Abstract
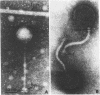
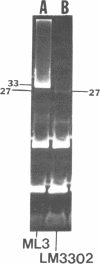
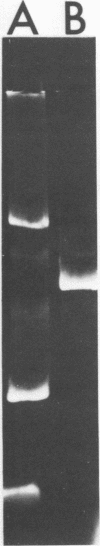
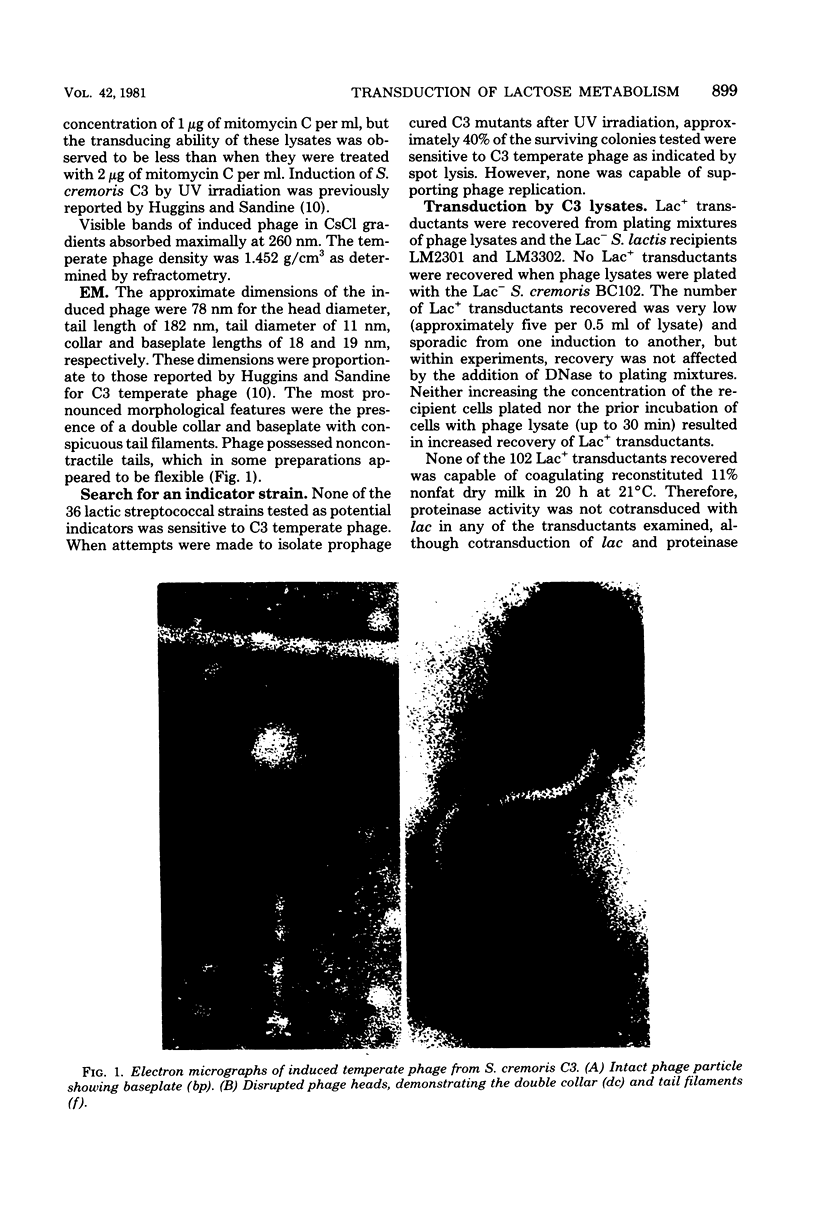
Temperate phage was induced from Streptococcus cremoris C3 and morphologically characterized by high-resolution electron micrographic techniques. Interspecies genetic transfer of lactose-fermenting ability by the temperate phage was demonstrated, using two lactose-negative (Lac−) S. lactis strains as recipients. Plasmid transfer was confirmed by agarose gel electrophoresis. Transductant plasmid profiles were of three types—those containing no visible plasmid deoxyribonucleic acid, those possessing a 23-megadalton (Mdal) plasmid, and those containing a 23-Mdal plasmid and a 30-Mdal plasmid. A Lac+ transductant could serve as a donor of the lac determinants during solid-surface matings. These results add to previously published reports of inter- and intraspecies genetic transfer in dairy starter cultures.
