Abstract
Constitutive expression of the cold-regulated COR15a gene of Arabidopsis thaliana results in a significant increase in the survival of isolated protoplasts frozen over the range of −4.5 to −7°C. The increased freezing tolerance is the result of a decreased incidence of freeze-induced lamellar-to-hexagonal II phase transitions that occur in regions where the plasma membrane is brought into close apposition with the chloroplast envelope as a result of freeze-induced dehydration. Moreover, the mature polypeptide encoded by this gene, COR15am, increases the lamellar-to-hexagonal II phase transition temperature of dioleoylphosphatidylethanolamine and promotes formation of the lamellar phase in a lipid mixture composed of the major lipid species that comprise the chloroplast envelope. We propose that COR15am, which is located in the chloroplast stroma, defers freeze-induced formation of the hexagonal II phase to lower temperatures (lower hydrations) by altering the intrinsic curvature of the inner membrane of the chloroplast envelope.
The ability to endure low temperatures and freezing is a major determinant of the geographical distribution and productivity of agricultural crops. Even in areas considered suitable for the cultivation of a given species or cultivar, decreases in yield and crop failure frequently occur as a result of aberrant, freezing temperatures. In spite of attempts to minimize damage to freezing-sensitive crops—primarily by using energy-costly practices to modify the microclimate—substantial economic losses resulting from freezing are incurred annually in a diverse array of agricultural crops.
Only modest increases (1–2°C) in the freezing tolerance of crop species would have a dramatic impact on agricultural productivity and profitability. The development of genotypes with increased freezing tolerance would provide a more reliable means to minimize crop losses from freezing stresses and greatly diminish the use of energy-costly practices to modify the microclimate. However, there has been little progress in improving the freezing tolerance of crop species by using classical plant breeding approaches. For example, the freezing tolerance of the best wheat varieties today is essentially the same as the most freezing-tolerant varieties developed in the early part of this century (1). Currently, there is considerable interest in the use of genetic engineering techniques for increasing the freezing tolerance of agricultural crop species.
Since 1985, when Guy et al. (2) first reported that gene expression is altered during cold acclimation, remarkable progress has been made in identifying an ever-increasing number of genes that are regulated by low temperatures (3, 4). Many of these genes encode hydrophilic polypeptides with little or no homology with previously described polypeptides. These include the COR and LTI genes of Arabidopsis thaliana (5–7), the COR and pao86 genes of barley (8, 9), and the A/ES genes of alfalfa (10). It is widely speculated that these genes might have roles in freezing tolerance because their level of expression is correlated positively with the freezing tolerance of several alfalfa cultivars (11), and the synthesis of the COR polypeptides coincides closely with increases in freezing tolerance (12–14). COR6.6, COR15a, COR47, and COR78 are among the genes that are most highly induced during cold acclimation of A. thaliana (3, 4), and the COR polypeptides have similarities with the “cryoprotective proteins” that reportedly protect chloroplast thylakoids from freeze-induced destabilization in vitro (15, 16).
The first direct evidence that any of the COR genes is functionally involved in the cold acclimation process was provided only recently (17). By constructing transgenic lines of A. thaliana that constitutively express the COR15a gene under nonacclimating conditions, we discovered that this gene affects the freezing tolerance of both chloroplasts frozen in situ and protoplasts frozen in vitro.
Nonetheless, the results were somewhat perplexing. Although constitutive expression of the COR15a gene resulted in a significant increase in protoplast survival over the range of −5 to −8°C, there was also a small decrease in survival over the range of −2 to −4°C. Furthermore, the mechanism by which the COR15a gene increased freezing tolerance was puzzling because the increase in survival was a manifestation of an increase in the cryostability of the plasma membrane and the COR15am polypeptide is located within the chloroplast stroma. We now present studies of the effect of the COR15a gene on specific freeze-induced lesions in the plasma membrane and chloroplast envelope that provide (i) an explanation for both the negative and positive effects on protoplast survival and (ii) a working hypothesis for the mechanism by which COR15am increases the cryostability of cellular membranes and freezing tolerance.
MATERIALS AND METHODS
Plant Materials.
A. thaliana (ecotype RLD) plants were cultivated for 17–20 days as described in ref. 18. Transgenic plants that constitutively synthesized COR15am were created by the procedures described in ref. 17.
Methods.
Protoplast isolation and determination of freezing tolerance were as described in ref. 17 with the modification that the protoplast suspensions were frozen at the various temperatures for 2.5 hr rather than 30 min. Freeze-fracture electron microscopy (FFEM) studies were conducted according to the procedures described in ref. 18 with the modification that the protoplast pellets were frozen at the various temperatures for 90 min before cryofixation.
