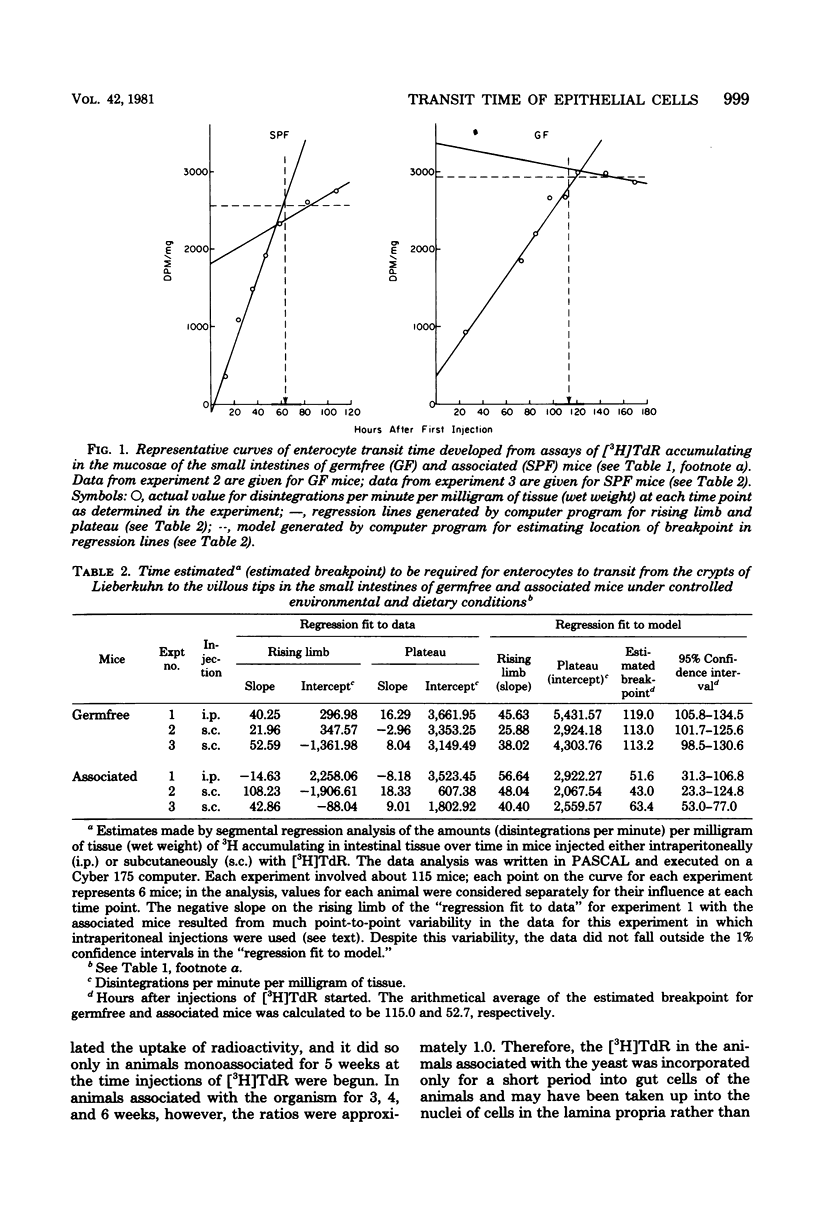
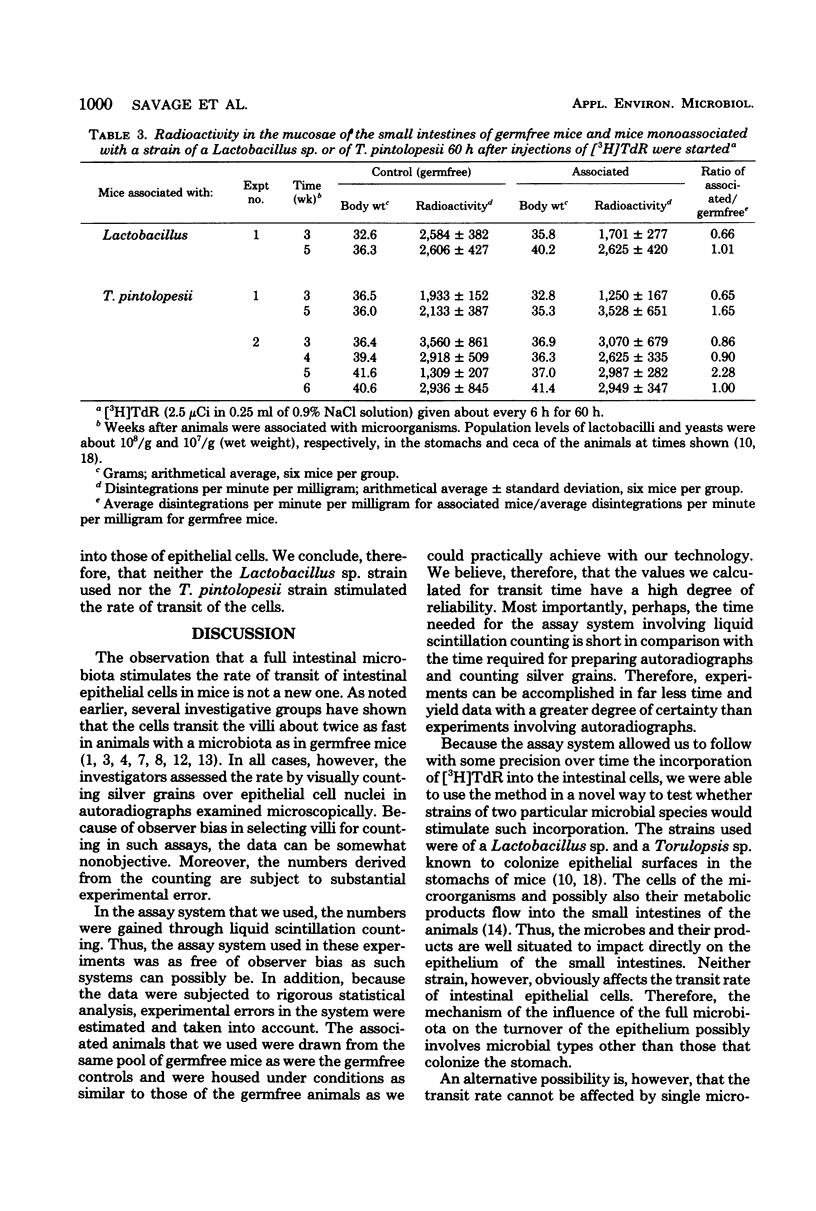
Abstract
Germfree mice housed in isolators under controlled environmental and nutritional conditions were associated with an intestinal microflora. These associated animals and germfree mice drawn from the same population were tested for the rate at which the epithelial cells transited from the crypts of Lieberkuhn to the tips of the villi in their small intestines. The method for estimating the rate of transit of epithelial cells involved the use of liquid scintillation counting to determine the amount of radioactivity entering the cells while the animals were being injected with [3H]thymidine and statistical analysis of th data with a computer program developed for the purpose. As estimated by that method, the cells transited from the crypts to the villous tips in germfree mice in about 115 h and in the associated animals in about 53 h. In monoassociated mice, a strain of a Lactobacillus sp. had no effect on the transit time of the epithelial cells. A strain of Torulopsis pintolopesii stimulated uptake of 3[H]thymidine by the small bowel mucosae in mice monoassociated with the organisms for 5 weeks. In animals monoassociated with the yeast fo 3, 4, and 6 weeks, however, the radioactive compound was incorporated into the bowel mucosae to the same extent as the mucosae of germfree mice. Therefore, similarly to the Lactobacillus strain, T. pintolopesii has no obvious influence on the transit rate of small bowel epithelial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS G. D., BAUER H., SPRINZ H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963 Mar;12:355–364. [PubMed] [Google Scholar]
- Fortin-Magana R., Hurwitz R., Herbst J. J., Kretchmer N. Intestinal enzymes: indicators of proliferation and differentiation in the jejunum. Science. 1970 Mar 20;167(3925):1627–1628. doi: 10.1126/science.167.3925.1627. [DOI] [PubMed] [Google Scholar]
