Abstract
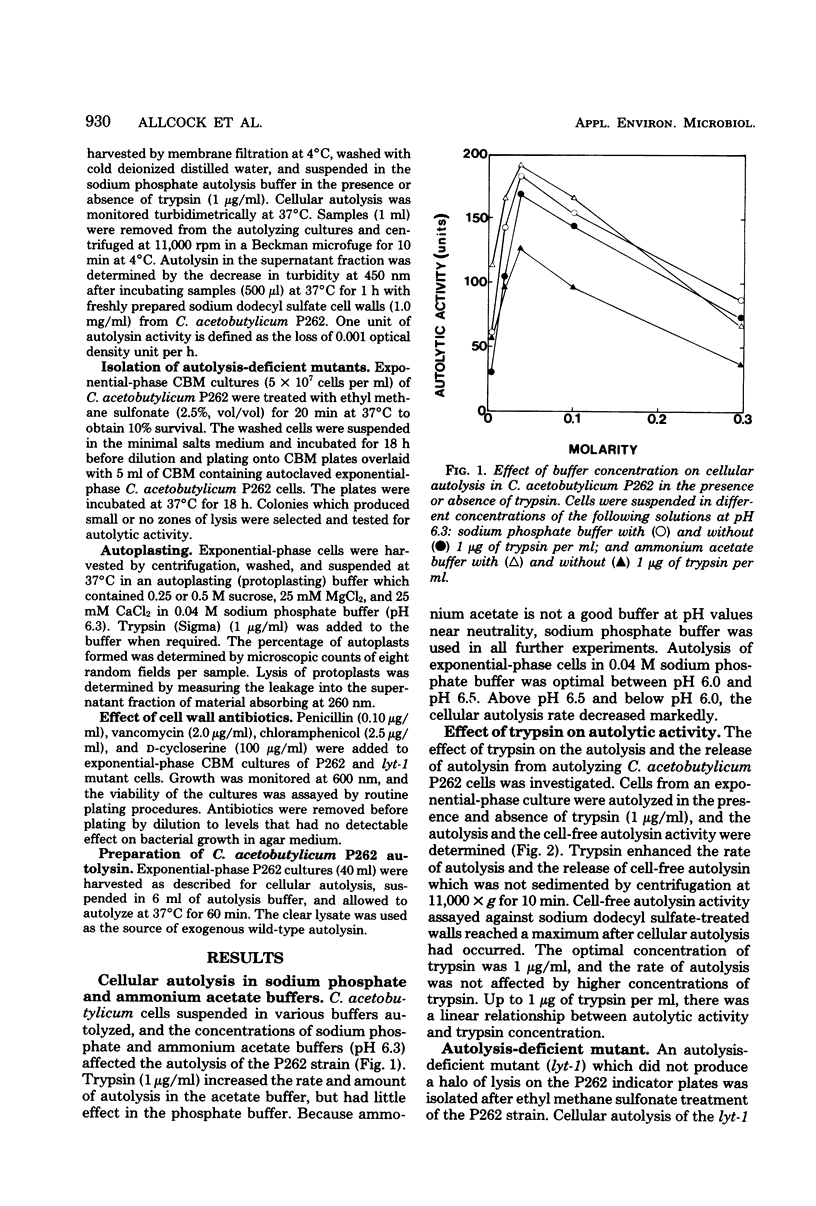
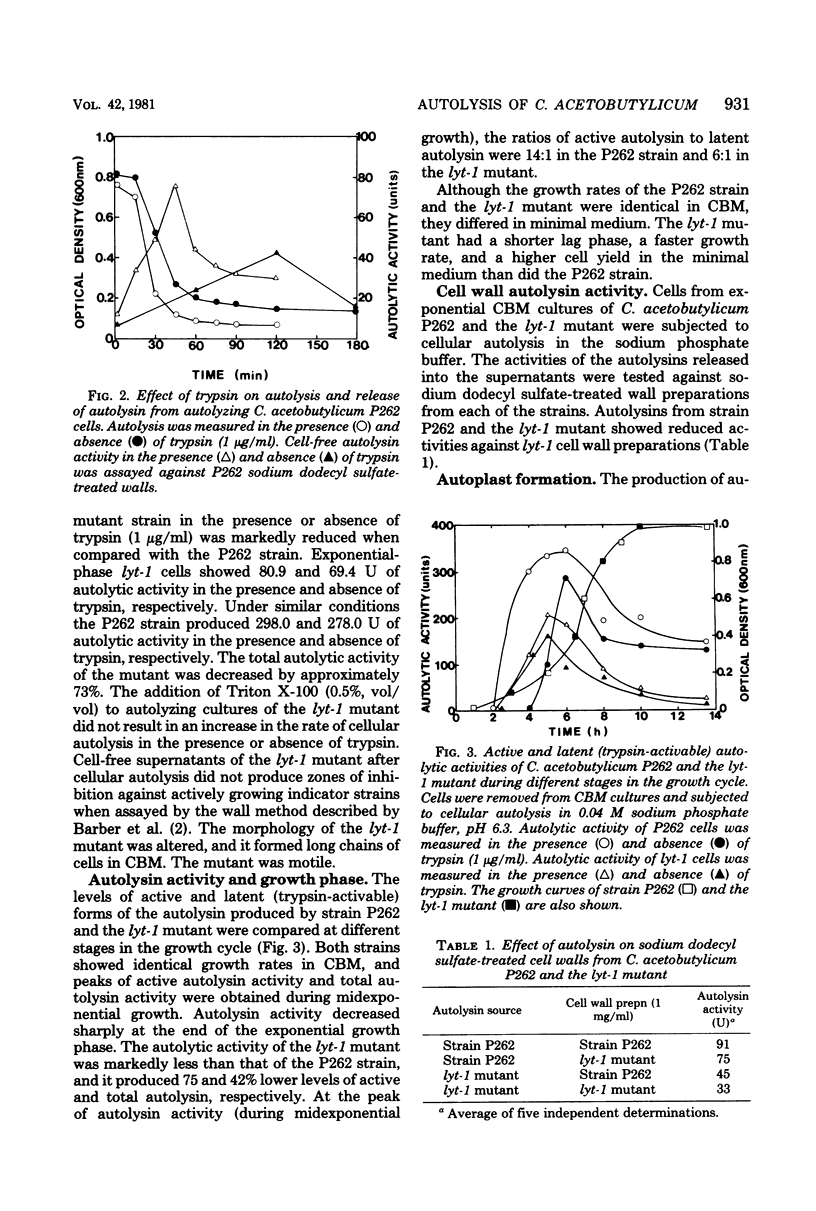
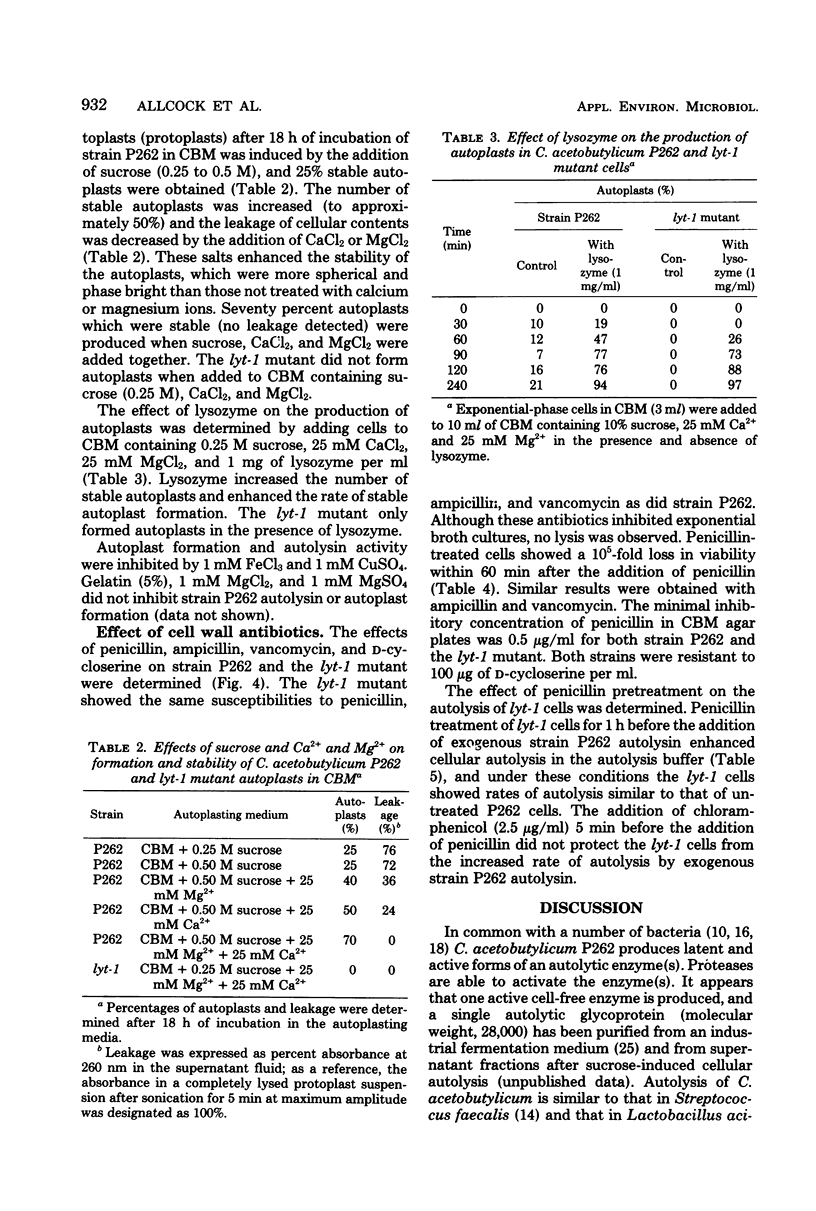
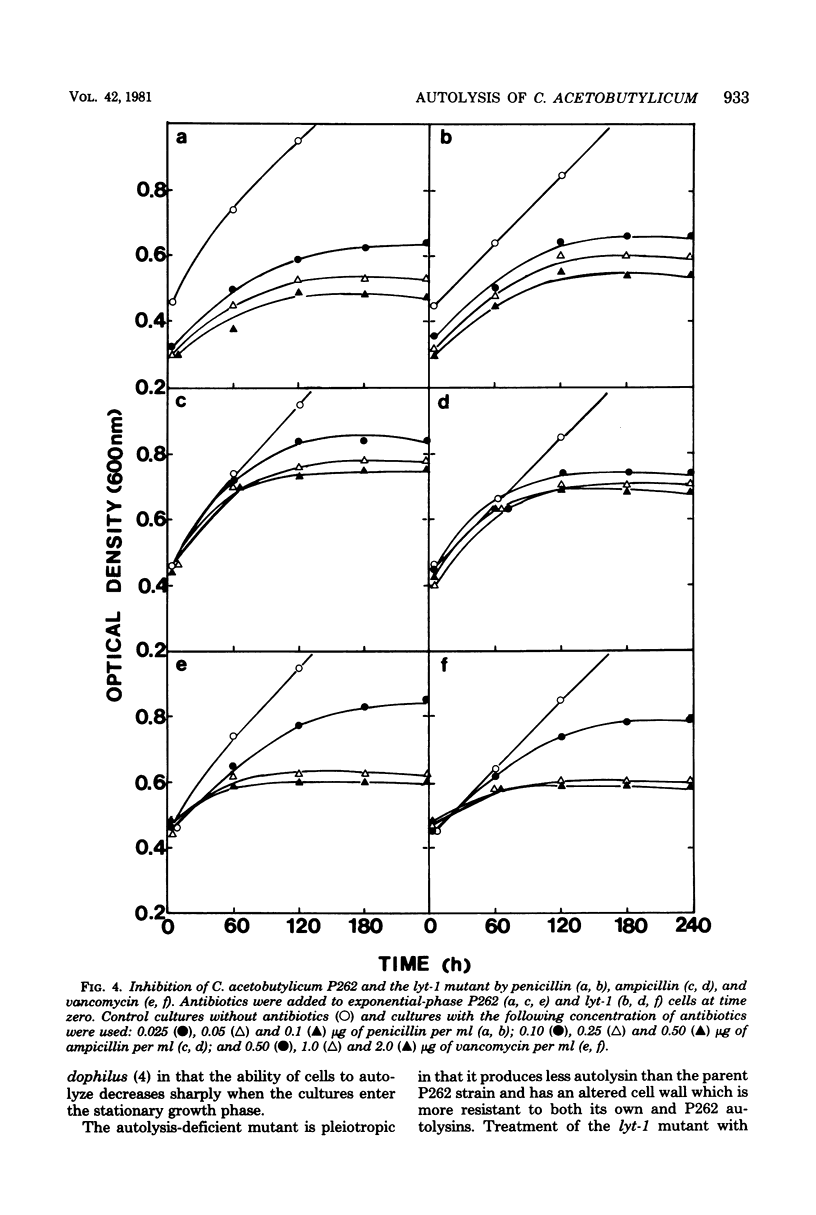
The optimum conditions for autolysis and autoplast formation in Clostridium acetobutylicum P262 have been defined. Autolysis was optimal at pH 6.3 in 0.04 M sodium phosphate buffer, and the bacterium produced latent and active forms of an autolytic enzyme. The ability of cells to autolyze decreased sharply when cultures entered the stationary phase. Autoplasts were induced by 0.25 to 0.5 M sucrose and were stable in media containing sucrose, CaCl2, and MgCl2. A pleiotropic autolysis-deficient mutant (lyt-1) was isolated. The mutant produced less autolysin than did the parent P262 strain, and it had an altered cell wall which was more resistant to both its own and P262 autolysins. The mutant formed long chains of cells, and lysozyme was required for the