Lipid dispersions for the NMR studies were prepared as follows. Dioleoylphosphatidylethanolamine (DOPE) in chloroform (Avanti Polar Lipids) was dried in vacuo overnight and then hydrated at 2°C with 20 mM Pipes/KOH (pH 7.4) at 10 wt% in the absence or presence of the COR15am polypeptide (2 wt%) and then kept on ice overnight. COR15am was purified by using the procedures described in ref. 19. Lipid dispersions composed of the major lipid species of the chloroplast envelope (monogalactosyldiacylglycerol, MGDG; digalactosyldiacylglycerol, DGDG; sulfoquinovosyldiacylglycerol, SQDG; phosphatidylcholine, PC; and phosphatidylglycerol, PG) were prepared from lipids isolated and purified from rye (Secale cereale L. cv Puma) leaves according to the procedures described in ref. 20.
1H-decoupled 31P-NMR spectra (161 MHz) were obtained with a Varian VXR-400 spectrometer. Samples were placed in the spectrometer at 0°C and equilibrated for 10 min. The temperature was then increased in increments of either 1°C (Fig. 3) or 5°C (Fig. 5) and equilibrated for 10 min at the indicated temperatures before recording the spectrum. X-ray diffraction powder patterns were obtained at the Cornell High Energy Synchrotron Source (CHESS). Samples were loaded in the instrument at −20°C and then warmed to the specified temperatures and equilibrated for 10 min before recording the powder patterns with an integrated CCD camera.
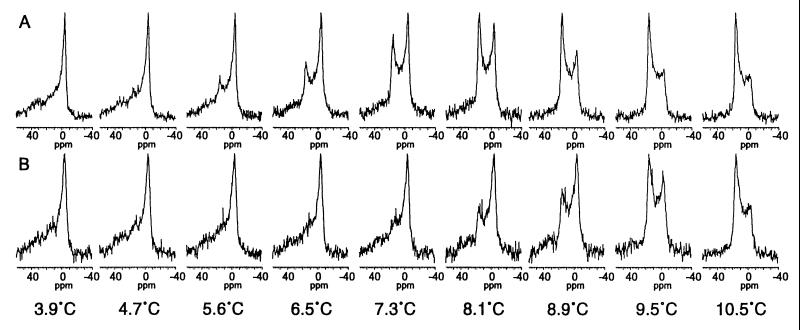
Figure 3.
31P-NMR spectra of DOPE suspended in Pipes buffer in either the absence (A) or the presence (B) of the COR15am polypeptide (2 mg/10 mg DOPE) at temperatures below and above the Tbh. The spectrum for the lamellar phase is characterized by a high-field peak from −2 to −6 ppm and a low-field shoulder from 25 to 35 ppm (e.g., the spectrum at 3.9°C) and the spectrum for the HII phase is characterized by a low-field peak from 15 to 18 ppm and a high-field shoulder from −3 to −5 ppm (e.g., the spectrum at 10.5°C) (38).
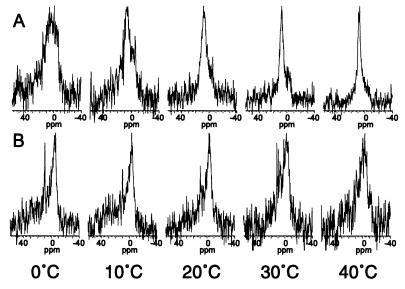
Figure 5.
31P-NMR spectra of a mixture of the major lipid species present in the chloroplast envelope (MGDG:DGDG:SQDG:PC:PG) in proportions (50:30:5:10:5 mol%) that resemble the inner membrane of the chloroplast envelope of rye leaves (27) in either the absence (A) or the presence (B) of the COR15am polypeptide (1 mg/10 mg lipid) at 10°C intervals from 0–40°C.
RESULTS AND DISCUSSION
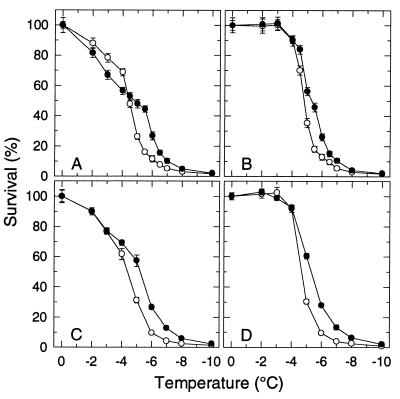
A comparison of the “survival signatures” (survival vs. freezing temperature) of protoplasts isolated from leaves of the transgenic COR15a plants (T8 line) with those isolated from the wild-type RLD demonstrates the negative and positive effects that expression of the COR15a gene under nonacclimating conditions has on the survival of isolated protoplasts (Fig. 1A) (17). Over the range of −2 to −4°C, survival of T8 protoplasts was less (6–12%) than that of the RLD protoplasts. Over the range of −5 to −7°C, survival of the T8 protoplasts was significantly greater than that of the RLD protoplasts, with the maximum difference (16% for RLD, 44% for T8) at −5.5°C. Both effects were very reproducible and also observed with lines of F3 families (obtained by crossing RLD with T8 plants) that either did or did not constitutively express COR15a (17).
