Abstract
Although the collecting duct is regarded as the primary site at which mineralocorticoids regulate renal sodium transport in the kidney, recent evidence points to the distal convoluted tubule as a possible site of mineralocorticoid action. To investigate whether mineralocorticoids regulate the expression of the thiazide-sensitive Na–Cl cotransporter (TSC), the chief apical sodium entry pathway of distal convoluted tubule cells, we prepared an affinity-purified, peptide-directed antibody to TSC. On immunoblots, the antibody recognized a prominent 165-kDa band in membrane fractions from the renal cortex but not from the renal medulla. Immunofluorescence immunocytochemistry showed TSC labeling only in distal convoluted tubule cells. Semiquantitative immunoblotting studies demonstrated a large increase in TSC expression in the renal cortex of rats on a low-NaCl diet (207 ± 21% of control diet). Immunofluorescence localization in tissue sections confirmed the strong increase in TSC expression. Treatment of rats for 10 days with a continuous subcutaneous infusion of aldosterone also increased TSC expression (380 ± 58% of controls). Furthermore, 7-day treatment of rats with an orally administered mineralocorticoid, fludrocortisone, increased TSC expression (656 ± 114% of controls). We conclude that the distal convoluted tubule is an important site of action of the mineralocorticoid aldosterone, which strongly up-regulates the expression of TSC.
Keywords: fludrocortisone/sodium chloride transport/distal convoluted tubule/collecting duct
The mineralocorticoid hormone aldosterone regulates urinary sodium excretion by increasing the rate of renal epithelial sodium absorption (1). It is widely held that the major site of aldosterone action in the mammalian kidney is the cortical collecting duct, where aldosterone regulates apical sodium entry via the amiloride-sensitive epithelial sodium channel (1–3). However, the results of recent renal micropuncture studies (4) and studies of [3H]metolazone binding in renal cortical membranes (4, 5) suggest that the distal convoluted tubule is an additional renal tubule site of mineralocorticoid action. Apical sodium entry into the distal convoluted tubule is mediated by a thiazide-sensitive Na–Cl cotransporter (TSC) (6). Thus, it is likely that putative actions of aldosterone to regulate sodium transport in the distal convoluted tubule would result from regulation of the thiazide-sensitive Na–Cl entry pathway.
Investigation of the function and regulation of the TSC has been facilitated by the recent cloning of cDNAs for the cotransporter from flounder bladder (7) and rat kidney (8), which has led to the development of molecular tools for localization of TSC expression. Based on experiments using in situ hybridization (9) and reverse transcription–PCR (10), it was concluded that TSC mRNA is found virtually exclusively in the distal convoluted tubule in the rat kidney. Immunohistochemical studies using fusion protein-derived antibodies to TSC also have demonstrated that expression of the TSC protein in the rat kidney is limited to the distal convoluted tubule cells (11).
We hypothesize here that aldosterone may act on the distal convoluted tubule to increase the expression of the TSC of the distal convoluted tubule. To address this hypothesis, we have developed a peptide-derived polyclonal antibody to TSC. Using this antibody, we have carried out immunoblotting and immunofluorescence experiments demonstrating that increases in circulating levels of mineralocorticoids, whether achieved by dietary NaCl restriction or mineralocorticoid administration, result in a marked increase in TSC protein expression in the distal convoluted tubule. Thus, the TSC of the renal distal convoluted tubule appears to be an important target for aldosterone-mediated regulation of renal sodium chloride excretion.
METHODS
Polyclonal Antibodies.
A 24-aa synthetic peptide corresponding to amino acids 104–126 of the amino-terminal tail of the rat TSC (with an added amino-terminal cysteine) was produced by standard solid-phase peptide synthesis techniques (sequence: NH2-DGRPGHELTDGLVEDETGANSEKC-COOH). Analysis using the blast computer program showed no significant overlap of the immunizing peptide with any known eukaryotic protein, including related cotransporters expressed in kidney and other epithelial tissues. The peptide was purified by HPLC and was conjugated to maleimide-activated keyhole limpet hemocyanin via covalent linkage to the amino-terminal cysteine. Two rabbits were immunized with this conjugate by using a combination of Freund’s complete and incomplete adjuvants. The rabbits developed ELISA titers >1:32,000 prior to exsanguination. One of these antisera (L573) was used for the present studies after affinity purification on a column made with the same synthetic peptide used for immunizations (SulfoLink Antibody Immobilization kit, Pierce). Initial characterization of the antibody was achieved by using membrane fractions obtained by differential centrifugation carried out as described (12). A previously characterized rabbit polyclonal antibody to the Na–K–2Cl cotransporter of the thick ascending limb (13) was used for control immunoblots. In addition, a rabbit polyclonal anti-TSC antibody (14) raised to a bacterial fusion protein corresponding to a portion of the amino-terminal tail of rat TSC (kindly provided by D. H. Ellison, University of Colorado) was used to confirm the key findings made with our anti-TSC antibody.
