Abstract
An isoform of the mammalian renal type II Na/Pi-cotransporter is described. Homology of this isoform to described mammalian and nonmammalian type II cotransporters is between 57 and 75%. Based on major diversities at the C terminus, the new isoform is designed as type IIb Na/Pi-cotransporter. Na/Pi-cotransport mediated by the type IIb cotransporter was studied in oocytes of Xenopus laevis. The results indicate that type IIb Na/Pi-cotransport is electrogenic and in contrast to the renal type II isoform of opposite pH dependence. Expression of type IIb mRNA was detected in various tissues, including small intestine. The type IIb protein was detected as a 108-kDa protein by Western blots using isolated small intestinal brush border membranes and by immunohistochemistry was localized at the luminal membrane of mouse enterocytes. Expression of the type IIb protein in the brush borders of enterocytes and transport characteristics suggest that the described type IIb Na/Pi-cotransporter represents a candidate for small intestinal apical Na/Pi-cotransport.
The kidney and the small intestine are important (externally oriented) control sites to maintain and balance the extracellular concentration of inorganic phosphate (Pi). In the kidney, reabsorption of filtered Pi occurs in the proximal tubule via apically located Na/Pi-cotransporters. Two dissimilar Na/Pi-cotransporters, named type I and type II, have been identified and have been shown to be expressed in the apical membrane of proximal tubular cells (1). As demonstrated recently by targeted inactivation, the type II Na/Pi-cotransporter represents the major pathway by which Pi is reabsorbed (1, 2). With the exception of osteoclasts (3), expression of the type II cotransporter has not yet been described other than in proximal tubules.
In addition to the well characterized renal handling of Pi, an understanding of whole body Pi-homeostasis necessitates elucidating the entry step of Pi in the small intestine (apical Na/Pi-cotransport). However, until now, the molecular identity of a mammalian small intestinal apical Na/Pi-cotransporter has not been described. Although expression of type III Na/Pi-cotransporter mRNA (retroviral receptors Glvr-1 and Ram-1; ref. 4) has been reported in small intestine, as yet, there is no evidence that these Na/Pi-cotransporters are expressed in the apical membrane. Rather, it seems that type III cotransporters are expressed ubiquitously in epithelial and nonepithelial cells.
Based on an expressed sequence tag (EST) clone derived from a cDNA library of murine embryonic cells, we have obtained a functional full length clone coding for a mammalian isoform of the renal type II Na/Pi-cotransporter, which was named type IIb Na/Pi-cotransporter. Expression of type IIb mRNA was found in a variety of tissues, including small intestinal mucosa. By immunohistochemistry, expression of the type IIb protein was localized at the brush border membrane of enterocytes. Transport characteristics of type IIb-mediated Na/Pi-cotransport were similar to the ones described for small intestinal Na/Pi-cotransport (5, 6). Our data suggest that the described type IIb Na/Pi-cotransporter may represent the (a) small intestinal apical Na/Pi-cotransporter.
MATERIALS AND METHODS
Sequencing and Rapid Amplification of 5′-cDNA Ends.
An EST-clone (Genome Systems, St. Louis; clone AA647858) with an insert of 3.4 kilobases (kb) was sequenced on both strands. Sequence comparison with the mouse renal type II Na/Pi-cotransporter (7, 8) suggested that ≈700 bp were missing at the 5′ end. To obtain the full length cDNA rapid amplification of 5′ cDNA ends was performed as follows: Total RNA (10 μg) from mouse small intestinal mucosa was retrotranscribed with 200 units MMLV-RT (GIBCO/BRL) by using an oligo-dT8 primer. Extension of the 5′ end of the cDNA was performed by polynucleotide transferase (30 units, GIBCO/BRL) in the presence of 0.4 mM dATP. PCR was performed with a specific antisense primer derived from the EST-sequence and a T17 primer containing a SalI adapter. A second round of PCR was performed with a nested antisense and a SalI-specific primer. The final PCR product was digested with SalI and Sau3a and was subcloned into pBluescript SK(+) (Stratagene). The same extension products were obtained by two independent rounds of reactions.