Figure 1.
Freezing tolerance of protoplasts isolated from leaves of nonacclimated plants of A. thaliana: RLD (○), the wild type, and T8 (•), which constitutively expresses the COR15a gene. Protoplasts were suspended in either a 0.400 (A and B) or 0.413 (C and D) osmol sorbitol solution before freezing. (A and C) Survival after a conventional freeze/thaw treatment. (B and D) Survival after a freeze/hypertonic-thaw treatment. Survival (percentage of the unfrozen control) was determined by staining with fluorescein diacetate (17). Results shown are the mean and SD of three experiments.
The apparent paradoxical effect of the COR15a gene on survival can be explained by consideration of the specific freeze-induced lesions that limit survival. Freezing injury in protoplasts isolated from nonacclimated leaves of A. thaliana is the result of two different lesions in the plasma membrane that occur at different subfreezing temperatures (18).
Over the range of −2 to −4°C, the predominant form of injury is expansion-induced lysis (EIL), which is a consequence of the osmotic excursions incurred during a freeze/thaw cycle (21). Freeze-induced osmotic contraction results in endocytotic vesiculation of the plasma membrane, and the surface area of the plasma membrane is decreased. Sufficiently large area reductions are irreversible, and the protoplasts lyse during subsequent osmotic expansion during thawing of the suspending medium.
At temperatures below −4°C, injury is manifested as a loss of osmotic responsiveness that is the result of freeze-induced lamellar-to-hexagonal II phase transitions (LOR-HII) that involve the plasma membrane and various endomembranes—most often the chloroplast envelope—in regions where they are brought into close apposition as a result of freeze-induced dehydration and the removal of water from the surfaces of membranes (22). Although the protoplasts are osmotically responsive during freezing of the suspending medium, they are osmotically unresponsive during subsequent thawing. Osmotic responsiveness requires maintenance of the physical continuity and semipermeable characteristics of the plasma membrane. Freeze-induced formation of the HII phase disrupts both. Although the HII phase reverts to the lamellar phase and bilayers are reformed upon rehydration after thawing, the original membrane individuality is not necessarily restored and the plasma membrane is likely to become melded with the endomembranes, especially the chloroplast envelope, such that leakage of cytoplasmic and organelle contents occurs (see ref. 22).
By comparing the survival signatures of T8 and RLD (Fig. 1A) from a perspective of these two lesions, we hypothesized that the lower survival of T8 protoplasts over the range of −2 to −4°C was the result of an increased incidence of EIL and that the higher survival over the range of −5 to −7°C was the result of a decreased incidence of LOR-HII. Both of these predictions were directly confirmed.
EIL can be precluded by limiting the extent of osmotic expansion after thawing of the suspending medium. This is accomplished by adding a hypertonic solution to the partially thawed suspension and is referred to as a “freeze/hypertonic thaw” treatment (23). Whereas survival of protoplasts subjected to a conventional freeze/thaw cycle (Fig. 1A) reflects the combined incidence of EIL and LOR-HII, survival of protoplasts subjected to a freeze/hypertonic thaw treatment (Fig. 1B) is limited only by LOR-HII. The incidence of EIL can be calculated as the difference in survival between a freeze/hypertonic thaw treatment and a conventional freeze/thaw cycle.
The incidence of EIL at −2, −3, −4, and −4.5°C, which was calculated from the results shown in Fig. 1 A and B, was indeed higher for T8 protoplasts (18, 33, 34, and 31%) than the RLD protoplasts (12, 22, 22, and 23%). However, the difference in the incidence of EIL between T8 and RLD could be eliminated by increasing the osmolality of the suspending medium in which the protoplasts were isolated and subsequently frozen from 0.400 to 0.413 (Fig. 1 C and D). In our initial studies (17), plasmolysis studies of thin sections of leaves indicated that 0.400 osmol was the optimum tonicity (slightly hypertonic) for isolation of RLD protoplasts, and it was assumed to be the same for T8. However, subsequent leaf plasmolysis studies revealed that the tonicity of the cytoplasm is slightly higher in T8 and that 0.413 is the optimum osmolality for isolating protoplasts from T8 leaves. When suspended and frozen in a 0.400 osmol solution, the plasma membrane of T8 protoplasts will undergo larger surface areal changes during freeze/thaw-induced osmotic contraction/expansion and increase the incidence of EIL (21). Thus, the negative effect (i.e., an increased incidence of EIL) observed with T8 protoplasts in the initial studies (17) when the protoplasts were suspended in a 0.400 osmol solution is an artifact due to the T8 protoplasts not being suspended in a solution of the appropriate osmolality. The reason for the small difference (0.013 osmol) in the intracellular osmolality is not known at this time.