For immunocytochemistry, the present studies also utilized monospecific affinity-purified antibodies to the bumetantide-sensitive Na+–K+–2Cl− cotransporter of the thick ascending limb and to the Na+–Ca2+ exchanger, a connecting-tubule marker. The Na+–K+–2Cl− cotransporter antibody is a chicken polyclonal antibody (LC20) raised to a synthetic peptide corresponding to amino acids 33–55 of the rat cotransporter, based on the sequence published by Gamba et al. (7). This antibody gave complete overlap of labeling with our previously characterized rabbit polyclonal antibody to the Na+–K+–2Cl− cotransporter (13) in double-labeling experiments in rat (data not shown). The antibody to the Na+–Ca2+ exchanger is mouse mAb (Affinity BioReagents, Golden, CO).
Animals and Experimental Protocols.
Pathogen-free male Sprague–Dawley rats (Taconic Farms) weighing 180–220 g were used in this study.
Dietary NaCl restriction study.
Dietary sodium restriction for 10 days was used to produce a physiological increase in circulating aldosterone level. All rats were maintained on a gelled diet, based on an approach originally described by Bouby et al. (15). The gelled diet contained all nutrients and all water provided to the rats each day plus a variable amount of NaCl. The base diet was a commercially available synthetic rat chow containing no added NaCl (Formula 53140000; Ziegler Brothers, Gardner, PA) to which was added agar (0.5%) and deionized water (25 ml/15 g of rat chow) for gelation. Prior to formation of the gel by addition of the water, 2 mmol of NaCl was added per 15 g of rat chow for control animals, and no NaCl was added for low-NaCl animals. All animals received the equivalent of 15 g/200 g BW per day synthetic chow and 25 ml/200 g BW per day water. The low-NaCl rats ingested 0.2 meq/200 g BW per day sodium (derived from Na present in the base diet), and the control rats took in 2.2 meq/200 g BW per day sodium. The rats were fed once daily for 10 days and ate the entire portion with little or no loss owing to the gelled state of the food. None of the rats showed any loss of body weight during the experiment. On the final day, a 24-hr urine collection was made for determination of the rates of excretion of sodium, chloride, and potassium (Monarch 2000; Instrumentation Laboratory, Lexington, MA). After 10 days of treatment, the rats were killed for semiquantitative immunoblotting as described below. Serum samples were collected at the time each rat was killed for determination of the serum aldosterone concentration by radioimmunoassay (Coat-A-Count; Diagnostic Products, Los Angeles).
Aldosterone infusion study.
Rats were anesthetized with methoxyflurane (metofane; Pitman–Moore, Mundelein, IL) and osmotic minipumps (model 2ML2; Alzet, Palo Alto, CA) were implanted subcutaneously to deliver 200 μg of aldosterone (Sigma) per day. This delivery rate for aldosterone was chosen to achieve plasma aldosterone levels of about 1000 ng/dl, based on published observations (16). Aldosterone was initially dissolved in dimethyl sulfoxide and diluted by isotonic saline before installation. Control rats were implanted with minipumps containing vehicle alone. Each rat was fed a gelled diet (prepared as described above) providing a daily, fixed amount of NaCl (58 mg/200 g BW), deionized water (25 ml/200 g BW), and the base rat chow (15 g/200 g BW) throughout the study period. The amount of Na in this diet was 1.0 meq/200 g BW per day. After 10 days of treatment, the rats were killed for semiquantitative immunoblotting as described below.
Fludrocortisone administration study.
Control and treated rats were fed once daily with a gelled diet containing a daily, fixed amount of deionized water (25 ml/200 g BW), NaCl (125 mg/200 g BW), the base rat chow (15 g/200 g BW), and 0.5% agar. For the treated rats, fludrocortisone (Sigma) was dissolved in water at a concentration of 50 mg/liter, and was used in making the gelled diet. The dose of fludrocortisone corresponded to 1.2 mg/200 g BW per day. After 7 days of treatment, the rats were killed for semiquantitative immunoblotting as described below.
Immunoblotting.
Semiquantitative immunoblotting was carried out as described (17) to assess relative expression levels of TSC using whole homogenates from whole kidneys of the rats prepared as described above under Animals and Experimental Protocols. In brief, whole kidneys were homogenized by using a tissue homogenizer (Omni 1000 fitted with a micro-sawtooth generator) in ice-cold isolation solution (pH 7.6) containing 250 mM sucrose, 10 mM triethanolamine (Calbiochem), 1 μg/ml leupeptin (Bachem), and 0.1 mg/ml phenylmethylsulfonyl fluoride (United States Biochemical), and total protein concentration (bicinchoninic acid (BCA) kit; Pierce) was adjusted to 1.2 μg/μl with isolation solution. The samples were solubilized in 5× Laemmli sample buffer (1 vol per 4 vol of sample) followed by heating to 60°C for 15 minutes. Initial Coomassie blue-stained SDS/polyacrylamide gels confirmed that samples were matched with regard to protein content.
