Abstract
Design
A retrospective medical-record review of evoked-potential and audiometric data was used to determine the accuracy with which click-evoked and toneburst-evoked ABR thresholds predict pure-tone audiometric thresholds.
Methods
The medical records were reviewed of a consecutive group of patients who were referred for ABR testing for audiometric purposes over the past four years. ABR thresholds were measured for clicks and for several tonebursts, including a single-cycle, Blackman-windowed, 250-Hz toneburst, which has a broad spectrum with little energy above 600 Hz. Typically, the ABR data were collected because the patients were unable to provide reliable estimates of hearing sensitivity, based on behavioral-test techniques, due to developmental level. Data were included only if subsequently obtained behavioral audiometric data were available to which the ABR data could be compared. Almost invariably, the behavioral data were collected after the ABR results were obtained. Because of this, data were included on only those ears for which middle-ear tests (tympanometry, otoscopic examination, pure-tone air- and bone-conduction thresholds) indicated that middle-ear status was similar at the times of both tests. With these inclusion criteria, data were available on 140 ears of 77 subjects.
Results
Correlation was 0.94 between click-evoked ABR thresholds and the average pure-tone threshold at 2 and 4 kHz. Correlations exceeded 0.92 between ABR thresholds for the 250-Hz toneburst and low-frequency behavioral thresholds (250 Hz, 500 Hz, and the average pure-tone thresholds at 250 and 500 Hz). Similar or higher correlations were observed when ABR thresholds at other frequencies were compared to the pure-tone thresholds at corresponding frequencies. Differences between ABR and behavioral threshold depended on behavioral threshold, with ABR thresholds overestimating behavioral threshold in cases of normal hearing and underestimating behavioral threshold in cases of hearing loss.
Conclusions
These results suggest that ABR thresholds can be used to predict pure-tone behavioral thresholds for a wide range of frequencies. Although controversial, the data reviewed in this paper suggest that click-evoked ABR thresholds result in reasonable predictions of the average behavioral thresholds at 2 and 4 kHz. However, there were cases for which click-evoked ABR thresholds underestimated hearing loss at these frequencies. There are several other reasons why click-evoked ABR measurements were made, including that they (1) generally result in well-formed responses, (2) assist in determining whether auditory neuropathy exists, and (3) can be obtained in a relatively brief amount of time. Low-frequency thresholds were predicted well by ABR thresholds to a single-cycle, 250-Hz toneburst. In combination, click-evoked and low-frequency toneburst-evoked ABR threshold measurements might be used to quickly provide important clinical information for both ends of the audiogram. These measurements could be supplemented by ABR threshold measurements at other frequencies, if time permits. However, it may be possible to plan initial intervention strategies based on data for these two stimuli.
INTRODUCTION
There has always been a need for objective measurements that can be used to predict the pure-tone audiogram in patients who cannot provide reliable behavioral responses to sound. This has been the case because the developmental level of some patients is such that they are unable to perform the behavioral tasks necessary to provide accurate estimates of threshold. The need for objective test procedures has become more pronounced with the advent of universal newborn hearing screening (UNHS). UNHS programs have resulted in the identification of hearing loss in infants and young children at ages for which behavioral audiological assessment maybe unreliable. Twenty-to-thirty years ago, click-evoked auditory brainstem response (ABR) measurements represented the primary tool for both identification and diagnosis of hearing loss (Galambos and Hecox, 1978; Schulman-Galambos and Galambos, 1979; Galambos et al., 1984). That is, click-evoked ABR thresholds were used in targeted newborn hearing screening programs (when screenings were reserved for infants at risk for hearing loss), but also were used to estimate the magnitude of hearing loss for the purposes of initiating intervention. Currently, clicks are used in some UNHS programs that screen with ABR measurements.
Several studies exist describing the relationship between click-evoked ABR thresholds and behavioral thresholds (e.g., Jerger and Mauldin, 1978; Gorga et al., 1985; van der Drift et al., 1987). In the above studies, the strongest correlations were observed between click-evoked ABR thresholds and pure-tone thresholds at 2 and 4 kHz. This finding may seem surprising, given the broad amplitude spectra of clicks (limited mainly by the frequency response of the transducer); however, this association between ABR and behavioral thresholds may be a consequence of the peripheral auditory system’s response to impulsive stimuli. When stimulated with a click, there is greater discharge synchrony among auditory-nerve fibers innervating the cochlear base or high-frequency region, compared to the more dispersive discharges that occur at its apex or low-frequency region (Kiang et al., 1965; Kiang, 1975). Although fibers innervating places along the entire cochlea respond when clicks are presented to the ear, there is greater synchrony for higher frequency fibers, which might account for the frequency-dependent nature of the correlations between electrophysiological thresholds for clicks and behavioral thresholds for pure tones. In contrast to the above ABR studies, others have reported less agreement between click-evoked responses and behavioral thresholds at these same frequencies (e.g., Stapells et al., 1994). This result has been attributed to the click’s broad spectrum, which, as stated above, results in the excitation of fibers innervating essentially all places along the cochlea. In this circumstance, the click-evoked threshold might relate to the frequency(ies) for which hearing is best. Given these conflicting results, there may be value in further exploring the relationship between click-evoked ABR thresholds and pure-tone thresholds, especially for frequencies for which it has been suggested that the two estimates are correlated.