A comparison of the survival signatures of RLD and T8 protoplasts suspended in a 0.413 osmol solution (Fig. 1C) or after a freeze/hypertonic thaw treatment (Fig. 1 B and D) demonstrates the positive effect of expression of the COR15a gene on freezing tolerance. Over the range of −4.5 to −7°C, survival of T8 protoplasts is greater than that of RLD because of a decrease in the incidence of LOR-HII. As a result, the survival curve is shifted by ∼1°C. The fact that the increase in survival occurred only over a limited range is not unexpected. With RLD protoplasts, the incidence of LOR-HII, which limits the maximum freezing tolerance of nonacclimated leaves, increases from <10% to >90% between −4 and −6°C (Fig. 1 B and D). Most important, the increased survival over the range of −4.5 to −7°C is not unique to isolated protoplasts and also is observed in excised leaves (M.U. and P.L.S., unpublished results).
FFEM studies have provided direct confirmation that the increased survival of the T8 protoplasts was associated with a decreased incidence of freeze-induced formation of the HII phase. The HII phase is a three-dimensional array of inverted cylindrical micelles that is characterized by periodicity in the two dimensions normal to the long axis of the cylinders. Formation of the HII phase is an interbilayer event and is observed most often in regions where the plasma membrane is brought into close apposition with the chloroplast envelope as a result of freeze-induced dehydration. (The thylakoids have never been observed to be involved.) However, because the HII phase is a nonlamellar phase, it does not occur in either the plasma membrane or chloroplast envelope per se. Participation of the plasma membrane and the chloroplast envelope in the formation of the HII phase is evident in Fig. 2A in which regions of the envelope and plasma membrane appear to be contiguous with the HII domain.
Figure 2.
FFEMs of protoplasts isolated from leaves of nonacclimated seedlings (17–20 days old) and suspended in a 0.40 osmol sorbitol solution and frozen to −6°C for 90 min. (A) Protoplasmic fracture face of the plasma membrane (pm) of a T8 protoplast showing a typical, lamellar region, which is characterized by a random distribution of intramembranous particles, melding into a region where the HII phase has formed. The plasma membrane, which also contains numerous aparticulate domains (apd), is overlaying the chloroplast envelope (ce) with the HII phase appearing in localized regions. Two other ultrastructural morphologies are commonly observed to be associated with freeze-induced formation of the HII phase in biological cells: well-ordered striations in the plasma membrane (pm-st) and the chloroplast envelope (ce-st) and loosely ordered swirls. V, vitreous layer of unfrozen suspending medium; i, ice. (Magnification: ×35,700; bar represents 0.5 nm; arrow indicates direction of shadowing). (B) High magnification (×65,200) micrograph of an RLD protoplast illustrating the tightly packed cylinders that are characteristic of the HII phase (HII), the well-ordered striations (pm-st), and the loosely ordered swirls (pm-sw). (Bar represents 0.25 nm; arrow indicates direction of shadowing).
In addition to the tightly packed cylinders characteristic of the HII phase, two other ultrastructural morphologies are commonly observed to be associated with freeze-induced formation of the HII phase: well-ordered striations and loosely ordered swirls in regions of the plasma membrane and chloroplast envelope (Fig. 2) (18, 22, 24). The origin of the swirls and striations is not known; however, it is possible that they are intermediate stages in the lyotropically induced lamellar-to-hexagonal II phase transition (23, 24).
The incidence of the HII morphologies determined in the FFEM studies closely paralleled the protoplast mortality attributed to LOR-HII in both RLD and T8 (Table 1). Also, the frequency of occurrence of the various ultrastructural morphologies induced by freeze-induced dehydration at a particular temperature was different for RLD and T8 protoplasts. Although the frequency of tightly packed cylinders increased with decreasing temperature in both RLD and T8, at any particular temperature, they occurred at a much higher frequency in the RLD protoplasts. Also, the frequency of the various morphologies in RLD at −5°C was similar to that of T8 at −6°C. That is, the incidence was shifted by ∼1°C, which is the same magnitude of the shift in the survival signature (Fig. 1).
