Abstract
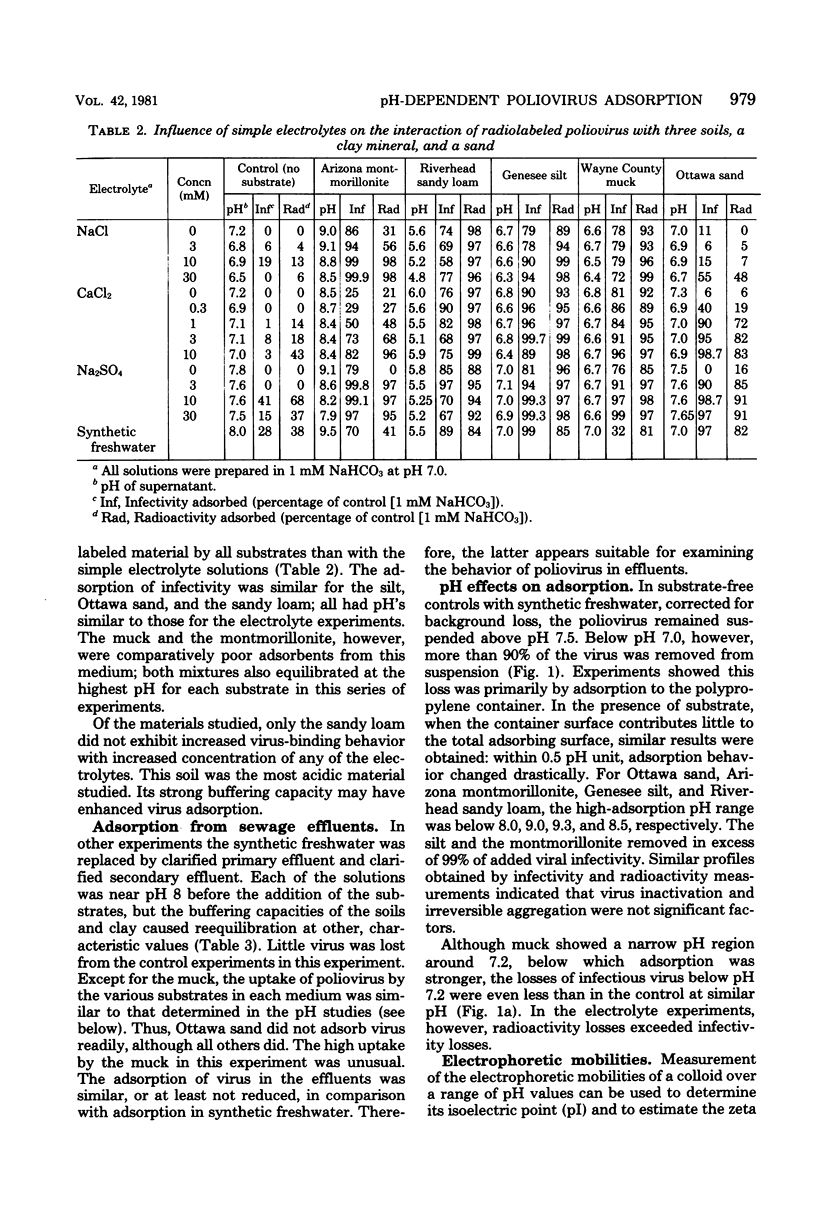
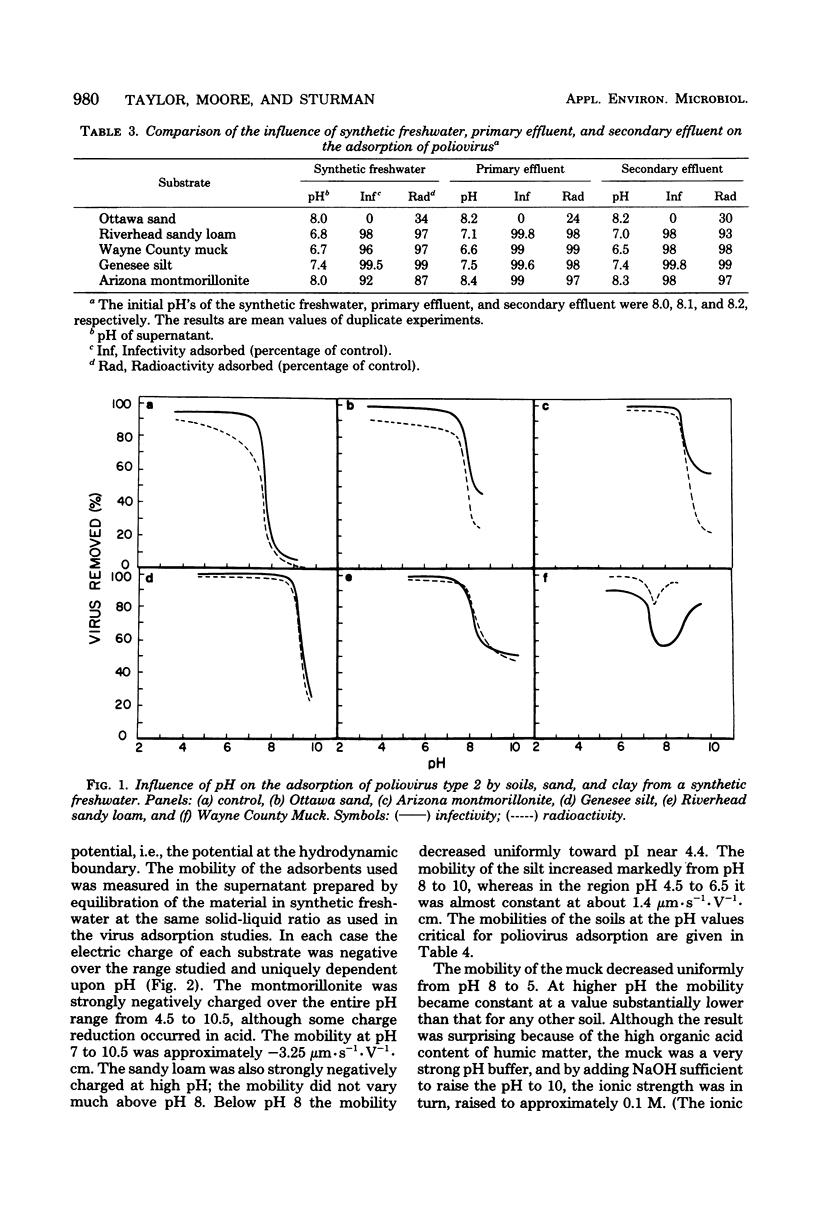
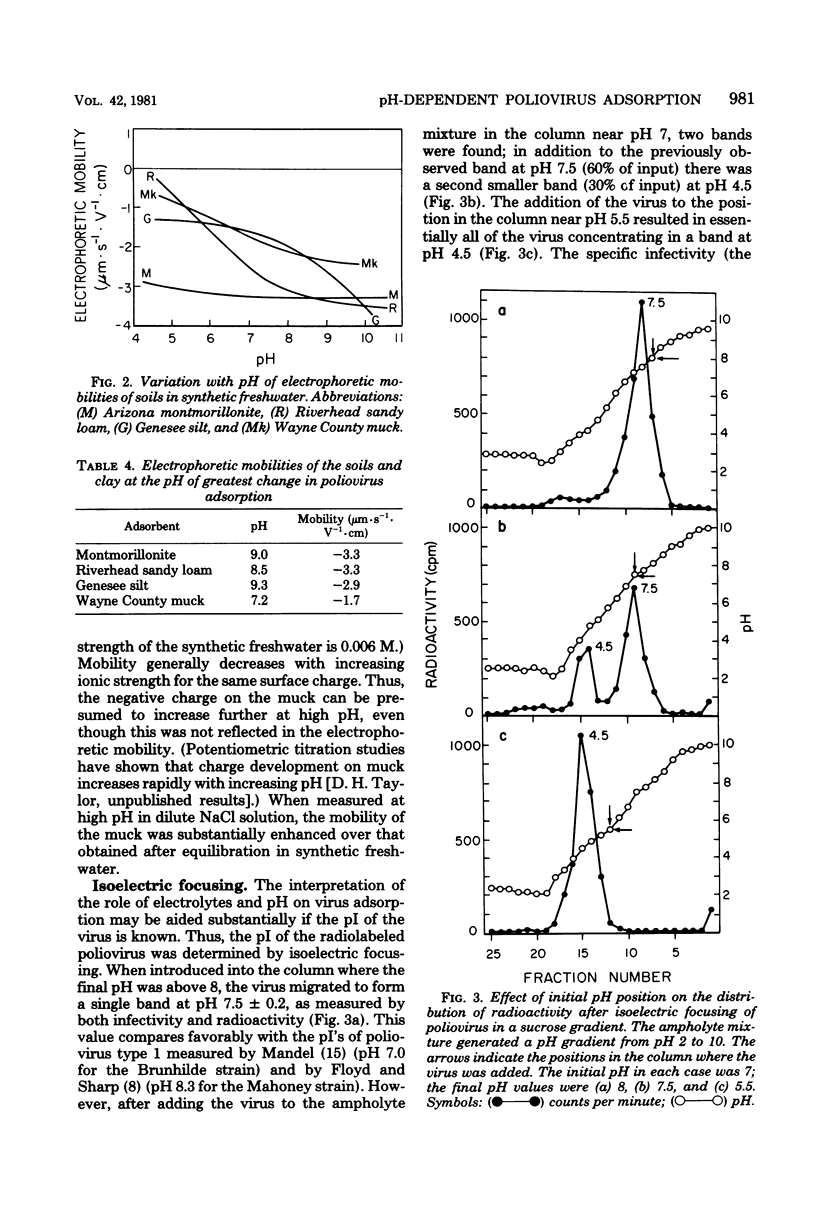
The pH and the nature an concentration of simple electrolytes influenced the interaction of poliovirus type 2 with three soils, a sand, and a clay mineral. In electrolytes above pH 9 the virus was not adsorbed extensively to the substrates, but below pH 7 almost all virus was bound. For each adsorbent there was a characteristic pH region of transition from strong to weak uptake. Differences between the soils in virus uptake were shown to parallel their pH-dependent mineral. In electrolytes above pH 9 the virus was not adsorbed extensively to the substrates, but below pH 7 almost all virus was bound. For each adsorbent there was a characteristic pH region of transition from strong to weak uptake. Differences between the soils in virus uptake were shown to parallel their pH-dependent mineral. In electrolytes above pH 9 the virus was not adsorbed extensively to the substrates, but below pH 7 almost all virus was bound. For each adsorbent there was a characteristic pH region of transition from strong to weak uptake. Differences between the soils in virus uptake