Construction of a Full Length cDNA.
Total RNA (10 μg) of mouse small intestine was retrotranscribed by using a dT8 primer. A PCR fragment was amplified by using a sense primer (nucleotides 8–28) and an antisense primer (nucleotides 961–980) and was cloned into the pGEM-T vector (GIBCO/BRL). The fragment corresponding to the 5′ end of the transporter was excised with BglII and NotI. To obtain the missing 3.2 kb of the transporter, the EST-clone was digested with BglII and SalI. Both parts were ligated into pSPORT1 (GIBCO/BRL), which was digested with NotI and SalI.
Reverse Transcription (RT)–PCR Analysis.
Total RNA (10 μg) isolated from different tissues was retrotranscribed with 200 units of reverse transcriptase (MMLV, GIBCO/BRL) by using a dT8 primer. PCR was performed with a sense (nucleotides 620–638) and an antisense (nucleotides 887–906) primer derived from the EST-clone. Amplification of the mouse kidney-specific type II Na/Pi-cotransporter was achieved by a primer pair derived from the mouse NaPi-7 cDNA (7, 8): sense position, 10–29; antisense position, 195–210.
Northern Blots.
Total RNA from mouse kidney cortex and upper small intestinal mucosa was isolated by the cesium trifluoroacetate/guanidinium thiocyanate method. Poly(A)+ RNA was obtained by oligo-dT cellulose chromatography. Poly(A)+RNA (5 μg) was separated on agarose gels (1.2%) and was transferred onto nylon membranes (BioDyn). Blots were hybridized with the following probes obtained by random priming in the presence of [α32P]dCTP: (i) A 5′ end fragment of 900 bp of the type IIb cDNA was obtained by restriction with NotI and BglII, and 9ii) a full length probe of the type IIa (NaPi-7; refs. 7 and 8) cDNA was obtained by restriction with NotI and SalI. Equal loadings were confirmed by using probes specific for the ribosomal protein L 28 (9). Hybridization was performed in 6× standard saline citrate (SSC), 5× Denhardt’s, 0.5% SDS, and herring sperm DNA (100 μg/ml) at 65°C. Blots were washed sequentially with 2× SSC/0.1% SDS (room temperature, 10 min), 1× SSC/0.1% SDS (10 min at 40°C), and 0.5× SSC/0.1% SDS (20 min at 55°C). After exposure, results were analyzed by the software package image quant (Molecular Dynamics).
Immunodetections.
Rabbit polyclonal antibodies were raised against a synthetic peptide close to the C terminus. Mouse small intestinal brush border membranes were isolated by a Mg2+-precipitation technique (10), and Western blots were performed as described (11). For gel electrophoresis, membranes were denatured in 2% SDS without heating. For immunohistochemistry, mouse duodenum was rinsed with 0.9% NaCl and was fixed by immersion in 3% paraformaldehyde, 0.05% picric acid in a 6:4 mixture of 0.1 M cacodylate buffer (pH 7.4). All other steps were performed as described (11). A swine anti-rabbit IgG conjugated to fluorescein isothiocyanate (Dakopatts, Glostrup, Denmark) was used as a secondary antibody. For peptide protections, the antigenic peptide was added at concentrations of 100 μg/ml.
Transport Assays in Oocytes of Xenopus laevis.
Isolation and handling of X. laevis oocytes has been described elsewhere (12). Oocytes were injected with 5 ng cRNA (in 50nl water). Transport was measured 3 days later, either by isotope flux as described (12, 13, 18) or by electrophysiological means under steady state, voltage clamped conditions (14).
