SUMMARY
Little data are available on cellular immune responses during infection with hepatitis E virus (HEV). We therefore mapped CD4 T-cell epitopes in open reading frame (ORF)2 and ORF3 proteins of HEV using lymphocyte proliferation assays and overlapping peptide libraries. Proliferation of peripheral blood mononuclear cells from 40 patients with acute hepatitis E and 21 healthy controls with recombinant HEV ORF2 protein or pools of overlapping HEV ORF2/ORF3 peptides was measured. HLA-DQB1 and HLA-DRB1 alleles were also determined. Mononuclear cells from patients with hepatitis E more often showed significant proliferation on stimulation with recombinant ORF2 protein than controls (32/40 vs 7/21), and had higher median (range) stimulation indices [2.6 (0.9–15.2) vs 1.3 (0.6–12.9)]. Peptide pools corresponding to amino acids 73–156, 289–372, 361–444 and 505–588 of HEV ORF2 protein were associated with significant proliferation. Individual peptides in these pools did not show a clear pattern of stimulation. HEV ORF3 peptide pools did not induce proliferative responses. Lymphocyte proliferation in response to the peptide pool corresponding to amino acids 289–372 of HEV ORF2 protein was associated with presence of HLA-DRB1 allele 010X. These data on mapping of T-cell epitopes in HEV proteins may prove useful for designing HEV vaccines and for studying the immunopathogenesis of hepatitis E.
Keywords: cellular immune response, immunopathogenesis, lymphocytes, lymphocyte proliferation assay, peripheral blood mononuclear cells, vaccination
INTRODUCTION
Hepatitis E virus (HEV) infection is a major cause of acute viral hepatitis in several developing countries, in particular those in South and Southeast Asia, North Africa, the Middle East, etc. [1,2]. The infection is responsible for large outbreaks of viral hepatitis, each affecting several hundred to several thousand subjects [3], and a large proportion of sporadic hepatitis E cases in endemic regions. Transmission is predominantly faecal–oral, usually through contaminated drinking water [1,2]. Person-to-person transmission is uncommon [4,5]. The disease is characterized by a high attack rate among young adults, a relative sparing of children, and a particularly high attack rate and mortality (up to 15–20%) among pregnant women [1,2]. Infection is usually self-limiting and chronicity is not known.
The HEV virions are small, nonenveloped, 32–34 nm diameter particles with icosahedral symmetry. The viral genome is approximately 7.2-kb long, single-stranded, positive-sense, polyadenylated RNA that contains three open reading frames (ORF) [6]. The ORF1 codes for nonstructural proteins and ORF2 for a 660-amino acid viral capsid protein. The ORF3 encodes a 123-amino acid phosphoprotein that appears to associate with the cytoskeleton [7], as well as the viral capsid protein [8]; this protein appears to play a role in modulation of cell signalling [9] and in the assembly of viral nucleocapsid [8].
Humoral immune responses against HEV have been studied in detail [10-16]. These studies show prominent antibody response directed against immunodominant antigenic epitopes in the ORF2 and ORF3 proteins [10-15]. In addition, several B-cell epitopes have also been identified in the ORF1 protein [16]. An IgM anti-HEV response appears during the early phase of clinical illness and disappears over 4–5 months [10]. The IgG antibodies follow a few days later, are longer lived, persisting for several years at least in some patients [17]. Humoral immune responses have been used for the diagnosis of acute infection or prior exposure to HEV.
Attempts have been made to use recombinant proteins corresponding to the viral capsid proteins as candidate vaccines against HEV infection. Animals vaccinated with these proteins develop anti-HEV antibodies, and are protected from disease, although not against infection [18,19]. However, such protection appears to be short lasting [20].
In contrast to humoral immune responses, only limited data [21,22] are available on cellular immune responses during HEV infection. Information on cellular immune responses in HEV infection may have several implications. First, T-cell immune responses may be helpful in providing protection against viruses. Thus, viral proteins that contain T-cell epitopes may prove useful as potential vaccines, by stimulating T-cell immunity and by providing T-cell help for antibody production. Further, activation of T-cell immune responses may downregulate viral replication through a cytokine-mediated, noncytolytic pathway, as has been shown to occur in hepatitis B and C virus infections [23]. Secondly, cellular immune responses may play a role in host cell injury. Thus, information on cellular responses may help us better understand the pathogenetic mechanisms in HEV infection. We have previously shown proliferation of lymphocytes from patients with acute hepatitis E in response to HEV peptides [21], and differences in cellular immune responses in pregnant and nonpregnant women with this infection [22]. In this report, we present our results on T-cell epitope mapping of HEV ORF2 and ORF3 proteins using overlapping peptides encompassing the entire lengths of these proteins.
METHODS
Patients and controls
Patients with acute hepatitis E, who had a characteristic clinical picture and biochemical evidence of acute hepatitis, and who tested positive for serum IgM anti-HEV antibodies and negative for HBsAg and anti-HCV antibody, were included in the study. Healthy adult subjects with no recent history of acute hepatitis and testing negative for serum IgG anti-HEV antibodies were studied as controls. Serum IgM and IgG anti-HEV antibodies were detected using commercial enzyme immunoassays (Genelabs Diagnostics, Singapore, Singapore).
An Institutional Ethics Committee approved the study protocol and all study subjects provided informed consent.
Hepatitis E virus open reading frame 2 protein and peptides
Overlapping 20-mer peptides corresponding to the amino acid sequences of the ORF2 and ORF3 proteins of Burmese strain of HEV (GenBank accession number M73218) were synthesized (Mimotopes, Clayton, Vic., Australia). These peptides were offset from each other by eight amino acids; thus, consecutive peptides had an overlap of 12 amino acids. A total of 81 and 14 peptides corresponding to ORF2 and ORF3 proteins, respectively, were synthesized (Appendix 1).
