Abstract
Native cylic nucleotide-gated (CNG) channels are composed of α and β subunits. Olfactory CNG channels were expressed from rat cDNA clones in Xenopus oocytes and studied in inside-out patches. Using tandem dimers composed of linked subunits, we investigated the stoichiometry and arrangement of the α and β subunits. Dimers contained three subunit types: αwt, βwt, and αm. The αm subunit lacks an amino-terminal domain that greatly influences gating, decreasing the apparent affinity of the channel for ligand by 9-fold, making it a reporter for inclusion in the tetramer. Homomeric channels from injection of αwtαwt dimers and from αwt monomers were indistinguishable. Channels from injection of αwtαm dimers had apparent affinities 3-fold lower than αwt homomultimers, suggesting a channel with two αwt and two αm subunits. Channels from coinjection of αwtαwt and ββ dimers were indistinguishable from those composed of α and β monomers and shared all of the characteristics of the α+β phenotype of heteromeric channels. Coinjection of αwtαm and ββ dimers yielded channels also of the α+β phenotype but with an apparent affinity 3-fold lower, indicating the presence of αm in the tetramer and that α+β channels have adjacent α-subunits. To distinguish between an α-α-α-β and an α-α-β-β arrangement, we compared apparent affinities for channels from coinjection of αwtαwt and βαwt or αwtαwt and βαm dimers. These channels were indistinguishable. To further argue against an α-α-α-β arrangement, we quantitatively compared dose–response data for channels from coinjection of αwtαm and ββ dimers to those from α and β monomers. Taken together, our results are most consistent with an α-α-β-β arrangement for the heteromeric olfactory CNG channel.
Cyclic nucleotide-gated (CNG) channels are best known for their role in sensory neurons in the transduction of external stimuli into an electrical signal (1–4). Since the initial cloning of CNG channels from retinal-rod photoreceptors (5) and from olfactory sensory neurons (6, 7), they have also been found to be expressed in testes, kidney, heart, and brain (8–11). It appears that many kinds of cells use CNG channels as part of their repertoire in shaping their response to extracellular signals and as a mode of Ca2+ entry (12). In olfactory receptor neurons, cell-surface receptors that recognize a vast array of odorants use a G protein-mediated signaling pathway to stimulate adenylyl cyclase, and the resulting increase in cAMP concentration opens CNG channels (4). Cation influx through CNG channels depolarizes the cell and increases intracellular Ca2+ concentrations (13). Both the olfactory and rod classes of CNG channels are also found in the hippocampus (11), where they may play a role in memory and learning (14).
The putative topology of all known CNG subunits is similar to voltage-gated K+ channels, with six membrane-spanning segments and a pore-forming P region. CNG channels, however, have an additional cyclic nucleotide-binding domain in their intracellular carboxyl-terminal region that exhibits sequence similarity to other cyclic nucleotide-binding proteins. Recently, the amino-terminal region of the olfactory α-subunit has been shown to potentiate channel activation by interaction with the carboxyl-terminal region (15). Ca2+/calmodulin inhibits these channels by binding to the amino-terminal domain and preventing this autoexcitatory interaction (15, 16).
Like K+ channels, CNG channels are thought to form as tetramers of four subunits arranged around a central, ion-conducting pore (17–19). Native rod and olfactory CNG channels are thought to be composed of at least two types of subunits, α and β (20–22). The olfactory β-subunit exhibits 52% sequence identity to the α-subunit and 30% sequence identity to the rod β-subunit. It is expressed throughout the nasal epithelium, especially in olfactory sensory neurons, and in the vomeronasal organ (20, 22, 23). The olfactory β-subunit does not form cyclic nucleotide-activatable channels by itself. However, incorporation of the β-subunit into heteromeric channels with the α-subunit has a profound effect on the behavior of the olfactory channel, greatly increasing the apparent affinity for cAMP, as well as altering its voltage-dependence and rectification properties (20, 22).
We wished to ascertain the stoichiometry and arrangement of the subunits of heteromeric α+β olfactory CNG channels. Our approach was to generate tandem dimer constructs containing two subunits placed in tandem in a single open reading frame, separated by a short linker. These dimers were constructed from two types of α-subunits and the β-subunit. One of the α-subunits (αm) was mutant, containing a deletion in the amino-terminal region that strongly affects its apparent affinity for agonist (15, 16) and makes it a robust reporter for inclusion in the channel tetramer. We injected a number of combinations of these tandem dimers, alone or in pairs, and observed the characteristics of the CNG channels that formed. The conclusions of this study are predicated on certain basic assumptions: (i) CNG channels are tetramers; (ii) there is a fixed or preferred assembly of subunits, and the phenotype of expressed channels arises from a single population of channels; (iii) linking subunits together as dimers has no effect on subunit behavior and does not force assembly of subunits into an arrangement different from their preferred arrangement; (iv) when both subunits of a dimer incorporate into the channel, the subunits are neighboring; and (v) although the first subunit may, under certain circumstances, incorporate into the channel without the second subunit, the second subunit never incorporates into the channel without the first subunit. In this study, we present evidence supporting many of these assumptions, suggesting that α+β heteromultimers have two α-subunits and two β-subunits and that they are arranged in an α-α-β-β configuration.