were shown to parallel their pH-dependent charge properties, as determined by whole-particle microelectrophoresis. Only when the pH was close to or above the critical region was uptake increased with electrolyte concentration. The transition region for all substrates was above pH 7.5 the isoelectric point of the virus. Thus, it appears that when both the virus and substrate are highly negative charged, repulsive electrostatic effects may exceed inherent attractive interactions, thereby inhibiting adsorption.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTELL P., PIERZCHALA W., TINT H. The adsorption of enteroviruses by activated attapulgite. J Am Pharm Assoc Am Pharm Assoc. 1960 Jan;49:1–4. [PubMed] [Google Scholar]
- Drewry W. A., Eliassen R. Virus movement in groundwater. J Water Pollut Control Fed. 1968 Aug;(Suppl):257–271. [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: effects of salts on the aggregation of poliovirus and reovirus at low pH. Appl Environ Microbiol. 1978 Jun;35(6):1084–1094. doi: 10.1128/aem.35.6.1084-1094.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979 Aug;38(2):241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Yin F. H., Noble-Harvey J. Fractionation of biologically active and inactive populations of human rhinovirus type 2. Virology. 1975 Feb;63(2):384–394. doi: 10.1016/0042-6822(75)90311-6. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K. Zonal electrophoresis and isoelectric focusing of proteins and virus particles in density gradients of small volume. Anal Biochem. 1974 May;59(1):75–82. doi: 10.1016/0003-2697(74)90011-6. [DOI] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Moore R. S., Taylor D. H., Sturman L. S., Reddy M. M., Fuhs G. W. Poliovirus adsorption by 34 minerals and soils. Appl Environ Microbiol. 1981 Dec;42(6):963–975. doi: 10.1128/aem.42.6.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. H., Bosmann H. B. Measurement of the electrokinetic properties of vaccinia and reovirus by laser-illuminated whole-particle microelectrophoresis. J Virol Methods. 1981 Apr;2(5):251–260. doi: 10.1016/0166-0934(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Fields J. E. Concentration and purification of viruses by adsorption to and elution from insoluble polyelectrolytes. Appl Microbiol. 1971 Apr;21(4):703–709. doi: 10.1128/am.21.4.703-709.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