The ORF2 protein was expressed in insect Tn5 cells using a recombinant baculovirus vector system and purified as described elsewhere [24]. When expressed in this manner, the full-length ORF2 protein is processed into an approximately 56 kDa form that encompasses amino acids 112–607 of this protein.
Lymphocyte proliferation studies [25]
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized (10 U/mL) venous blood by density gradient centrifugation using Histopaque (Sigma, St Louis, MO, USA); washed in phosphate-buffered saline and resuspended in complete RPMI-1640 containing 10% heat-inactivated foetal calf serum (Sigma), 2 mM glutamine and antibiotic and antimycotic mixture (Invitrogen, Carlsbad, CA, USA).
Triplicate 200-μL cultures were set up in flat-bottom 96-well tissue culture plates (Nunc, Roskilde, Denmark) using 105 cells per well and either phytohaemagglutinin (PHA, 1:200; Gibco-BRL, Rockville, MD, USA) or purified recombinant ORF2 protein of HEV (2 μg/well) or a pool of peptides corresponding to the HEV ORF2 or ORF3 protein (2 μg/well of each peptide dissolved in DMSO). A total of 11 pools were used (Appendix 1); nine of these pools (1–9) contained nine consecutive peptides each corresponding to the ORF2 protein (10 μg/mL of each peptide) and two (10 and 11) contained seven peptides each corresponding to the ORF3 protein.
Cells were cultured at 37 °C in a 5% CO2 atmosphere for 3 days in case of PHA and for 5 days in case of HEV protein or HEV peptide pools. The concentration of DMSO in cultures was ≤1:100. Tritiated thymidine (0.5 μCi/well) was added during the last 18 h of culture, cells were harvested and radioactivity measured by liquid scintillation counting. The results were expressed as stimulation indices (SI) where SI = count per min for test culture/count per min for unstimulated control culture. A significant proliferation response was defined as SI ≥ 2.0. For experiments in which controls showed reactivity, additional analysis was carried out, considering SI values exceeding the 95th percentile of those in controls as significant.
In case of some patients who showed reactivity to peptide pools and consented to providing repeat blood specimens, proliferation assays were set up using PBMCs and individual peptides contained in these pools.
HLA-DR genotype determination
Genomic DNA was extracted using the salting-out method [26]. HLA-DRB1 and DQB1 low-resolution typing was carried out using sequence-specific primer kits (Deutsche Dynal, Hamburg, Germany), as per the manufacturer’s protocol. The frequencies of various HLA alleles among patients showing reactivity to HEV ORF2 protein or to specific peptide pools were compared with those not showing such reactivity.
Statistical methods
For intergroup comparisons of categorical data, chi-square test (with Yates’ correction, where applicable) was used. For continuous data, Wilcoxon’s rank sum test or Student’s t-test was used, depending on whether the data were normally distributed or not. P-values below 0.05 were taken as significant.
RESULTS
Study groups
Forty patients with acute hepatitis E, aged 18-76 (median 23) were studied. The time duration from the onset of acute hepatitis symptoms to the time of specimen collection was 6–46 days (median 18). The serum bilirubin and alanine aminotransferase levels ranged from 3.0 to 30.0 (median 10.6) mg/dL and from 234 to 2321 (579) IU/L, respectively. Of the 40 patients, two had fulminant hepatic failure and 38 had acute uncomplicated illness. The control subjects (n = 21) were aged 19–54 (median 28) years.
Lymphocyte proliferation assays using recombinant full-length hepatitis E virus open reading frame 2 protein
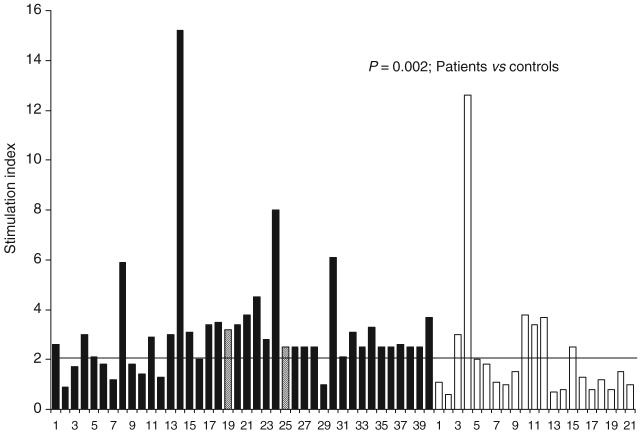
On stimulation with recombinant HEV ORF2 protein (10 μg/mL), 32 of the 40 patients (80%) showed significant stimulation. In contrast, only 7 (33%) of the 21 controls showed similar stimulation (P < 0.001). The SI values observed in patients (range 0.9–15.2; median 2.6) were higher than those observed in healthy controls (range 0.6–12.9; median 1.3; P = 0.002; Wilcoxon’s rank sum test) (Fig. 1). However, only 5 of 40 (13%) patients and 1 of 21 (5%; P = ns) controls had SI values exceeding 3.8 (95th percentile values of among controls).
Fig. 1.
Results of lymphocyte proliferation assays in response to recombinant HEV ORF2 protein among patients with acute hepatitis E (solid bars) and healthy controls (empty bars). The bars indicate stimulation indices in the presence of recombinant hepatitis E virus ORF2 protein (10 μg/mL). The horizontal line indicates the cut-off (2.0), above which stimulation indices were considered as significant. Stimulation indices in patients were higher than those among controls (medians 2.6 and 1.3, respectively; P = 0.002, Wilcoxon’s rank sum test). The numbers on X-axis represent serial numbers for patients and controls. Hatched lines represent the two patients (19 and 25) who had fulminant hepatic failure.