MATERIALS AND METHODS
The cDNA for the α-subunit and the β-subunit of the rat olfactory CNG channel were kindly provided by the laboratories of R. R. Reed (The Johns Hopkins School of Medicine, Baltimore, MD) and Kai Zinn (California Institute of Technology, Pasadena, CA), respectively. These cDNAs were separately subcloned into a high-expression vector (kindly provided by E. R. Liman, Harvard University, Boston, MA) that contains the untranslated sequences of the Xenopus β-globin gene (24). In general, the oocyte expression, solutions, and electrophysiology were like those previously described (25). Briefly, Xenopus oocytes were injected with in vitro-transcribed RNA coding for channel subunits, incubated for 3–7 days, and patch-clamped in the inside-out configuration. Intracellular and extracellular solutions contained 130 mM NaCl, 0.2 mM EDTA, and 3 mM Hepes (pH 7.2). For some experiments, niflumic acid (500 μM) was added to the pipette (extracellular) solution to reduce endogenous Ca2+-activated Cl− currents. Cyclic nucleotides were added to the internal solution at the concentrations indicated. The αm subunit has a deletion from E50 to R92. It was generated by using a method based on PCR like that previously described (26) and was verified by sequence analysis. The first subunit of a tandem dimer was wild-type and joined to the following subunit by a 21-aa linker consisting of the sequence QQQQQQQQIEGRQQQQQQQQA. The α-subunit as the second subunit of a dimer was the αwt or αm subunit with an M2V mutation to create an NcoI cut/splice site. The β-subunit as the second subunit of a dimer had an S2G mutation to create an NcoI cut/splice site.
For the data presented here, dimer coinjections were done at a ratio of 1:1, and monomer coinjections at a ratio of 4:1 or 2:1 (α:β). Data from coinjection of αα and ββ dimers at a ratio of 4:1 were very similar to those at a ratio of 1:1, and data from coinjection of α and β monomers at a ratio of 20:1 were similar to those at 4:1 (data not shown), indicating the ample supply of β-subunits to form channels of the preferred arrangement for the experiments in this study. Because of the large effect of the β-subunit on the apparent affinity of channels for cAMP, we can estimate an upper limit for the fractional population of channels that could have been α-homomultimers in experiments that produced α+β heteromultimers. We modeled the dose–response data from the αα+ββ coinjections as resulting from a mixed population of these two channel types, and estimate that no more than 25% of the channels could have been α-homomultimers (data not shown). All currents were tested for desensitization as shown in Fig. 2D. For those without desensitization, voltage pulses were applied every 3–5 sec. For those that desensitized, cyclic nucleotide-free solution was perfused for a minimum of 20 sec between each application of ligand. Voltage pulses were applied every 1 sec, and on application of ligand, the largest current was used for the measurement. Using this protocol, we estimate that errors from desensitization were seldom greater than 15% for any given measurement.
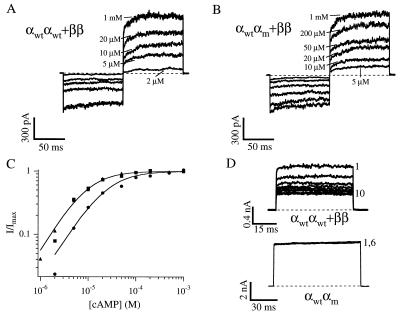
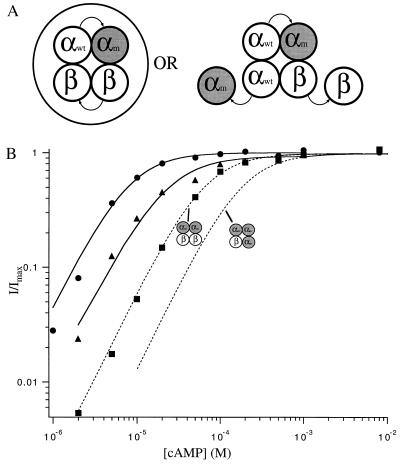
Figure 2.
Currents from coinjection of αwtαwt and ββ (A) or αwtαm and ββ (B) dimers activated by a range of concentrations of cAMP. The pulse protocol is as in Fig. 1. (C) Data are normalized dose–response relations at 60 mV from patches with channels from coinjection of α and β monomers (triangles), αwtαwt and ββ dimers (squares), or αwtαm and ββ dimers (circles) for activation of the channels by cAMP. Superimposed on the data are fits of the dimer data to Hill equations of the form in Fig. 1. The fitted values of K1/2 are 9.2 μM and 25 μM, and of n are 1.3 and 1.4 for channels from αwtαwt and ββ and αwtαm and ββ coinjections, respectively, and Imax was set at unity. (D) Desensitization is shown for currents with successive brief depolarizations to 60 mV given every 1 s, in the presence of saturating cAMP, for channels from αwtαwt and ββ or αwtαm injections. The former desensitize, like channels from α+β monomers, but the latter do not, like channels from α monomers.
RESULTS
We studied cloned rat olfactory CNG channels expressed in Xenopus oocytes. Several days after injecting oocytes with RNA coding for either subunit monomers or tandem dimers, we recorded CNG currents from inside-out patches pulled from oocyte membranes. Cyclic nucleotides were applied to the intracellular side of the patch, and the currents in the absence of cyclic nucleotides were subtracted. Currents were usually recorded by using a successive-pulse protocol to −60 and 60 mV from a holding potential of 0 mV (Fig. 1 Inset). Oocytes injected with RNA coding for wild-type α-subunit monomers produced CNG currents with properties very similar to those previously described for olfactory α-homomultimers (Table 1; refs. 6, 27). The αm subunit lacks amino acids 50–92 in the amino-terminal region, shown to contain an autoexcitatory region that potentiates CNG channel gating and the site of modulation by Ca2+/calmodulin (15, 16). Dose–response data for activation of αm homomultimers by cAMP showed that the concentration of ligand that activates half the maximum current (K1/2) is shifted to higher concentrations by about 9-fold relative to αwt channels. Additionally, for these channels, saturating cAMP can activate only 61 ± 1% (n = 6) of the current activated by cGMP, compared with αwt channels that can produce as much current by saturating cAMP as by cGMP (Imax, cAMP/Imax, cGMP = 0.98 ± 0.02, n = 12) (Fig. 1D; Table 1). These data indicate that expression of αm momomers yields homomeric channels with properties very similar to αwt channels in the presence of Ca2+/calmodulin; that is, with reduced apparent affinities for cyclic nucleotide and a reduction in the maximum current elicited by saturating cAMP (15, 16).