Table 1.
Protoplast mortality and the incidence of the HII phase (tightly packed cylinders) and associated ultrastructural morphologies (well-ordered striations and loosely ordered swirls) in protoplasts isolated from leaves of nonacclimated RLD and T8 plants
| RLD | T8 | ||
|---|---|---|---|
| −5.0°C | Protoplast mortality (LOR-HII) | 65% | 44% |
| Incidence of HII morphologies | 63% (32/51) | 37% (33/89) | |
| loosely ordered swirls | 33% (17/51) | 26% (23/89) | |
| well-ordered striations | 33% (17/51) | 15% (13/89) | |
| tightly packed cylinders | 22% (11/51) | 7% (6/89) | |
| −5.5°C | Protoplast mortality (LOR-HII) | 83% | 56% |
| Incidence of HII morphologies | 76% (82/108) | 57% (100/176) | |
| loosely ordered swirls | 15% (16/108) | 45% (80/176) | |
| well-ordered striations | 22% (24/108) | 17% (30/176) | |
| tightly packed cylinders | 44% (47/108) | 9% (16/176) | |
| −6.0°C | Protoplast mortality (LOR-HII) | 87% | 74% |
| Incidence of HII morphologies | 91% (19/21) | 74% (84/113) | |
| loosely ordered swirls | 62% (13/21) | 44% (50/113) | |
| well-ordered striations | 67% (14/21) | 42% (48/113) | |
| tightly packed cylinders | 52% (11/21) | 21% (24/113) |
Protoplast mortality (LOR-HII) was calculated from the survival data after a freeze/hypertonic thaw treatment shown in Fig. 1B. The number before the slash represents the number of protoplasts in which the particular morphology was observed; the number after the slash represents the total number of protoplasts observed.
What Is the Mechanism by Which the COR15a Gene Decreases the Incidence of Freeze-Induced Formation of the HII Phase?
The COR15a gene encodes a 15-kDa polypeptide, COR15a, that is targeted to the chloroplast (5). During import into the chloroplast, the signal sequence is removed resulting in the mature (9.4 kDa) polypeptide designated COR15am. On first consideration, it is very puzzling how COR15am, which is located in the chloroplast stroma, can minimize the incidence of freeze-induced formation of the HII phase, which is an interbilayer phenomenon that involves the chloroplast envelope and the plasma membrane and other membranes that are exterior to the chloroplast envelope. As a working hypothesis, we propose that: (i) the onset (freezing temperature) of the formation of the HII phase is determined by the membrane that has the greatest propensity to form the HII phase, i.e., the “weak link” in the ensemble. In our FFEM studies (18, 22, 24–26), the chloroplast envelope, which is comprised of an inner and outer membrane, and the plasma membrane are most often involved in freeze-induced formation of the HII phase. If the inner membrane of the chloroplast envelope is the weak link in the ensemble, then COR15am, which is located within the chloroplast stroma (5), could decrease the incidence of the HII phase by affecting only the propensity of the inner membrane of the chloroplast envelope to form the HII phase.
The supposition that the inner membrane of the chloroplast envelope is the “weak link” in the ensemble that undergoes the lamellar-to-hexagonal II phase transition derives from an analysis of the lipid composition of the inner and outer membranes of the chloroplast envelope of rye (27). MGDG, which has a very high propensity to form the HII phase, comprises 48 mol% of the total lipids in the inner membrane, but only 20 mol% in the outer membrane. The proportion of PC, which would stabilize the bilayer configuration and decrease the propensity to form the HII phase, is much lower in the inner (8 mol%) than in the outer (32 mol%) membrane. The proportion of DGDG, which also has a low propensity to form the HII phase, is similar in both (30 mol%). Thus, the ratio of nonbilayer to bilayer-forming lipids (MGDG:DGDG + PC) is 1.21 in the inner membrane and 0.32 in the outer membrane. This difference in the lipid composition of the inner and outer membranes of the chloroplast envelope also occurs in spinach (28) and pea (29). A priori, there is no reason to suggest that A. thaliana is any different.
31P-NMR studies of the phase behavior of lipid mixtures composed of the major lipid classes that comprise the chloroplast envelope (MGDG:DGDG:SQDG:PC) in proportions similar to those present in the outer and inner membranes have confirmed that the inner membrane has a greater propensity to form nonlamellar phases than does the outer membrane (30).