RESULTS
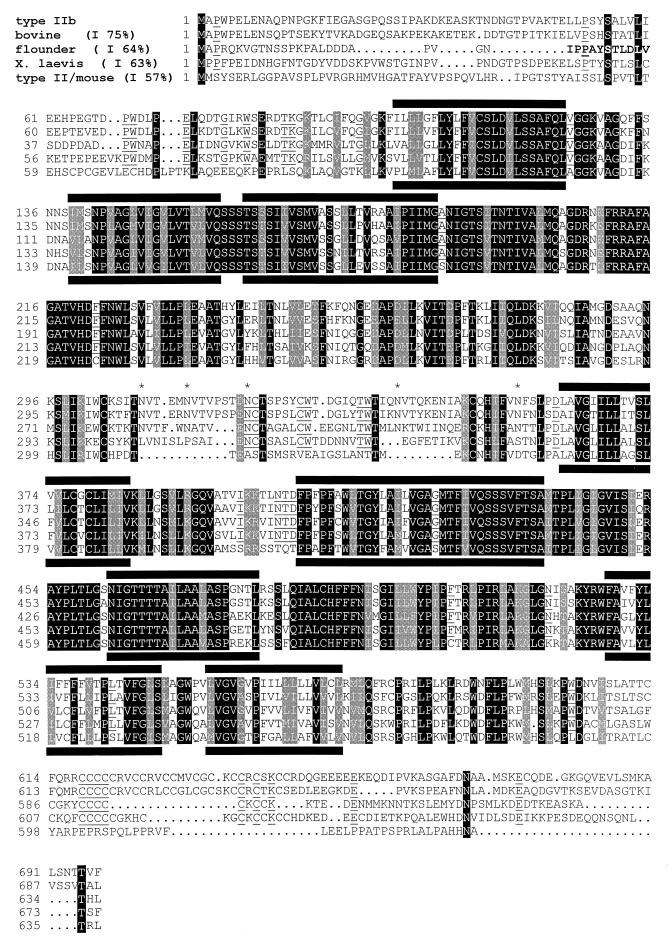
By databank search, an EST cDNA clone (AA647858) prepared from mouse two-cell stage embryos was found that showed 73% homology over a length of 312 bp to the mouse renal type II Na/Pi-cotransporter (7, 8). Preliminary analysis by Northern blotting indicated that a related mRNA species is expressed in small intestinal mucosa (data not shown). Full length sequencing of the EST clone (3.5 kb) suggested that, at the 5′ end, 700 bp were missing. By rapid amplification of 5′ cDNA ends, a full length cDNA (4,039 bp) was obtained (GenBank accession no. AF081499) containing an ORF (positions 45–2,137) coding for a protein of 697 amino acids (Fig. 1).
Figure 1.
Amino acid sequence comparisons. Sequences of type II Na/Pi-cotransporters were aligned by pileup (sequence analysis software package; Genetics Computer Group, Madison, WI). Shaded boxes indicate consensus residues in all species listed. Additionally, equal residues among the type IIb, bovine, flounder, and X. laevis isoforms are underlined. Predicted transmembrane regions are indicated by bars, and potential N-glycosylation sites of the proposed extracellular loop are indicated by an asterisk. Numbers given in parentheses (x%) indicate the percentage of overall homologies to the type IIb sequence.
Amino acid comparisons revealed that the newly identified protein is 57–75% homologous to Na/Pi-cotransporters identified in bovine NBL cells (15), flounder kidney and intestine (16), and intestine and lung of X. laevis (17) and to the renal type II Na/Pi-cotransporter (NaPi-7; refs. 7 and 8) (Fig. 1). Overall homology to type I (1) and type III Na/Pi-cotransporters (4) was ≈20%. As illustrated, highest homologies among the listed Na/Pi-cotransporters are seen in regions that also have been proposed to represent transmembrane regions (1). The most striking difference of the newly identified protein compared with the mouse renal type II Na/Pi-cotransporter is found in the C-terminal region containing clusters of cysteine residues. A similar clustering of cysteine residues is also present in the Na/Pi-cotransporters of bovine cells, flounder kidney/intestine, and Xenopus intestine. Therefore, we propose to subdivide type II Na/Pi-cotransporters into a subfamily type IIa (represented by the renal isoforms of mouse, rat, rabbit, opossum kidney cells, and human; ref. 1) and type IIb (represented by the isoforms of bovine, flounder, and Xenopus and the one described here).