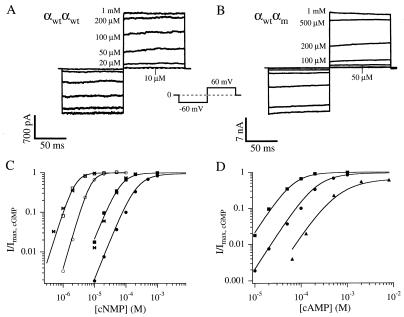
Figure 1.
Currents from injection of RNA for wild-type olfactory αwtαwt homodimers (A) and for αwtαm heterodimers (B) activated by a range of concentrations of cAMP. The voltage-pulse protocol is shown in the Inset. (C) Data are normalized dose–response relations at 60 mV from patches with channels from injection of αwt monomers (bows), αwtαwt homodimers (squares), or αwtαm heterodimers (circles) for activation of the channels by cAMP (filled symbols) or cGMP (open symbols). Superimposed on the data are fits to the dimer data of Hill equations of the form I = Imax {[cNMP]n/(K1/2n + [cNMP]n)}, where [cNMP] is the concentration of ligand, K1/2 is the concentration that produces half-maximal current, and n is the Hill slope. For αwtαwt, K1/2 = 70 μM and n = 1.9 for activation by cAMP and K1/2 = 2.4 μM, and n = 2.5 for activation by cGMP and Imax was set to unity. For αwtαm, K1/2 = 269 μM, n = 2.0 and Imax = 0.89 for activation by cAMP, and K1/2 = 7.8 μM and n = 2.7 for activation by cGMP and Imax was set to unity. (D) The dose–response data for activation of αwtαwt dimers (squares) and αwtαm dimers (circles) by cAMP are replotted from (C). Also plotted are dose–response data for activation of αm monomers (triangles) by cAMP. Superimposed on the data are fits to Scheme 1. K = 1050 M−1 for all three curves. For αwtαwt, L = 200, and for αm, L = 1.86. The prediction of Scheme 1 for the case of a channel with two αwt-subunits and two αm-subunits is based on the assumption of a concerted final transition among the subunits from closed to open (19), which predicts L = 19.4. The curve superimposed on that data is this prediction.
Table 1.
Gating paramaters of channels from injections of the indicated RNA
| RNA injected |
K1/2, μM
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| cAMP
|
cGMP
|
n (60 mV)
|
I−60mV/I60 mV
|
Desensitization | |||||
| 60 mV | −60 mV | 60 mV | −60 mV | cAMP | cGMP | cAMP | cGMP | ||
| αwt mono | 83 ± 3 | 89 ± 3 | 2.77 ± 0.13 | 2.85 ± 0.11 | 2.33 ± 0.06 | 2.56 ± 0.07 | 0.79 ± 0.01 | 0.81 ± 0.02 | No |
| n = 26 | n = 26 | n = 10 | n = 10 | n = 25 | n = 10 | n = 28 | n = 12 | ||
| αm mono | 715 ± 51 | ND | ND | ND | 1.77 ± 0.14 | ND | ND | ND | No |
| n = 5 | n = 3 | ||||||||
| αwt + β mono | 10.5 ± 0.8 | 16.9 ± 1.0 | 5.2 ± 1.2 | 12.8 ± 1.6 | 1.5 ± 0.1 | 1.3 ± 0.1 | 0.51 ± 0.01 | 0.49 ± 0.02 | Yes |
| n = 20 | n = 20 | n = 4 | n = 4 | n = 20 | n = 4 | n = 44 | n = 29 | ||
| αm + β mono | 53 ± 3 | 82 ± 10 | 12 ± 1 | 21 ± 1 | 1.46 ± 0.05 | 1.36 ± 0.07 | 0.60 ± 0.02 | 0.54 ± 0.02 | Yes |
| n = 5 | n = 5 | n = 2 | n = 2 | n = 5 | n = 5 | n = 4 | n = 2 | ||
| αwtαwt | 65 ± 2 | 76 ± 5 | 2.3 ± 0.2 | 2.4 ± 0.2 | 2.06 ± 0.07 | 2.56 ± 0.08 | 0.78 ± 0.1 | 0.81 ± 0.01 | No |
| n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | ||
| αwtαm | 257 ± 8 | 274 ± 8 | 8.9 ± 0.3 | 9.5 ± 0.4 | 2.30 ± 0.04 | 2.79 ± 0.04 | 0.77 ± 0.01 | 0.79 ± 0.01 | No |
| n = 16 | n = 16 | n = 15 | n = 15 | n = 16 | n = 15 | n = 15 | n = 15 | ||
| αwtαwt + ββ | 8.5 ± 0.4 | 18.5 ± 1.5 | 5.6 ± 0.3 | 9.9 ± 1.2 | 1.36 ± 0.05 | 1.19 ± 0.06 | 0.57 ± 0.01 | 0.52 ± 0.02 | Yes |
| n = 14 | n = 14 | n = 11 | n = 10 | n = 14 | n = 14 | n = 13 | n = 9 | ||
| αwtαm + ββ | 28.4 ± 3.7 | 47.6 ± 4.4 | 9.1 ± 1.1 | 9.3 ± 1.1 | 1.21 ± 0.07 | 1.61 ± 0.10 | 0.62 ± 0.02 | 0.57 ± 0.04 | Yes |
| n = 14 | n = 14 | n = 10 | n = 9 | n = 14 | n = 10 | n = 14 | n = 9 | ||
| αwtαwt + βαwt | 15.3 ± 1.7 | 21.4 ± 1.9 | 3.5 ± 0.5 | 4.4 ± 0.5 | 1.26 ± 0.11 | 1.16 ± 0.06 | 0.55 ± 0.02 | 0.60 ± 0.02 | Yes |
| n = 7 | n = 5 | n = 5 | n = 4 | n = 7 | n = 5 | n = 5 | n = 4 | ||
| αwtαwt + βαm | 11.8 ± 1.3 | 19.6 ± 2.2 | 4.6 ± 0.5 | 8.1 ± 1.1 | 1.41 ± 0.07 | 1.50 ± 0.08 | 0.54 ± 0.02 | 0.54 ± 0.03 | Yes |
| n = 10 | n = 7 | n = 4 | n = 5 | n = 8 | n = 4 | n = 7 | n = 4 | ||
| αwtαm + βαm | 33.6 ± 5.2 | 46.9 ± 3.6 | 7.4 ± 0.3 | 8.4 ± 0.6 | 1.25 ± 0.04 | 1.18 ± 0.04 | 0.64 ± 0.01 | 0.62 ± 0.02 | Yes |