All the patients with acute hepatitis E showed good stimulation with PHA, with median SI of 163 (range 15–599); the corresponding median SI in healthy controls was 161 (26–462) (P = ns; Wilcoxon’s rank sum test).
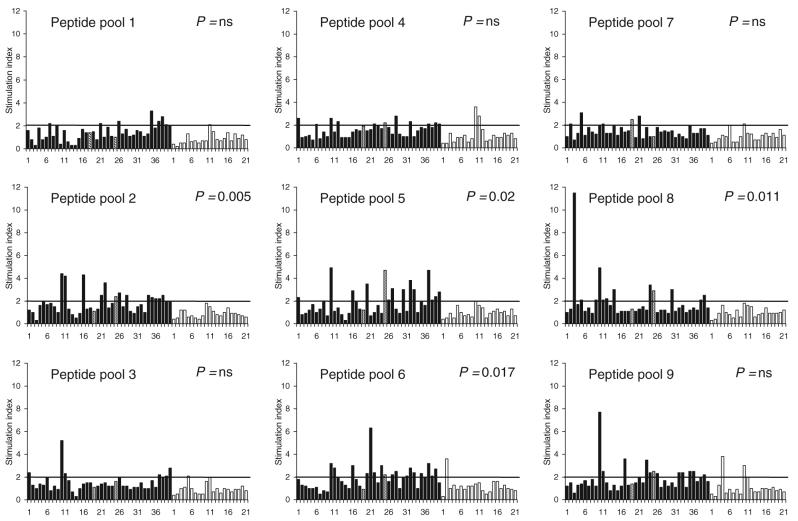
Lymphocyte proliferation using peptide pools
Table 1 shows the number of patients and controls in whom significant stimulation was observed with each of the peptide pools studied. For four of the nine peptide pools corresponding to HEV ORF2 protein (pools 2, 5, 6 and 8; corresponding to amino acids 73–156, 289–372, 361–444 and 505–588, respectively, of the ORF2 protein), reactivity was observed more often among patients with hepatitis E than among controls. Figure 2 shows the SI values observed among patients and controls with these peptide pools. The median (range) SI values among patients and controls were as follows: pool 2–1.7 (0.3–4.4) vs 0.8 (0.4–1.8); pool 5–1.5 (0.3–4.9) vs 0.9 (0.4–2.0); pool 6–1.8 (0.5–6.3) vs 1.0 (0.3–3.6) and pool 8–1.3 (0.9–11.5) vs 0.9 (0.3–1.8). Reactivity to pools 4 and 9 was also common among patients; however, the difference between the proportions of patients and controls showing reactivity to these pools was not statistically significant (P = ns; chi-square test with Yates’ correction).
Table 1.
Number of patients with acute hepatitis E and healthy negative controls showing significant proliferation of peripheral blood mononuclear cells with various pools of peptides corresponding to hepatitis E virus open reading frames (ORF)2 and ORF3 proteins
| ORF | Peptide pool | Amino acid region of the protein | Patients (n = 40) |
Healthy controls (n = 21) |
P-value* |
|---|---|---|---|---|---|
| ORF2 | Pool 1 | 1–84 | 9 | 1 | ns |
| Pool 2 | 73–156 | 14 | 0 | 0.005 | |
| Pool 3 | 145–228 | 9 | 1 | ns | |
| Pool 4 | 217–300 | 12 | 2 | ns | |
| Pool 5 | 289–372 | 15 | 1 | 0.02 | |
| Pool 6 | 361–444 | 17 | 1 | 0.01 | |
| Pool 7 | 433–516 | 5 | 2 | ns | |
| Pool 8 | 505–588 | 12 | 0 | 0.01 | |
| Pool 9 | 577–660 | 14 | 3 | ns | |
| ORF3 | Pool 10 | 1–68 | 8 | 2 | ns |
| Pool 11 | 49–123 | 7 | 0 | ns |
Patients vs controls using chi-square test (with Yates’ correction, where applicable).
Fig. 2.
Results of lymphocyte proliferation assays in response to pools of peptides corresponding to HEV ORF2 protein among patients with acute hepatitis E (solid bars) and healthy controls (empty bars). The bars indicate stimulation indices in the presence of pools of nine 20-mer peptides (10 μg/mL each). The horizontal line indicates the cut-off of 2.0, above which stimulation indices were considered as significant. For four pools (pools 2, 5, 6 and 8), the proportion of patients with significant stimulation was higher among patients than in controls. The numbers on X-axes represent serial numbers for patient and controls. Hatched lines represent the two patients (19 and 25) who had fulminant hepatic failure.
There was no difference in the proportion of patients and healthy controls who showed lymphoproliferative response to the peptide pools corresponding to HEV ORF3 protein (pools 10 and 11; Fig. 3).
Fig. 3.
