Abstract
We report here that the rat heart is a site of oxytocin (OT) synthesis and release. Oxytocin was detected in all four chambers of the heart. The highest OT concentration was in the right atrium (2128 ± 114 pg/mg protein), which was 19-fold higher than in rat uterus but 3.3-fold lower than in the hypothalamus. OT concentrations were significantly greater in the right and left atria than in the corresponding ventricles. Furthermore, OT was released into the effluent of isolated, perfused rat heart (34.5 ± 4.7 pg/min) and into the medium of cultured atrial myocytes. Reverse-phase HPLC purification of the heart extracts and heart perfusates revealed a main peak identical with the retention time of synthetic OT. Southern blots of reverse transcription–PCR products from rat heart revealed gene expression of specific OT mRNA. OT immunostaining likewise was found in atrial myocytes and fibroblasts, and the intensity of positive stains from OT receptors paralleled the atrial natriuretic peptide stores. Our findings suggest that heart OT is structurally identical, and therefore derived from, the same gene as the OT that is primarily found in the hypothalamus. Thus, the heart synthesizes and processes a biologically active form of OT. The presence of OT and OT receptor in all of the heart’s chambers suggests an autocrine and/or paracrine role for the peptide. Our finding of abundant OT receptor in atrial myocytes supports our hypothesis that OT, directly and/or via atrial natriuretic peptide release, can regulate the force of cardiac contraction.
Vasopressin and oxytocin (OT) are synthesized predominantly in the magnocellular neurons of the supraoptic nucleus and paraventricular nucleus as well as in the parvocellular neurons within the paraventricular nucleus as parts of larger precursor molecules (1). The precursors are modified posttranslationally and are transported to the posterior pituitary, where the final bioactive peptide products are stored until they are released into the blood stream. Despite being the first peptide hormone to be characterized and synthesized, the effects of oxytocin long were considered to be restricted to stimulation of uterine contractions during labor and milk ejection during lactation. However, OT is found in equivalent concentrations in the neurohypophysis and plasma of both sexes, which suggests that it also may have other physiological roles (2). Moreover, in the central nervous system, OT-containing axons terminate in several brain stem nuclei known to be involved in cardiovascular control, suggesting a potential role for OT in central cardiovascular regulation (3, 4). Indeed, decreased blood pressure may be observed in response to oxytocin given intracerebroventricularly (5), and the inhibition of brain OT synthesis by an antisense oligonucleotide increased blood pressure in rats (6). In primates or humans, the administration of oxytocin often is associated with a decrease in blood pressure (7, 8). Peripherally injected OT decreases mean arterial pressure in rats by unknown mechanisms (5, 9). Petty et al. (9) reported that i.v. injection of OT caused biphasic dose-dependent changes in mean arterial pressure consisting of an initial pressor effect, accompanied by bradycardia and a decrease in cardiac output, followed by a prolonged fall in mean arterial pressure, which reached a maximum after 30 min and was accompanied by an increase in cardiac output.
It recently has been reported that OT may play a role in blood volume regulation via its natriuretic properties and in the modulation of blood pressure by stimulating the release of atrial natriuretic peptide (ANP) (10–12). Blood volume expansion caused a concomitant release of both OT and ANP, which is believed to be important in induction of the subsequent natriuresis and diuresis and in the reduction of blood volume (12). Furthermore, elevated plasma OT concentrations are correlated with increased sodium excretion (13). In genetically AVP-deficient Brattleboro rats, dehydration natriuresis is OT related (14). This natriuretic effect of OT may be mediated via ANP because we have shown that OT increases urinary excretion of cGMP and that this effect was abolished by ANP-specific antibodies (data not shown).
In previous experiments, the reduction in effective circulating blood volume that followed volume expansion occurred too rapidly to result from natriuresis (12). This finding implied that ANP might have physiologically significant negative chronotropic and inotropic effects to reduce cardiac output that would further reduce circulating blood volume. Indeed, in further experiments, oxytocin reduced the heart rate and the force of atrial contractions in isolated atria from perfused hearts in the absence of a central control mechanism (10, 11). Moreover, the addition of oxytocin (10−6 M) significantly stimulated ANP release, and an oxytocin receptor antagonist (10−7 and 10−6 M) caused dose-related inhibition of oxytocin-induced ANP release and, in the last few minutes of perfusion, decreased ANP release below that of control hearts, suggesting that intracardiac oxytocin stimulates ANP release (11). For this study, the hypothesis that the OT produced and secreted in the heart can stimulate ANP release was tested by determining the presence of OT and the expression of the OT gene in the chambers of the rat heart. Our results indicate that the atria already known to synthesize and store ANP are also the site not only of OT synthesis and release but also of oxytocin receptor (OTR) gene expression.