Regardless of which view is correct, there remains a need to provide threshold estimates for frequencies other than 2 and 4 kHz. Even among those studies that report good correlations between click-evoked ABR thresholds and high-frequency behavioral thresholds, the limitations of these predictions for lower frequencies is not disputed. As a consequence of all of these issues, several efforts have been undertaken to provide frequency-specific (and perhaps place-specific1) evoked-potential estimates of auditory function. One approach to achieving this goal is referred to as the derived-response technique, originally described in animal studies by Teas et al. (1962) and extended to humans by Don and Eggermont (1978) and Eggermont and Don (1978). In this approach, clicks are presented with high-pass noise maskers that are used to prevent high-frequency (basal) cochlear regions from responding. The high-pass frequency of the masker is varied, and responses in the presence of adjacent high-pass maskers are subtracted, resulting in a “derived response” that is thought to include responses coming from neurons innervating cochlear regions limited by the two adjacent cut-off frequencies. Although accurate predictions of pure-tone thresholds have been reported (Don, Eggermont, and Brackman, 1979), this technique apparently is not in widespread clinical use for the purposes of estimating threshold. The procedure, however, is now being considered as part of an approach that can be used to detect small vestibular schwannomas and cochlear hydrops (e.g., Don et al., 1997).
An alternative approach has been to generate stimuli that have sufficiently rapid onsets that they can effectively elicit an ABR (which is an onset response requiring neural synchrony), while at the same time maintaining a relatively well-defined stimulus in the frequency domain. The most common approach to generating stimuli with these two characteristics involves the use of gated sinusoids or tonebursts. The rapid onsets and short durations of these stimuli result in energy centered over the nominal stimulus frequency, but dispersed towards both higher and lower frequencies. Stimulus duration, rise-fall time and the manner in which these stimuli are gated will determine the spectral spread in the stimulus. Several studies have been described in which pure-tone thresholds and toneburst-evoked ABR thresholds have been compared (e.g., Kodera et al., 1977; Munnerly et al., 1991; Purdy and Abbas, 2002; Stapells, 2000; Suzuki et al., 1977). The agreement between the two threshold measures suggest that toneburst-evoked ABR thresholds can be used to predict the magnitude and configuration of hearing loss (Suzuki et al., 1982). It is difficult to know the extent to which tonebursts are used clinically; however, there are anecdotal indications to suggest that toneburst stimuli are in common use when ABRs are measured in efforts to predict the pure-tone audiogram.
Others have argued that, due to cochlear mechanical responses and the spread of spectral energy that is a characteristic of brief sinusoids, the use of toneburst stimuli alone is insufficient to generate a response whose threshold can then be correlated with behavioral thresholds at the nominal test frequency (Picton et al., 1979; Stapells, 1984; Stapells et al., 1994). As a consequence, it has been suggested that tonebursts should be combined with a notched-noise masker, whose notch occurs at the toneburst frequency. In this paradigm, the hypothesis is that the notched noise would prevent fibers innervating distant cochlear regions from responding to the stimulus, with only fibers innervating frequency regions within the notch contributing to the response. In one of the most comprehensive descriptions of behavioral threshold predictions from ABR measurements, Stapells et al. (1995) demonstrated correlations ≥ 0.94 for frequencies of 0.5, 2, and 4 kHz, using the notched-noise technique. Despite this report, the procedure apparently is not in widespread clinical use. A recent study by Johnson and Brown (in press) compared the accuracy of behavioral threshold predictions from ABR thresholds evoked using two different stimulation paradigms, tonebursts presented alone or tonebursts presented in combination with notched noise. The results from that study indicated that there was no difference in the accuracy with which either tonebursts alone or tonebursts plus notched noise predicted behavioral threshold.