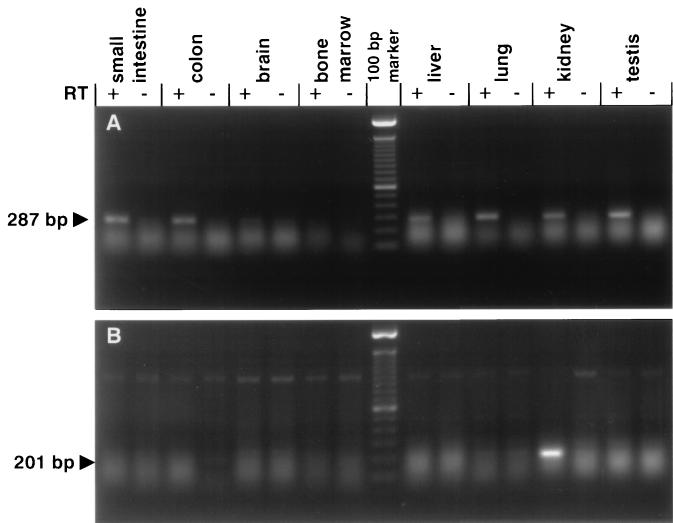
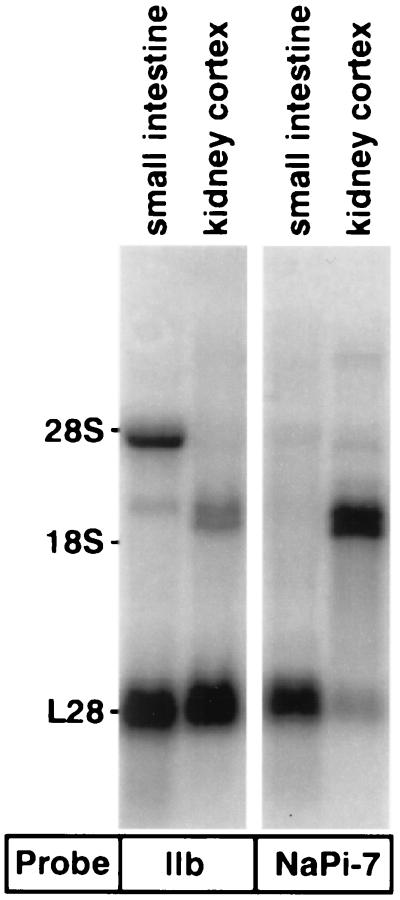
Expression of type IIb mRNA was analyzed by RT-PCR using total RNA (Fig. 2) and Northern blots using poly(A)+RNA (Fig. 3). By RT-PCR using primers positioned within the ORF, expression of type IIb mRNA was indicated in the mucosa of the upper small intestine, colon, liver, lung, kidney, and testis. As a control, the same RNA samples were subjected to RT-PCR analysis for the renal type IIa cotransporter NaPi-7. As indicated, a type IIa-related PCR product was found only in RNA isolated from kidney, confirming the kidney specific expression of the type IIa cotransporter (1, 18). Northern blots performed with poly(A)+RNA isolated of mouse kidney cortex and small intestine are shown in Fig. 3. By using a 5′-end probe of 900 bp, the major mRNA species detected in small intestinal mRNA was at ≈4 kb. In mRNA of kidney cortex, no such signal was detectable. In addition, in small intestinal mRNA, a faint signal at ≈2.5 kb was evident. By using the same probe, two signals at ≈2.5 kb also were detected with poly(A)+RNA of mouse kidney cortex. To verify a possible crossreaction with the renal type II cotransporter, the same blots were hybridized with probes derived from the NaPi-7 cDNA (Fig. 3B). As illustrated, no signals with small intestinal mRNA were detected by this probe, but, with mouse kidney cortex mRNA, a strong signal (double band at ≈2.5 kb) was observed representing the renal type II Na/Pi-cotransporter. This suggested that the double band seen in kidney mRNA with the type IIb probe represents a crossreaction with the type II (NaPi-7) cotransporter. Confirmation for such a crossreaction was obtained with poly(A)+RNA isolated from kidney cortex of mice in which the renal type II Na/Pi-cotransporter has been knocked out (19).