(ii) The COR15am polypeptide, which is predicted to be composed largely of amphipathic α-helical regions (5, 31), alters the intrinsic curvature of the monolayers that comprise the inner membrane of the chloroplast envelope such that its propensity to form the HII phase is decreased and deferred to lower freezing temperatures (lower hydrations). This supposition is based on reports that amphipathic polypeptides that form an α-helix can have a strong effect on the intrinsic curvature of monolayers and that a shift in the lamellar-to-hexagonal II phase transition temperature (Tbh) is an extremely sensitive test of an effect on monolayer curvature (32).
To test this hypothesis experimentally, we first conducted 31P-NMR studies of the effect of COR15am on the Tbh of DOPE. In these studies, the onset of the Tbh occurred between 4.7 and 5.6°C, with the proportion of the lipid in the HII phase increasing between 5.6 and 10.5°C (Fig. 3A). When the DOPE liposomes were prepared in the presence of COR15am, the onset of the Tbh occurred between 7.3 and 8.1°C, with the proportion of the HII phase progressively increasing between 8.1 and 10.5°C (Fig. 3B). Thus, COR15am increased the onset Tbh of DOPE by ∼2°C, which was similar in magnitude to both the shift in the survival signature of the T8 protoplasts relative to that of the RLD protoplasts (Fig. 1) and the shift in the incidence of the freeze-induced formation of the HII phase determined in the FFEM studies (Table 1). We observed no effect of BSA when it was included at the same concentration that was used for COR15am.
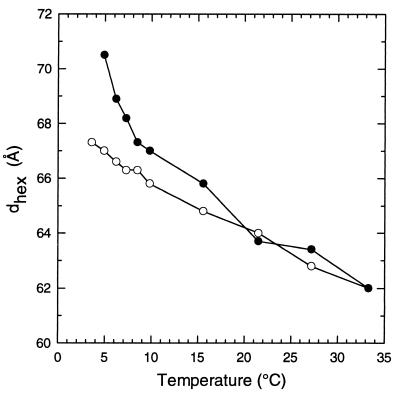
X-ray diffraction studies revealed that COR15am also increased the hexagonal lattice spacing of the HII phase of DOPE between 5 and 15°C (Fig. 4). In these studies, the onset Tbh of DOPE was 3.6°C in the absence and 4.9°C in the presence of COR15am.
Figure 4.
X-ray diffraction measurements of the hexagonal lattice spacing of the HII phase formed by DOPE in Pipes buffer (20 mM, pH 7.4) in either the absence (○) or the presence (•) of the COR15am polypeptide (2 mg/10 mg DOPE).
We next determined the effect of COR15am on the phase behavior of a complex mixture composed of the major lipid species in the inner membrane of the chloroplast envelope. At 0°C, the 31P-NMR spectrum (Fig. 5A) of this mixture consisted of a broad peak between ∼12 and −6 ppm and appeared to be a composite of several phases (lamellar, nonlamellar, and possibly cubic), which is commonly reported for mixtures of MGDG:DGDG and the lipid extract of chloroplasts (33–36). Cullis et al. (37) have suggested that a symmetrical peak that is intermediate between the peaks of the lamellar (from −2 to −6 ppm) and the HII phase (from 15 to 18 ppm) but not as narrow as one from a pure cubic phase or small vesicles can be due to isotropic motion averaging of lipids primarily in a bilayer configuration experiencing rapid exchange with a small proportion of lipids in micelles or the cubic phase.
When the liposomes were prepared in the presence of COR15am, the 31P-NMR spectrum at 0°C (Fig. 5B) was characteristic of the lamellar phase (a high-field peak from −2 to −6 ppm and a low-field shoulder from 25 to 35 ppm) (38). In addition, a narrow, isotropic motion peak at 10 ppm, which may be the result of a small proportion of the lipid in either the cubic phase, inverted micelles, or small vesicles, also was observed. Thus, upon hydration, the lipid mixture formed a mixture of lamellar and nonlamellar phases; however, addition of COR15am stabilized the lipid mixture in the lamellar phase.
COR15am also affected the thermotropic phase behavior of the inner membrane lipid mixture during heating (Fig. 5). After heating to 40°C, the 31P-NMR spectrum of the lipid mixture without the COR15am polypeptide (Fig. 5A) consisted of a narrow, nearly symmetrical peak at 9 ppm, which is characteristic of isotropic motion averaging of lipids in either the cubic or inverted micellar phase (38). In contrast, when the lipid mixture containing COR15am was heated, the lamellar phase was still present at 40°C (Fig. 5B). Collectively, the results of the NMR and x-ray diffraction studies support the hypothesis that COR15am alters the intrinsic curvature of the inner membrane of the chloroplast envelope.