| n = 10 | n = 10 | n = 9 | n = 9 | n = 10 | n = 10 | n = 10 | n = 9 | ||
The first six paramaters are from fits of the data to Hill equations of the form in Fig. 1. The current ratios are ratios of the Imax from the Hill equations fits, and are thus saturating currents elicited by cAMP or cGMP. Desensitization was measured by observing any current decline during successive brief depolarizations to 60 mV, applied 1/sec. Injections of αwtβ, βαwt, βαm, ββ, and coinjections of αwtβ and βαwt, and αwtβ and βαm dimers failed to elicit CNG currents, with a minimum of 10 oocytes tested for each. ND, not done.
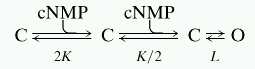
We can understand these gating alterations by considering a simplified model of CNG channel gating (25):
 |
 |
where C and O represent closed and open channels and K and L are the equilibrium constants for the initial binding of ligand and for the allosteric conformational change, respectively. This model considers that the opening of the channel involves the independent and identical binding of two cyclic nucleotides followed by a concerted allosteric opening transition. At saturating concentrations of cyclic nucleotide, at which the channels are fully ligand-bound, the open probability (Po) is given by L/(L + 1) where, in general, L depends on the channel and on the ligand. For homomeric αwt and homomeric αm channels, L is so large for cGMP-bound channels that at saturating cGMP, the fractional activation is very near unity. The potentiation by the autoexcitatory region contained within the deletion in αm involves an interaction of this region with the cyclic nucleotide-binding region that increases by about 100-fold the equilibrium constant, L, of the allosteric conformational change (15). Thus, this deletion, or the presence of Ca2+/calmodulin, strongly decreases L, and this reduction is manifested in two ways: (i) a shift in the apparent affinity for activation of the channel by ligand to higher concentrations and (ii) for a case like cAMP, in which L is smaller, a marked reduction in the maximum current at saturating ligand. Thus, for unmodulated cAMP-bound wild-type α-channels, L has a value of about 100–200 (28), and so a 100-fold reduction in L, as for αm channels or wild-type channels in the presence of Ca2+/calmodulin, gives an L near 1, and thus a fractional activation around half-maximal.
We constructed tandem dimers from three different rat olfactory CNG channel subunits: wild-type α-subunits (αwt), mutant α-subunits (αm), and wild-type β-subunits. Injection of RNA for tandem αwtαwt dimers produced currents indistinguishable from those produced by αwt monomers in all respects, including the K1/2 for activation by cAMP and cGMP, the slope of the dose–response relation, and rectification (Fig. 1 A and C; Table 1). Injection of RNA for tandem αwtαm dimers produced currents similar to those from αwtαwt dimers or αwt monomers, but with apparent affinities for both cAMP and cGMP shifted to about 3-fold higher concentrations, and a small reduction in the maximum current elicited by saturating cAMP relative to cGMP (Imax, cAMP/Imax, cGMP = 0.84 ± 0.02, n = 15) (Fig. 1 B and C; Table 1). In Fig. 1D are plotted dose–response data for αwtαwt dimers, αwtαm dimers, and αm monomers for activation by cAMP. Superimposed on the data are curves for fits of the data to the model of Scheme 1. The αm data were fit with a value of L reduced by about a factor of 100, relative to that for αwtαwt, while keeping K the same. The αwtαm data were fit with K again kept constant and L set to the geometric mean between its values for αwtαwt and αm, which is just what Scheme 1 predicts for a channel with a concerted opening transition that contains two wild-type and two mutant subunits (19). Thus, the altered gating of the αm subunits acts as a reporter, verifying their inclusion in the tetramer, and the physical link between the αwt and αm subunits makes a αwt-αm-αwt-αm arrangement the most likely possibility (Fig. 5).
