial communities. To analyze the similarity matrices for the occurrence of a seasonal cycle, they were compared to a ranked matrix of sampling points in time representing a seasonal cycle in which adjacent months are most similar and samples 6 months apart least similar.
To investigate which combination of environmental variables related best to the viral, cyanobacterial, and heterotrophic bacterial community assemblages, the Bio-Env procedure from Clarke and Ainsworth was used [10]. The spearman rank correlations (ρw) between the Bray-Curtis dissimilarity matrix for the community composition and the Euclidean distance matrix for all possible different subsets of the environmental variables were determined. The highest coefficients at each level of complexity were tabulated and can be interpreted as the ‘best explanatory environmental variables’ of the community assemblage. The statistical significance of the optimal combination of environmental variables was not tested because the Bio-Env procedure is mainly an exploratory technique, which is based on a large number of strongly interdependent similarity calculations [10]. Environmental variables tested were viral, bacterial, cyanobacterial, prochlorophyte, algal, and flagellate abundances, as well as the abiotic factors pH, water temperature, and concentrations of oxygen, phosphate, ammonium, nitrate, and bicarbonate.
Results
Abiotic Conditions
The environmental parameters given in Table 1 reflect the nature of Lake Loosdrecht as a highly eutrophic and turbid shallow lake [8]. Concentrations of the potentially growth-limiting nutrients dissolved reactive phosphate (DRP), nitrate and ammonium [19] were highest in March and below detection limits for a large part of the study period (Table 1). The pH and water temperature were both highest in summer, whereas Secchi-disk depth was highest in March and lowest in August.
Table 1.
Ranges of physical, chemical and biological parameters observed in surface water of Lake Loosdrecht in 2003
| Lake Loosdrecht 2003 | |
|---|---|
| Parameter | Range |
| Mean depth m | 1.9 to 1.9 |
| Secchi depth m | 0.4 to 0.6 |
| Temperature °C | 3 to 23 |
| Chl-α μg/l | 44.0 to 88.8 |
| O2 mg/l | 9.3 to 13.5 |
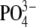 mg/l mg/l |
<0.005 to 0.218 |
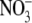 mg/l mg/l |
<0.1 to 0.24 |
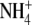 mg/l mg/l |
<0.05 to 0.218 |
| Ptotal mg/l | 0.04 to 0.07 |
| Ntotal mg/l | 16.1 to 45.5 |
Microbial and Viral Abundance
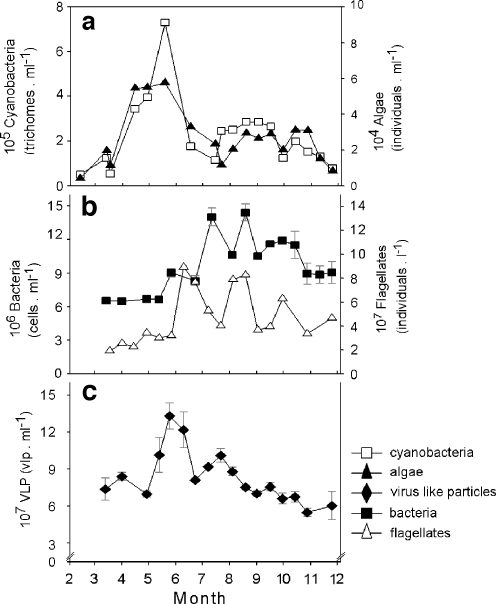
From March onwards algae and in particular cyanobacteria increased in numbers (Fig. 2a). The plankton community was dominated by large filamentous cyanobacteria, with average concentrations of 2.2 × 105 cyanobacterial trichomes ml−1, which corresponds to about 1 × 107 cyanobacterial cells ml−1. Highest filamentous cyanobacterial abundances (7.3 × 105 trichomes ml−1) were detected in May, after which cyanobacterial numbers showed a rapid 6.3-fold decrease in June. Relatively high cyanobacterial numbers reoccurred from the end of July until the onset of autumn. The eukaryotic algae followed this pattern, however, at much lower abundances. Cyanobacterial numbers showed a strong positive correlation to algal numbers and a negative correlation with Secchi-disk depth (Table 2).
Figure 2.
Algal, cyanobacterial, bacterial, nanoflagellate, and viral abundances during the study period. White squares, black triangles, black squares, white triangles and black rhombus represent cyanobacteria, algae, bacteria, nanoflagellates and virus like particles, respectively. a Flow cytometric counts of total cyanobacterial (including prochlorophytes) and algal communities. b Epifluorescence microscopic counts of heterotrophic bacterial community and light-microscopic counts of nanoflagellate community. c Epifluorescence microscopic counts of viral abundances. Error bars show standard deviation (n = 3)
Table 2.
Pearson linear correlation coefficients and level of significance for different variables
| Lake Loosdrecht 2003 | ||||||
|---|---|---|---|---|---|---|
| Variable | Viruses | Flagellates | Cyanobacteria | Prochlorophytes | Algae | Bacteria |
| Viruses | 0,10 | 0,25 | 0,59* | 0,16 | −0,09 | |
| Flagellates | 0,10 | −0,25 | 0,29 | −0,23 | 0,58* | |
| Cyanobacteria | 0,25 | −0,25 | 0,61* | 0,79*** | −0,37 | |
| Prochlorophytes | 0,59* | 0,29 | 0,61* | 0,22 | 0,18 | |
| Algae | 0,16 | −0,23 | 0,79*** | 0,22 | −0,35 | |
| Bacteria | −0,09 | 0,58* | −0,37 | 0,18 | −0,35 | |
| Chl-a | 0.52* | 0.45 | 0.48 | 0.70** | 0.36 | 0.11 |
| Secchi | −0,58* | −0,40 | −0,53* | −0,60* | −0,54* | −0,14 |
| Temperature | 0,48 | 0,67** | 0,13 | 0,75** | 0,00 | 0,40 |
| Cyanobacterial richness | −0,73* | −0,28 | −0,47 | −0,61 | −0,30 | 0,14 |
| Bacterial richness | −0,24 | −0,17 | 0,01 | −0,20 | 0,31 | −0,20 |
| Viral richness | 0,02 | −0,25 | 0,64 | 0,21 | 0,38 | −0,82* |
*p < 0.05, **p < 0.01, ***p < 0.001
The heterotrophic bacterial community started to increase in late spring, when the cyanobacterial community was already peaking (Fig. 2b). This bacterial community reached high abundances of 14.0 and 14.4 × 107 bacterial cells ml−1 in July and August, respectively, and showed a 2.3-fold fluctuation during the study period. Bacterial numbers were negatively correlated with viral richness (number of PFGE bands) and positively with flagellate abundances (Table 2). Nutrients were not significantly correlated to any of the other measured variables in Lake Loosdrecht (data not shown).