MATERIALS AND METHODS
Preparation of Heart Homogenates.
Experiments were performed in accordance with the Canadian Guidelines on Animal Care and with the approval of the Bioethics Committee of Centre Hospitalier de l’Université de Montréal. Female Sprague-Dawley rats (225–250 g) were killed by decapitation. The hearts were collected, and the atria and ventricles were dissected rapidly, were frozen in liquid nitrogen, and were kept at −80°C until needed. The frozen tissue then was homogenized in an ice-cold acid solution (1 M HCl/1% formic acid/1% trifluoroacetic acid/1% NaCl). The homogenates were centrifuged at 1,500 × g for 15 min at 4°C, and the supernatants were extracted with heat-activated Vycor glass beads (Corning) or Sep-Pak cartridges (Millipore).
Extraction of Immunoreactive Oxytocin.
Oxytocin was extracted from the heart tissue with heat-activated Vycor glass beads. These were washed extensively with 60% acetone and then were activated for 1 h at 600°C before being stored at 100°C until used. The heart homogenates (300 μl) were mixed with the Vycor glass beads and were suspended in 50 mg/ml H20 (500 μl). The suspension was rotated vertically for 30 min at 4°C, then was centrifuged for 3 min at low speed. The supernatant then was discarded. The glass beads were washed with bidistilled water (1.5 ml), and the adsorbed material was eluted with 60% acetonitrile in 0.05 M HCl. The solvent was evaporated by SpeedVac (Savant) centrifugation.
Extraction by Vycor glass was compared with the extraction by Sep-Pak C-18 cartridges (Millipore). The Sep-Pak cartridges were activated by 100% acetonitrile and washed with 0.2% ammonium acetate (pH 4.0). The homogenates were applied on the column, and, after washing with 0.2% ammonium acetate, the adsorbed material was eluted with 2 ml of 80% acetonitrile in 0.1% BSA. After vacuum centrifugation, the residue was dissolved in radioimmunoassay buffer, and the concentration of OT was quantified in 50- and 100-μl aliquots. The extraction procedure was monitored with 125I-OT. About 2,000 cpm of 125I-OT was added to 300 μl of homogenate and was processed separately. Both extraction procedures gave ≈85% recovery of OT.
Isolated Heart Perfusion.
Heart perfusion was performed as described (11, 15). On the day of the study, the animals were heparinized (1,000 units i.p.) and anesthetized with sodium pentobarbital (40 mg/kg i.p.). The hearts were excised rapidly and immediately were placed in an ice-cold Krebs–Henseleit solution saturated with oxygen. The heart then was mounted rapidly on a perfusion system and was perfused retrogradely via the aorta at 37°C and at a pressure of 80 cmH2O (59 mmHg; 1 mmHg = 133 Pa). The Krebs–Henseleit perfusion solution (in mmol/liter: NaCl 117, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, Na2EDTA 0.5 and dextrose 11) had a pH of 7.4 when gassed with 95% O2 and 5% CO2. The base of the pulmonary artery was incised to allow efficient drainage of the right ventricle. Coronary flow was measured every minute for 10 min by timed collections of the effluent collected in polystyrene tubes containing 150 μl of phenylmethylsulfonyl fluoride, 150 μl of EDTA, and 100 μl of 1% BSA. Samples were stored at −80°C until the OT was assayed by RIA.
Oxytocin Radioimmunoassay.
The radioimmunoassay was developed in our laboratory by using specific antibodies generated against oxytocin (generous gift from Mariana Morris, Wright State University, Dayton, Ohio). The labeling of synthetic OT (Peninsula Laboratories) with 125I-Na by using the lactoperoxidase method was similar to that described for ANP (16). The iodinated tracer was purified by HPLC (Waters) on a C18 Bondapak column. The monoiodinated OT was eluted with 35% acetonitrile (CH3CN) in 0.1% trifluoroacetic acid (TFA). Standard curves generated with 125I-OT demonstrated a sensitivity of 0.1 pg and linearity in the range of 0.25–200 pg/ml. The cross-reactivity of the antibody was <1% with AVP and vasotocin. The radioimmunoassay was performed in RIA buffer (50 mM NaP04, pH 7.5/0.1 M EDTA/0.1% BSA/0.01% sodium azide). Two-hundred microliters of sample or standards (0–200 pg) were incubated with 100 μl of antibody (1:80,000) for 24 h at 4°C. Then, 100 μl of 125I-OT (3,000 cpm) was added to each tube and was incubated for 48 h at 4°C. The antibody-bound radioactivity was separated from free radioactivity by adding 1 ml of dextran-coated charcoal and centrifuging for 45 min at low speed at 4°C. The radioactivity in the supernatant then was measured in a γ counter.
