Abstract
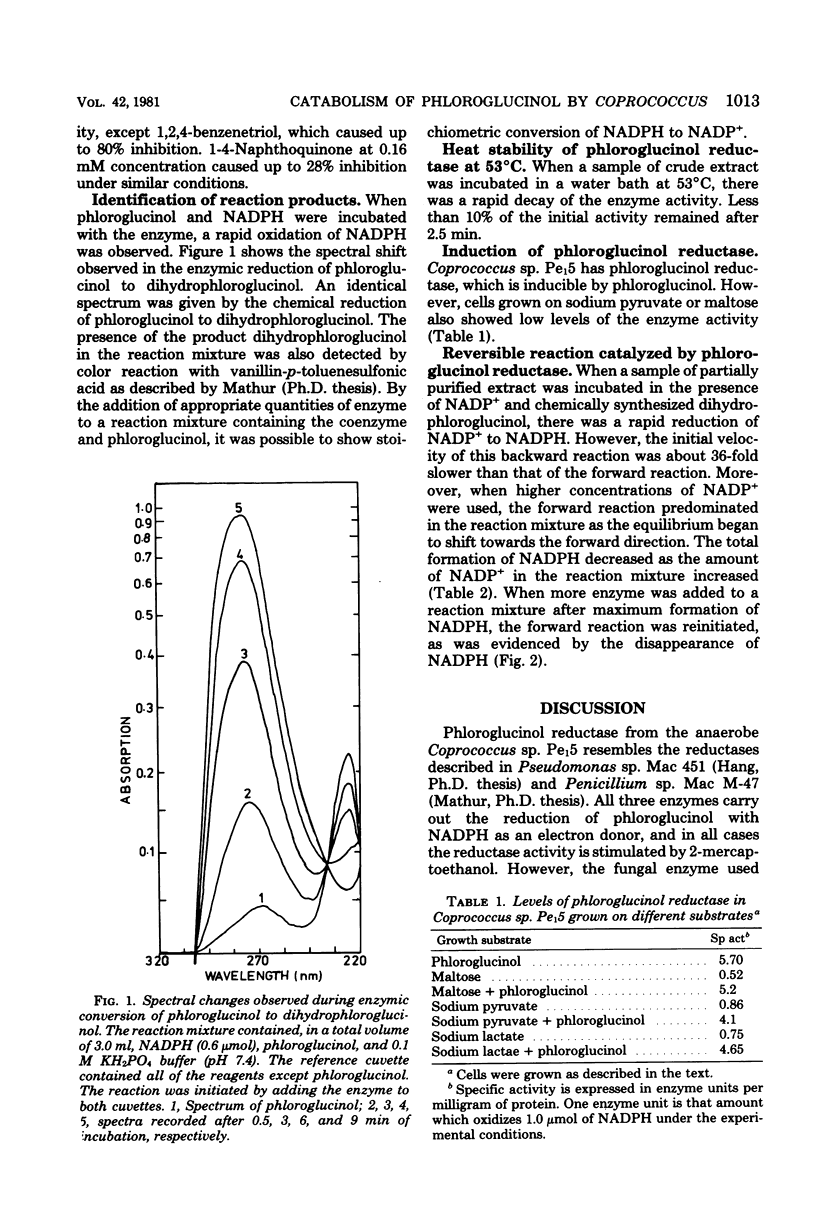
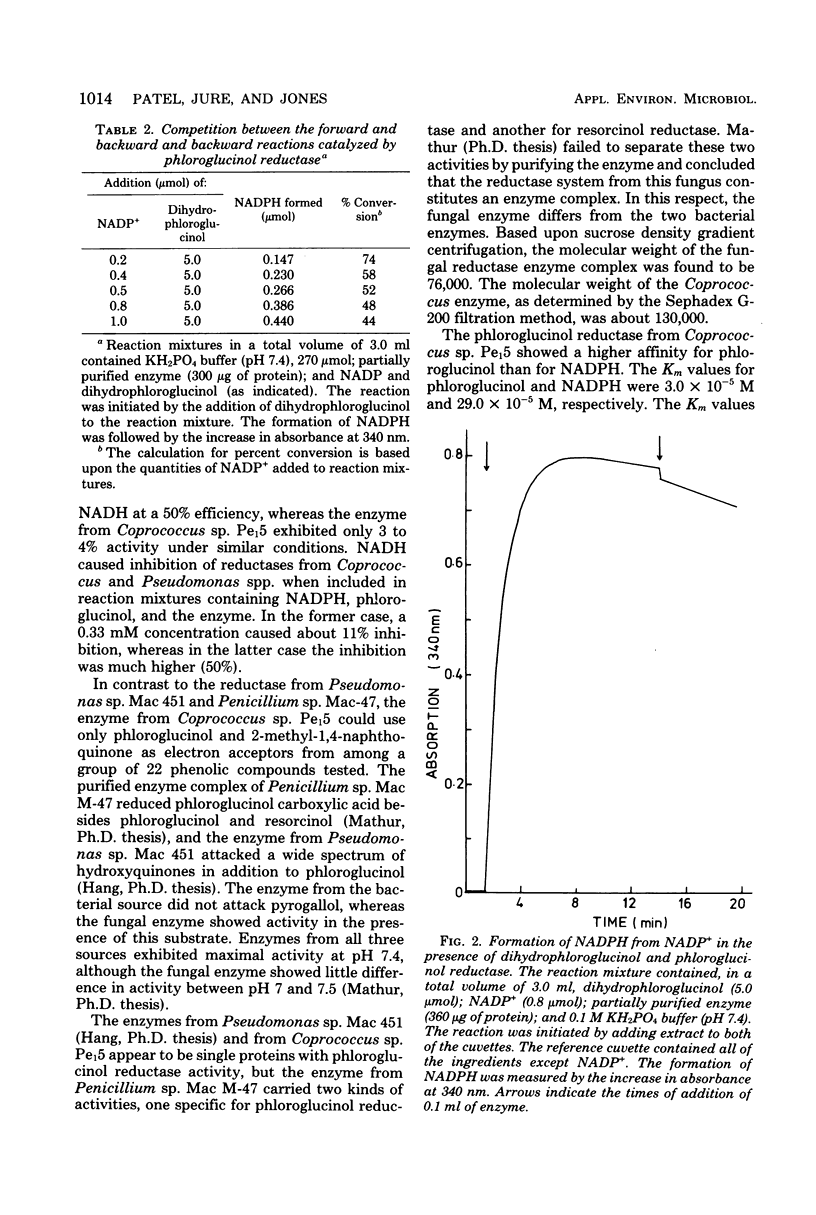
A rumen isolate, Coprococcus, sp. Pe15, was found to carry phloroglucinol reductase, which catalyzed the initial step in the breakdown of phloroglucinol. The organism uses phloroglucinol as the sole source of carbon and energy when grown in the absence of oxygen. Induced levels of enzyme were detected in cells grown either on phloroglucinol or on other carbon sources in the presence of limiting quantities of phloroglucinol. Although the organism is a strict anaerobe, the enzyme from anaerobically grown cells was insensitive to air. The partially purified enzyme required reduced nicotinamide adenine dinucleotide phosphate as an electron donor and was specific for phloroglucinol. However, partial enzyme activity (14 to 17%) was also detected in the presence of 2-methyl-1,4-naphthoquinone but not in the presence of several other phenolic compounds. The enzyme exhibited a higher affinity for phloroglucinol than for reduced nicotinamide adenine dinucleotide phosphate, with Km values of 3.0 × 10−5 M and 29.0 × 10−5 M, respectively. The optimum pH for maximal enzyme activity was 7.4, and the molecular weight of the native protein was about 130,000, as determined by the Sephadex gel filtration technique.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIM F. Formation in a Mycobacterium of an adaptive enzyme for oxidation of phloroglucinol. Proc Soc Exp Biol Med. 1956 May;92(1):150–151. doi: 10.3181/00379727-92-22414. [DOI] [PubMed] [Google Scholar]
- Balba M. T., Evans W. C. The methanogenic fermentation of aromatic substrates. Biochem Soc Trans. 1977;5(1):302–304. doi: 10.1042/bst0050302. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Keeney M., Van Soest P. J. Effects of carbon dioxide on growth and maltose fermentation by Bacteroides amylophilus. J Bacteriol. 1969 May;98(2):668–676. doi: 10.1128/jb.98.2.668-676.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., PATEL M. D. Oxidation of p-cresol and related compounds by a Pseudomonas. Biochem J. 1957 Jun;66(2):227–233. doi: 10.1042/bj0660227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. Dissimilation of aromatic substrates by Rhodopseudomonas palustris. Biochem J. 1967 Aug;104(2):30P–31P. [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- FINA L. R., FISKIN A. M. The anaerobic decomposition of benzoic acid during methane fermentation. II. Fate of carbons one and seven. Arch Biochem Biophys. 