Viral numbers in Lake Loosdrecht showed a 2.5-fold fluctuation during the study period (Fig. 2c). The most pronounced change in viral numbers was observed between April and May, when a 1.9-fold increase in VLP was observed up to a maximum number of 1.3 × 108 VLP ml−1. Another marked increase in viral numbers was observed in July, when viral numbers reached 1.0 × 108 VLP ml−1. Viral numbers were observed to gradually decrease towards the end of the experimental period, with 6.0 × 107 VLP ml−1 observed in November. High viral numbers in May coincided with maximum cyanobacterial numbers, whereas the viral increase in July closely followed high bacterial numbers. Throughout the study period, viral numbers were positively correlated to prochlorophytes and Chl-α. Viral numbers were negatively correlated to cyanobacterial richness (number of DGGE phylotypes) and Secchi-disk depth (Table 2).
The nanoflagellate community in Lake Loosdrecht showed large seasonal fluctuations, reaching high numbers of 8.94 × 104 ml−1 in June after a 2.8-fold increase; 8.26 × 104 ml−1 in August after a 2.1-fold increase and 6.3 × 104 ml−1 at the end of September after a 1.6-fold increase (Fig. 2b). High nanoflagellate numbers in June and July coincided with decreasing viral and bacterial numbers during these periods. Flagellate numbers were correlated with both bacterial abundances and water temperature (Table 2). Abundances for bacteria (12-02, 15-04, and 22-07), phytoplankton (23-06), viruses (12-02, 11-11), and flagellates (12-02, 17-10 and 11-11) are missing from the current study due to methodological problems.
Viral and Microbial Community Composition
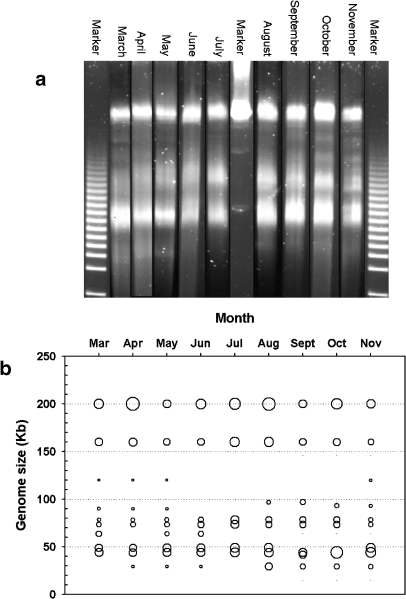
The viral community composition in Lake Loosdrecht was determined monthly based on differences in total viral genome size using PFGE. Difficulties were experienced when applying the PFGE technique on eutrophic lake water, probably due to high concentrations of polymeric materials. This methodological problem resulted in low band resolution and high background staining of the PFGE gels (Fig. 3). To improve the gel quality, cleaning procedures were tested as reported by Riemann and Middelboe [39]. These cleaning procedures improved the gel quality but also led to a high loss of viral numbers, especially in the larger viral size classes and were therefore chosen not to be applied in this study. Finally, it should be noted that the high background staining on the gels may have caused incomplete detection of small, faint bands and thus to an underestimation of viral richness.
Figure 3.
a PFGE gel of viral population, b and bubble plot representation of PFGE gel. Viral richness was determined using samples pooled per month for the study period. Day when samples were taken from experiment is indicated on x-axis; M, marker. Bubble position indicates position PFGE band and corresponding genome size, bubble size indicates relative band intensity. The DNA standards used in the PFGE were a 5 kb and a λ marker
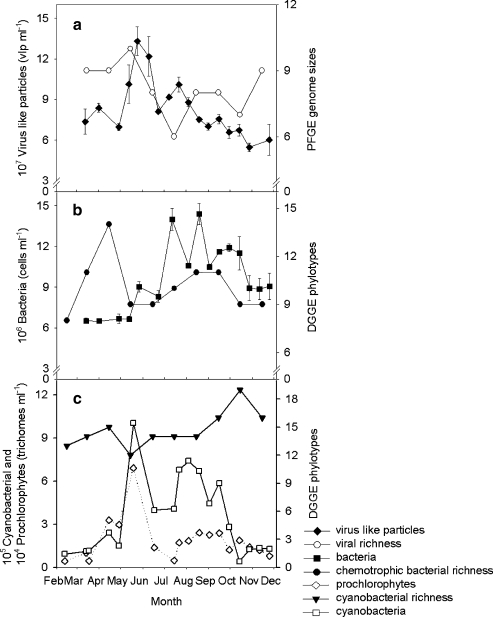
Throughout the experimental period, a total of 13 different viral populations were observed with genome sizes varying between 30 and 200 kb (Fig. 3). Genome sizes of 44, 49, 74, 78 and 163 kb were most abundant with average relative abundances of 17, 15, 12, 11 and 14%, respectively. The high band intensities at 200 kb were due to a combination of viral genomes larger than 200 kb, which were not separated with the PFGE settings that we used. The 200 kb band was thus counted as 1 viral population, by which the actual viral richness was probably underestimated. The number of observed viral genome sizes varied per month with highest virioplankton richness (10 genome sizes) during the peak in viral numbers in May (Fig. 4a). Viral richness decreased along with the cyanobacterial numbers, reaching a minimum of six viral genome sizes during the second viral peak in July. Viral genome sizes of >200, 160, 78, and 44 kb varied in intensity but persisted throughout the study period.