In summary, it appears that there are many ways in which ABR thresholds can be used to predict pure-tone thresholds. There are proponents of each of the above techniques, although disagreement remains as to which technique is most applicable in the clinic. One factor that must be considered in the clinical application of any technique relates to the time it takes to collect the data. These procedures are often performed when the infant is in a state of natural sleep, a condition that is needed for accurate ABR threshold measurements. However, there is no guarantee that the infant will sleep long enough to complete measurements for a large number of stimuli. In older children, the tests are frequently performed while the patient is sedated. While the sedatives that are typically administered are relatively safe, sedation requires access to on-site medical staff, monitoring equipment and additional precautions; as a consequence, its use results in increased costs. Even with sedation, there is no guarantee that the child will sleep long enough to complete the test for a wide range of stimuli. Test time remains an important factor when performing an ABR for threshold purposes.
The purpose of the present study was to describe the accuracy with which ABR thresholds can be used to predict pure-tone thresholds. Data were collected in response to a variety of stimuli, including clicks and tonebursts, much as has been reported previously. New to this paper are the results that were obtained with a specially constructed low-frequency toneburst (Gorga et al., 1991). All of the evoked-potential data reported in this paper were collected as part of routine clinical ABR assessments, in which the order of stimuli is prioritized to provide important information in the least amount of time. In the ideal clinical evaluation, the goal is to obtain information about low-frequency, mid-frequency and high-frequency sensitivity bilaterally in a single test session. Unfortunately, it is not always possible to collect data for all stimulus conditions in a single session; thus, some prioritization is needed, especially since patients in some areas travel considerable distances to receive these ABR tests. Although not a new idea, the present paradigm follows a plan in which priority is given to stimuli according to the information they will provide; thus, click-evoked thresholds are measured first, followed by threshold measurements for the single-cycle, low-frequency toneburst. Once these data are collected, ABR thresholds are next measured for a mid-frequency toneburst. If the child remains asleep, additional, high-frequency stimuli are included. This sequence, in many ways, mimics the approach that is taken when young children receive a behavioral audiometric evaluation, in which measurements are prioritized according to stimulus frequency. It represents a prioritization of the ABR data-collection process, with the hope that we obtain the most important information first, followed by complimentary or relatively less important information as we move through the paradigm. It is a compromise between the need for information for a wide range of frequencies and time efficiency. However, this paradigm would be useful only if the approach results in accurate estimates of pure-tone thresholds, which is the focus of this paper.
METHODS
A. Subjects
In total, data are reported from 140 ears of 77 subjects, who ranged in age from 5 days to 20 years at the time of the ABR test (with 71 of the subjects being less than 5 years of age). The 20-year old subject was tested as part of a genetic study on hearing loss. The average age at the time of the ABR test for the entire group was 30.4 months. When restricted to the 71 subjects less than 5 years of age, the mean age at the time of the ABR was 21.7 months. Due primarily to subject state, complete sets of data were not always available for both ears of all subjects. Otoscopic examinations and tympanometric measurements (226- or 1000-Hz probe tones, depending on age) were used to describe middle-ear function. Behavioral audiometric assessments typically were conducted after the ABR test. The average age at the time of the behavioral tests for the entire sample was 36.5 months. When restricted to the 71 subjects who were less than 5 years of age, the mean age at which behavioral data were obtained was 28.7 months. Thus, the difference between the average age for ABR testing and the average age for behavioral tests was 6.3 months when all subjects were included and 7 months when restricted to the group of children less than 5 years of age.
Data are reported only for those ears for which both sets of threshold measurements were available. In some cases, limited behavioral-threshold data were available. These cases were included as long as there were conditions for which ABR and behavioral thresholds could be compared. There were several reasons why data were not included from every ear of every infant who was initially assessed by ABR. In an effort to control subject conditions as much as possible, data were included only if the middle-ear findings were the same at the time of both the ABR and behavioral-hearing assessment. For example, if a child had a normal otoscopic and/or tympanometric examination at the time of the ABR, data were included only if the child also had a normal otoscopic and/or tympanometric examination at the time of the behavioral hearing test. Some patients did not return to our hospital for follow-up services beyond the initial ABR; in these instances, audiometric data were not available. Subjects were excluded if there was evidence of auditory neuropathy (cochlear microphonic was present and/or normal otoacoustic emissions were observed in the presence of a grossly abnormal ABR waveform). In these cases, the ABR is not a good indicator of pure-tone sensitivity. Finally, the developmental level of some patients was such that only ABR thresholds were available. Thus, we could not evaluate the accuracy of the ABR test relative to a pure-tone threshold standard for these exceptions.