Figure 2.
RT-PCR analysis using primers specific for the type IIb (A) or the renal type II (NaPi-7) (B) Na/Pi-cotransporter. All reactions were performed in the presence or absence of reverse transcriptase (RT; +, −). Integrity of the RNA preparations was confirmed by Northern blots using probes specific for β-actin (not shown).
Figure 3.
Northern blot analysis of poly(A)+RNA isolated from mouse upper small intestinal mucosa and kidney cortex. Blots were hybridized with probes derived from a 900-bp 5′ end fragment of type IIb cDNA or from the full length cDNA of the mouse renal type II Na/Pi-cotransporter. Hybridization to the ribosomal protein L28 mRNA was used to confirm equal loadings. In the case of the NaPi-7 probe, five times less poly(A)+RNA of kidney cortex was loaded.
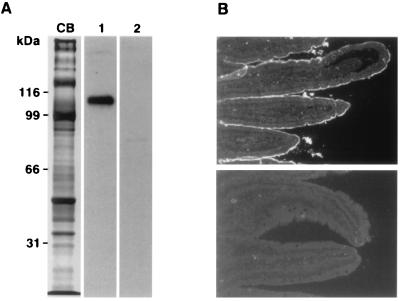
Expression of the type IIb protein was analyzed by immunoblotting and immunofluorescence using a polyclonal antibody raised against a synthetic C-terminal peptide. On Western blots performed with isolated small intestinal brush border membranes, a reaction with a single band of ≈108 kDa was observed that could be protected completely by inclusion of the antigenic peptide (Fig. 4A). Because four potential N-glycosylation sites are contained in a region representing an extracellular loop and because, in the case of the renal type II cotransporter, N-glycosylation has been demonstrated in this loop (20), the molecular mass of 108 kDa likely represents the glycosylated form of the type IIb protein (unglycosylated Mr 78). In cryostat sections of mouse duodenum, specific reaction was observed at the apical membrane of enterocytes that was prevented by the antigenic peptide (Fig. 4B).
Figure 4.
Immunodetection of the type IIb Na/Pi-cotransporter. (A) Western blots of isolated mouse small intestinal brush border membranes (35 μg protein per lane). CB, Coomassie blue staining. Incubation with the first antibody was performed in the absence (lane 1) or presence (lane 2) of the antigenic peptide. (B) Immunofluorescence detection of the type IIb cotransporter in the apical membrane of enterocytes. Incubation with the primary antibody was performed in the absence (Upper) or presence (Lower) of antigenic peptide (100 μg/ml).