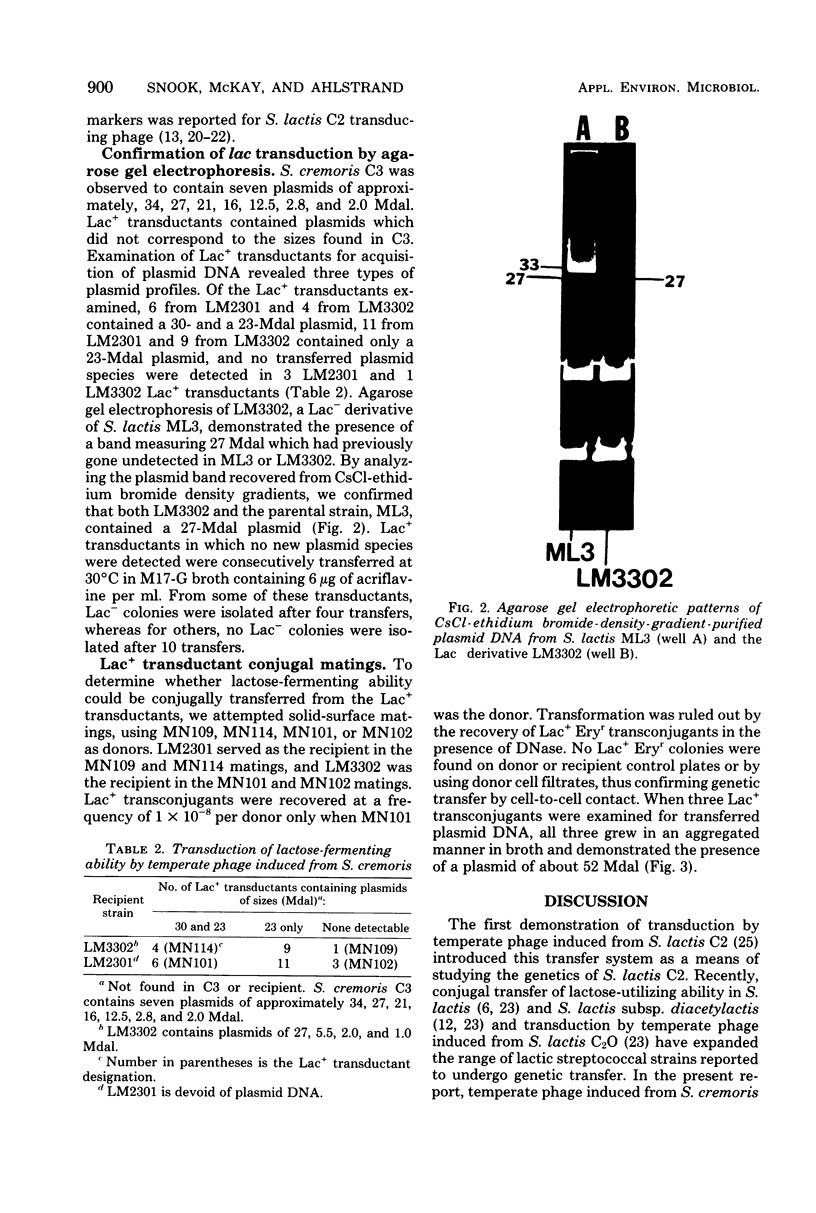
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann H. W., Audurier A., Berthiaume L., Jones L. A., Mayo J. A., Vidaver A. K. Guidelines for bacteriophage characterization. Adv Virus Res. 1978;23:1–24. doi: 10.1016/s0065-3527(08)60096-2. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. High-frequency conjugation associated with Streptococcus lactis donor cell aggregation. J Bacteriol. 1980 Sep;143(3):1260–1264. doi: 10.1128/jb.143.3.1260-1264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. Prophage-Cured Derivatives of Streptococcus lactis and Streptococcus cremoris. Appl Environ Microbiol. 1980 Nov;40(5):964–966. doi: 10.1128/aem.40.5.964-966.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins A. R., Sandine W. E. Incidence and properties of temperate bacteriophages induced from lactic streptococci. Appl Environ Microbiol. 1977 Jan;33(1):184–191. doi: 10.1128/aem.33.1.184-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Genetic Evidence for Plasmid-Linked Lactose Metabolism in Streptococcus lactis subsp. diacetylactis. Appl Environ Microbiol. 1979 May;37(5):1041–1043. doi: 10.1128/aem.37.5.1041-1043.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L. Isolation and examination of transducing bacteriophage particles from Streptococcus lactis C2. J Dairy Sci. 1976 Mar;59(3):396–404. doi: 10.3168/jds.s0022-0302(76)84219-1. [DOI] [PubMed] [Google Scholar]
- Kozak W., Rajchert-Trzpil M., Zajdel J., Dobrzański W. T. Lysogeny in lactic streptococci producing and not producing nisin. Appl Microbiol. 1973 Feb;25(2):305–308. doi: 10.1128/am.25.2.305-308.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L. D., McKay L. L. Isolation and characterization of plasmid DNA in Streptococcus cremoris. Appl Environ Microbiol. 1978 Dec;36(6):944–952. doi: 10.1128/aem.36.6.944-952.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie R. J. Lysogenic strains of group N lactic streptococci. Appl Microbiol. 1974 Jan;27(1):210–217. doi: 10.1128/am.27.1.210-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl Microbiol. 1973 Apr;25(4):682–684. doi: 10.1128/am.25.4.682-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Simultaneous loss of proteinase- and lactose-utilizing enzyme activities in Streptococcus lactis and reversal of loss by transduction. Appl Microbiol. 1974 Sep;28(3):342–346. doi: 10.1128/am.28.3.342-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Stabilization of Lactose Metabolism in Streptococcus lactis C2. Appl Environ Microbiol. 1978 Aug;36(2):360–367. doi: 10.1128/aem.36.2.360-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaneva K. P. Electron microscopy of virulent phages for Streptococcus lactis. Appl Environ Microbiol. 1976 Apr;31(4):590–601. doi: 10.1128/aem.31.4.590-601.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]