B. ABR Procedures
For infants less than 3 months of age, tests were performed during natural sleep. For infants older than 3 months, chloral hydrate (50 mg/kg) was used to sedate the infant. The oldest subject (20 years of age) was tested without sedation. For other patients who were sedated, the sedation order was written by a pediatrician who evaluated the patient prior to testing, and the sedative was administered by a nurse. Sedated patients were monitored throughout testing, using pulse oxymetry. Following the end of testing (which sometimes occurred because of the patient’s state of arousal), sedated patients were re-examined by a nurse prior to hospital discharge. Although a less frequent occurrence with sedation, patients did not always remain asleep long enough to test all stimulus conditions.
ABRs were recorded with electrodes placed at the high forehead and on each mastoid. The electrode contralateral to the ear of stimulation served as ground. Electrode impedances were roughly equivalent and were < 5 kilohms at the start of the test. Responses to either 1000 (clicks) or 2000 (tonebursts) stimuli were averaged, and each response was replicated. Response filter settings depended slightly on the stimuli, which are described below. For clicks, 1-, 2-, and 4-kHz tonebursts, the ABR was filtered between 100 and 3000 Hz; for the 250-Hz toneburst, responses were filtered from 30 to 3000 Hz.
Both clicks and tonebursts were used as stimuli, all of which were generated by the same clinical system that was used to record the response (Navigator, Bio-logic Systems Corp.). One-hundred μs clicks (rarefaction polarity), presented at a rate of either 17/s or 27/s, were sent to an insert earphone (ER-3A), whose frequency response shaped the stimulus spectrum. Responses always were measured for clicks first for several reasons. Clicks almost invariably result in the most robust responses, allowing us to make these measurements in a short amount of time. They provide a template response that could be used for comparison with the responses that were obtained with other stimuli that typically do not result in as robust a response. Finally, they provide a quick way to determine whether ABR-threshold estimates were affected by the presence of a neurological condition, such as auditory neuropathy (AN). If click-evoked measurements indicated that AN was present, ABR-threshold testing was terminated. During click-evoked measurements, the response window was set to 15 ms. Clinically, we view the ABR threshold for clicks as an estimate of high-frequency sensitivity.
Following click-evoked ABR measurements, several toneburst stimuli were used, time permitting. The next stimulus in our paradigm was a 250-Hz, Blackman-windowed toneburst having 2 ms on both the rise and fall with no plateau. Thus, its total duration was 4 ms, or equal to a single cycle at this frequency. The spectrum of this stimulus is broad, having relatively constant energy up to 600 Hz, beyond which the energy falls off rapidly as a consequence of the Blackman window. The time waveform and amplitude spectrum of this stimulus have been described elsewhere (Gorga et al., 1991). This toneburst was presented at a rate of 37/s and had an initial negative-going excursion, meaning that the first half cycle resulted in reduced pressure in the ear canal (akin to a rarefaction click). Its polarity was not alternated because previous work has shown that the response is less well defined when alternating polarity is used (Gorga et al., 1991). This occurs because the negative trough following wave V with one polarity (180 degrees phase) tends to line up with the positive peak of wave V for the opposite polarity (0 degrees phase), thus resulting in some cancellation of the response. In turn, this effect presumably is due to the phase-locking ability of the auditory system, which is evident in the discharge patterns of auditory neurons (e.g., Rose et al., 1971). A response window of 25 ms was used when responses were recorded for all toneburst stimuli. We viewed ABR thresholds for the 250-Hz toneburst as providing an estimate of low-frequency sensitivity.
If ABR thresholds were measured to clicks and the 250-Hz toneburst, and the patient remained asleep, a 1-kHz toneburst was used next. This stimulus was also gated with a 4-ms Blackman window. The initial phase (polarity) of this stimulus was 180 degrees, although phase is less of an issue in relation to response identification at this frequency, compared to 250 Hz (Gorga et al., 1991; Fowler, 1992). The ABR threshold for this stimulus was used to estimate sensitivity for mid frequencies.