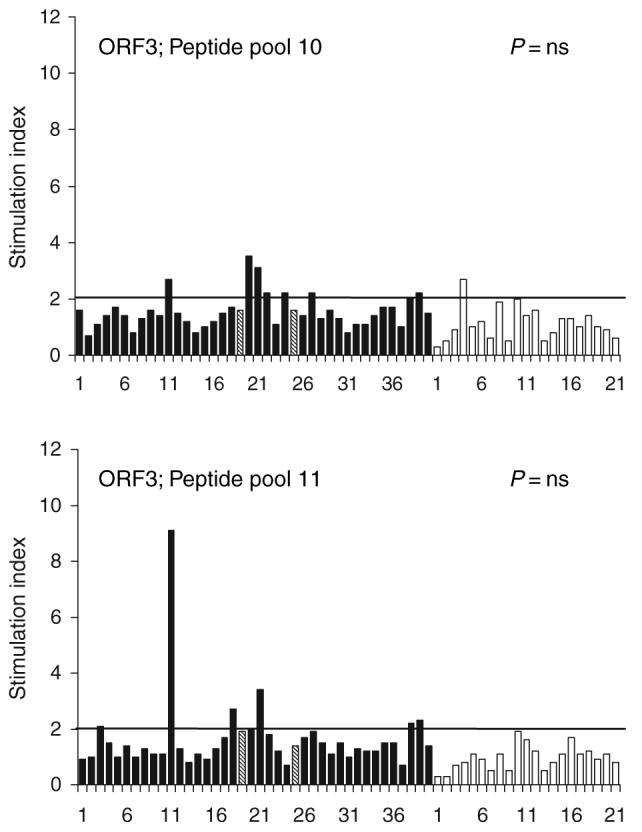
Results of lymphocyte proliferation assays in response to pools of peptides corresponding to HEV ORF3 protein among patients with acute hepatitis E (solid bars) and healthy controls (empty bars). The bars indicate stimulation indices in the presence of pools of seven 20-mer peptides (10 μg/mL each) each. The horizontal line indicates the cut-off of 2.0, above which stimulation indices were considered as significant. For both pools, the proportion of patients with significant stimulation was similar among patients and controls. The numbers on X-axes represent serial numbers for patient and controls. Hatched lines represent the two patients (19 and 25) who had fulminant hepatic failure.
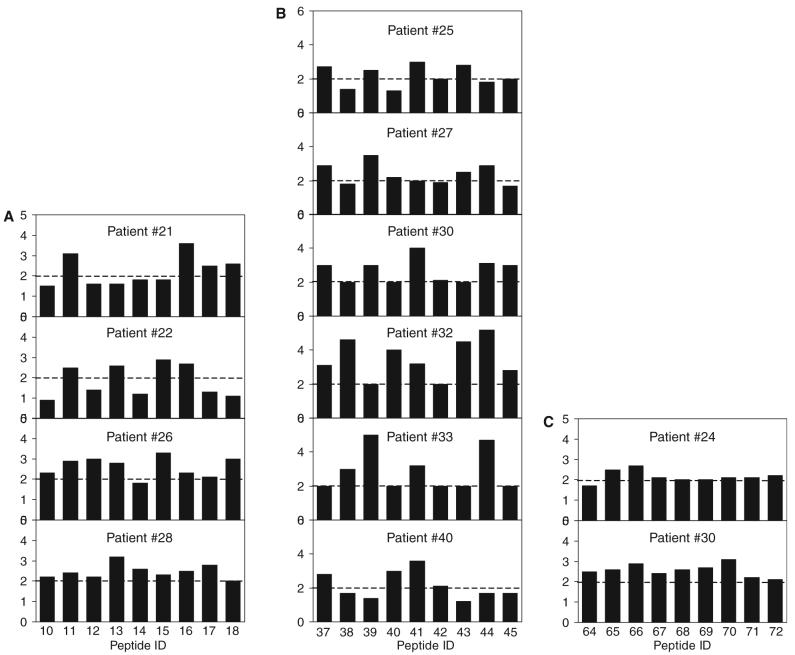
Lymphocyte proliferation using individual peptides
Lymphocyte proliferation assays were carried out with individual peptides contained in peptide pools 2, 5 and 8 in four, six and two patients, respectively (Fig. 4). In these experiments, no consistent pattern of reactivity to individual peptides was observed.
Fig. 4.
Results of lymphocyte proliferation assays in response to individual peptides contained in pools 2, 5 and 8 of peptides corresponding to HEV ORF2 protein among patients with acute hepatitis E. The bars indicate stimulation indices in the presence of individual 20-mer peptides (10 μg/mL). Panel A indicates stimulation indices with peptides 10 to 18 in four patients, panel B those with peptides 37 to 45 in six patients with acute hepatitis E and panel C those with peptides 64 to 72 in two patients with acute hepatitis E. The horizontal line indicates the cut-off of 2.0, above which stimulation indices were considered as significant.
HLA alleles
Table 2 shows the frequency of various HLA-DR and -DQ alleles among patients who showed proliferation in response to ORF2 protein and to various peptide pools and those who did not show such proliferation. The only significant association observed was between the reactivity to peptide pool 5 and the presence of HLA-DRB1 allele 010X.
Table 2.
Frequency of various HLA-DRB1 and HLA-DQB1 alleles among patients with acute hepatitis E reactive to or non-reactive to HEV ORF2 protein or peptide pools corresponding to this protein
| HEV ORF2 protein |
Pool 2 |
Pool 5 |
Pool 6 |
Pool 8 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | Allele | R (n = 32) | NR (n = 8) | R (n = 14) | NR (n = 26) | R (n = 15) | NR (n = 25) | R (n = 17) | NR (n = 23) | R (n = 12) | NR (n = 28) |
| HLA-DRB1 | 010X | 4 | 0 | 2 | 2 | 4 | 0* | 3 | 1 | 1 | 3 |
| 030X | 2 | 3 | 2 | 3 | 0 | 5 | 1 | 4 | 1 | 4 | |
| 040X | 5 | 0 | 2 | 3 | 2 | 3 | 3 | 2 | 1 | 4 | |
| 0701 | 10 | 2 | 5 | 7 | 3 | 9 | 4 | 8 | 4 | 8 | |
| 080X | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | |
| 090X | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | |
| 1001 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | |
| 110X | 5 | 0 | 3 | 2 | 1 | 3 | 2 | 3 | 1 | 4 | |
| 120X | 6 | 2 | 2 | 6 | 5 | 3 | 5 | 3 | 2 | 6 | |
| 130X | 8 | 3 | 4 | 7 | 3 | 8 | 3 | 8 | 4 | 7 | |
| 140X | 4 | 0 | 0 | 4 | 3 | 1 | 2 | 2 | 1 | 3 | |
| 150X | 13 | 3 | 7 | 9 | 5 | 11 | 8 | 8 | 5 | 11 | |
| HLA-DQB1 | 0201 | 11 | 4 | 5 | 10 | 4 | 11 | 4 | 11 | 3 | 12 |
| 020X | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | |
| 0301 | 5 | 0 | 2 | 3 | 3 | 2 | 2 | 3 | 2 | 3 | |
| 030X | 10 | 0 | 5 | 5 | 4 | 6 | 6 | 4 | 4 | 6 | |
| 0401 | 2 | 0 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 2 | |
| 0501 | 4 | 1 | 1 | 4 | 3 | 2 | 3 | 2 | 2 | 3 | |
| 0503 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 1 | |
| 0504 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | |
| 050X | 6 | 2 | 2 | 6 | 3 | 5 | 2 | 6 | 1 | 7 | |
| 0601 | 6 | 1 | 3 | 4 | 2 | 5 | 4 | 2 | 2 | 4 | |
| 0603 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | |
| 060X* | 10 | 4 | 4 | 10 | 4 | 10 | 4 | 10 | 5 | 9 | |
Data are shown as number of patients with a particular HLA allele. R, reactive; NR, nonreactive.