Figure 4.
Viral, bacterial and cyanobacterial abundances and richness over the study period. a Number of viral genome sizes per month observed with PFGE and viral abundances. b Number of heterotrophic bacterial DGGE phylotypes (DGGE bands) observed per month and bacterial abundances. c Number of cyanobacterial DGGE phylotypes observed per month and cyanobacterial abundances. The flow cytometric cyanobacterial counts are split up in the phycocyanin-containing filamentous cyanobacterial species (cyanobacterial) and filamentous cyanobacteria not containing phycocyanin (prochlorophytes). Error bars show standard deviation (n = 3)
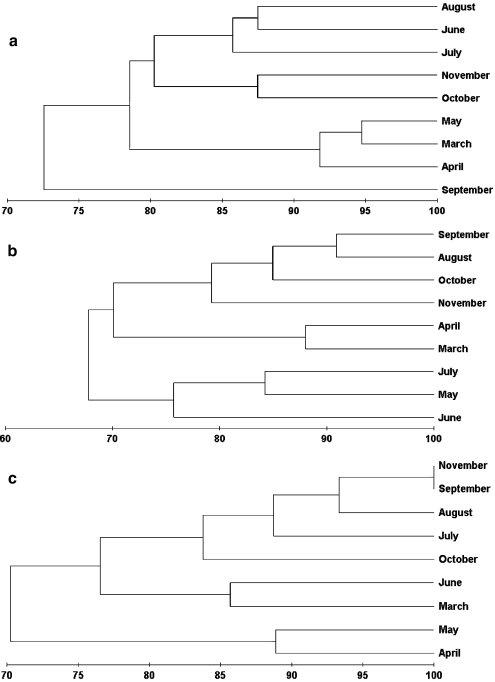
Cluster analysis based on Bray-Curtis similarity indices of the monthly PFGE banding patterns puts the viral community in September on a separate branch, implying low similarity to the viral community of all other samples (Fig. 5a). This difference in viral community composition was mainly due to the absence of a PFGE band of 49 kb and the presence of bands in the 40- and 97-kb range (Fig. 3). The successive months May and June clustered separately, indicating changes in viral community composition during this period. The separate clustering of spring, summer, and autumn samples suggests a seasonal trend in viral community composition. Mantel tests indeed confirmed the presence of a significant annual cycle in viral community composition (ρ = 0.542, p = 0.002). A Spearman rank correlation was performed to test which (combination of) environmental variables correlated best with the viral community assemblages observed in Lake Loosdrecht throughout the experimental period (Table 3). The environmental variable which best grouped the months, in a manner consistent with the viral community pattern, was bacterial abundance (ρ = 0.37); next best was the pH value. A Mantel test indeed confirmed that the overall pattern in bacterial abundances and viral community composition were significantly correlated (p = 0.024). Increasing the subset of environmental variables (k) did not raise the matching coefficient (ρ; Table 3).
Figure 5.
Similarity analysis of the viral, bacterial and cyanobacterial community profiles. All dendrograms were constructed using group-average linking. Bray-Curtis similarity matrices were obtained based on presence–absence analysis of bands. a Dendrogram showing degree of similarity between viral community profiles of the different months based on PFGE analysis. b Dendrogram showing degree of similarity between heterotrophic bacterial community profiles of the different months based on DGGE analysis. c Dendrogram showing degree of similarity between cyanobacterial community profiles of the different months based on DGGE analysis
Table 3.
Bio-Env procedure. Combinations of variables, k at a time, giving the largest rank correlations (ρw) between similarity matrices of plankton community assemblage and environmental variables
| Spearman rank correlation | |
|---|---|
| K | Best variable combinations (ñw) |
| Viral community structure (PFGE) | |
| 1 | Bact (0.37), pH (0.28), Temp (0.12), NH4 (0.11)... |
| 2 | Bact, pH (0.35); Bact, Temp (0.29); Bact, HCO3 (0.29); pH, NH4 (0.27) ... |
| 3 | Bact, Temp, pH (0.34); Bact, pH, NH4 (0.30); Bact, pH, NO3 (0.30) ... |
| 4 | Bact, pH, NO3, NH4 (0.34); Bact, Temp, pH, NO3 (0.30) ... |
| Heterotrophic bacterial community structure (DGGE) | |
| 1 | VLP (0.37); Algae (0.22); Flag (0.21); Cyano (0.10) |
| 2 | VLP, Algae (0.445); VLP, Flag (0.41); VLP, O2 (0.38); VLP, NH4 (0.34) |
| 3 | VLP, Flag, Algae (0.448); VLP, Algae, NH4 (0.43); VLP, Algae, NO3 (0.43) |
| 4 | VLP, Algae, NO3, NH4 (0.42); VLP, Flag, Algae, O2 (0.39) |
| Cyanobacterial community structure (DGGE) | |
| 1 | Algae (0.59); VLP (0.27); Flag (0.23); Bact (0.13) |
| 2 | VLP, Algae (0.61); Flag, Algae (0.56); Algae, Bact (0.55); VLP, Bact (0.47) |
| 3 | VLP, Algae, Bact (0.70); VLP, Flag, Algae (0.59); Flag, Algae, Bact (0.57) |
| 4 | VLP, Flag, Algae, Bact (0.66); VLP, Algae, Bact, PH (0.53) |
Bold variables indicate the combination of environmental variables, which best match the pattern in viral, heterotrophic bacterial and cyanobacterial community structure. Bact, bacterial abundances; Temp, water temperature; Flag, nanoflagellate abundances
Analysis of the heterotrophic bacterial community composition revealed a diverse bacterial community with a total of 18 different DGGE phylotypes (DGGE bands) observed throughout the experimental period. The highest bacterial richness of 14 DGGE phylotypes was observed in April, when the bacterial abundance was still low (Fig. 4b). Analysis of the monthly DGGE profiles revealed separate clustering of the late spring to early summer samples from the rest of the year, indicating that changes in the bacterial community structure occurred in both May and August (Fig. 5b). A Mantel test confirmed a significant seasonal cycle in the bacterial community profile (ρ = 0.483, p = 0.006). Virus-like particle abundance was the variable which, on its own, best grouped the months in a manner consistent with the heterotrophic community composition (ρ = 0.37; Table 3); second best was algal abundance (ρ = 0.22). The subset of VLP, flagellate and algal abundances correlated best with heterotrophic bacterial community structure (ρ = 0.448). A Mantel test indicated that patterns in heterotrophic bacterial community composition were significantly correlated to VLP abundance (p = 0.028) but not to changes in viral community composition (ρ = 0.122; p = 0.25).