For immunoblotting, SDS/PAGE was carried out using minigels of 7.5% polyacrylamide, and proteins were transferred electrophoretically to nitrocellulose membranes as described (13). After blocking with 5 g/dl nonfat dry milk, membranes were probed with the affinity-purified polyclonal antibody to TSC (L573) at an IgG concentration of 0.2 μg/ml in an antibody dilution buffer solution containing 150 mM NaCl, 50 mM sodium phosphate, 10 mg/dl sodium azide, 50 mg/dl Tween 20, and 1 g/dl BSA (pH 7.5). The secondary antibody was donkey anti-rabbit IgG conjugated to horseradish peroxidase (Pierce; catalog no. 31458) used at a concentration of 0.16 μg/ml. Sites of antibody–antigen reaction were visualized by using luminol-based enhanced chemiluminescence (LumiGLO; Kirkegaard and Perry Laboratories) before exposure to x-ray film (Kodak no. 165-1579 scientific imaging film). Controls were carried out as described in Results, using the affinity-purified antiserum preadsorbed with the immunizing peptide.
Relative quantitation of the band densities from immunoblots and preliminary Coomassie-stained gels were carried out by using a laser densitometer (Molecular Dynamics) and imagequant software (Molecular Dynamics). To facilitate comparisons, we normalized the densitometry values, defining the mean for the control group as the 100% value. P < 0.05 was considered statistically significant (unpaired t test or Welch t test).
Immunofluorescence Immunocytochemistry.
Tissue for immunocytochemistry was taken from 140- to 200-g male Sprague–Dawley rats. The localizations obtained were from rat kidneys fixed with 2% paraformaldehyde by perfusion (2 min with PBS, 5 min with paraformaldehyde, 2 min with 10% EDTA in 0.1 M Tris). Fixed kidneys were rinsed in PBS, added to cryoprotectant, and frozen, as described by Ginns et al. (18). Cryostat sections (12–15 μm thick) were made and picked up on coverslips coated with HistoGrip (Zymed). Sections were treated with 6 M guanidine for 10 min to uncover antigenic sites and washed three times with high-salt solution (50 ml of PBS, 0.5 g of BSA, and 1.13 g of NaCl). A blocking agent for nonspecific binding sites (50 ml of PBS, 0.5 g of BSA, and 0.188 g of glycine, pH 7.2) was then added to the sample for 20 min followed by the diluted primary antibody for overnight incubation at 4°C. Antibodies were diluted to 10 μg/ml with incubation medium (50 ml of PBS, 0.05 g of BSA, 200 ml of 5% NaN3). During the second day, these sections were rinsed three times for 5 min, once for 15 min, and once for 30 min with high-salt solution before incubation with the secondary antibody for 2 hr at 4°C. Appropriate species-specific fluorophor-conjugate antibodies prepared specifically for multilabeling (Jackson ImmunoResearch) were diluted 1:100 with incubation medium. These samples were again washed five times with high-salt solution over the course of 1 hr and then in PBS to remove the excess salt before mounting.
RESULTS
Anti-TSC Antibody.
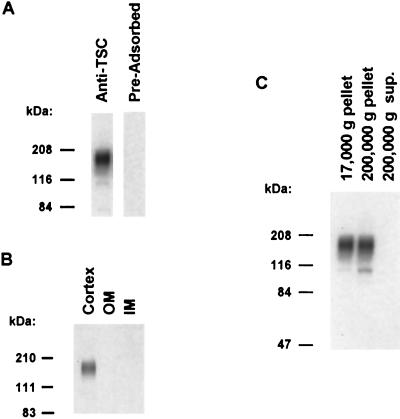
A rabbit polyclonal antibody (L573) was raised to a carrier-conjugated synthetic peptide corresponding to a 23-aa portion of the amino-terminal tail of rat TSC. Fig. 1A shows that this antibody recognizes a broad band centered at 165 kDa on an immunoblot run using a crude membrane fraction of rat renal cortex (Left lane), consistent with that seen in prior studies (11). When an identical blot was probed with the same antibody preadsorbed with the immunizing peptide, the band was ablated, indicating that the recognition of the band by the antibody is via an epitope present in the peptide. Fig. 1B shows an immunoblot run with whole homogenates of rat renal cortex, outer medulla, and inner medulla. The 165-kDa TSC band was seen only in the cortex, in accord with its expected localization only in cortical distal segments of the nephron (11). Fig. 1C shows an immunoblot run with samples from differential centrifugation of a renal cortical homogenate. The 165-kDa TSC band is limited to the membrane fractions, consistent with its identification as an integral membrane protein (7). Note that a second band of approximately 110 kDa is seen in the high-speed membrane fraction. This band most likely represents the nonglycosylated form of the TSC protein.