Purification of Heart OT by HPLC.
The dried acetone extracted homogenates were dissolved in an aqueous solution of 20% acetonitrile containing 0.1% of TFA and were applied to a C18 Bondapak column (4.6 × 250 mm) of a reverse-phase HPLC (Waters). The column was eluted with a linear gradient of 20–50% CH3CN/0.1% TFA at a flow rate of 1 ml/min. Thirty fractions (1 ml each) were collected and lyophilized. Direct RIA after reconstitution in RIA buffer determined the presence of OT in the HPLC fractions. The synthetic 9-aa OT, as well as pituitary gland extracts, chromatographed under identical conditions served as standards.
Reverse Transcription–PCR (RT-PCR) Analysis.
Total RNA was extracted from rat heart compartments by the acid guanidinium-thiocyanate-phenol-chloroform method. RQ1 DNase (Promega, Fisher Scientific) was used to digest genomic DNA in the RNA extracts. The integrity of the RNA preparations was verified by gel electrophoresis and ethidium bromide staining of the gels. RNA concentrations were measured by UV spectrophotometry. First-strand cDNA was synthesized in a final volume of 40 μl containing first strand buffer, 2 μg of rat cardiac or uterine RNA, 2 μg of hexanucleotide primer (Pharmacia), and avian myeloblastosis virus reverse transcriptase (12 units/μg RNA; Life Sciences, St. Petersburg, FL). First strand cDNA (10 μl) then was used for PCR amplification with different combinations of OT exon-specific oligonucleotide primers. The specific sequence of these primers has been described by Lefebvre et al. (17, 18). A+ was a sense-strand primer corresponding to a sequence in exon A, starting 3 bp downstream of the initiation codon. B+ and B− primers were sense- and antisense-strand primers located at the 5′ and 3′ ends of exon B, respectively. C− was an antisense primer complementary to a sequence in exon C, terminating the stop codon. Amplification was performed for 36 cycles. Each cycle involved the following: 94°C for 1 min, 59°C for 1 min, and 72°C for 2 min followed by a 5-min final extension at 72°C. Ten microliters of the PCR reactions were electrophoresed on 1.5% agarose gel and were transferred onto Hybond N+ nylon membrane (Amersham) by vacuum transfer in 1.5 M NaCl and 0.5 M NaOH. The probe used for Southern blot analysis of PCR products was the OT cDNA fragment corresponding to the sequences between primers B+ and B− on exon B of the OT gene. The RT-PCR for detection of OTR mRNA was reported previously (11).
Immunolocalization of OT, OTR, and ANP in the Rat Heart.
Animals were killed by decapitation. Hearts were excised immediately and were washed in fresh ice-cold PBS, then were fixed in 4% paraformaldehyde for 18 h at 4°C. The hearts were washed in PBS twice, then were incubated overnight in 0.5 M sucrose in PBS. The tissue was frozen in isopentane prechilled over dry ice and then was stored at −80°C until processed.
The ANP antiserum was produced in New Zealand white rabbits by immunization, with the antigen obtained by coupling 26-aa carboxyterminal ANP (Arg101-Tyr126) with thyroglobulin as described (16). This antibody is specific for C-terminal ANP peptides. The crossreactivity with Ser99-Tyr126 circulating peptide is 100%. The antibody also recognizes the 126-aa prohormone Asn1-Tyr126. The ANP antibody was used in a final dilution of 1:500, and oxytocin and oxytocin receptor antibodies were used in final dilutions of 1:250 in PBS-BSA.
Cryostat whole heart sections (6–7 μm thick) were cut and mounted on poly-l-lysine coated glass slides and were placed overnight in a partial vacuum at 4°C. Slides were stored in boxes with Drierite at −80°C until the immunolocalization procedures were performed as described by Al Kawas et al. (19). In brief, slides were treated with 95% methanol supplemented with 0.3% H2O2 for 30 min at room temperature. The sections then were washed in PBS and were preincubated with 2.5% BSA-PBS for 30 min, and then were incubated with the respective primary antibodies or with nonimmune serum for the control sections. After washing with PBS, the sections were incubated with F(ab′)2 fragments of horseradish peroxidase-conjugated goat anti-rabbit IgG (Calbiochem) at a dilution of 1:100 for 1 h at room temperature. The sections were reacted in 0.05% diaminobenzidine (Sigma) in 0.05 M Tris⋅HCl buffer (pH 7.4) in the presence of H2O2. The sections were counterstained faintly with hematoxylin for light microscopy observation.