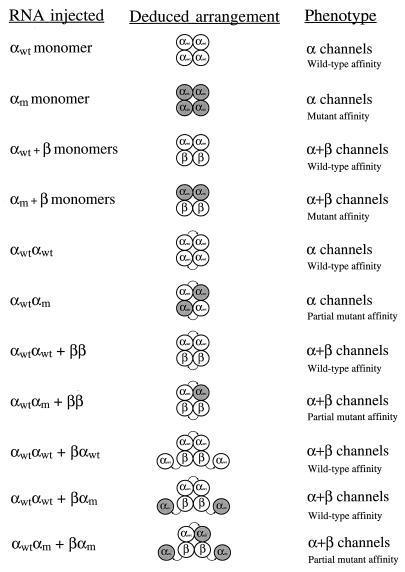
Figure 5.
Deduced arrangement of the channels formed from all of the injections and coinjections. The assignment of phenotype was based on the evidence discussed in the text. “Wild-type affinity,” “mutant affinity,” and “partial mutant affinity” refer to channels that show behavior consistent with none, all, or half of their α-subunits being αm-subunits. The αm-subunits are shaded.
Fig. 2A shows data from an experiment with coinjection of αwtαwt and ββ dimers into the same oocyte. Heteromeric α+β channels differ from homomeric α channels in a number of signature characteristics (Table 1) (20, 22). Channels formed by αwtαwt and ββ dimers were indistinguishable from channels formed by α and β monomers in all of the characteristics that distinguish them from homomeric α channels (Fig. 2 A, C, and D; Table 1). These characteristics include (i) an eightfold shift in the K1/2 for cAMP to lower concentrations, (ii) a reduction in the slope of the dose–response curve (Hill equation slope 1.2–1.6 vs. 2.1–2.7), (iii) a more pronounced time-dependence of the current on voltage steps, (iv) desensitization when expressed in oocytes (Fig. 2D), and (v) a more pronounced rectification of the current at saturating concentrations. Collectively, these identifying characteristics clearly distinguish between channels composed of α-subunits and those composed of both α- and β-subunits and form the basis of the assigned phenotype for each combination of injected dimers (Fig. 5). Like heteromultimers formed by α and β monomers, α+β channels formed by dimers produce as much current with saturating cAMP as with saturating cGMP (Imax, cAMP/Imax, cGMP = 1.06 ± 0.06, n = 8). Thus, coinjection of an αα dimer with a ββ dimer yields channels indistinguishable from α+β heteromultimers formed by monomers.
We asked whether substitution of an αm for an αwt subunit as the second subunit of an αα dimer shifts apparent affinities for ligand of phenotypic α+β channels, like the results in Fig. 1 for α-channels. Channels formed from coinjection of αwtαm and ββ dimers resulted in currents with these same α+β signature characteristics but with the K1/2 values for activation by cAMP and cGMP shifted to higher concentrations relative to channels created by coinjection of αwtαwt and ββ dimers (Fig. 2 B and C; Table 1). This shift in the apparent affinities indicates that these channels have incorporated the αm-subunit half of the injected αwtαm dimer. As with channels from αwtαm dimers alone, the shift in the K1/2 value for α+β channels suggests that both α-subunits of the dimer are being incorporated and that they are adjacent. Thus, a tetrameric arrangement of α and β subunits with two adjacent α-subunits yields channels very similar to channels formed from α and β monomers, suggesting that α+β heteromultimers have adjacent α-subunits.
Singly injecting all dimers consisting of one α-subunit and one β-subunit failed to yield CNG currents (Table 1). These dimers would be predicted to form channels with an alternating αβ arrangement (α-β-α-β). Nonexpression by itself is not strong evidence, but this result is consistent with functional α+β channels not consisting of alternating α and β subunits. Coinjections of αβ dimers and βα dimers also failed to yield CNG currents.
Having demonstrated that α+β channels have adjacent α-subunits, we turned our attention to discriminating between the two remaining alternatives for subunit arrangement in a tetrameric heteromultimer containing adjacent α-subunits: a channel with three α-subunits and one β-subunit (α-α-α-β) or a channel with two adjacent α-subunits and two adjacent β-subunits (α-α-β-β). To distinguish between these two possibilities, we coinjected a dimer with two α-subunits (αα) with a dimer with a β-subunit followed by an α-subunit (βα) (Table 1). Channels formed from these injections were α+β-like and had behavior indistinguishable from that of channels created by coinjections of αα and ββ dimers (Table 1; Fig. 3). Furthermore, similar to the results obtained with αα (Fig. 1) or αα and ββ (Fig. 2) injections, channels formed always displayed the effect of the presence of the αm-subunit when it was the second subunit of the αα dimer. This effect was easily observable by the presence of the telltale shift in the apparent affinity. We also tested coinjections of αα and αβ dimers. Channels formed from these experiments acted much like those from injection of αα dimers or α monomers; that is, of the α-channel phenotype (data not shown). However, because we cannot detect the presence of the αβ dimers either alone or in coinjections, we lack any independent evidence that these dimers are functional and are being expressed. We therefore must attach less weight to this result. The uncertain expression of αβ dimers also reduces the weight we attach to the result that coinjection of αβ and βα dimers did not yield functional channels.
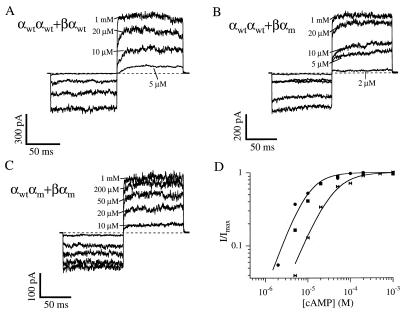
Figure 3.