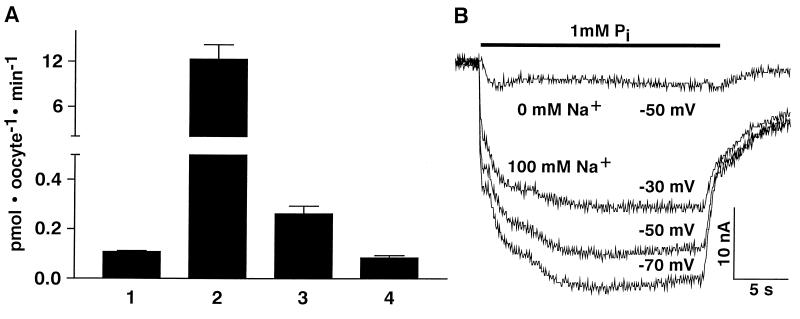
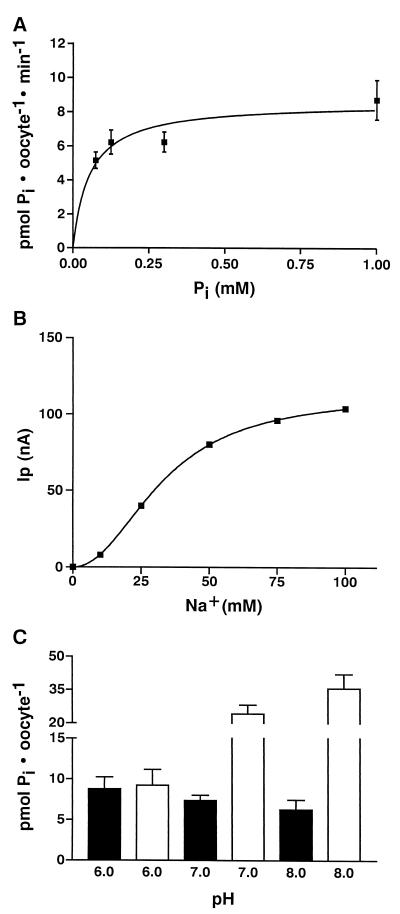
Type IIb cRNA was injected into oocytes of X. laevis, and transport of phosphate was measured in the presence and absence of sodium by isotope flux (Fig. 5A). Compared with oocytes injected with water, oocytes injected with type IIb cRNA exhibited a large expression of Pi-transport, which depended on the presence of sodium and which was not observed after injection of antisense cRNA (data not shown). Furthermore, oocytes injected with type II cRNA did not take up sulfate (SO42=), suggesting that the type IIb cotransporter exhibits similar specificity as described for the renal type IIa cotransporter (13, 18). As reported for the renal type II Na/Pi-cotransporter (8, 14), superfusion of oocytes expressing the type IIb cotransporter with phosphate exhibited an inwardly directed current that depended on the presence of sodium and the steady state holding potential (Fig. 5B). This indicated that, like the type II cotransporter, Na/Pi-cotransport by the type IIb cotransporter is electrogenic. As observed in isotope flux measurements (Fig. 5A), there was also evidence for a small contribution of a Na-independent Pi-transport. Based on the results shown in Fig. 5, transport characteristics of type IIb-induced Na/Pi-cotransport were determined by either isotope flux or by electrophysiological measurements. By both methods an apparent Km(Pi) of ≈50 μM and a Km(Na) of ≈30 mM was determined (Fig. 6). Because pH dependence is a hallmark of the renal type II Na/Pi-cotransporter, type IIb-mediated Na/Pi-cotransport was determined at different pH-values (Fig. 6C). In contrast to the renal type II isoform, type IIb-associated Na/Pi-cotransport was less dependent of the pH and was slightly higher at more acid pH-values.
Figure 5.
Characterization of Na/Pi-cotransport in oocytes injected with type IIb cRNA. (A) Isotope flux measurements performed at 0.5 mM phosphate (1, 2, 3) or 1 mM sulfate (4) (mean ± SD of 8–10 oocytes; two experiments). Bar 1: Pi-uptake in the presence of NaCl into oocytes injected with water; bars 2 and 3: Pi-uptake into oocytes injected with type IIb cRNA in the presence of NaCl (2) or choline-Cl (3). (B) Inwardly directed currents measured under steady state conditions by using the two-electrode voltage clamp. Oocytes were voltage clamped at indicated voltages and were superfused with 1 mM phosphate in the absence or presence of NaCl (see refs. 8 and 14).
Figure 6.
Characterization of type IIb-mediated Na/Pi-cotransport. (A) Isotope flux measurements were performed at different Pi concentrations in the presence of sodium and were corrected by the values obtained from oocytes injected with water. The calculated value for Km(Pi) was 50 μM. (B) Electrophysiological determination of the Km(Na). The calculated Km(Na) was 33 mM, and the stoichiometry was >2. (C) pH dependence of type IIb (black bars) and the renal type II (NaPi-7) Na/Pi-cotransport (open bars). Values were corrected with uptake in the presence of choline-Cl, which was not changed by the different pH values. The data represent the mean ± SD of 8–10 oocytes. All experiments have been performed at least twice. The data given in C were derived from one oocyte. The same result was obtained with different oocytes from different batches.