Cell Culture.
Atrial myocytes from 4-day-old Sprague–Dawley rats were prepared. The pups were decapitated, and their hearts were removed aseptically. The atria were dissected and washed in Jokklik medium supplemented with 25 units/ml of heparin to remove erythrocytes. Isolated atrial cardiocytes were prepared by repeated digestion with collagenase and mechanical dispersion as described (20). Cells were counted in a hematocymeter and were diluted and seeded in plastic multiwell dishes at a density of 1.25 × 105 cells/cm2. The cells were cultured in DMEM containing 20% fetal calf serum at 37°C for 2 days in atmosphere of 5% CO2. Cells then were cultivated further in complete serum-free medium 1 as described (20). The studies were performed 7 days after isolation, when cells had reached confluence and were beating synchronously. The cells were washed three times and then were incubated for 1 h at 37°C, after which, the medium was collected, and the cells were scrapped and frozen. OT was measured by RIA in the medium and in the cells.
RESULTS
Immunoreactive OT in the Rat Heart.
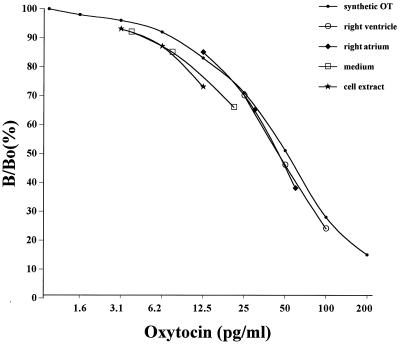
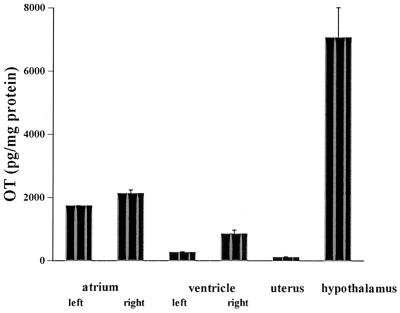
Immunoreactive oxytocin (ir-OT) was detected by RIA in all chambers of the heart. Serial dilutions of rat atrial and ventricular homogenates revealed a good parallelism with the standard curve obtained by using synthetic OT. This parallelism indicates that the peptide present in the homogenates is indistinguishable from synthetic oxytocin and that measurement is not affected by cross-reacting contaminants, as illustrated with homogenates from the left atrium and right ventricle (Fig. 1). Similar parallelism was observed with the other chambers as well as in control homogenates from the neural lobe of the pituitary gland, hypothalamus, and uterus. The highest OT concentration was found in the right atrium (2,128 ± 114 pg/mg protein) (Fig. 2), which was 19-fold higher than in the rat uterus (112 ± 29 pg/mg protein) but 3.3-fold lower than in the hypothalamus (7,061 ± 950 pg/mg protein). Oxytocin levels were significantly higher in the right atria than in the left atria (1,739 ± 16 pg/mg protein) and in the right (853 ± 118 pg/mg protein) and left (267 ± 30 pg/mg protein) ventricles. Perfusion of three isolated hearts with Krebs–Henseleit buffer resulted in nearly constant release of oxytocin, measured every minute over a 10-min experimental period. The OT concentration in the effluent averaged 34.5 ± 4.7 pg/min. This result suggests that OT is not only present in the heart but that it also can be secreted from this organ.
Figure 1.
Oxytocin immunoreactivity of serial dilutions (1:80; 1:40; 1:20) of rat extracts from atrial and ventricular homogenates as well as medium and cell extracts from cultures of atrial myocytes in vitro were measured by radioimmunoassay. Results are compared with the oxytocin standard curve. The ordinate shows the ratio (expressed as the percentage) of bound 125I-labeled oxytocin in the presence (B) and in the absence (Bo) of synthetic oxytocin.
Figure 2.
Oxytocin concentration in rat tissues obtained by RIA after prior extraction by Vycor heat-activated glass beads.
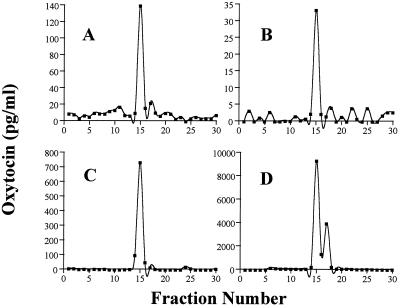
To determine whether the OT detected by RIA in heart extracts represents an intact hormone, we applied a reverse-phase HPLC separation procedure that revealed two distinct immunoreactive peaks. Representative results obtained in the right atrium, heart perfusate, and pituitary extracts and in synthetic OT are shown in Fig. 3. The main peak, separated at 15 min of elution time (35% acetonitrile), had a retention time identical to that of synthetic OT. The second peak that eluted at 17 min was not identified, but it represented <15% of total immunoreactivity found. It is possible that this peak is a degradation product of OT because trace amounts also were found in the oxytocin standard. These data indicate that the OT present in the heart is identical to that found in the pituitary gland and to synthetic OT.