P < 0.05 (Yates' corrected chi-square test).
DISCUSSION
Our data show that patients with acute hepatitis E mount a proliferative T-cell immune response to the ORF2 protein of HEV. The immunodominant T-cell epitopes of HEV ORF2 protein were located in the regions encompassed by amino acids 73–156, 289–444 and 505–588. Except for association of reactivity with HLA-DRB1 allele 010X with pool 5 (amino acids 289–372), immune response to various regions of ORF2 protein did not show any relationship with the presence of a specific HLA allele. In comparison to ORF2, there was no significant lymphoproliferative response to peptides corresponding to the HEV ORF3 protein.
HEV infection is common in several parts of the world [1,2]. Antibody responses to this infection have been well characterized [10-16]. These responses develop early during the course of the infection and are directed against several epitopes distributed over all the three viral proteins [10-16]. Based on this information, diagnostic tests have been developed using recombinant HEV proteins and peptides [10,14]. In contrast, little data are available regarding the presence and nature of T-cell responses during HEV infection.
We have previously reported the presence of lymphoproliferative responses in nearly half of the adult patients with acute hepatitis E, including pregnant women with this disease [21,22]. However, these studies used only a limited panel of seven synthetic peptides corresponding to HEV proteins that had originally been chosen to study humoral immune responses [15,27]. Our current data are based on the use of peptide libraries that covered the entire lengths of HEV ORF2 and ORF3 proteins and a recombinant ORF2 protein. In the current study, we found lymphocyte proliferation in nearly 80% of patients with acute hepatitis E using the recombinant ORF2 protein. This higher frequency may represent the presence of additional T-cell stimulatory regions in the ORF2 protein beyond the peptides used in our previous study. In addition, the doses of peptides used in the current study (2 μg/well) were much higher than those used previously (1–100 ng/well). The current data indicate that activation of T-cell responses is common during acute hepatitis E. However, it may be worthwhile to point out that the SI values exceeded 4.0 in only five of the 32 patients who showed a proliferative response. This is partly because of the presence of proliferative responses in our control population, an issue that is discussed later. It may also reflect sequestration of antigen-specific cells into liver, the major site of inflammation, leading to a relatively lower level of detectable immune reactivity in the peripheral blood.
In our previous study [21], proliferation responses were more frequent among patients with hepatitis E than among controls against a peptide corresponding to amino acids 613–627 of HEV ORF2. In the current study, 14 of the 40 patients with acute hepatitis E had proliferative responses to peptide pool 9 that included this region; however, this frequency was not different from that among healthy subjects, as some (3/21) of the latter group also showed proliferative responses to this pool.
We observed lymphoproliferative responses to three widely scattered regions in the HEV ORF2 protein in patients with acute hepatitis E. The ongoing attempts to develop a vaccine against HEV have mostly included recombinant proteins corresponding to parts of the HEV ORF2 protein [18-20]. A candidate vaccine, which includes amino acids 112–607 of this protein, has been shown to prevent liver injury but not viraemia and faecal excretion of the virus in animal model [28,29]; however, the protection appears to be short lasting [20]. This vaccine includes two of the three immunostimulatory regions in entirety but does not include a part (amino acids 73–111) of the third T-cell stimulatory region identified in the current study. It is possible that inclusion of additional T-cell epitopes corresponding to this third immunostimulatory region may improve the efficacy of this vaccine and increase the duration of protection provided by it. As truncation of amino acids 1–111 appears to be important for the assembly of ORF2 protein as virus-like particles [30], this may imply inclusion of peptides or proteins corresponding to this region of ORF2 either as an additional constituent of the vaccine or as a booster, or use of DNA-based vaccines, attempts for which have already been made [31,32].
Recently, a neutralization epitope that spans from amino acids 458–607 of the HEV ORF2 has been identified [33]; it includes one of the three regions (505–588) of the HEV ORF2 protein to which proliferative responses were found in our study. This suggests that this region of ORF2 may be particularly important in protection against HEV, as it may provide T-cell help for the development of protective antibodies.
In hepatitis B and hepatitis C virus infections, cellular immune responses, although important for elimination of the virus from the host, have also been shown to mediate host cell injury [23,34,35]. It is thus important to determine whether cytotoxic T-cell responses directed against HEV proteins could play a role in the induction of host liver cell injury. This would be particularly important for those epitopes that induce proliferative responses and are therefore likely candidates for inclusion in future vaccines. Further work is also needed to identify the CD8+ cytotoxic T-cell responses to HEV.