Because of practical issues associated with test time, ABR threshold estimates were more frequently available for clicks, 250 Hz, and 1 kHz, compared to other stimuli. However, there were cases in which time permitted measurements of ABR thresholds at either 2 or 4 kHz. These stimuli also were played at a rate of 37/s. For 2 kHz, the rise/fall times were 1.5 ms, while at 4 kHz, the rise/fall times were 1 ms. These stimuli were gated with a Blackman window, as was the case for other toneburst stimuli. For 1, 2, and 4 kHz, the temporal characteristics of the stimuli represent a compromise between uniform energy spread in linear versus logarithmic frequency. If the rise/fall times were fixed in time for all stimulus frequencies, then the energy spread would have been uniform in linear frequency, which is not how frequency is organized within the cochlea. However, the absolute rise times would have been the same at each frequency, which would be expected to result in similar onset synchrony for all frequencies (within the limits imposed by the mechanics of the cochlea as a function of frequency). If rise/fall times were fixed in terms of sinewave cycles, then the energy spread would be uniform in logarithmic frequency, which is how frequency is organized within the cochlea. However, this would have resulted in greater onset synchrony at high frequencies (due to their shorter periods which would result in more rapid absolute rise times), compared to low frequencies. By choosing rise/fall times that compromise between these two conditions, we hoped to achieve stimuli that would successfully elicit responses for a wide range of frequencies, while resulting in energy spread (in log coordinates) that was only slightly greater for low frequencies, compared to high frequencies. None of these rules were used in the generation of the 250-Hz tone burst, which is viewed as a unique stimulus. Temporal characteristics and stimulus levels for ABR stimuli are provided in Table 1. The levels represent the peak SPL for 0 dB nHL. For a more complete description of the approach we took to determine these reference levels, please see Gorga et al. (1993). For all stimuli, ABR threshold was defined as the lowest level at which a response was observed, with the following exception. In the interest of clinical-test efficiency, measurements for a specific stimulus terminated if an ABR was observed at 20 dB nHL. The impact of this practical decision on threshold agreements will be described later.
TABLE 1.
Temporal characteristics and reference equivalent SPL for the five stimuli used during ABR measurements. The temporal characteristics for the click were defined by the electrical waveform prior to transduction by the earphone
| Stimulus | Rise/Fall Time (ms) | Plateau (ms) | Total Duration (ms) | pSPL for 0 dB nHL |
|---|---|---|---|---|
| 250 Hz | 2.0 | 0 | 4 | 43 |
| 1000 Hz | 2.0 | 0 | 4 | 24 |
| 2000 Hz | 1.5 | 0 | 3 | 28 |
| 4000 Hz | 1.0 | 0 | 2 | 32 |
| Clicks | 0 | 0.1 | 0.1 | 35 |
C. Behavioral threshold measurements
Pure-tone audiometric data typically were collected after the ABR thresholds were measured. These tests were performed in sound-treated environments, using primarily insert earphones and audiometers calibrated to ANSI standards (S3.2, 1996), and behavioral test procedures appropriate for the developmental level of the patient (i.e., visual reinforcement audiometry, conditioned play audiometry). Under no circumstances, however, was behavioral observation used to estimate thresholds. It may be important to note that the data used in this report were taken from the medical records of patients who were followed clinically. As a result, the clinicians performing the behavioral hearing tests were familiar with the results from the previously performed ABR evaluation. The implications of this potential bias are described below.
Table 2 describes the distribution of pure-tone thresholds (divided into categories of magnitude of hearing loss) at each octave frequency. We were not able to measure behavioral pure-tone thresholds at all six octave frequencies in both ears of all subjects, for reasons related to patient testability. This problem was greatest at 8 kHz, a frequency at which behavioral threshold data were available in only 48 ears. The low number of observations at this frequency is a result of its low priority in audiometric evaluations in children, although recent data suggest that high-frequency information may be important for speech and language development (Stelmachowicz et al., 2004). For the purposes of this paper, however, this was not viewed as a significant problem, since none of the ABR data were compared to behavioral thresholds at this frequency.
TABLE 2.