These studies establish that expression of the COR15a gene decreases the propensity for freeze-induced formation of the HII phase (LOR-HII)—the lesion that limits the freezing tolerance of nonacclimated leaves of A. thaliana and winter cereals (rye, wheat, barley, and oat). In these species, the propensity for freeze-induced formation of the HII phase is decreased during the initial stages of cold acclimation such that LOR-HII does not occur in cold-acclimated leaves (22). Although the COR15a gene is involved in this process other factors also must be involved. Whereas LOR-HII is completely precluded after cold acclimation, constitutive expression of the COR15a gene only defers the occurrence of the freeze-induced formation of the HII phase to lower temperatures. Alterations in membrane lipid composition and the accumulation of sugars also contribute to the decreased propensity for formation of the HII phase (22). It also is possible that other COR genes are involved.
Obviously, transformation with the COR15a gene will have little effect on the maximum freezing tolerance of species that undergo moderate to large increases in freezing tolerance during cold acclimation (e.g., oat, barley, wheat, rye). Nevertheless, transformation with the COR15a gene may increase their tolerance to freezing in early fall or late spring when their freezing tolerance is limited by LOR-HII.
More important, these findings have potential application for increasing the freezing tolerance of crop species in which LOR-HII limits maximum freezing tolerance, e.g., extremely frost-sensitive species that undergo little, if any, increase in freezing tolerance. With such species, the construction of transgenic plants that express the COR15a gene could result in an increase in freezing tolerance that is of practical significance—even if it is only 1–2°C. Such species, however, must first be identified. So, just as elucidation of the role of the COR15a gene in the cold acclimation process has required the identification of specific lesions that limit freezing tolerance, so too will the development of rational strategies for the use of molecular biology approaches to improve the freezing tolerance of agricultural crop species.
Acknowledgments
We thank Ernest Fontes (Cornell High Energy Synchrotron Source) and Cathy Lester and David J. Fuller (NMR Facility, Dept. of Chemistry) for their technical assistance. Supported by grants from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (96–35100-3163), the U.S. Department of Energy (DE-FG01–84ER13214) (P.L.S.), the National Science Foundation (IBN-9307348), and the Michigan Agricultural Experiment Station (M.F.T.).
ABBREVIATIONS
- COR
cold-regulated
- EIL
expansion-induced lysis
- HII
hexagonal II phase
- LOR-HII
loss of osmotic responsiveness that is associated with freeze-induced formation of the hexagonal II phase
- Tbh
lamellar-to-hexagonal II phase transition temperature
- DOPE
dioleoylphosphatidylethanolamine
- osmol
osmolal
- FFEM
freeze-fracture electron microscopy
- MGDG
monogalactosyldiacylglycerol
- DGDG
digalactosyldiacylglycerol
- SQDG
sulfoquinovosyldiacylglycerol
- PC
phosphatidylcholine
- PG
phosphatidylglycerol
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Limin A E, Fowler D B. Field Crops Res. 1991;27:201–218. [Google Scholar]
- 2.Guy C L, Niemi K J, Brambl R. Proc Natl Acad Sci USA. 1985;82:3673–3677. doi: 10.1073/pnas.82.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomashow M F. Adv Gen. 1990;28:99–131. [Google Scholar]
- 4.Thomashow M F. In: Advances in Low-Temperature Biology. Steponkus P L, editor. Vol. 2. London: JAI Press; 1993. pp. 183–210. [Google Scholar]
- 5.Lin C, Thomashow M F. Plant Physiol. 1992;99:519–525. doi: 10.1104/pp.99.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmour S J, Artus N N, Thomashow M F. Plant Mol Biol. 1992;18:13–22. doi: 10.1007/BF00018452. [DOI] [PubMed] [Google Scholar]
- 7.Nordin K, Vahala T, Palva E T. Plant Mol Biol. 1993;21:641–653. doi: 10.1007/BF00014547. [DOI] [PubMed] [Google Scholar]
- 8.Cattivelli L, Bartels D. Plant Physiol. 1990;93:1504–1510. doi: 10.1104/pp.93.4.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosatti C, Nevo E, Stanca A M, Cattivelli L. Theor Appl Genet. 1996;93:975–981. doi: 10.1007/BF00224101. [DOI] [PubMed] [Google Scholar]