DISCUSSION
Intake and extrusion of inorganic phosphate is determined by the intestinal and renal handling of phosphate. In recent years, some renal phosphate transporters and their roles in the regulation of the renal handling of Pi have been described (1, 2, 4, 7, 8, 18). However, small intestinal phosphate cotransporters expressed in the mammalian small intestine are characterized far less.
With respect to the renal handling of Pi, three dissimilar types of sodium-dependent phosphate cotransporters expressed in the plasma membrane have been described so far: a type I, a type II, and a type III (1). It could be shown that the type II Na/Pi-cotransporter plays a major role in proximal tubular reabsorption of Pi and that proximal tubular capacity to reabsorb Pi to a large part depends on the net abundance of type II cotransporters in the apical membrane of proximal tubules (1, 2). The role of type I and III cotransporters is less clear.
So far, expression of type II mRNA has been described in kidney cortex only. Expression of type I mRNA also is found in liver and brain, and expression of type III mRNA seems to occur in almost every tissue (1, 4), notably, also, in the intestine. The nonspecific tissue expression pattern of type III cotransporters rules out the possibility of type III Na/Pi-cotransporters being candidates for small intestinal apical Na/Pi-cotransporters.
Derived from an EST clone from an embryonic mouse cDNA library, we obtained the full length cDNA coding for a type II Na/Pi-cotransporter. The deduced protein showed high homology to described type II Na/Pi-cotransporters. Highest homologies were found in regions that most likely represent transmembrane segments, and the most striking differences between the identified cotransporter, the bovine, flounder, and X. Laevis isoforms, and the renal type Na/Pi-cotransporters are represented by cysteine clusters at the C-terminal ends. Therefore, we propose the name type IIb Na/Pi-cotransporter for the identified mammalian isoform and propose to extend this nomenclature also for the bovine (15), the flounder, (16) and the X. laevis (17) isoforms.
Injection of type IIb cRNA into oocytes of X. laevis resulted in expression of Na-dependent phosphate transport with characteristics similar to that observed for Na/Pi-cotransport mediated by the renal type II Na/Pi-cotransporter (8, 14, 18). However, the most striking difference of type IIb-mediated Na/Pi-cotransport was its pH dependence.
So far, proteins involved in mammalian small intestinal Na/Pi-cotransport have not been described. In nonruminants, highest rates of Pi-reabsorption are observed in the upper small intestine (5). Na/Pi-cotransport in mouse small intestine is highest at a more acidic pH and exhibits a Km value for Pi of ≈50 μM (5, 6).The functional characteristics observed for type IIb-mediated Na/Pi-cotransport are in agreement with these data and support the notion that the type IIb cotransporter may represent a candidate for a small intestinal Na/Pi-cotransporter. This is supported further by the observation that both type IIb mRNA and protein are expressed in mouse small intestinal mucosa and, notably, that the type IIb protein is localized at the brush border membrane of the enterocytes.
In summary, we have identified a mammalian Na/Pi-cotransporter with high homology to described type II Na/Pi-cotransporters. Expression of the mRNA and the protein of such a transport protein was demonstrated in the mammalian small intestine. Kinetic properties and pH dependence of type IIb-associated Na/Pi-cotransport favor this protein as a candidate for a Na/Pi-cotransporter involved in intestinal Pi-reabsorption. Apart from the small intestine, expression of type IIb mRNA also was recognized in other tissues, such as lung, colon, liver, kidney, and testis. The physiological role of Na/Pi-cotransport mediated by the type IIb isoform in small intestine as well as in the other tissues remains to be determined.