We found association between only one HLA-DR allele and reactivity to a particular peptide pool. This association will need confirmation in future studies. Our failure to find more widespread associations between HLA alleles and proliferative responses may be related either to small sample size or to the use of peptide pools instead of specific peptides.
An important observation in our study was that while lymphoproliferative responses to ORF2 peptide pools were marked, those to ORF3 peptide pools were virtually nonexistent. Although we did not use the recombinant ORF3 protein, it appears that T-cell responses are more often directed against the viral capsid protein than against the ORF3 protein. This is particularly interesting, as humoral immune responses are directed against both ORF2 and ORF3, especially the immunodominant C-terminal end of the ORF3 protein.
Our study is limited by the fact that our control subjects were residents of an HEV-endemic region and may have had prior subclinical exposure to HEV. Despite being negative for anti-HEV antibodies, 7 of our 21 healthy control subjects showed proliferative responses to ORF2 protein, suggesting that anti-HEV screening may not be adequate to exclude prior HEV exposure. In our previous studies too, we found reactivity to HEV peptides in a proportion of healthy subjects [21]. This is supported by the previous observation of disappearance of anti-HEV with time in at least half of the subjects infected during an outbreak [17].
To conclude, we have shown activation of T-cell responses in patients with acute hepatitis E and mapped the regions of HEV ORF2 protein that might contain lymphoproliferative T-cell epitopes. Further work is needed to narrow down these T-cell epitopes to identify the relationship of specific HLA alleles with these epitopes and to determine the role of these epitopes in the development of a vaccine and in better understanding the mechanism of liver cell damage during HEV infection.
ACKNOWLEDGEMENTS
This work was supported by a research grant from the Indian Council of Medical Research, New Delhi to RA, and also received partial support from the Wellcome Trust, UK through a Senior Research Fellowship to SJ. The authors thank Mr Srikant Srivastava for help in specimen collection.
Abbreviations
- HEV
hepatitis E virus
- PBMC
peripheral blood mononuclear cells
- ORF
open reading frame
- PHA
phytohaemagglutinin
- SI
stimulation indices
APPENDIX
APPENDIX 1.
Amino acid sequence of various peptides corresponding to hepatitis E virus ORF2 (peptides 1-81) and ORF3 (peptides 82–95) used for lymphocyte proliferation assays
| Pool number | Peptide number | Amino acid sequence |
|---|---|---|
| 1 | 1 | H–MRPRPILLLLLMFLPMLPAP–OH |
| 2 | H–LLLMFLPMLPAPPPGQPSGR–OH | |
| 3 | H–LPAPPPGQPSGRRRGRRSGG–OH | |
| 4 | H–PSGRRRGRRSGGSGGGFWGD–OH | |
| 1 | 5 | H–RSGGSGGGFWGDRVDSQPFA–OH |
| 6 | H–FWGDRVDSQPFAIPYIHPTN–OH | |
| 7 | H–QPFAIPYIHPTNPFAPDVTA–OH | |
| 8 | H–HPTNPFAPDVTAAAGAGPRV–OH | |
| 9 | H–DVTAAAGAGPRVRQPARPLG–OH | |
| 2 | 10 | H–GPRVRQPARPLGSAWRDQAQ–OH |
| 11 | H–RPLGSAWRDQAQRPAVASRR–OH | |
| 12 | H–DQAQRPAVASRRRPTTAGAA–OH | |
| 13 | H–ASRRRPTTAGAAPLTAVAPA–OH | |
| 14 | H–AGAAPLTAVAPAHDTPPVPD–OH | |
| 15 | H–VAPAHDTPPVPDVDSRGAIL–OH | |
| 16 | H–PVPDVDSRGAILRRQYNLST–OH | |
| 17 | H–GAILRRQYNLSTSPLTSSVA–OH | |
| 18 | H–NLSTSPLTSSVATGTNLVLY–OH | |
| 3 | 19 | H–SSVATGTNLVLYAAPLSPLL–OH |
| 20 | H–LVLYAAPLSPLLPLQDGTNT–OH | |
| 21 | H–SPLLPLQDGTNTHIMATEAS–OH | |
| 22 | H–GTNTHIMATEASNYAQYRVA–OH | |
| 23 | H–TEASNYAQYRVARATIRYRP–OH | |
| 24 | H–YRVARATIRYRPLVPNAVGG–OH | |
| 25 | H–RYRPLVPNAVGGYAISISFW–OH | |
| 26 | H–AVGGYAISISFWPQTTTTPT–OH | |
| 27 | H–ISFWPQTTTTPTSVDMNSIT–OH | |
| 4 | 28 | H–TTPTSVDMNSITSTDVRILV–OH |
| 29 | H–NSITSTDVRILVQPGIASEL–OH | |
| 30 | H–RILVQPGIASELVIPSERLH–OH | |
| 31 | H–ASELVIPSERLHYRNQGWRS–OH | |
| 32 | H–ERLHYRNQGWRSVETSGVAE–OH | |
| 33 | H–GWRSVETSGVAEEEATSGLV–OH | |
| 34 | H–GVAEEEATSGLVMLCIHGSL–OH | |
| 35 | H–SGLVMLCIHGSLVNSYTNTP–OH | |
| 36 | H–HGSLVNSYTNTPYTGALGLL–OH | |
| 5 | 37 | H–TNTPYTGALGLLDFALELEF–OH |
| 38 | H–LGLLDFALELEFRNLTPGNT–OH | |
| 39 | H–ELEFRNLTPGNTNTRVSRYS–OH | |
| 40 | H–PGNTNTRVSRYSSTARHRLR–OH | |
| 41 | H–SRYSSTARHRLRRGADGTAE–OH | |
| 42 | H–HRLRRGADGTAELTTTAATR–OH | |
| 43 | H–GTAELTTTAATRFMKDLYFT–OH | |
| 44 | H–AATRFMKDLYFTSTNGVGEI–OH | |
| 45 | H–LYFTSTNGVGEIGRGIALTL–OH | |
| 6 | 46 | H–VGEIGRGIALTLFNLADTLL–OH |