1960 Dec;91:163–165. doi: 10.1016/0003-9861(60)90483-5. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O. Crystalline oxygenases of pseudomonads. Bacteriol Rev. 1966 Dec;30(4):720–731. doi: 10.1128/br.30.4.720-731.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson W. D., Taylor A., Mathur D. K., Blackwood A. C. Identification of an intermediate of phloroglucinol degradation by mass spectroscopic analysis. Can J Biochem. 1970 Feb;48(2):215–216. doi: 10.1139/o70-036. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nottingham P. M., Hungate R. E. Methanogenic fermentation of benzoate. J Bacteriol. 1969 Jun;98(3):1170–1172. doi: 10.1128/jb.98.3.1170-1172.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Evans W. C. Oxidative metabolism of phthalic acid by soil pseudomonads. Biochem J. 1960 Aug;76(2):310–318. doi: 10.1042/bj0760310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F. J., Jones G. A., Wolin E. A. Anaerobic degradation of some bioflavonoids by microflora of the rumen. Can J Microbiol. 1969 Aug;15(8):972–974. doi: 10.1139/m69-173. [DOI] [PubMed] [Google Scholar]
- Takeda H., Hayaishi O. Crystalline L-lysine oxygenase. J Biol Chem. 1966 Jun 10;241(11):2733–2736. [PubMed] [Google Scholar]
- Tsai C. G., Gates D. M., Ingledew W. M., Jones G. A. Products of anaerobic phloroglucinol degradation by Coprococcus sp. Pe15. Can J Microbiol. 1976 Feb;22(2):159–164. doi: 10.1139/m76-022. [DOI] [PubMed] [Google Scholar]
- Tsai C. G., Jones G. A. Isolation and identification of rumen bacteria capable of anaerobic phloroglucinol degradation. Can J Microbiol. 1975 Jun;21(6):794–801. doi: 10.1139/m75-117. [DOI] [PubMed] [Google Scholar]
- Whittle P. J., Lunt D. O., Evans W. C. Anaerobic photometabolism of aromatic compounds by Rhodopseudomonas sp. Biochem Soc Trans. 1976;4(3):490–491. doi: 10.1042/bst0040490. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Evans W. C. The metabolism of benzoate by Moraxella species through anaerobic nitrate respiration. Evidence for a reductive pathway. Biochem J. 1975 Apr;148(1):1–10. doi: 10.1042/bj1480001a. [DOI] [PMC free article] [PubMed] [Google Scholar]