Figure 1.
Characterization of anti-TSC antibody by immunoblotting. (A) Immunoblot of crude membrane fraction (17,000 × g pellet from supernatant of 4,000 × g centrifugation) of rat renal cortex (10 μg of total protein per lane) probed with either affinity-purified anti-TSCs [Ig G concentration, 0.2 μg/ml, Left lane] or the same antibody preadsorbed with 1 mg of the immunizing peptide (Right lane). (B) Immunoblot showing regional localization of TSC in rat kidney. Each lane was loaded with a crude membrane fraction obtained from cortex, outer medulla (OM), and inner medulla (IM), respectively (10 μg of total protein per lane). (C) Immunoblot showing results of differential centrifugation of cortical homogenate from rat carried out as described (12). First lane, pellet from centrifugation at 17,000 × g for 20 min after initial centrifugation at 1,000 × g for 10 min at 4°C. Second lane, pellet from centrifugation at 200,000 × g for 1 hr. Third lane, supernatant from 200,000 × g centrifugation. Each lane was loaded with 10 μg of total protein. The predominant 165-kDa band is shown in the membrane fractions (pellets from 17,000 × g and 200,000 × g centrifugation), whereas it is absent from the cytosolic fraction (final supernatant).
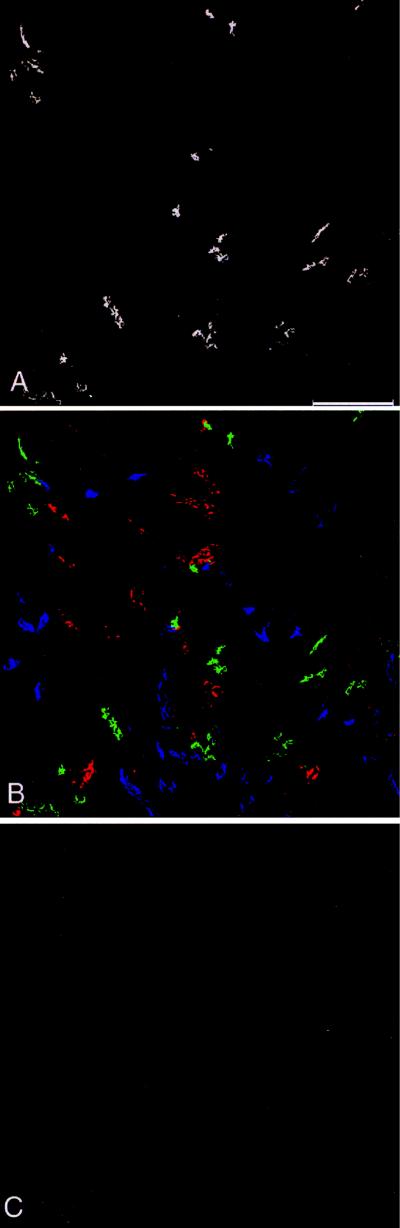
Fig. 2 shows very-low-power survey images of the renal cortex of an untreated control rat prepared to assess the general distribution of anti-TSC labeling. Fig. 2A shows labeling with the anti-TSC antibody alone. Note that the anti-TSC antibody labels short tubule segments in the cortex that are distributed anisotropically, consistent with the prior demonstration that TSC is expressed chiefly, if not exclusively, in the distal convoluted tubule (9–11). Fig. 2B shows same region as Fig. 2A with triple-labeling for TSC, the absorptive Na–K–2Cl transporter of the thick ascending limb, and the Na–Ca exchanger, a connecting-tubule marker. Note that there is little or no overlap of labeling seen for these three antibodies, suggesting that the anti-TSC labeling does not extend to either the thick ascending limb or the connecting tubule. Fig. 2C is a labeling control showing that labeling with the anti-TSC antibody was ablated when the antibody was preadsorbed with an excess of the immunizing peptide.
Figure 2.
Very-low-power immunofluorescence localization of TSC in rat renal cortex. (A) TSC labeling only. (B) Triple-labeling with the anti-TSC rabbit polyclonal antibody (green), a chicken polyclonal antibody to thick ascending limb Na+–K+–2Cl− cotransporter (blue), and a mouse monoclonal antibody to Na+–Ca2+ exchanger, a con necting tubule marker (red). C shows that no labeling was detected when the anti-TSC antibody was preadsorbed with an excess of the immunizing peptide. (Bar, 250 μm.)
Effect of Dietary Salt Restriction on TSC Expression.