Figure 3.
Reverse-phase HPLC elution profile of oxytocin immunoreactivity in rat tissues. Tissue extracts, lyophilized and reconstituted in 20% acetonitrile in 0.1% TFA, were applied to a C18 Bondapak column and were eluted with acetonitrile gradient (20–50%) in 0.1% TFA. (A) The extract from the right atrium. (B) The extract from the heart perfusates. (C) The HPLC profile of OT synthetic standard. (D) The extract from the pituitary gland.
OT Gene Transcripts in Rat Heart.
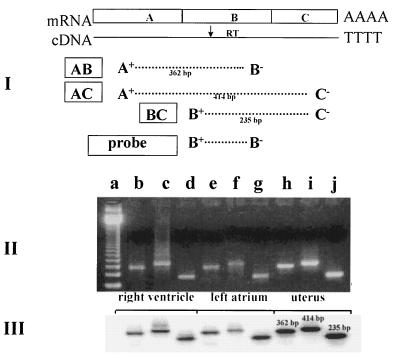
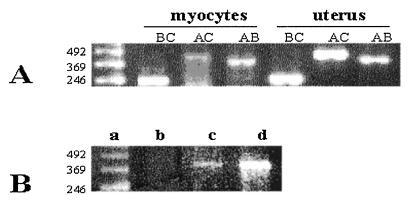
The specific OT transcript was demonstrated by amplification of rat heart cDNA by PCR. For PCR amplification, we applied OT gene-specific primers homologous to sequences on three exons of the OT gene, located at the two ends and in the middle of coding region (Fig. 4). These primers range from the signal sequence in exon A to the stop codon in exon C of the OT gene. This strategy was chosen because it was expected to reveal any structural differences that might exist between the OT coding regions in the heart and in the uterus, known to be identical to those in the hypothalamic OT gene. After PCR amplification of the cDNA and electrophoresis in agarose gel, the single co-migrating bands were obtained from both rat atria and ventricles as well as from the uterus samples. As shown in Fig. 4II, the amplified products generated from the uterus or the different chambers of the heart yielded products identical in size to the various combinations of primers. In addition, the specificity of the PCR products was verified by Southern blotting. All PCR bands hybridized to the probe of the OT gene cDNA (Fig. 4III). These results suggest that the coding regions of the heart OT transcripts are structurally identical to the uterine OT transcript.
Figure 4.
RT and PCR analysis of rat cardiac OT mRNA based on the concept presented by Lefebvre et al. (17). (I) Schematic representation of the approximate positions of the primers used in the RT-PCR amplification and the probe used to determine the specificity of the PCR products. A+, exon A-specific primer sense-strand, primer corresponding to sequences +3 and +26 OT gene; B+, exon B-specific sense-strand primer (+416 to + 434); B−, exon B-specific antisense-strand primer (+559 to +584); C−, exon C-specific antisense-strand primer (+838 to +864). (II) Ethidium bromide-stained agarose gel showing the PCR amplification products obtained with OT exon-specific primers by using reverse-transcribed mRNA from rat right ventricle, left atrium, and uterus, used as control organ. Lanes: a, 123-bp ladder of GIBCO/BRL; b, e, and h, AB OT cDNA products of PCR; c, f, and I, AC OT cDNA products of PCR; d, g, and j, BC OT cDNA products of PCR. (III) Southern blot analysis of PCR amplification products resolved in an agarose gel, transferred to nylon membrane, and hybridized with B+B− OT cDNA probe.
Immunocytochemical Localization of Cardiac OT and OTR.