| 47 | H–ALTLFNLADTLLGGLPTELI–OH | |
| 48 | H–DTLLGGLPTELISSAGGQLF–OH | |
| 49 | H–TELISSAGGQLFYSRPVVSA–OH | |
| 50 | H–GQLFYSRPVVSANGEPTVKL–OH | |
| 51 | H–VVSANGEPTVKLYTSVENAQ–OH | |
| 52 | H–TVKLYTSVENAQQDKGIAIP–OH | |
| 53 | H–ENAQQDKGIAIPHDIDLGES–OH | |
| 54 | H–IAIPHDIDLGESRVVIQDYD–OH | |
| 7 | 55 | H–LGESRVVIQDYDNQHEQDRP–OH |
| 56 | H–QDYDNQHEQDRPTPSPAPSR–OH | |
| 57 | H–QDRPTPSPAPSRPFSVLRAN–OH | |
| 58 | H–APSRPFSVLRANDVLWLSLT–OH | |
| 59 | H–LRANDVLWLSLTAAEYDQST–OH | |
| 60 | H–LSLTAAEYDQSTYGSSTGPV–OH | |
| 61 | H–DQSTYGSSTGPVYVSDSVTL–OH | |
| 62 | H–TGPVYVSDSVTLVNVATGAQ–OH | |
| 63 | H–SVTLVNVATGAQAVARSLDW–OH | |
| 8 | 64 | H–TGAQAVARSLDWTKVTLDGR–OH |
| 65 | H–SLDWTKVTLDGRPLSTIQQY–OH | |
| 66 | H–LDGRPLSTIQQYSKTFFVLP–OH | |
| 67 | H–IQQYSKTFFVLPLRGKLSFW–OH | |
| 68 | H–FVLPLRGKLSFWEAGTTKAG–OH | |
| 69 | H–LSFWEAGTTKAGYPYNYNTT–OH | |
| 70 | H–TKAGYPYNYNTTASDQLLVE–OH | |
| 71 | H–YNTTASDQLLVENAAGHRVA–OH | |
| 72 | H–LLVENAAGHRVAISTYTTSL–OH | |
| 9 | 73 | H–HRVAISTYTTSLGAGPVSIS–OH |
| 74 | H–TTSLGAGPVSISAVAVLAPH–OH | |
| 75 | H–VSISAVAVLAPHSALALLED–OH | |
| 76 | H–LAPHSALALLEDTLDYPARA–OH | |
| 77 | H–LLEDTLDYPARAHTFDDFCP–OH | |
| 78 | H–PARAHTFDDFCPECRPLGLQ–OH | |
| 79 | H–DFCPECRPLGLQGCAFQSTV–OH | |
| 80 | H–LGLQGCAFQSTVAELQRLKM–OH | |
| 81 | H–QSTVAELQRLKMKVGKTREL–OH | |
| 10 | 82 | H–MNNMSFAAPMGSRPCALGLF–OH |
| 83 | H–PMGSRPCALGLFCCCSSCFC–OH | |
| 84 | H–LGLFCCCSSCFCLCCPRHRP–OH | |
| 85 | H–SCFCLCCPRHRPVSRLAAVV–OH | |
| 86 | H–RHRPVSRLAAVVGGAAAVPA–OH | |
| 87 | H–AAVVGGAAAVPAVVSGVTGL–OH | |
| 88 | H–AVPAVVSGVTGLILSPSQSP–OH | |
| 11 | 89 | H–VTGLILSPSQSPIFIQPTPS–OH |
| 90 | H–SQSPIFIQPTPSPPMSPLRP–OH | |
| 91 | H–PTPSPPMSPLRPGLDLVFAN–OH | |
| 92 | H–PLRPGLDLVFANPPDHSAPL–OH | |
| 93 | H–VFANPPDHSAPLGVTRPSAP–OH | |
| 94 | H–SAPLGVTRPSAPPLPHVVDL–OH | |
| 95 | H–RPSAPPLPHVVDLPQLGPRR–OH |
REFERENCES
- 1.Aggarwal R, Krawczynski K. Hepatitis E: an overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15:9–20. doi: 10.1046/j.1440-1746.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- 2.Krawczynski K, Aggarwal R. Hepatitis E. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff’s Diseases of the Liver. 9th edn. Philadelphia, PA: Lippincott-Raven; 2002. pp. 877–889. [Google Scholar]
- 3.Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal R, Naik SR. Hepatitis E: intrafamilial transmission versus waterborne spread. J Hepatol. 1994;22:718–723. doi: 10.1016/s0168-8278(94)80229-7. [DOI] [PubMed] [Google Scholar]
- 5.Somani SK, Aggarwal R, Naik SR, Srivastava S, Naik S. A serological study of intra-familial spread from patients with sporadic hepatitis E virus infection. J Viral Hepat. 2003;10:446–449. doi: 10.1046/j.1365-2893.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 6.Tam AW, Smith MM, Guerra ME, et al. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71:9045–9053. doi: 10.1128/jvi.71.12.9045-9053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyagi S, Korkaya H, Zafrullah M, Jameel S, Lal SK. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its non-glycosylated form of the major capsid protein, ORF2. J Biol Chem. 2002;277:22759–22767. doi: 10.1074/jbc.M200185200. [DOI] [PubMed] [Google Scholar]
- 9.Korkaya H, Jameel S, Gupta D, et al. The ORF3 protein of hepatitis E virus binds to Src homology 3 domains and activates MAPK. J Biol Chem. 2001;276:42389–42400. doi: 10.1074/jbc.M101546200. [DOI] [PubMed] [Google Scholar]
- 10.Favorov MO, Fields HA, Purdy MA, et al. Serologic identification of hepatitis E virus infections in epidemic and endemic settings. J Med Virol. 1992;36:246–250. doi: 10.1002/jmv.1890360403. [DOI] [PubMed] [Google Scholar]
- 11.Khudyakov YE, Khudyakova NS, Fields HA, et al. Epitope mapping in proteins of hepatitis E virus. Virology. 1993;194:89–96. doi: 10.1006/viro.1993.1238. [DOI] [PubMed] [Google Scholar]
- 12.Khudyakov YE, Lopareva EN, Jue DL, Crews TK, Thyagarajan SP, Fields HA. Antigenic domains of the open reading frame 2-encoded protein of hepatitis E virus. J Clin Microbiol. 1999;37:2863–2871. doi: 10.1128/jcm.37.9.2863-2871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddell MA, Li F, Anderson DA. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J Virol. 2000;74:8011–8017. doi: 10.1128/jvi.74.17.8011-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson GJ, Chau KH, Cabal CM, Yarbough PO, Reyes GR, Mushahwar IK. Solid-phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J Virol Methods. 1992;38:175–186. doi: 10.1016/0166-0934(92)90180-l. [DOI] [PubMed] [Google Scholar]