The cyanobacterial community structure in Lake Loosdrecht was very diverse with in total 25 different DGGE phylotypes observed throughout the entire experimental period (Fig. 6). The highest cyanobacterial richness was recorded in October when cyanobacterial abundances decreased (Fig. 4c). At the time of the cyanobacterial peak in May, the cyanobacterial richness was observed to be at its minimum, with 12 different phylotypes. Analysis of the monthly cyanobacterial DGGE profiles revealed that the cyanobacterial community in April and May differed from communities in the previous and subsequent months (Fig. 5c). Changes in cyanobacterial community composition were observed from June to July as well. A significant annual cycle could also be detected in the cyanobacterial community pattern (Mantel test; ρ = 0.32, p = 0.04). Of all the environmental variables tested, the pattern in algal abundances provided the best match to cyanobacterial community structure (ρ = 0.59; Table 3); VLP abundances provided the second best match (ρ = 0.27). The subset of VLP, algal, and bacterial abundances ‘explain’ most of the pattern in cyanobacterial community composition (ρ = 0.7). No significant relation could be detected between the cyanobacterial community profile and VLP abundances (p = 0.078) or VLP community profile (ρ = 0.01, p = 0.44).
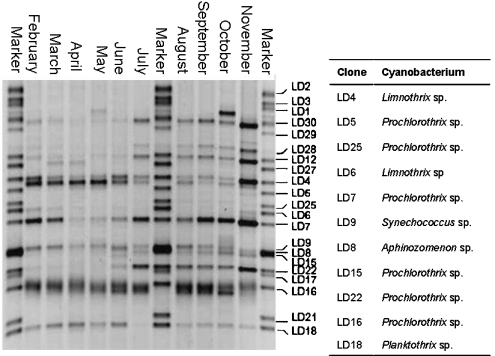
Figure 6.
Denaturing gradient gel electrophoresis (DGGE) gel showing cyanobacterial community profiles over the study period. M, marker composed of cyanobacterial clone library from lake Loosdrecht. Labels indicate the corresponding clone label of the marker band. Clone-labels of marker bands corresponding to cyanobacteria are identified
Discussion
Due to the selective and parasitic nature of viral infection, it is expected that viral and host community dynamics will covary in predator–prey type oscillations, both in abundances and community composition [17, 33, 57, 59]. We aimed to study the temporal dynamics of the total viral community in relation to environmental parameters and in particular to bacterial and cyanobacterial community dynamics, as potential viral hosts in Lake Loosdrecht.
The virioplankton in Lake Loosdrecht showed marked temporal changes in abundance and community composition, indicating viruses to be a dynamic component of the aquatic community. By applying the Sybr Green I dye, which has a high sensitivity for dsDNA, we especially observed dynamics of dsDNA viruses during the present study. Viral abundances observed during spring and summer were significantly higher compared to the rest of the year (Student t test, p = 0.01), indicating that viral abundances followed a seasonal pattern. Especially between May and August strong fluctuations in viral abundances could be observed. The most pronounced increase in viral numbers in May coincided with a peak in filamentous cyanobacterial abundances, and the viral increase in July closely followed high bacterial numbers, suggesting a tight interaction between the viral and both the cyanobacterial and bacterial communities during this period. In spite of this, no significant correlation could be detected when comparing the temporal fluctuations in viral abundances with fluctuations in bacterial and dominant filamentous cyanobacterial abundances observed throughout the experimental period.
Viral abundances did show a significant positive correlation to Chl-α concentrations, suggesting that a significant proportion of the viruses in Lake Loosdrecht may be phytoplankton viruses. A significant correlation between viral abundances and Chl-a has previously been reported for a variety of freshwater environments [30, 45, 55], including eutrophic shallow lakes [29]. Viral numbers also showed a significant correlation to the second most abundant group of filamentous cyanobacteria present in Lake Loosdrecht, the filamentous prochlorophytes [12, 36]. Prochlorophytes are filamentous cyanobacteria lacking phycobilins, but possessing Chl-b as accessory pigment [49]. Viruses have been isolated and identified for the marine prochlorophyte Prochlorococcus [43], but for freshwater environments no viruses of prochlorophytes have been reported, so far. During the present study, prochlorophytes reached maximum numbers in May, which was followed by a rapid decrease in June, as observed for the phycobilin-containing cyanobacteria (Fig. 4c). The prochlorophyte community in Lake Loosdrecht is mainly composed of Prochlorothrix hollandica, but recently the presence of a taxon closely related to P. hollandica has been described by Zwart et al. [63]. It is unknown if this taxon is included in our flow cytometric prochlorophyte counts, since it does not have a cultivated representative, and we do not yet know if its pigmentation and shape resembles P. hollandica.