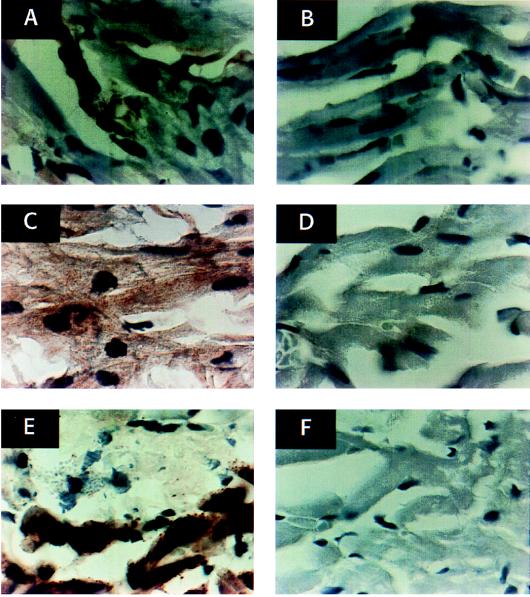
The presence of ir-OT was determined by immunocytochemistry. OT was present in fibroblasts and atrial myocytes (Fig. 5A). The staining of ir-OT in the ventricle (Fig. 5B) was very low to negative and paralleled the low content of OT in this chamber as estimated by RIA. The atrial myocytes also stained positively for OTR (Fig. 5C) in parallel with the ANP stores (Fig. 5E). OTR staining appeared to be more intense in atria (Fig. 5C) than in ventricles (Fig. 5D), consistent with the higher amounts of OTR mRNA in atria than in ventricles (11).
Figure 5.
Immunocytochemical staining of sections of rat atrium (A) and ventricle (B) with rabbit anti-OT antibody at final dilution of 1/250 and atrium (C) and ventricle (D) with rabbit anti-OTR antibody diluted 1/250. The results are related to control sections cut on the border of the atrium (lower part of images) and the ventricle (upper part of images) reacted with anti-ANP rabbit antibody diluted 1/500 (E) and normal rabbit serum (F). The specific immunostaining is visible as a yellow-brownish color after incubation with F(ab′)2 fragments of horseradish peroxidase-conjugated goat anti-rabbit IgG and diaminobenzidine. The sections were counterstained with hematoxylin.
OT and OTR in Cultured Atrial Myocytes.
The immunolocalization of abundant OTR in atrial myocytes from rat atria and the presence of OT in these cells prompted us to investigate whether these cells express OT and OTR mRNA in vitro and whether they synthesize ir-OT. Fig. 1 shows that both medium and atrial myocytes contain OT, which is detected specifically by RIA, and that its serial dilution shows parallelism to the standard curve. The OT content measured in seven independent cell cultures and collected after 1 h of incubation averaged 228 ± 55 pg/ml of medium and 53 ± 3 pg/mg protein in the cells. RT-PCR revealed the presence of both OTR mRNA (Fig. 6A) as well as OT mRNA (Fig. 6B). However the amplifications were decidedly weaker than those observed in the control cDNA sample from the uterus.
Figure 6.
Detection of oxytocin mRNA and oxytocin receptor mRNA in rat atrial myocytes by RT-PCR. (A) Amplification of cDNA with OT-exon-specific primers (as shown in Fig. 4). The photograph presents RT-PCR products after electrophoresis on 2% agarose in ethidium bromide. Lanes: a, 123-bp ladder GIBCO/BRL; b, c, and d, OT-exon-specific PCR product amplified from the cDNA of atrial myocytes; e, f, and g, the corresponding PCR OT gene products amplified from uterine cDNA. (B) OTR RT-PCR products after electrophoresis on 2% agarose. Lanes, a, 123-bp ladder GIBCO/BRL; b, not reverse-transcribed total RNA from atrial myocytes; c, atrial myocytes; d, uterus.
DISCUSSION
We report here that the heart is a site of OT synthesis and release. The presence of OT was detected by radioimmunoassay in all four chambers of the rat heart, and its gene expression was determined by the presence of specific OT mRNA after PCR analyses. Furthermore, we have shown the presence of OT in the effluent of isolated rat heart perfusates and in the medium of cultured atrial myocytes, which confirms that OT is synthesized and released from the heart. In addition, the presence of OT and ANP in atrial myocytes paralleled that of OTR localized by immunocytochemical methods. These data support the hypothesis that the OT gene is transcribed and translated in the rat heart. Our analysis suggests that heart OT is structurally identical to, and therefore derived from the same gene as, the OT mRNA found primarily in the hypothalamus.
Translation of the OT transcript found in heart tissue was ascertained by measuring the OT content in the tissue extracts from RIA, and the specificity of the measured peptide was confirmed by HPLC analysis. The concentration of OT in atria and ventricles was higher than in uterus. This profile may reflect specific posttranscription processing of OT peptide within the heart tissue because the presence of low OT mRNA in the heart and relatively high OT mRNA in the uterus argues against the abundant biosynthesis of cardiac OT. On the other hand, a special regulatory mechanism suppressing the translation of the uterine OT gene, and therefore preventing uterine contraction, could exist in addition to the already known uterine OT regulation on the level of gene expression (17). However, some OT molecules detected by RIA in cardiac tissue may have been receptor bound or else were internalized OT originating from plasma. Present and recent findings (11) that heart contains specific OTR support this point of view.