production of autoplasts. Growth of the P262 strain or the lyt-1 mutant was inhibited by the same concentrations of penicillin, ampicillin, and vancomycin. The lyt-1 mutant strain treated with the minimum growth-inhibitory concentration of penicillin autolyzed upon the addition of wild-type autolysin to the autolysis buffer at the same rate as did the untreated P262 strain. Chloramphenicol did not protect the penicillin-treated lyt-1 cells against autolysis enhanced by exogenous wild-type autolysin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J. M., Robb F. T., Webster J. R., Woods D. R. Bacteriocin production by Clostridium acetobutylicum in an industrial fermentation process. Appl Environ Microbiol. 1979 Mar;37(3):433–437. doi: 10.1128/aem.37.3.433-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M. Wall autolysin of Lactobacillus acidophilus strain 63 AM gasser. Biochemistry. 1970 Jul 21;9(15):2952–2955. doi: 10.1021/bi00817a003. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckmann M. M. Mutant of Bacillus subtilis with a temperature-sensitive autolytic amidase. J Bacteriol. 1973 May;114(2):798–803. doi: 10.1128/jb.114.2.798-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rogers H. J. Characterization of Bacillus licheniformis 6346 mutants which have altered lytic enzyme activities. J Bacteriol. 1974 May;118(2):358–368. doi: 10.1128/jb.118.2.358-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Galli E., Hughes D. E. The autolysis of Clostridium sporogenes. J Gen Microbiol. 1965 Jun;39(3):345–353. doi: 10.1099/00221287-39-3-345. [DOI] [PubMed] [Google Scholar]
- Kawata T., Takumi K., Sato S., Yamashita H. Autolytic formation of spheroplasts and autolysis of cell walls in Clostridium botulinum type A. Jpn J Microbiol. 1968 Dec;12(4):445–455. doi: 10.1111/j.1348-0421.1968.tb00418.x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970 Aug;103(2):457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Reversal of cycloserine inhibition by D-alanine. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:693–695. doi: 10.3181/00379727-101-25064. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Lipids and lipoteichoic acid of autolysis-defective Streptococcus faecium strains. J Bacteriol. 1980 Jun;142(3):741–746. doi: 10.1128/jb.142.3.741-746.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J. R., Reid S. J., Jones D. T., Woods D. R. Purification and Characterization of an Autolysin from Clostridium acetobutylicum. Appl Environ Microbiol. 1981 Feb;41(2):371–374. doi: 10.1128/aem.41.2.371-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Ward J. B. Characterization of the autolytic enzymes of Clostridium perfringens. J Gen Microbiol. 1979 Oct;114(2):349–354. doi: 10.1099/00221287-114-2-349. [DOI] [PubMed] [Google Scholar]