Restriction of dietary NaCl intake normally triggers a homeostatic decrease in renal NaCl excretion associated with a rise in circulating aldosterone levels. To assess the effect of dietary NaCl restriction on TSC expression in the kidney, we ran semiquantitative immunoblots using whole-kidney homogenates from six control rats receiving 2.2 meq/200 g BW per day sodium and from six low-NaCl rats receiving 0.2 meq/200 g BW per day sodium for 10 days. The rates of urinary sodium and chloride excretion were markedly lower in rats fed the low-NaCl diet, as expected. [Sodium excretion rates: low-NaCl diet, 42 ± 4 μeq/day; control diet, 1400 ± 120 μeq/day (P < 0.001). Chloride excretion rates: low-NaCl diet, 96 ± 8 μeq/day; control diet, 1390 ± 130 μeq/day (P < 0.001).] However, urinary potassium excretion was not significantly different between the two groups (low-NaCl diet, 800 ± 30 μeq/day; control diet, 710 ± 60 μeq/day). Serum aldosterone levels were markedly elevated in the rats fed the low-NaCl diet [low-NaCl diet, 3,500 ± 1,300 pM; control diet, 780 ± 320 pM (P < 0.05)].
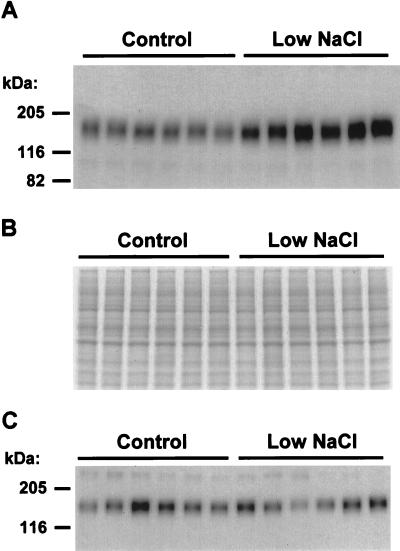
Fig. 3A shows an immunoblot comparing the relative expression levels of TSC in kidneys of control rats (Left six lanes) and rats fed the low-NaCl diet (Right six lanes). Each lane was loaded with an equal quantity of total protein from a whole-kidney homogenate from a different rat. The abundance of TSC protein in kidneys of rats of the low-NaCl diet was markedly increased relative to that of kidneys from control rats [normalized band densities: low-NaCl diet, 207 ± 21%; control diet, 100 ± 5% (P < 0.005)]. Fig. 3B shows a loading control for the immunoblot achieved by running an identically loaded polyacrylamide gel and staining it with Coomassie blue. Inspection of Fig. 3B reveals that there is little or no variation in the densities of the major bands stained with Coomassie blue, demonstrating that the marked increase in TSC band density seen in Fig. 3A was not due to unequal loading. Fig. 3C shows an immunoblot using the same samples but probed with a polyclonal antibody to the Na–K–2Cl cotransporter of the thick ascending limb. In contrast to TSC, the expression level of the Na–K–2Cl cotransporter was not systematically altered by dietary NaCl restriction.
Figure 3.
Effect of dietary NaCl restriction on TSC expression in rat kidney. (A) Immunoblot of whole-kidney homogenates from Sprague–Dawley rats ingesting dietary Na of 2.2 meq/200 g BW per day (control) or 0.2 meq/200 g BW per day (low NaCl). Each lane was loaded with a protein sample from a different rat. The blot was loaded with 10 μg of total protein per lane. (B) Coomassie blue-stained SDS/12% polyacrylamide gel using the same samples as used in the immunoblot in A. This loading gel established that the accompanying immunoblot (loaded identically, Fig. 3A) was uniformly loaded. (C) Control immunoblot using the same samples but probed with a rabbit polyclonal antibody to Na-K-2Cl cotransporter protein (13). The blot was loaded with 7 μg of total protein per lane.
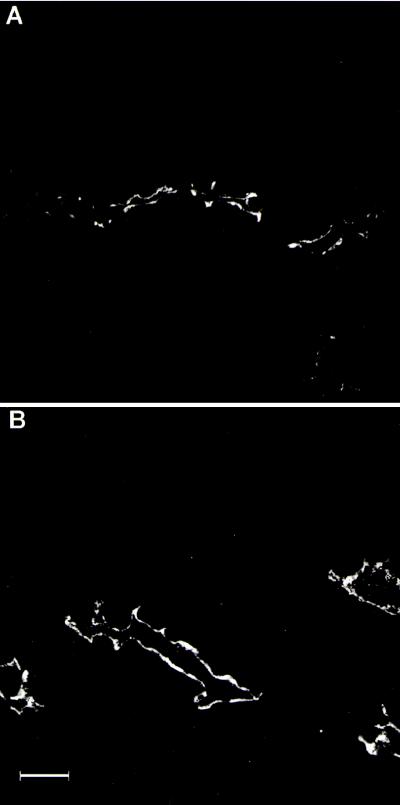
Fig. 4 shows immunofluorescence labeling of representative distal convoluted tubules in cortical sections from rats fed the control diet (Fig. 4A) and the low-NaCl diet (Fig. 4B). The labeling is predominantly apical under both conditions. Thus, the increased cortical expression seen in immunoblots is not associated with a major redistribution of TSC within distal convoluted cells.