The results suggest that oxytocin in the heart is rather constitutively secreted than stored because (i) immunocytochemistry did not reveal abundant OT tissue deposits, but faint staining was localized in small groups or single cells randomly distributed throughout the atrium; (ii) OT was present in the heart perfusate and therefore was secreted from the heart; (iii) and the OT concentration was relatively high in the incubation medium of cultured atrial myocytes and low within these cells, which suggests immediate secretion of OT by the cells after its synthesis and posttranslational processing.
The presence of OT and OT mRNA in atrial myocytes and fibroblasts is an unexpected finding; however, Horackova et al. (21) showed that ganglia isolated from the heart and cultured in vitro contain ir-OT as well as other neuropeptides. Our results indicate that synthesis of OT in the heart is not limited to neurons. In addition, in situ hybridization and immunocytochemistry studies revealed that cardiac tissues, including atrial and ventricular cardiomyocytes, contain high levels of the prohormone convertases (22), which participate in processing various neuropeptides including OT (23). Thus, the heart has the potential to process a biologically active form of OT and secrete OT into the circulation as shown in our experiments.
Although the concentration of OT peptide found in the heart chambers is relatively small, it is similar to OT content already found in other peripheral tissues such as thymus (24), amnion, or placenta (18). These low concentrations are compatible with paracrine or autocrine effects of cardiac OT because receptors for the peptide also have been localized in the various chambers of the heart (11) and because we already have observed that OT causes the release of ANP from isolated perfused hearts (11) and atria (10). Furthermore, OT exerted negative chrono and inotropic effects, probably mediated by ANP release. The doses required for these effects were high but might be in the range that would be present at the OTR, particularly in the right atrium. Furthermore, the effects were blocked by an OTR blocker (10, 11).
It is possible that locally released oxytocin may stimulate an ANP release that might play a physiological role after blood volume expansion in reducing the rate and force of cardiac contraction, and thereby cardiac output, resulting in a rapid reduction in the effective circulating blood volume. Blood volume expansion as a result of increased venous return to the heart would stretch the cardiac myocytes activating oxytocin release from them just as can occur for ANP release (25). OT released locally by paracrine or autocrine activation of the OT receptors would release ANP that in turn acts on its receptors to activate guanylyl cyclase and reduce intracellular calcium release (26). The decreased intracellular calcium concentration would produce a negative inotropic and chronotropic response that rapidly would reduce cardiac output and, thereby, affect blood volume.
Oxytocin also was released from the perfused heart, albeit in small quantities that would probably not significantly increase plasma concentrations unless there was a marked increase in release after stretch or other stimuli to the cardiac myocytes. It already has been hypothesized that volume expansion by baroreceptor input to the hypothalamus causes a release of oxytocin, which accounts for the increased circulating oxytocin concentrations found after volume expansion (12), and that this may play a physiological role by acting on the OTR in the heart to cause the release of ANP. If a significant release of cardiac OT occurs, this could further increase circulating OT concentrations, already increased by neurophypophyseal secretion of the peptide to magnify the natriuretic action of OT. Further studies are necessary to determine whether this sequence of events occurs in vivo. The actions of oxytocin that we have described here may account for the abolition by OT of ventricular arrhythmias in vivo (27).
Acknowledgments
We express gratitude to Céline Coderre and Nathalie Charron for their technical assistance. These studies were supported by The Medical Research Council of Canada Grants MT-10337 (to J.G.) and MT-11674 (to J.G. and S.M.D.), a grant from the Heart and Stroke Foundation of Canada (to J.G.), and Institute of Mental Health Grant MH51853 (to S.M.M.).