High nanoflagellate numbers in June and July coincided with decreasing viral numbers in these periods, suggesting that nanoflagellates could be a source of viral removal in both marine and freshwater environments. Previous research has shown that grazing of viral particles by nanoflagellates could be a source of viral removal in both marine and freshwater environments [4, 21, 31]. Besides the above association, no significant correlation between viral and nanoflagellate abundances could be detected during the present study however. Nanoflagellate abundances did reflect changes in bacterial abundances, suggesting bacteria as probable food source for the nanoflagellate community in Lake Loosdrecht.
Microbial Community Composition
To describe the bacterial and cyanobacterial community composition in Lake Loosdrecht, we applied a PCR-DGGE protocol that has been thoroughly tested and described for Lake Loosdrecht water [61–63]. To enable the comparison between the heterotrophic bacterial community and the cyanobacterial community, we omitted bands corresponding to cyanobacterial band positions in the total bacterial DGGE pattern from further analysis. This procedure could have led to an underestimation of the heterotrophic bacterial richness since heterotrophic bacterial and cyanobacterial bands may migrate to the same position. This situation was not encountered, however, when testing the protocol and sequencing a subsample of excised DGGE bands [63].
Changes in heterotrophic bacterial community composition followed a striking seasonal pattern. Seasonality in freshwater bacterial assemblages has also been reported for the eutrophic Lake Plußsee [25] and two hypereutrophic lakes in Belgium [51]. Two marked shifts in heterotrophic bacterial community composition were apparent in Lake Loosdrecht, the first shift was mainly due to a decrease in heterotrophic bacterial richness during the cyanobacterial peak in May. The second shift occurred between July and August, implying that the two bacterial peaks observed during these months differed in community composition. It might very well be that these shifts in community assemblage were due to changes in the phytoplankton community, since Van Hannen and coworkers [52, 53] demonstrated that the bacterial community structure in Lake Loosdrecht is tightly linked to phytoplankton community composition. Our data indeed indicate algal and cyanobacterial abundances among the four environmental variables which, on their own, best relate to the heterotrophic bacterial community assemblage, as well as viral and flagellate abundances. Viral abundance was the only variable that significantly related to heterotrophic bacterial community composition, however, suggesting an interaction between bacterial and virioplankton communities. No significant correlation between viral and bacterial abundances could be detected during the present study, though.
Cyanobacterial richness was higher than the richness observed for the heterotrophic bacterial community, which was probably due to the higher overall cyanobacterial abundances (up to 3.3 × 107 cyanobacterial cells ml−1) and to the difference in resolution of the two applied DGGE protocols, since the cyanobacterial DGGE protocol was specifically designed to detect cyanobacteria. The cyanobacterial community followed an annual cycle, with the largest shifts in community composition occurring during spring and early summer. The low number of DGGE phylotypes and the joint clustering of April and May indicate that the cyanobacterial peak in May was probably due to the numerical increase in cyanobacterial phylotypes that were already present in April. Analysis of the DGGE gel confirmed that in May no new DGGE phylotypes appeared, whereas three phylotypes disappeared and seven phylotypes increased in band intensity as compared to the pattern in April. After the cyanobacterial decline in May, a marked shift in cyanobacterial community assemblage occurred, with several new DGGE phylotypes appearing. Both flow cytometric counts and DGGE analysis showed that several of these newly appearing phylotypes can be attributed to prochlorophytes.
Zwart et al. [63] already demonstrated that the dominant, seemingly uniform filamentous cyanobacterial community in Lake Loosdrecht is actually a diverse assemblage of different cyanobacteria, composed of at least five different taxa belonging to the Limnothrix/Pseudoanabaena group. It might be that viral infection enables the stable coexistence of this assemblage of different cyanobacteria, in accordance with the ‘killing the winner’ theory. This theory predicts that viral infection can control the abundance of the competitive dominant, thereby enabling the stable coexistence of less competitive species and enhancing microbial diversity [46, 47]. Viral abundances were indeed found to be among the environmental variables which best explained the cyanobacterial community assemblage, but no significant correlation between viral and cyanobacterial community composition could be detected. In addition, the viral community did not seem to enhance microbial diversity during the present study, since viral abundance was negatively correlated to bacterial and especially cyanobacterial richness. Bouvier and Del Giorgio [5] recently provided evidence that viral regulation may in fact decrease the overall prokaryotic diversity, by maintaining competitively strong phylogenetic groups at such low densities, that they are below the detection threshold.
Viral Community Composition
PFGE is now a commonly applied method in aquatic ecology for analysis of total viral community diversity based on differences in total genome size [27, 39, 42, 60]. This study is one of the first applying PFGE to describe viral community composition in a freshwater setting [2]. With the PFGE settings used in this study, we were able to detect viral genome sizes between 30 and 200 kb, the size region in which most bacteriophages can be found [1]. We therefore did not detect the large genome size viruses, which are often attributed to algal viruses [27, 28]. PFGE studies to date report most viral DNA to be present in the smaller genome size range between 30 and 70 kb [2, 27, 42, 59]. Furthermore, in the present study, most viral genome sizes clustered within this range around 45 kb. A second cluster of genome sizes was observed around 75 kb, thus somewhat larger than the 70 kb cluster reported by Larsen et al. [22].
A substantial 39% of the detected viral genome sizes persisted throughout the experiment, only varying in viral numbers per genome size (detected as relative PFGE band intensity) between the different months. Stability of virioplankton genome groups has also been reported for a range of marine environments [39, 42] and in the Charente Estuary [2], when applying PFGE. It might very well be that the actual viral community is more dynamic than detected with PFGE, since different viruses can have the same genome size and would therefore not be detected as different using PFGE. Although an underestimation of viral richness due to low quality PFGE gels may have occurred in this study, between 6 to 10 different PFGE bands could be observed per sample, which is in the range of other studies [28, 42] and higher than the viral richness observed by PFGE in the Charente river [2]. However, there is still room for further improvement of the applied PFGE technique on highly eutrophic lake water.
Changes in viral community composition followed a clear seasonal pattern, reflecting the seasonality detected in bacterial and cyanobacterial community composition. Viruses are generally believed to follow the seasonal distribution of their host organisms, due to their host specificity [59]. Seasonal changes in viral community composition have previously been demonstrated in both freshwater and marine studies [2, 13, 33, 60].