Counts of the number of ears with pure-tone thresholds in each of five major categories of hearing loss at each of six octave frequencies. Threshold definitions for each group were as follows: Normal ≤ 20 dB HL; Mild > 20 dB HL ≤ 40 dB HL; Moderate > 40 dB HL ≤ 60 dB HL; Severe > 60 dB HL ≤ 80 dB HL; Profound > 80 dB HL
| Frequency | Normal | Mild | Moderate | Severe | Profound | Total |
|---|---|---|---|---|---|---|
| 250 | 12 | 31 | 21 | 14 | 15 | 93 |
| 500 | 25 | 42 | 17 | 15 | 24 | 123 |
| 1000 | 29 | 28 | 21 | 17 | 21 | 116 |
| 2000 | 34 | 28 | 20 | 20 | 13 | 115 |
| 4000 | 40 | 21 | 32 | 18 | 12 | 123 |
| 8000 | 18 | 12 | 10 | 6 | 2 | 48 |
RESULTS
A. ABR waveforms for clicks and for toneburst stimuli
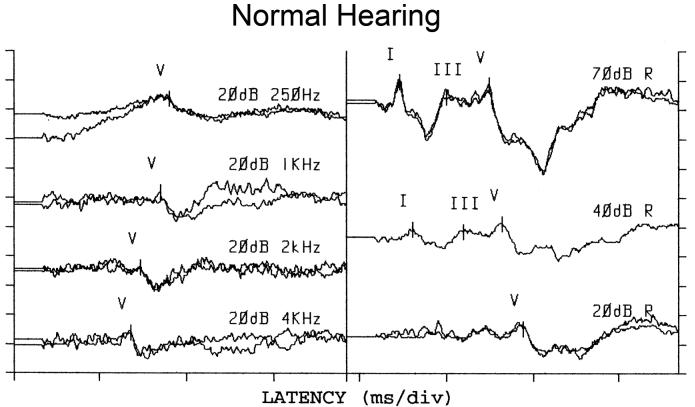
Figure 1 provides examples of ABR waveforms from one ear of an infant with normal hearing. This child (who was sedated) was quiet for a long enough period of time that responses could be measured for the full range of stimuli. Responses to tone bursts are shown in the left column, while responses to clicks are shown in the right column. Although the waveforms are plotted on the same X axis, the response windows (time base) differed across stimuli, with a 25-ms epoch for toneburst stimuli and a 15-ms epoch for clicks. As a result, absolute response latencies, based on these waveforms plots, can be compared within a column but should not be compared across columns. This is also the case for the waveforms shown in Fig. 2. For all stimuli, no measurements were made below 20 dB nHL, which represents our minimum response level. In order to provide examples of responses for all stimuli, an intensity series is shown only for clicks; for tonebursts, the responses are shown only at the minimum response level. This child produced responses for clicks and for a wide range of toneburst frequencies.
Figure 1.
ABR waveforms for toneburst stimuli (left column) and clicks (right column) in a patient with normal hearing. For toneburst stimuli, responses are only shown at the minimum response level (20 dB nHL). For clicks, responses are shown at several levels, including the minimum response level. The time base varied, depending on stimulus. Thus, one cannot directly compare response latencies based on these plots. For tonebursts (left column), the response window was 25 ms in length. For clicks (right column), the response epoch was 15 ms.
Figure 2.
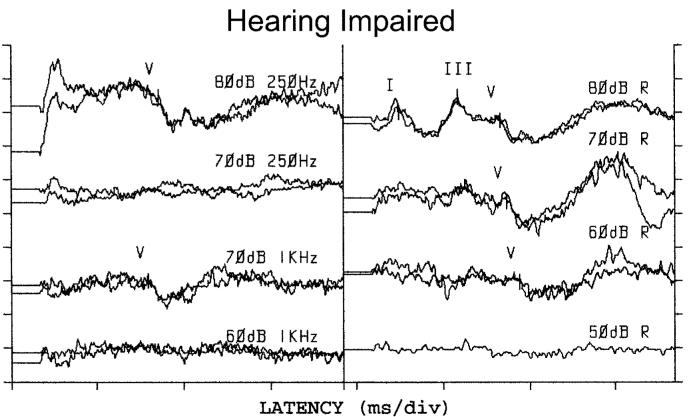
ABR waveforms in a patient with moderate-to-severe hearing loss, following the convention used in Fig. 1. In this case, however, waveforms are shown at the lowest level at which a response was evident and then at the next lower level. Response epochs for toneburst (left column) and click (right column) responses were 25 ms and 15 ms, respectively.
Figure 2 provides examples of waveforms from one ear of a child with moderate- to-severe hearing loss. In this case, data were collected for clicks, and for a 250-Hz and a 1-kHz toneburst. ABR waveforms are shown at the lowest level at which a response was observed and then at the next lower level tested in order to provide measurements at and below threshold. The click-evoked response (right column) was present down to 60 dB nHL, but there was no response at 50 dB nHL. At 250 Hz, a response was observed at 80 dB nHL, but not at 70 dB nHL. At 1 kHz, a response was evident 70 dB nHL, but not at 60 dB nHL. While responses could not be measured down to the minimum response levels, due to the presence of hearing loss, it was possible to estimate thresholds from these data.