- 15.Coursaget P, Buisson Y, Depril N, et al. Mapping of linear B cell epitopes on open reading frame 2- and 3-encoded proteins of hepatitis E virus using synthetic peptides. FEMS Microbiol Lett. 1993;109:251–255. doi: 10.1111/j.1574-6968.1993.tb06176.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaur M, Hyams KC, Purdy MA, et al. Human linear B-cell epitopes encoded by the hepatitis E virus include determinants in the RNA-dependent RNA polymerase. Proc Natl Acad Sci USA. 1992;89:3855–3858. doi: 10.1073/pnas.89.9.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khuroo MS, Kamili S, Dar MY, Moecklii R, Jameel S. Hepatitis E and long-term antibody status. Lancet. 1993;341:1355–1355. [PubMed] [Google Scholar]
- 18.Tsarev SA, Tsareva TS, Emerson SU, et al. Recombinant vaccine against hepatitis E: dose response and protection against heterologous challenge. Vaccine. 1997;15:1834–1838. doi: 10.1016/s0264-410x(97)00145-x. [DOI] [PubMed] [Google Scholar]
- 19.Purdy MA, McCaustland KA, Krawczynski K, Spelbring J, Reyes GR, Bradley DW. Preliminary evidence that a trp-EHEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV) J Med Virol. 1993;41:90–94. doi: 10.1002/jmv.1890410118. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Emerson SU, Nguyen H, et al. Recombinant vaccine against hepatitis E: duration of protective immunity in rhesus macaques. Vaccine. 2002;20:3285–3291. doi: 10.1016/s0264-410x(02)00314-6. [DOI] [PubMed] [Google Scholar]
- 21.Naik S, Aggarwal R, Naik SR, et al. Evidence for activation of cellular immune responses in patients with acute hepatitis E. Indian J Gastroenterol. 2002;21:149–152. [PubMed] [Google Scholar]
- 22.pal R, Aggarwal R, Naik SR, Das V, Das S, Naik SR. Immunological alterations in pregnant women with acute hepatitis E. J Gastroenterol Hepatol. 2005;20:1094–1101. doi: 10.1111/j.1440-1746.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari C, Missale G, Boni C, Urbani S. Immunopathogenesis of hepatitis B. J Hepatol. 2003;39(Suppl. 1):S36–S42. doi: 10.1016/s0168-8278(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 24.Zafrullah M, Khursheed Z, Yadav S, Sehgal D, Jameel S, Ahmad F. Acid pH enhances structure and structural stability of the capsid protein of hepatitis E virus. Biochem Biophys Res Commun. 2003;313:67–73. doi: 10.1016/j.bbrc.2003.11.088. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Botran R, Vetvicka V. Methods in Cellular Immunology. 2nd edn. Boca Raton, FL: CRC Press; 2002. pp. 53–57. [Google Scholar]
- 26.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215–1218. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coursaget P, Depril N, Buisson Y, Molinie C, Roue R. Hepatitis type E in a French population: detection of anti-HEV by a synthetic peptide-based enzyme-linked immunosorbent assay. Res Virol. 1994;145:51–57. doi: 10.1016/s0923-2516(07)80007-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Emerson SU, Nguyen H, et al. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine. 2001;20:853–857. doi: 10.1016/s0264-410x(01)00399-1. [DOI] [PubMed] [Google Scholar]
- 29.Purcell RH, Nguyen H, Shapiro M, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607–2615. doi: 10.1016/s0264-410x(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 30.Li TC, Yamakawa Y, Suzuki K, et al. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamili S, Spelbring J, Carson D, Krawczynski K. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis. 2004;189:258–264. doi: 10.1086/380801. [DOI] [PubMed] [Google Scholar]
- 32.He J, Hayes CG, Binn LN, et al. Hepatitis E virus DNA vaccine elicits immunologic memory in mice. J Biomed Sci. 2001;8:223–226. doi: 10.1007/BF02256416. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y-H, Purcell RH, Emerson SU. An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotypes 1, 2, 3 and 4. Vaccine. 2004;22:2578–2585. doi: 10.1016/j.vaccine.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis. 2002;2:43–50. doi: 10.1016/s1473-3099(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 35.Rosen HR. Hepatitis C pathogenesis: mechanisms of viral clearance and liver injury. Liver Transpl. 2003;9:S35–S43. doi: 10.1053/jlts.2003.50253. [DOI] [PubMed] [Google Scholar]