Figure 4.
Immunofluorescence localization of anti-TSC antibody in cortical sections from rats fed control (A) and low-NaCl (B) diets. An increase in TSC labeling of distal convoluted tubules from rats on the low-NaCl diet is detected compared with rats fed the control diet. Labeling conditions and confocal microscope settings are identical for A and B. (Bar, 25 μm.)
Effect of Administration of Exogenous Mineralocorticoids on TSC Expression.
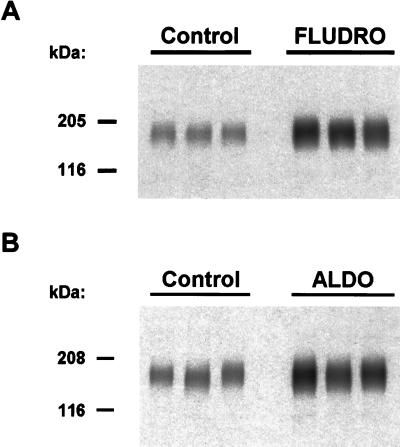
To provide a direct test of the role of mineralocorticoids in the regulation of TSC expression, two experiments were done testing separately the effects of long-term administration of the mineralocorticoids fludrocortisone and aldosterone. In each experiment, dietary intake of NaCl, water, and nutrients was identical in control and mineralocorticoid groups (see Methods). Fig. 5A is an immunoblot showing the effect of 7-day oral administration of fludrocortisone (see Methods) on TSC expression in whole-kidney homogenates. Loading controls were carried out as for the dietary-NaCl-restriction experiment (data not shown). As can be appreciated from Fig. 5A, long-term administration of fludrocortisone (1.2 mg/day in diet for 7 days) resulted in a marked increase in TSC abundance [normalized band densities: fludrocortisone, 656 ± 114%; control, 100 ± 1% (P < 0.05)]. Fig. 5B shows an immunoblot illustrating the effect of a 10-day subcutaneous infusion of aldosterone (200 μg/day for 10 days) via osmotic minipump on TSC expression in whole-kidney homogenates. As seen with fludrocortisone, aldosterone markedly increased renal TSC expression [normalized band densities: aldosterone, 380 ± 58%; control, 100 ± 8% (P < 0.05)]. Thus, both mineralocorticoids markedly increased TSC expression.
Figure 5.
Effect of exogenous mineralocorticoids on TSC expression in rat kidney. Immunoblots were run with 10 μg of protein per lane of whole-kidney homogenates from Sprague–Dawley rats and was probed with anti-TSC. Preliminary SDS/12% polyacrylamide gels were run and were stained with Coomassie blue to confirm equality of loading in each lane (not shown). (A) TSC protein level was significantly increased by 7-day oral administration of fludrocortisone (Fludro) compared with control. (B) TSC protein level was significantly increased by 10-day subcutaneous infusion of aldosterone (ALDO) compared with vehicle-administered control.
DISCUSSION
TSC is expressed in the distal convoluted tubule of the kidney and is responsible for a large fraction of the net sodium and chloride reabsorption that occurs in the distal portion of the mammalian renal tubule (6). Based on the observed effects of thiazides on NaCl excretion and on the salt-wasting phenotype seen in association with mutations of its gene (Gitelman’s syndrome) (19, 20), TSC is believed to be essential for normal NaCl conservation by the kidney. However, the investigation of its regulation has been limited by the fact that it has not been practical to isolate and perfuse distal convoluted tubules in vitro, leaving technically difficult in vivo techniques as the only means of evaluating its function (see Introduction). In general, a more distal renal tubule segment, namely the collecting duct, has been inferred to be the site at which sodium reabsorption is regulated by aldosterone (1–3), and the distal convoluted tubule has not been widely recognized as a major site of aldosterone action. The current study provides evidence, however, that physiologically important regulation by aldosterone occurs in the distal convoluted tubule and that TSC is a target for regulation by aldosterone.
A preliminary step necessary for the present study was the development and characterization of a rabbit polyclonal antibody that recognizes the TSC protein with a high degree of specificity. To achieve this, an HPLC-purified synthetic peptide (linked to an appropriate carrier protein) was used as the immunogen. The immunizing peptide was chosen to maximize specificity of the antibody through use of the blast computer program to compare candidate peptides to all of the proteins in the combined protein databases. The sequence chosen showed no significant overlap with any known eukaryotic protein, including related cotransporters expressed in kidney and other epithelial tissues. A series of preliminary experiments using immunoblotting, differential centrifugation, and immunofluorescence immunolocalization ensured that this antibody indeed specifically recognizes the thiazide-sensitive cotransporter of the renal distal convoluted tubule.