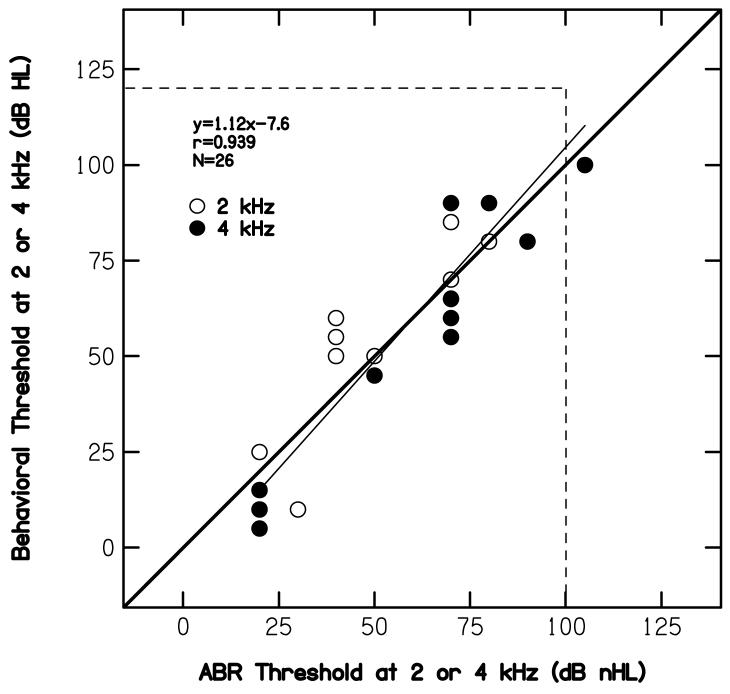
B. ABR thresholds for clicks compared to behavioral thresholds at 2 and 4 kHz
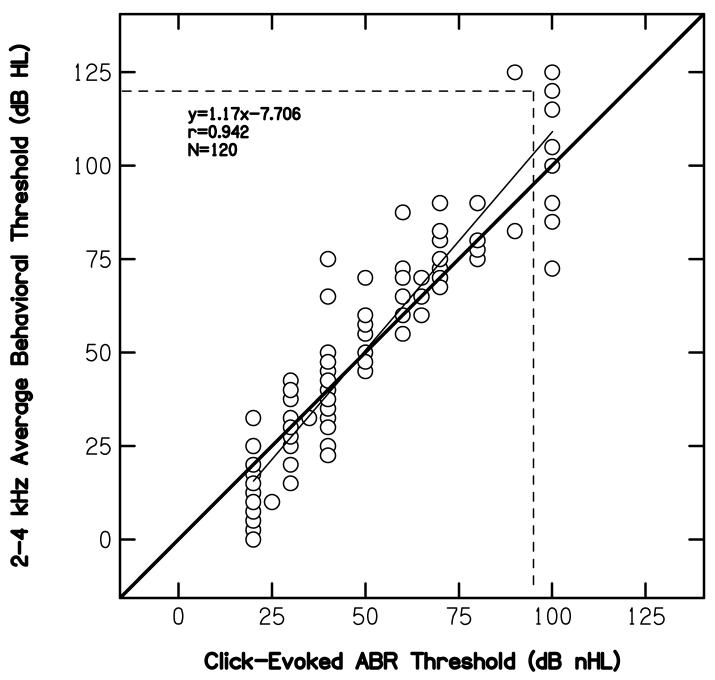
Figure 3 plots the average pure-tone threshold (in dB HL) at 2 and 4 kHz as a function of click-evoked ABR threshold (in dB nHL). The solid heavy line serves as a point of reference; it represents the case when there were no differences between the two threshold estimates. The thin, near-diagonal line represents a least-squares, linear fit to the data, the equation for which is provided in the panel. The horizontal and vertical dashed lines represent the limits of our audiometric and electrophysiologic instrumentation, respectively. Overlapping data points were not offset. Data points falling outside the space limited by the two dashed lines represent conditions for which no response was measured. These points, however, were included in the linear regression. Whether included or not had little effect on the correlation here or in the figures to follow. ABR data at the minimum response level of 20 dB nHL also were included in the regression, even though these data do not represent threshold in a traditional sense. The influence of the inclusion of data points at the minimum response level is described later. Finally, the number of ears on which data are reported and the correlation between behavioral and ABR thresholds are reported within the panel. While prediction errors occurred (the worst of which was 35 dB), it is clear that a relationship exists between the average pure-tone threshold at 2 and 4 kHz and the click-evoked ABR threshold. Nearly 89% of the variance in behavioral threshold can be accounted for in the variability in ABR thresholds. Thus, at least for the group data, it would appear that the pure-tone threshold can be predicted from the evoked-potential threshold.
Figure 3.