- GORDON H. A., BRUCKNER-KARDOSS E. Effect of normal microbial flora on intestinal surface area. Am J Physiol. 1961 Jul;201:175–178. doi: 10.1152/ajplegacy.1961.201.1.175. [DOI] [PubMed] [Google Scholar]
- Hagemann R. F., Sigdestad C. P., Lesher S. A quantitative description of the intestinal epithelium of the mouse. Am J Anat. 1970 Sep;129(1):41–51. doi: 10.1002/aja.1001290104. [DOI] [PubMed] [Google Scholar]
- Hughes W. L., Bond V. P., Brecher G., Cronkite E. P., Painter R. B., Quastler H., Sherman F. G. CELLULAR PROLIFERATION IN THE MOUSE AS REVEALED BY AUTORADIOGRAPHY WITH TRITIATED THYMIDINE. Proc Natl Acad Sci U S A. 1958 May;44(5):476–483. doi: 10.1073/pnas.44.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H., Ishihara Y., Yamanaka J., Ito T. Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn J Exp Med. 1973 Aug;43(4):297–305. [PubMed] [Google Scholar]
- Khoury K. A., Floch M. H., Hersh T. Small intestinal mucosal cell proliferation and bacterial flora in the conventionalization of the germfree mouse. J Exp Med. 1969 Sep 1;130(3):659–670. doi: 10.1084/jem.130.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Savage D. C. Models for study of the specificity by which indigenous lactobacilli adhere to murine gastric epithelia. Infect Immun. 1979 Dec;26(3):966–975. doi: 10.1128/iai.26.3.966-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESHER S., FRY R. J., KOHN H. I. Age and the generation time of the mouse duodenal epithelial cell. Exp Cell Res. 1961 Aug;24:334–343. doi: 10.1016/0014-4827(61)90436-0. [DOI] [PubMed] [Google Scholar]
- LESHER S., WALBURG H. E., Jr, SACHER G. A., Jr GENERATION CYCLE IN THE DUODENAL CRYPT CELLS OF GERM-FREE AND CONVENTIONAL MICE. Nature. 1964 May 30;202:884–886. doi: 10.1038/202884a0. [DOI] [PubMed] [Google Scholar]