Currents from coinjection of αwtαwt and βαwt (A), αwtαwt and βαm (B), or αwtαm and βαwt (C) dimers activated by a range of concentrations of cAMP. The pulse protocol is as in Figs. 1 and 2. (D) Data are normalized dose–response relations at 60 mV for patches with channels from coinjections of αwtαwt and βαm (circles), αwtαwt and βαwt (squares), and αwtαm and βαm (bows). Superimposed are fits of the αwtαwt and βαm and αwtαm and βαm data to Hill equations of the form as in Fig. 1. The fitted values of K1/2 are 9.8 μM and 29 μM, and of n are 1.4 and 1.1 for channels from αwtαwt and βαm, and αwtαm and βαm injections, respectively, and Imax was set at unity.
Other investigators using tandem dimers have shown that they may sometimes incorporate only the leading subunit into the channel tetramer, leaving the second subunit outside of the channel (29). We asked whether the same thing could happen here. To test for this behavior, we focused on the αα and βα coinjections that produced channels closely matching the α+β phenotype whose stoichiometry and arrangement we wished to determine. In this scenario, coinjection of αα and βα dimers could result in a channel with two α and two β subunits, with the α subunits of the βα dimers unincorporated (Fig. 5).
Using the reporting ability of the αm subunit, we examined channels formed by coinjection of αwtαwt and βαm dimers. If the resulting channels were of the α-α-α-β arrangement, and thus were incorporating the αm subunit, we would expect to detect it by its effect on the apparent affinity. Fig. 3 shows data from coinjections of αwtαwt and βαwt (A), αwtαwt and βαm (B), or αwtαm and βαm (C) dimers. All three coinjection experiments produced currents with all of the signature features of α+β channels, including increased cAMP affinity, decreased dose–response relation slope, increased time-dependence and rectification, and desensitization (Table 1). Plotted in Fig. 3D are dose–response curves for activation of the resulting channels by cAMP. There was no significant difference between the data for the channels from αwtαwt and βαm coinjections and those from αwtαwt and βαwt coinjections, strongly suggesting that the α-subunits of the βα dimers were not being incorporated. Data from the αwtαm and βαm coinjections show that when the αm is the second subunit of the αα dimer, the dose–response curve is clearly shifted to higher concentrations. They were indistinguishable from those from αwtαm and ββ coinjections (Table 1); that is, as with channels from αwtαwt and ββ dimer or α and β monomer coinjections but with an apparent affinity shifted to about 3-fold higher concentrations. We were thus able to discriminate between the two alternatives by looking for the telltale shift in the apparent affinity caused by the αm subunit. The results from this experiment further suggest that α+β heteromultimers have two α-subunits and two β-subunits in an α-α-β-β arrangement.
However, the data in Fig. 3 are not inconsistent with an α-α-α-β arrangement if a third α-subunit were to come from an αα dimer instead of the βα dimer. To further examine this possibility, we recorded currents resulting from coinjections of αm and β monomers, and compared dose–response data from these channels with those from coinjection of αwt and β monomers or αwtαm and ββ dimers (Fig. 4). The implications of this experiment for the α-α-β-β and α-α-α-β scenarios are depicted in Fig. 4A. Shown schematically are the two possibilities for the case of coinjection of αwtαm and ββ dimers. If an α+β channel indeed has two α-subunits and two β-subunits, then coinjection of αwt and β monomers would yield a channel with two αwt-subunits; coinjection of αm and β monomers, a channel with two αm-subunits; and coinjection of αwtαm and ββ dimers, a channel with one αwt-subunit and one αm-subunit. Thus, like the comparison of α channels with zero, two, or four αm-subunits (Fig. 1D), an α-α-β-β arrangement predicts that the apparent affinity for a channel with one αwt-subunit and one αm-subunit would be the geometric mean between that for a channel with zero and two αm-subunits (Fig. 4B). On the other hand, for coinjection of αα and ββ dimers to yield an α+β channel with three α-subunits and one β-subunit (Fig. 4A, Right), the first α-subunit only of an αα dimer, but not the second, and the first β-subunit only of a ββ dimer, but not the second, would have to incorporate. Thus, this scenario predicts that coinjection of αwt and β monomers would yield a channel with three αwt-subunits; coinjection of αm and β monomers, a channel with three αm-subunits; and coinjection of αwtαm and ββ dimers, a channel with two αwt-subunits and one αm-subunit. This scenario would thus require that the ratio of the K1/2 values of channels from coinjections of αwt and β monomers and αm and β monomers not be the square of the ratio of its values from coinjections of αwt and β monomers and αwtαm and ββ dimers, but the cube of that ratio (Fig. 4B). However, the data from coinjection of αm and β monomers indicate a change in the apparent affinity very nearly the square of the ratio of its values from coinjections of αwt and β monomers and αwtαm and ββ dimers. Thus, the model prediction from the α-α-β-β arrangement fits the data well. This experiment further argues against a α-α-α-β configuration and further supports the α-α-β-β arrangement.
Figure 4.