ABBREVIATIONS
- OT
oxytocin
- ANP
atrial natriuretic peptide
- OTR
OT receptor
- TFA
trifluoroacetic acid
- RT
reverse transcription
- ir-OT
immunoreactive OT
References
- 1.Swanson L W, Kuypers H G J M. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 2.Heller H. In: Occurrence, Storage and Metabolism of Oxytocin. Caldeyro-Barcia R, Heller H, editors. New York: Pergamon; 1961. pp. 3–23. [Google Scholar]
- 3.Chalmers J, Pilowsky P. J Hypertens. 1991;9:675–694. doi: 10.1097/00004872-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jirikowski G F, Back H, Forssmann W G, Stumpf W E. Neuropeptides. 1986;8:243–249. doi: 10.1016/0143-4179(86)90051-x. [DOI] [PubMed] [Google Scholar]
- 5.Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K S. Physiol Behav. 1996;60:1311–1315. doi: 10.1016/s0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- 6.Maier T, Dai W J, Csikos T, Jirikowski G F, Unger T, Colman J. Hypertension. 1998;31:480–486. doi: 10.1161/01.hyp.31.1.480. [DOI] [PubMed] [Google Scholar]
- 7.Hendricks C H, Brenner W E. Am J Obstet Gynecol. 1970;108:751–754. doi: 10.1016/0002-9378(70)90542-9. [DOI] [PubMed] [Google Scholar]
- 8.Sercher N J, Amso P, Wallin L. Acta Obstet Gynecol Scand. 1978;57:97–107. doi: 10.3109/00016347809155884. [DOI] [PubMed] [Google Scholar]
- 9.Petty M A, Lang R E, Unger T, Ganten D. Eur J Pharmacol. 1985;112:203–210. doi: 10.1016/0014-2999(85)90497-2. [DOI] [PubMed] [Google Scholar]
- 10.Favaretto A L V, Ballejo G O, Albuquerque-Araujo W I, Gutkowska J, Antunes-Rodrigues J, McCann S M. Peptides. 1997;18:1377–1381. doi: 10.1016/s0196-9781(97)00209-x. [DOI] [PubMed] [Google Scholar]
- 11.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg H H, McCann S M. Proc Natl Acad Sci USA. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haanwinckel M A, Elias L K, Favaretto A L V, Gutkowska J, McCann S M, Antunes-Rodrigues J. Proc Natl Acad Sci USA. 1995;92:7902–7906. doi: 10.1073/pnas.92.17.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards B R, LaRochelle J F T. Am J Physiol. 1984;247:F453–F465. doi: 10.1152/ajprenal.1984.247.3.F453. [DOI] [PubMed] [Google Scholar]
- 14.Conrad K P, Gellai P M, North W G, Valtin H. Ann N Y Acad Sci. 1993;689:346–362. doi: 10.1111/j.1749-6632.1993.tb55559.x. [DOI] [PubMed] [Google Scholar]
- 15.Lambert C, Mossiat C, Tanniere-Zeller M, Maupoil V, Rochette L. Cardiovasc Res. 1990;24:653–658. doi: 10.1093/cvr/24.8.653. [DOI] [PubMed] [Google Scholar]
- 16.Gutkowska J. Nucl Med Biol. 1987;14:323–333. [Google Scholar]
- 17.Lefebvre D L, Giaid A, Bennett H, Lariviere R, Zingg H H. Science. 1992;256:1553–1555. doi: 10.1126/science.1598587. [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre D L, Lariviere R, Zingg H H. Biol Reprod. 1993;48:632–639. doi: 10.1095/biolreprod48.3.632. [DOI] [PubMed] [Google Scholar]
- 19.Al Kawas S, Amizuka N, Bergeron J J M, Warshawsky H. Calcif Tissue Int. 1996;59:192–199. doi: 10.1007/s002239900108. [DOI] [PubMed] [Google Scholar]
- 20.Oparil S, Wyss J M. News Physiol Sci. 1993;8:223–228. [Google Scholar]
- 21.Horackova M, Croll R P, Hopkins D A, Losier A M, Armour J A. Tissue Cell. 1996;28:411–425. doi: 10.1016/s0040-8166(96)80027-9. [DOI] [PubMed] [Google Scholar]
- 22.Beaubien G, Schafer M K, Weihe E, Dong W, Chretien M, Seidah N G, Day R. Cell Tissue Res. 1995;279:539–549. doi: 10.1007/BF00318166. [DOI] [PubMed] [Google Scholar]
- 23.Dong W, Seidel B, Marcinkiewicz M, Chretien M, Seidah N G, Day R. J Neurosci. 1997;17:563–575. doi: 10.1523/JNEUROSCI.17-02-00563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geenen V, Legros J J, Franchimont P, Baudrichaye M, Defresne M P, Boniver J. Science. 1986;232:508–511. doi: 10.1126/science.3961493. [DOI] [PubMed] [Google Scholar]
- 25.Lang R E, Thölken H, Ganten D, Luft F C, Ruskoaho H, Unger T. Nature (London) 1986;314:264–266. doi: 10.1038/314264a0. [DOI] [PubMed] [Google Scholar]
- 26.Doyle D D, Ambler S K, Upshaw-Earley J, Bastawrous A, Goings G E, Page E. Circ Res. 1997;81:86–91. doi: 10.1161/01.res.81.1.86. [DOI] [PubMed] [Google Scholar]
- 27.Bieniarz J. In: Occurrence, Storage and Metabolism of Oxytocin. Caldeyro-Barcia R, Heller H, editors. New York: Pergamon; 1961. pp. 80–83. [Google Scholar]