The viral peak detected in May differed in community composition from the viral peak detected in July. This suggests that the viral community in May consisted for a considerable part of viruses infecting different hosts than the viral community present in July. This shift in viral community assemblage between May and June was accompanied by marked changes in viral richness and abundances, and cooccurred with shifts in bacterial and especially cyanobacterial community composition. Strong fluctuations in viral abundance and community composition in spring and summer were thus in accordance with changes observed in bacterial and cyanobacterial communities, suggesting a tight linkage between viral and microbial community dynamics during that period of time. Shifts in viral community composition were not always correlated to shifts in the microbial community, however. The separate clustering of the viral community in September compared to the other months was not accompanied by marked changes in bacterial or cyanobacterial community composition. Neither was it reflected by strong shifts in viral richness or abundances. When comparing temporal changes in viral community composition with changes in cyanobacterial and bacterial community assemblages over the entire experimental period, no significant relationship could be detected. We could thus only detect a tight linkage between viral and microbial community composition during periods of strong fluctuations in the bacterial and cyanobacterial community. Most virus–host dynamics in natural aquatic environments have so far been demonstrated during extreme situations, such as during the collapse of phytoplankton blooms [9, 27, 44].
Bacterial abundance was the only environmental variable that was significantly correlated with the temporal variation in viral community assemblage during the present study, suggesting a tight interaction between viruses and bacteria in Lake Loosdrecht. The tested environmental variables could only explain a small part of the fluctuations in viral community composition (best variable combination: ρ = 0.37), however. It could of course be that the majority of the viral community in Lake Loosdrecht infects nonbacterial and cyanobacterial hosts and is not affected by the environmental variables measured in this study. Since cyanobacteria and bacteria are highly abundant in Lake Loosdrecht, and the chance of viral propagation is linearly dependent on the density of susceptible hosts [24, 34], this would be unlikely however. An alternative explanation would be that viral infection is not as host specific as always assumed. The extent of host specificity of viral infection is indeed under discussion. Some viruses have been reported to exhibit a wide range of hosts [32]. For example, phages of Synechococcus can infect up to ten different strains [56]. In addition, some cyanomyoviridae have been observed by Sullivan et al. [43] to cross-infect hosts of different cyanobacterial genera.
A probable explanation for the lack in correlation between viral and microbial community composition would be that we were not able to detect the more subtle virus–host interactions with the applied technique to detect viral richness, and more specific techniques with a higher resolution might be necessary to reveal virus–host interactions in natural assemblages. Applying a cyanophage specific DGGE protocol, Mühling et al. [33] demonstrated a significant relationship between cyanophage and Synechococcus community composition over an annual cycle in the Red Sea.
In conclusion, the virioplankton was found to be a dynamic component of the aquatic community, with changes in viral community composition following a distinct seasonal pattern. Nutrients related very poorly to temporal changes in viral, bacterial and cyanobacterial community abundances and composition. Contrary to expectations viral abundances were not correlated to abundances of the most dominant plankton groups in Lake Loosdrecht, the bacteria and the filamentous cyanobacteria. In addition, we could only detect a linkage between the composition of viral and both bacterial and cyanobacterial community during periods of strong fluctuations in plankton abundances, whereas during the overall period no such correlation could be detected. Viral abundances did however relate well to cyanobacterial community assemblage and showed a significant positive correlation to Chl-a and prochlorophytes, suggesting that a significant proportion of the viruses in Lake Loosdrecht may be phytoplankton and more specific cyanobacterial viruses.
Acknowledgement
We thank Gabriel Zwart (NIOO-CL), Corina Brussaard, Judith van Bleijswijk, and Harry Witte (NIOZ) for helpful discussions and advice on the PFGE and DGGE techniques. Furthermore, we would like to thank DWR for their cooperation. We also like to thank three anonymous reviewers for helpful comments on the manuscript. This work was supported by grant 809.34.006 from the NWO sector of Earth and Life Sciences (ALW) for M. Tijdens. The contribution of S.G.H. Simis was funded by grant EO-053 from the User Support Programme managed by the programme office External Research of the Netherlands Organization for Scientific Research (NWO)—National Institute for Space Research (SRON). A-C. Baudoux was funded by NWO-ALW project 811.33.002. Publication No 4162 Netherlands Institute of Ecology (NIOO-KNAW) Centre for Limnology.