(A) Schematic diagram of how αwtαm and ββ dimer coinjections could produce channels with an arrangement of two α-subunits and two β-subunits (Left) or three α-subunits and one β-subunit (Right). (B) Dose–response data for activation by cAMP of channels from coinjection of αwt and β monomers (circles), αwtαm and ββ dimers (triangles), and αm and β monomers (squares). The solid lines are the Hill fits to the αwt and β monomer data and the αwtαm and ββ dimer data. For αwt and β monomers, K1/2 = 7.7 μM and n = 1.5. For αwtαm and ββ dimers, K1/2 = 22 μM and n = 1.4. For αm and β monomers (Hill fit not shown for clarity), K1/2 = 63 μM and n = 1.5. The dashed line on the left is the prediction (see Methods) for the α-α-β-β arrangement shown on the Left in A and is a Hill equation curve with K1/2 = 63 μM and n = 1.5. The dotted line on the right is the prediction for the α-α-α-β arrangement shown on the right in (A), and is a Hill equation curve with K1/2 = 178 μM and n = 1.5. The encircled arrangement is the one best supported by the data.
DISCUSSION
Tandem subunits have also been used to show the tetrameric nature of voltage-gated K+ channels (24), inward rectifier K+ channels (30), and CNG channels (17–19). In all of these cases, and in the present work, a mutant subunit was used whose presence in the channel produces obvious effects and so acted as a reporter for its incorporation into the tetramer. We define two channel phenotypes, α channels and α+β channels, based on a set of defining characteristics that differentiate the two. We exploited the characteristic fingerprint of α+β channels to distinguish which dimer injections produce α+β heteromeric channels and which produce α homomeric channels. In addition, the shift of the apparent affinity of the mutant α-subunit made it a reporter for incorporation of the second subunit of a dimer in the channel tetramer, the major uncertainty of the tandem dimer approach. An advantage of this study is that it determined the arrangement of functional CNG channels; that is, those that produced current on application of cyclic nucleotides. Broillet and Firestein (31) showed that the olfactory β-subunit can form homomeric channels gated not by cyclic nucleotides but by nitric oxide. Thus, even if multimers with arrangments other than those suggested here form in the oocyte membrane, we did not observe evidence that they are cyclic nucleotide-activatible channels.
Fig. 5 shows a summary of the results of the experiments presented in this work. For each injection or coinjection, we assign a phenotype of α channels or α+β channels based on their collection of signature differences. Shown in the center column is the arrangement of the subunits for the channels formed, as deduced from the data. For all injections involving αα dimers, both subunits of the αα dimers incorporated in the channel tetramer. For those involving coinjections of αα dimers and βα dimers, we deduce that only the initial β-subunit of the βα dimer is being incorporated, forming an α+β channel with two α subunits and two β subunits. If the αβ dimers are indeed functional and being expressed, then only the initial α-subunit of the αβ dimer is being incorporated, forming a tetramer with four α-subunits. Thus, taken together, all of the evidence indicates that α+β channels are composed of subunits in an α-α-β-β arrangement.
These experiments also provide evidence supporting the assumptions of a fixed or preferred assembly of α+β subunits and that the phenotype of expressed channels arises from a single population. This evidence includes (i) the lack of variability in the K1/2 for cAMP for channels of the α+β phenotype, even for different α:β injection ratios of monomer and dimer RNA (data not shown), (ii) the close similarity of the data for channels from αwtαwt and ββ dimers and α and β monomers (Fig. 2; Table 1), and (iii) the lack of any effect caused by the α-subunit of the βα dimer being wild-type or mutant (Fig. 3). Our results also show that dimerization of subunits has no significant effect on α- and β-subunit types and does not force assembly of subunits into an arrangement different from their preferred arrangement. We feel that the close similarity of dose–response data between αwtαwt dimers and αwt monomers (Fig. 1) and between αwtαwt and ββ dimers and α and β monomers (Fig. 2) makes an effect of dimerization, per se, unlikely. We also never saw evidence that the second subunit of a dimer can incorporate into the channel without the first subunit. This conclusion has been reached by others. Liu et al. (17), using tandem dimers of rod and fish olfactory CNG α-subunits, tested for preferential incorporation and found that both subunits of their dimers incorporated into channel tetramers. McCormack et al. (29), using Shaker-type K+ channels, found preferential incorporation of the leading subunit. Thus, no study using tandem dimers (including this work) reports preferential incorporation of the second subunit of a dimer in the channel.
Recently, it has been suggested that CNG channels gate not as a concerted opening transition involving all of the subunits, but rather that the four subunits in the tetramer may associate and activate as two independent dimers (32). For the case of α channels the difference in channel behavior predicted by a strictly concerted gating scheme and the coupled dimer scheme of Liu et al. (32) may be relatively subtle. However, for the general case of α+β heteromultimers, channel gating as two independent dimers may explain our finding that heteromultimers are composed of adjacent pairs of like subunits. That is, if α+β channels do indeed open via this mechanism, it may be that only adjacent, like subunits can undergo the opening conformational change as a coupled dimer, but not αβ or βα dimers. Thus, a tetramer with an alternating α-β-α-β arrangement would not yield functional CNG channels.
The question of what mechanism constrains subunit assembly in CNG channels is an interesting, and open, one. In voltage-gated K+ channels, hydrophilic amino-terminal domains guide assembly of subunits within subfamilies, and restrict cross-subfamily assembly (33). However, biochemical experiments on CNG channels similar to those in K+ channels fail to show amino-terminal–amino-terminal interactions of this kind (15, 34). Recently, it has been shown that the native olfactory channel may contain an alternatively spiced variant of the rod β-subunit, in addition to the olfactory β-subunit (35), and that olfactory heteromultimers containing the rod β-subunit behave remarkably similar to channels containing only olfactory α and β-subunits (35, 36). Indeed, the apparent lack of a subunit assembly-guiding domain on CNG channels may explain why many different types of CNG channel subunits can coassemble to form functional channels (36). Our experiments indicate that heteromeric α+β channels preferentially assemble in an α-α-β-β arrangement. The structural mechanism determining this preferential arrangement remains unknown.