- Ranken R., Wilson R., Bealmear P. M. Increased turnover of intestinal mucosal cells of germfree mice induced by cholic acid. Proc Soc Exp Biol Med. 1971 Oct;138(1):270–272. doi: 10.3181/00379727-138-35876. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigdestad C. P., Bauman J., Lesher S. W. Diurnal fluctuations in the number of cells in mitosis and DNA synthesis in the jejunum of the mouse. Exp Cell Res. 1969 Nov;58(1):159–162. doi: 10.1016/0014-4827(69)90126-8. [DOI] [PubMed] [Google Scholar]
- Sigdestad C. P., Lesher S. Photo-reversal of the circadian rhythm in the proliferative activity of the mouse small intestine. J Cell Physiol. 1971 Aug;78(1):121–126. doi: 10.1002/jcp.1040780115. [DOI] [PubMed] [Google Scholar]
- Suegara N., Siegel J. E., Savage D. C. Ecological determinants in microbial colonization of the murine gastrointestinal tract: adherence of Torulopsis pintolopesii to epithelial surfaces. Infect Immun. 1979 Jul;25(1):139–145. doi: 10.1128/iai.25.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen J. M., Kooyman J., Visser W. J., Holt S. J., Galjaard H. The effect of increased crypt cell proliferation on the activity and subcellular localization of esterases and alkaline phosphatase in the rat small intestine. Histochem J. 1977 Jan;9(1):61–75. doi: 10.1007/BF01007009. [DOI] [PubMed] [Google Scholar]
- Whitt D. D., Savage D. C. Kinetics of changes induced by indigenous microbiota in the activity levels of alkaline phosphatase and disaccharidases in small intestinal enterocytes in mice. Infect Immun. 1980 Jul;29(1):144–151. doi: 10.1128/iai.29.1.144-151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Savage D. C. Influence of certain indigenous gastrointestinal microorganisms on duodenal alkaline phosphatase in mice. Appl Environ Microbiol. 1976 Jun;31(6):880–888. doi: 10.1128/aem.31.6.880-888.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Stanley C., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal alkaline phosphatase activity in mice. Infect Immun. 1971 Jun;3(6):768–773. doi: 10.1128/iai.3.6.768-773.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