The chief conclusion from the present study is that mineralocorticoids strongly up-regulate the expression of the TSC in the distal convoluted tubule, an effect that is likely to have a major impact on the NaCl-absorption capacity of the distal convoluted tubule. The results of three different experiments support this conclusion. First, dietary NaCl restriction for 10 days in rats increased the serum aldosterone level by 5-fold and concomitantly increased TSC abundance in the kidney by more than 100% of the control value, based on immunoblotting (Fig. 3A). This effect appeared to be selective, because the abundance of the thick ascending limb Na–K–2Cl cotransporter did not increase. Second, aldosterone infusion for 10 days reproduced the increase in TSC expression seen in the dietary NaCl restriction studies (Fig. 5B). Third, oral administration of a synthetic mineralocorticoid, fludrocortisone, for 7 days also resulted in a marked up-regulation of TSC expression (Fig. 5A). Thus, the present studies provide direct evidence that TSC is an important aldosterone-induced protein.
The present studies complement several prior studies that have pointed to the distal convoluted tubule as a potential target site for the action of aldosterone. First, the presence of mineralocorticoid receptors in the distal convoluted tubule has been demonstrated by autoradiography of microdissected renal tubule segments (21, 22) and immunocytochemistry (14, 23, 24). In addition, the expression of mineralocorticoid receptor mRNA in the distal convoluted tubule has also been shown by using in situ hybridization (25). Early micropuncture experiments using the standing-droplet technique demonstrated that adrenalectomy in rats markedly decreases the ability of the distal tubule (including the distal convoluted tubule) to lower the luminal sodium concentration, an effect that is reversed by administration of aldosterone (26). More recent in vivo distal-tubule microperfusion studies in rat kidneys have demonstrated that dietary NaCl restriction (27) and aldosterone administration (4) increase TSC capacity in the distal convoluted tubule. Moreover, studies of [3H]metolazone binding in kidney cortical membranes (an index of TSC expression) have shown that both glucocorticoid and mineralocorticoid hormones increase the number of [3H]metolazone binding sites (4, 5). However, dietary NaCl restriction failed to elicit an increase in [3H]metolazone binding in kidney cortical membranes (28) in contrast to the rise in TSC abundance demonstrated in the present study, suggesting that factors other than the absolute abundance of TSC may influence [3H]metolazone binding capacity.
The present study also has important implications regarding the regulation of chloride excretion. It is widely held that renal sodium reabsorption is directly regulated by aldosterone and that associated changes in chloride excretion occur secondary to primary effects on sodium transport. The present results, coupled with observations cited above, imply that both sodium and chloride excretion can be directly regulated by aldosterone, as postulated by Crabbe (29).
Micropuncture studies of superficial nephron segments of the rat have demonstrated that the distal tubule of the rat is responsible for reabsorption of 6–8% of the filtered load of sodium, whereas the collecting duct system normally reabsorbs 1% or less of the filtered load (30). Although the portion of the distal tubule normally assessed by micropuncture is a combination of the distal convoluted tubule, connecting tubule, and initial collecting tubule (31), most of the observed distal sodium absorption occurs in the first half of the micropuncture-accessible distal tubule (30), i.e., in the distal convoluted tubule per se. Therefore, the distal convoluted tubules probably account for considerably more sodium absorption than occurs in the collecting ducts. Hence, in view of the marked increases in TSC abundance demonstrated here in response to aldosterone admininstration, a major component of the action of aldosterone in decreasing renal sodium excretion is likely to be a consequence of aldosterone’s action on the distal convoluted tubule.
Acknowledgments
The authors thank David H. Ellison for providing his anti-TSC antibody used to confirm key results of this study and W. Saul and J. Carducci for technical assistance. Funding for this study was received from the intramural budget of the National Heart, Lung and Blood Institute (National Institutes of Health Project Z01-HL-01282-KE to M.A.K.) and National Institutes of Health Grant DK27847 to J.B.W. The Confocal Microscope Facility use for the immunolocalizations was funded by National Science Foundation Grant BIR9318061.