Footnotes
†Deceased
References
- 1.Ackermann HW, DuBow MS (1987) General properties of bacteriophages. In: Viruses of prokaryotes. CRC Press, Boca Raton, FL
- 2.Auguet JC, Montanie H, Lebaron P (2006) Structure of virioplankton in the Charente Estuary (France): transmission electron microscopy versus pulsed field gel electrophoresis. Microb Ecol 51:197–208 [DOI] [PubMed]
- 3.Bergh O, Borsheim KY, Bratbak G, Heldal M (1989) High abundance of viruses found in aquatic environments. Nature 340:467–468 [DOI] [PubMed]
- 4.Bettarel Y, Sime-Ngando T, Bouvy M, Arfi R, Amblard C (2005) Low consumption of virus-sized particles by heterotrophic nanoflagellates in two lakes of the French Massif Central. Aquat Microb Ecol 39:205–209 [DOI]
- 5.Bouvier T, del Giorgio PA (2007) Key role of selective viral-induced mortality in determining marine bacterial community composition. Environ Microbiol 9:287–297 [DOI] [PubMed]
- 6.Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349 [DOI]
- 7.Breitbart M, Salamon P, Andresen B, Mahaffy JM, Segall AM, Mead D, Azam F, Rohwer F (2002) Genomic analysis of uncultured marine viral communities. Proc Natl Acad Sci USA 99:14250–14255 [DOI] [PMC free article] [PubMed]
- 8.Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22:361–369
- 9.Castberg T, Larsen A, Sandaa RA, Brussaard CPD, Egge JK, Heldal M, Thyrhaug R, van Hannen EJ, Bratbak G (2001) Microbial population dynamics and diversity during a bloom of the marine coccolithophorid Emiliania huxleyi (Haptophyta). Marine Ecol Prog Series 221:39–46 [DOI]
- 10.Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Marine Ecol Prog Series 92:205–219 [DOI]
- 11.Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. Primer-E, Plymouth
- 12.Dignum, M (2003) Phosphate uptake proteins as markers for the nutrient status of freshwater cyanobacteria. In: University of Amsterdam, Amsterdam
- 13.Dorigo U, Jacquet S, Humbert JF (2004) Cyanophage diversity, inferred from g20 gene analyses, in the largest natural lake in France, Lake Bourget. Appl Environ Microbiol 70:1017–1022 [DOI] [PMC free article] [PubMed]
- 14.Fischer UR, Velimirov B (2002) High control of bacterial production by viruses in a eutrophic oxbow lake. Aquat Microb Ecol 27:1–12 [DOI]
- 15.Fisher SG, Lerman LS (1979) Length-independent separation of DNA restriction fragments in two-dimensional gel eclectrophoresis. Cell 16:191–200 [DOI] [PubMed]
- 16.Frederickson CM, Short SM, Suttle CA (2003) The physical environment affects cyanophage communities in British Columbia inlets. Microb Ecol 46:348–357 [DOI] [PubMed]
- 17.Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548 [DOI] [PubMed]
- 18.Goddard VJ, Baker AC, Davy JE, Adams DG, De Ville MM, Thackeray SJ, Maberly SC, Wilson WH (2005) Temporal distribution of viruses, bacteria and phytoplankton throughout the water column in a freshwater hypereutrophic lake. Aquat Microb Ecol 39:211–223 [DOI]
- 19.Goldman CR, Marzolf ER, Elser JJ (1990) Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Can J Fish Aquat Sci 47:1468–1477
- 20.Gons HJ, Ebert J, Hoogveld HL, van den Hove L, Pel R, Takkenberg W, Woldringh CJ (2002) Observations on cyanobacterial population collapse in eutrophic lake water. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol 81:319–326 [DOI] [PubMed]
- 21.Gonzalez JM, Suttle CA (1993) Grazing by marine nanoflagellates on viruses and virus-sized particles—ingestion and digestion. Mar Ecol Prog Ser 94:1–10 [DOI]
- 22.Gulati RD, Ooms-Wilms AL, Van Tongeren OFR, Postema G, Siewertsen K (1992) The dynamics and role of limnetic zooplankton in Loosdrecht Lakes (the Netherlands). Hydrobiologia 233:69–86 [DOI]
- 23.Hennes KP, Simon M (1995) Significance of bacteriophages for controlling bacterioplankton growth in a mesotrophic lake. Appl Environ Microbiol 61:333–340 [DOI] [PMC free article] [PubMed]
- 24.Hennes KP, Suttle CA, Chan AM (1995) Fluorescently labeled virus probes show that natural virus populations can control the structure of marine microbial communities. Appl Environ Microbiol 61:3623–3627 [DOI] [PMC free article] [PubMed]
- 25.Hofle MG, Haas H, Dominik K (1999) Seasonal dynamics of bacterioplankton community structure in a eutrophic lake as determined by 5S rRNA analysis. Appl Environ Microbiol 65:3164–3174 [DOI] [PMC free article] [PubMed]
- 26.Jacquet S, Heldal M, Iglesias-Rodriguez D, Larsen A, Wilson W, Bratbak G (2002) Flow cytometric analysis of an Emiliana huxleyi bloom terminated by viral infection. Aquat Microb Ecol 27:111–124 [DOI]
- 27.Larsen A, Castberg T, Sandaa RA, Brussaard CPD, Egge J, Heldal M, Paulino A, Thyrhaug R, van Hannen EJ, Bratbak G (2001) Population dynamics and diversity of phytoplankton, bacteria and viruses in a seawater enclosure. Mar Ecol Prog Ser 221:47–57 [DOI]
- 28.Larsen A, Flaten GAF, Sandaa RA, Castberg T, Thyrhaug R, Erga SR, Jacquet S, Bratbak G (2004) Spring phytoplankton bloom dynamics in Norwegian coastal waters: microbial community succession and diversity. Limnol Oceanogr 49:180–190
- 29.Liu YM, Zhang QY, Yuan XP, Li ZQ, Gui JF (2006) Seasonal variation of virioplankton in a eutrophic shallow lake. Hydrobiologia 560:323–334 [DOI]
- 30.Madan NJ, Marshall WA, Laybourn-Parry J (2005) Virus and microbial loop dynamics over an annual cycle in three contrasting Antarctic lakes. Freshwater Biol 50:1291–1300 [DOI]
- 31.Manage PM, Kawabata Z, Nakano S, Nishibe Y (2002) Effect of heterotrophic nanoflagellates on the loss of virus-like particles in pond water. Ecol Res 17:473–479 [DOI]
- 32.Mann NH (2003) Phages of the marine cyanobacterial picophytoplankton. Fems Microbiol Rev 27:17–34 [DOI] [PubMed]