Acknowledgments
We thank Drs. R. Wenger and M. Gassmann for providing RNA samples of different mouse tissues. The art work of C. Gasser is gratefully acknowledged. Financial support was provided by the grants of the Swiss National Fonds to J.B. and H.M. and from the University of Zürich (Stiftung für wissenschaftliche Forschung).
ABBREVIATION
- EST
expressed sequence tag
- kb
kilobase
- RT
reverse transcription
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF081499).
References
- 1.Murer H, Biber J. Eur J Physiol. 1997;433:379–389. doi: 10.1007/s004240050292. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Kempson S A, Lötscher M, Biber J, Murer H. J Membr Biol. 1996;154:1–9. doi: 10.1007/s002329900127. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Guo X L, Alvarez U M, Hruska K A. J Clin Invest. 1997;100:539–549. doi: 10.1172/JCI119563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavanaugh M P, Kabat D. Kidney Int. 1996;49:959–963. doi: 10.1038/ki.1996.135. [DOI] [PubMed] [Google Scholar]
- 5.Cross H S, Debiec H, Peterlik M. Miner Electrolyte Metab. 1990;16:115–124. [PubMed] [Google Scholar]
- 6.Nakagawa N, Ghishan F K. Proc Soc Exp Biol Med. 1993;203:328–335. doi: 10.3181/00379727-203-43607. [DOI] [PubMed] [Google Scholar]
- 7.Collins J F, Ghishan F K. FASEB J. 1994;8:862–868. doi: 10.1096/fasebj.8.11.8070635. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann C, Wagner C A, Busch A E, Markovich D, Biber J, Lang F, Murer H. Pflügers Arch. 1995;430:830–836. doi: 10.1007/BF00386183. [DOI] [PubMed] [Google Scholar]
- 9.Burke P S, Lium E, Lin C S. Gene. 1994;142:315–316. doi: 10.1016/0378-1119(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 10.Stieger B, Murer H. Eur J Biochem. 1983;135:95–101. doi: 10.1111/j.1432-1033.1983.tb07622.x. [DOI] [PubMed] [Google Scholar]
- 11.Custer M, Lötscher M, Biber J, Murer H, Kaissling B. Am J Physiol. 1994;266:F767–F774. doi: 10.1152/ajprenal.1994.266.5.F767. [DOI] [PubMed] [Google Scholar]
- 12.Werner A, Biber J, Forgo J, Palacin M, Murer H. J Biol Chem. 1990;265:12331–12336. [PubMed] [Google Scholar]
- 13.Markovich D, Forgo J, Stange G, Biber J, Murer H. Proc Natl Acad Sci USA. 1993;90:8073–8077. doi: 10.1073/pnas.90.17.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster I C, Wagner C A, Busch A E, Lang F, Biber J, Hernando N, Murer H, Werner A. J Membr Biol. 1997;160:9–25. doi: 10.1007/s002329900291. [DOI] [PubMed] [Google Scholar]
- 15.Helps C, Murer H, McGivan J. Eur J Biochem. 1995;228:927–930. doi: 10.1111/j.1432-1033.1995.tb20341.x. [DOI] [PubMed] [Google Scholar]
- 16.Kohl B, Herter P, Hulseweh B, Elger M, Hentschel H, Kinne R K, Werner A. Am J Physiol. 1996;270:F937–F944. doi: 10.1152/ajprenal.1996.270.6.F937. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuya-Oka A, Stolow M A, Ueda S, Shi Y B. Dev Genetics. 1997;20:53–66. doi: 10.1002/(SICI)1520-6408(1997)20:1<53::AID-DVG7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Magagnin S, Werner A, Markovich D, Sorribas V, Biber J, Murer H. Proc Natl Acad Sci USA. 1993;90:5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck L, Karaplis A C, Amizuka N, Hewson A S, Ozawa H, Tenenhouse H S. Proc Natl Acad Sci USA. 1998;95:5327–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes G, Busch A, Lötscher M, Waldegger S, Lang F, Verrey F, Biber J, Murer H. J Biol Chem. 1994;269:24143–24149. [PubMed] [Google Scholar]