ABBREVIATIONS
- TSC
thiazide-sensitive Na–Cl cotransporter
- BW
body weight
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rossier B C, Palmer L G. In: The Kidney: Physiology and Pathophysiology. Seldin D W, Giebisch G, editors. New York: Raven; 1992. pp. 1373–1409. [Google Scholar]
- 2.Gruender S, Rossier B C. Curr Opin Nephrol Hypertension. 1997;6:35–39. doi: 10.1097/00041552-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 4.Velazquez H, Bartiss A, Bernstein P, Ellison D H. Am J Physiol. 1996;270:F211–F219. doi: 10.1152/ajprenal.1996.270.1.F211. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Vaughn D A, Blakely P, Fanestil D D. J Am Soc Nephrol. 1994;5:1361–1368. doi: 10.1681/ASN.V561361. [DOI] [PubMed] [Google Scholar]
- 6.Ellison D H, Velazquez H, Wright F S. Am J Physiol. 1987;253:F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 7.Gamba G, Saltzberg S N, Lombardi M, Miyanoshita A, Lytton J, Hediger M A, Brenner B M, Hebert S C. Proc Natl Acad Sci USA. 1993;90:2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee W-S, Hediger M A, Hebert S C. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 9.Obermuller N, Bernstein P, Velazquez H, Reilly R F, Moser D, Ellison D H, Bachman S. Am J Physiol. 1995;269:F900–F910. doi: 10.1152/ajprenal.1995.269.6.F900. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Huang Y G, Singh I, Schnermann J, Briggs J P. Am J Physiol. 1996;271:F931–F939. doi: 10.1152/ajprenal.1996.271.4.F931. [DOI] [PubMed] [Google Scholar]
- 11.Plotkin M D, Kaplan M R, Verlander J W, Lee W-S, Brown D, Poch E, Gullans S R, Hebert S C. Kidney Int. 1996;50:174–183. doi: 10.1038/ki.1996.300. [DOI] [PubMed] [Google Scholar]
- 12.Ecelbarger C A, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper M A. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 13.Ecelbarger C A, Terris J, Hoyer J R, Nielsen S, Wade J B, Knepper M A. Am J Physiol. 1996;271:F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 14.Bostonjoglo M, Reeves W B, Reilly R F, Velazquez H, Robertson N, Litwack G, Morsing P, Doerup J, Bachman S, Ellison D H. J Am Soc Nephrol. 1998;9:1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- 15.Bouby N, Bachmann S, Bichet D, Bankir L. Am J Physiol. 1990;258:F973–F979. doi: 10.1152/ajprenal.1990.258.4.F973. [DOI] [PubMed] [Google Scholar]
- 16.Will P C, Cortright R N, DeLisle R C, Douglas J G, Hopfer U. Am J Physiol. 1985;248:G124–G132. doi: 10.1152/ajpgi.1985.248.1.G124. [DOI] [PubMed] [Google Scholar]
- 17.Ecelbarger C A, Nielsen S, Olson B R, Murase T, Baker E A, Knepper M A, Verbalis J G. J Clin Invest. 1997;99:1852–1863. doi: 10.1172/JCI119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginns S M, Knepper M A, Ecelbarger C A, Terris J, He X, Coleman R A, Wade J B. J Am Soc Nephrology. 1996;7:2533–2542. doi: 10.1681/ASN.V7122533. [DOI] [PubMed] [Google Scholar]
- 19.Mount D B, Hoover R S, Hebert S C. J Membr Biol. 1997;158:177–186. doi: 10.1007/s002329900255. [DOI] [PubMed] [Google Scholar]
- 20.Simon D B, Lifton R P. Curr Opin Nephrol Hypertension. 1998;7:43–47. doi: 10.1097/00041552-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Vandewalle A, Farman N, Bencsath P, Bonvalet J P. Am J Physiol. 1981;240:F172–F179. doi: 10.1152/ajprenal.1981.240.3.F172. [DOI] [PubMed] [Google Scholar]
- 22.Farman N, Bonvalet J P. Am J Physiol. 1983;245:F606–F614. doi: 10.1152/ajprenal.1983.245.5.F606. [DOI] [PubMed] [Google Scholar]
- 23.Krozowski Z S, Rundle S E, Wallace C, Castell M J, Shen J H, Dowling J, Funder J W, Smith A I. Endocrinology. 1989;125:192–198. doi: 10.1210/endo-125-1-192. [DOI] [PubMed] [Google Scholar]
- 24.Lombes M, Farman N, Oblin M E, Baulieu E E, Bonvalet J P, Erlanger B F, Gasc J M. Proc Natl Acad Sci USA. 1990;87:1086–1088. doi: 10.1073/pnas.87.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escoubet B, Coureau C, Blot-Chabaud M, Bonvalet J P, Farman N. Am J Physiol. 1996;270:C1343–C1353. doi: 10.1152/ajpcell.1996.270.5.C1343. [DOI] [PubMed] [Google Scholar]
- 26.Hierholzer K, Wiederholt M, Holzgreve H, Giebisch G, Klose R M, Windhager E E. Pflügers Archiv. 1965;285:193–210. [Google Scholar]
- 27.Ellison D H, Velazquez H, Wright F S. J Clin Invest. 1989;83:113–126. doi: 10.1172/JCI113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z F, Vaughn D A, Beaumont K, Fanestil D D. J Am Soc Nephrol. 1990;1:91–98. doi: 10.1681/ASN.V1191. [DOI] [PubMed] [Google Scholar]
- 29.Crabbe J. Mol Cell Endocrinol. 1992;90:C11–C13. doi: 10.1016/0303-7207(92)90093-l. [DOI] [PubMed] [Google Scholar]
- 30.Giebisch G, Windhager E E. Am J Med. 1964;36:643–669. doi: 10.1016/0002-9343(64)90178-0. [DOI] [PubMed] [Google Scholar]
- 31.Good D W, Wright F S. Am J Physiol. 1979;236:F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]