Acknowledgments
We thank G. Eaholtz, A. Fodor, K. Matulef, L. Sunderman, and M. Varnum for comments on the manuscript, K. Black and H. Utsugi for technical assistance, and LeGay Sheridan for clerical assistance. This work was supported by the Human Frontiers Science Program and by Grant 5R01-EY10329-04 from the National Institutes of Health. W.N.Z. is an assistant investigator for the Howard Hughes Medical Institute.
ABBREVIATION
- CNG
cyclic nucleotide-gated
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Fesenko E E, Kolesnikov S S, Lyubarsky A L. Nature (London) 1985;313:310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Gold G H. Nature (London) 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 3.Yau K W, Baylor D A. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 4.Zufall F, Firestein S, Shepherd G M. Annu Rev Biophys Biomol Struct. 1994;23:577–607. doi: 10.1146/annurev.bb.23.060194.003045. [DOI] [PubMed] [Google Scholar]
- 5.Kaupp U B, Niidome T, Tanabe T, Terada S, Bonigk W, Stuhmer W, Cook N J, Kangawa K, Matsuo H, Hirose, et al. Nature (London) 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- 6.Dhallan R S, Yau K W, Schrader K A, Reed R R. Nature (London) 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig J, Margalit T, Eismann E, Lancet D, Kaupp U B. FEBS Lett. 1990;270:24–29. doi: 10.1016/0014-5793(90)81226-e. [DOI] [PubMed] [Google Scholar]
- 8.McCoy D E, Guggino S E, Stanton B A. Kidney Int. 1995;48:1125–1133. doi: 10.1038/ki.1995.396. [DOI] [PubMed] [Google Scholar]
- 9.Biel M, Zong X, Distler M, Bosse E, Klugbauer N, Murakami M, Flockerzi V, Hofmann F. Proc Natl Acad Sci USA. 1994;91:3505–3509. doi: 10.1073/pnas.91.9.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyand I, Godde M, Frings S, Weiner J, Muller F, Altenhofen W, Hatt H, Kaupp U B. Nature (London) 1994;368:859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- 11.Zufall F, Shepherd G M, Barnstable C J. Curr Opin Neurobiol. 1997;7:404–412. doi: 10.1016/s0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]
- 12.Rieke F, Schwartz E A. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 13.Anholt R R. Crit Rev Neurobiol. 1993;7:1–22. [PubMed] [Google Scholar]
- 14.Parent A, Schrader K, Munger S D, Reed R R, Linden D J, Ronnett G V. J Neurophysiol. 1998;79:3295–3300. doi: 10.1152/jn.1998.79.6.3295. [DOI] [PubMed] [Google Scholar]
- 15.Varnum M D, Zagotta W N. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Chen T Y, Ahamed B, Li J, Yau K W. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 17.Liu D T, Tibbs G R, Siegelbaum S A. Neuron. 1996;16:983–990. doi: 10.1016/s0896-6273(00)80121-x. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S E, Zagotta W N. Proc Natl Acad Sci USA. 1995;92:10222–10226. doi: 10.1073/pnas.92.22.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varnum M D, Zagotta W N. Biophys J. 1996;70:2667–2679. doi: 10.1016/S0006-3495(96)79836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley J, Li J, Davidson N, Lester H A, Zinn K. Proc Natl Acad Sci USA. 1994;91:8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T Y, Peng Y W, Dhallan R S, Ahamed B, Reed R R, Yau K W. Nature (London) 1993;362:764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- 22.Liman E R, Buck L B. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 23.Berghard A, Buck L B, Liman E R. Neuron. 1997;18:951–958. [Google Scholar]
- 24.Liman E R, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 25.Gordon S E, Zagotta W N. Neuron. 1995;14:857–864. doi: 10.1016/0896-6273(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S E, Zagotta W N. Neuron. 1995;14:177–183. doi: 10.1016/0896-6273(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 27.Zagotta W N, Siegelbaum S A. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 28.Fodor A A, Black K D, Zagotta W N. J Gen Physiol. 1997;110:591–600. doi: 10.1085/jgp.110.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormack K, Lin L, Iverson L, Tanouye M, Sigworth F. Biophys J. 1992;63:1406–1411. doi: 10.1016/S0006-3495(92)81703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Jan Y N, Jan L Y. Neuron. 1995;15:1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 31.Broillet M C, Firestein S. Neuron. 1997;18:951–958. doi: 10.1016/s0896-6273(00)80334-7. [DOI] [PubMed] [Google Scholar]
- 32.Liu D T, Tibbs G R, Paoletti P, Siegelbaum S A. Neuron. 1998;21:235–248. doi: 10.1016/s0896-6273(00)80530-9. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Jan Y N, Jan L Y. Science. 1992;257:1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S E, Varnum M D, Zagotta W N. Neuron. 1997;19:431–441. doi: 10.1016/s0896-6273(00)80951-4. [DOI] [PubMed] [Google Scholar]
- 35.Sautter A, Zong X, Hofmann F, Biel M. Proc Natl Acad Sci USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn J T, Krautwurst D, Schroeder J E, Chen T-Y, Reed R R, Yau K-W. Biophys J. 1998;74:1333–1345. doi: 10.1016/S0006-3495(98)77846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]