- 33.Muhling M, Fuller NJ, Millard A, Somerfield PJ, Marie D, Wilson WH, Scanlan DJ, Post AF, Joint I, Mann NH (2005) Genetic diversity of marine Synechococcus and co-occurring cyanophage communities: evidence for viral control of phytoplankton. Environ Microbiol 7:499–508 [DOI] [PubMed]
- 34.Murray AG, Jackson GA (1992) Viral dynamics—a model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar Ecol Prog Ser 89:103–116 [DOI]
- 35.Noble RT, Fuhrman JA (1998) Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat Microb Ecol 14:113–118 [DOI]
- 36.Pel R, Floris V, Hoogveld H (2004) Analysis of planktonic community structure and trophic interactions using refined isotopic signatures determined by combining fluorescence-activated cell sorting and isotope-ratio mass spectrometry. Freshwater Biol 49:546–562 [DOI]
- 37.Pel R, Hoogveld H, Floris V (2003) Using the hidden isotopic heterogeneity in phyto- and zooplankton to unmask disparity in trophic carbon transfer. Limnol Oceanogr 48:2200–2207
- 38.Proctor LM, Fuhrman JA (1990) Viral mortality of marine-bacteria and cyanobacteria. Nature 343:60–62 [DOI]
- 39.Riemann L, Middelboe M (2002) Stability of bacterial and viral community compositions in Danish coastal waters as depicted by DNA fingerprinting techniques. Aquat Microb Ecol 27:219–232 [DOI]
- 40.Short SM, Suttle CA (2003) Temporal dynamics of natural communities of marine algal viruses and eukaryotes. Aquat Microb Ecol 32:107–119 [DOI]
- 41.Simis SGH, Tijdens M, Hoogveld H, Gons HJ (2005) Optical changes associated with cyanobacterial bloom termination by viral lysis. J Plankton Res 27:937–949 [DOI]
- 42.Steward GF, Montiel JL, Azam F (2000) Genome size distributions indicate variability and similarities among marine viral assemblages from diverse environments. Limnol Oceanogr 45:1697–1706
- 43.Sullivan MB, Waterbury JB, Chisholm SW (2003) Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature 424:1047–1051 [DOI] [PubMed]
- 44.Tarutani K, Nagasaki K, Yamaguchi M (2000) Viral impacts on total abundance and clonal composition of the harmful bloom-forming phytoplankton Heterosigma akashiwo. Appl Environ Microbiol 66:4916–4920 [DOI] [PMC free article] [PubMed]
- 45.Taylor GT, Hein C, Labichella M (2003) Temporal variations in viral distributions in the anoxic Cariaco Basin. Aquat Microb Ecol 30:103–116 [DOI]
- 46.Thingstad TF (2000) Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr 45:1320–1328
- 47.Thingstad TF, Lignell R (1997) Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat Microb Ecol 13:19–27 [DOI]
- 48.Tomaru Y, Tarutani K, Yamaguchi M, Nagasaki K (2004) Quantitative and qualitative impacts of viral infection on a Heterosigma akashiwo (Raphidophyceae) bloom in Hiroshima Bay, Japan. Aquat Microb Ecol 34:227–238 [DOI]
- 49.Turner S, Burgerwiersma T, Giovannoni SJ, Mur LR, Pace NR (1989) The relationship of a prochlorophyte prochlorothrix-hollandica to green chloroplasts. Nature 337:380–382 [DOI] [PubMed]
- 50.Utermöhl H (1931) Neue Wege in der quantitativen Erfassung des Planktons. Verh int Ver Limnol 5:567–597
- 51.Van der Gucht K, Sabbe K, De Meester L, Vloemans N, Zwart G, Gillis M, Vyverman W (2001) Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ Microbiol 3:680–690 [DOI] [PubMed]
- 52.Van Hannen EJ, Mooij W, Van Agterveld MP, Gons HJ, Laanbroek HJ (1999) Detritus-dependent development of the microbial community in an experimental system: a qualitative analysis using denaturing gradient gel electrophoresis. Appl Environ Microbiol 65:2478–2484 [DOI] [PMC free article] [PubMed]
- 53.Van Hannen EJ, Zwart G, van Agterveld MP, Gons HJ, Ebert J, Laanbroek HJ (1999) Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl Environ Microbiol 65:795–801 [DOI] [PMC free article] [PubMed]
- 54.Van Tongeren OFR, Van Liere L, Gulati RD, Postema G, Boesewinkel-de Bruyn PJ (1992) Multivariate-analysis of the plankton communities in the Loosdrecht Lakes—relationship with the chemical and physical-environment. Hydrobiologia 233:105–117 [DOI]
- 55.Vrede K, Stensdotter U, Lindstrom ES (2003) Viral and bacterioplankton dynamics in two lakes with different humic contents. Microb Ecol 46:406–415 [DOI] [PubMed]
- 56.Waterbury JB, Valois FW (1993) Resistance to cooccurring phages enables marine synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol 59:3393–3399 [DOI] [PMC free article] [PubMed]
- 57.Weinbauer MG (2004) Ecology of prokaryotic viruses. Fems Microbiol Rev 28:127–181 [DOI] [PubMed]
- 58.Weinbauer MG, Hofle MG (1998) Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl Environ Microbiol 64:431–438 [DOI] [PMC free article] [PubMed]
- 59.Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114 [DOI] [PMC free article] [PubMed]
- 60.Wommack KE, Ravel J, Hill RT, Chun JS, Colwell RR (1999) Population dynamics of Chesapeake bay virioplankton: total-community analysis by pulsed-field gel electrophoresis. Appl Environ Microbiol 65:231–240 [DOI] [PMC free article] [PubMed]
- 61.Zwart G, Hiorns WD, Methe BA, Van Agterveld MP, Huismans R, Nold SC, Zehr JP, Laanbroek HJ (1998) Nearly identical 16S rRNA sequences recovered from lakes in North America and Europe indicate the existence of clades of globally distributed freshwater bacteria. Syst App Microbiol 21:546–556 [DOI] [PubMed]
- 62.Zwart G, Huismans R, van Agterveld MP, Van de Peer Y, De Rijk P, Eenhoorn H, Muyzer G, van Hannen EJ, Gons HJ, Laanbroek HJ (1998) Divergent members of the bacterial division Verrucomicrobiales in a temperate freshwater lake. FEMS Microbiol Ecol 25:159–169 [DOI]
- 63.Zwart G, Kamst-van Agterveld MP, van der Werff-Staverman I, Hagen F, Hoogveld HL, Gons HJ (2005) Molecular characterization of cyanobacterial diversity in a shallow eutrophic lake. Environ Microbiol 7:365–377 [DOI] [PubMed]