- 10.Luo M, Liu J-H, Mohapatra S, Hill R D, Mohapatra S S. J Biol Chem. 1992;267:15367–15374. [PubMed] [Google Scholar]
- 11.Mohapatra S S, Wolfraim L, Poole R J, Dhindsa R S. Plant Physiol. 1989;89:375–380. doi: 10.1104/pp.89.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy C L, Haskell D L. Plant Physiol. 1987;84:872–878. doi: 10.1104/pp.84.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohapatra S S, Poole R J, Dhindsa R S. Plant Physiol. 1987;84:1172–1176. doi: 10.1104/pp.84.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomashow M F, Gilmour S J, Hajela R, Horvath D, Lin C, Guo W. In: Horticultural Biotechnology. Bennett A B, O’Neill S D, editors. New York: Wiley-Liss; 1990. pp. 305–314. [Google Scholar]
- 15.Volger H G, Heber U. Biochim Biophys Acta. 1975;412:335–349. doi: 10.1016/0005-2795(75)90048-3. [DOI] [PubMed] [Google Scholar]
- 16.Hincha D K, Sieg F, Bakaltcheva I, Köth H, Schmitt J M. In: Advances in Low-Temperature Biology. Steponkus P L, editor. Vol. 3. London: JAI Press; 1996. pp. 141–183. [Google Scholar]
- 17.Artus N N, Uemura M, Steponkus P L, Lin C, Thomashow M F. Proc Natl Acad Sci USA. 1996;93:13404–13409. doi: 10.1073/pnas.93.23.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uemura M, Joseph R A, Steponkus P L. Plant Physiol. 1995;109:15–30. doi: 10.1104/pp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmour S J, Lin C, Thomashow M F. Plant Physiol. 1996;111:293–299. doi: 10.1104/pp.111.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch D V, Steponkus P L. Plant Physiol. 1987;83:761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steponkus P L. In: Endocytosis, Exocytosis, and Vesicle Traffic in Plants. Hawes C R, Coleman J O D, Evans D E, editors. Cambridge: Soc. Exp. Biol. Sem.; 1991. , Series 45, pp. 103–128. [Google Scholar]
- 22.Steponkus P L, Uemura M, Webb M S. In: Advances in Low-Temperature Biology. Steponkus P L, editor. Vol. 2. London: JAI Press; 1993. pp. 211–312. [Google Scholar]
- 23.Uemura M, Steponkus P L. Plant Physiol. 1989;91:1131–1137. doi: 10.1104/pp.91.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb M S, Steponkus P L. Plant Physiol. 1993;101:955–963. doi: 10.1104/pp.101.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb M S, Uemura M, Steponkus P L. Plant Physiol. 1994;104:467–478. doi: 10.1104/pp.104.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steponkus P L, Uemura M, Webb M S. In: Permeability and Stability of Lipid Bilayers. Disalvo E A, Simon S A, editors. Boca Raton, FL: CRC Press; 1995. pp. 77–104. [Google Scholar]
- 27.Uemura M, Steponkus P L. Plant Physiol. 1997;114:1493–1500. doi: 10.1104/pp.114.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block M A, Dorne A J, Joyard J, Douce R. J Biol Chem. 1983;258:13281–13286. [PubMed] [Google Scholar]
- 29.Cline K, Andrews J, Mersey B, Newcomb E H, Keegstra K. Proc Natl Acad Sci USA. 1981;78:3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uemura M, Steponkus P L. In: Plant Cold Hardiness Molecular Biology, Biochemistry, and Physiology. Li P H, Chen T T, editors. New York: Plenum; 1997. pp. 171–179. [Google Scholar]
- 31.Wilhelm K S. Ph.D. thesis. East Lansing, MI: Michigan State Univ.; 1996. [Google Scholar]
- 32.Epand R M, Shai Y, Segrest J P, Anantharamaiah G M. Biopolymers. 1995;37:319–338. doi: 10.1002/bip.360370504. [DOI] [PubMed] [Google Scholar]
- 33.Quinn P J, Williams W P. Biochim Biophys Acta. 1983;737:223–266. [Google Scholar]
- 34.Brentel I, Selstam E, Lindblom G. Biochim Biophys Acta. 1985;812:816–826. [Google Scholar]
- 35.Borovyagin V L, Tarakhovsky Y S, Vasilenko I A. Biochim Biophys Acta. 1988;939:111–123. [Google Scholar]
- 36.Gounaris K, Sen A, Brain A P R, Quinn P J, Williams W P. Biochim Biophys Acta. 1983;728:129–139. [Google Scholar]
- 37.Cullis P R, van Dijck P W M, de Kruijff B, de Gier J. Biochim Biophys Acta. 1978;513:21–30. doi: 10.1016/0005-2736(78)90108-6. [DOI] [PubMed] [Google Scholar]
- 38.Cullis P R, de Kruijff B. Biochim Biophys Acta. 1979;559:399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]