Average pure-tone behavioral thresholds at 2 and 4 kHz (dB HL) as a function of click-evoked ABR threshold (dB nHL). Horizontal and vertical dashed lines represent cases for which no response was measured. The heavy diagonal line serves as a point of reference, representing the case when there was one-to-one correspondence between pure-tone and ABR thresholds. The thinner, near-diagonal solid line represents a least-squares, linear fit to the data. The equation describing the best-fit line is provided within the panel, along with correlation (r), and the number of cases for which data were available.
C. ABR versus behavioral thresholds at low frequencies
Figure 4 compares behavioral thresholds for low-frequency pure tones to ABR thresholds for the 250-Hz toneburst. From top to bottom, comparisons are made to the behavioral threshold at 250 Hz, 500 Hz, and the average threshold at 250 and 500 Hz. The heavy diagonal solid, thin near-diagonal solid, and vertical and horizontal dashed lines in this figure serve the same purposes as they did in Fig. 4. Within each panel, the linear equation, number of ears, and correlations are provided for the comparison represented within that panel. ABR thresholds at 250 Hz are reasonably correlated with behavioral thresholds, regardless of the pure-tone threshold to which the ABR threshold is compared. Under the worst circumstance (predicting the pure-tone threshold at 250 Hz), nearly 86% of the variance in behavioral threshold can be accounted for by the variability in ABR threshold. Thus, it would appear that an accurate prediction of low-frequency sensitivity is possible with this single-cycle, 250-Hz toneburst.
Figure 4.
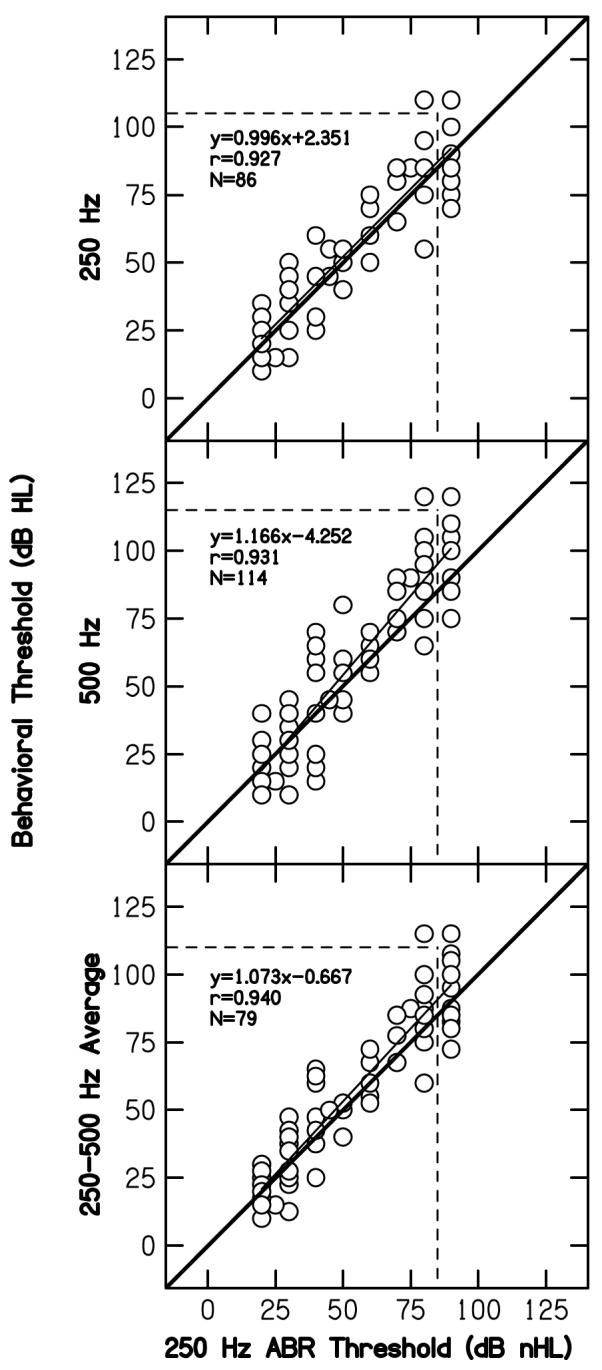
Pure-tone behavioral thresholds (dB HL) at 250 Hz (top), 500 Hz (middle) and the average of 250 and 500 Hz (bottom) as a function of ABR threshold (dB nHL) to a 250-Hz toneburst. Dashed horizontal and vertical lines, and the solid diagonal line serve the same purpose here as in Fig. 1. The best-fit line, correlation, and number of cases are shown in each panel.
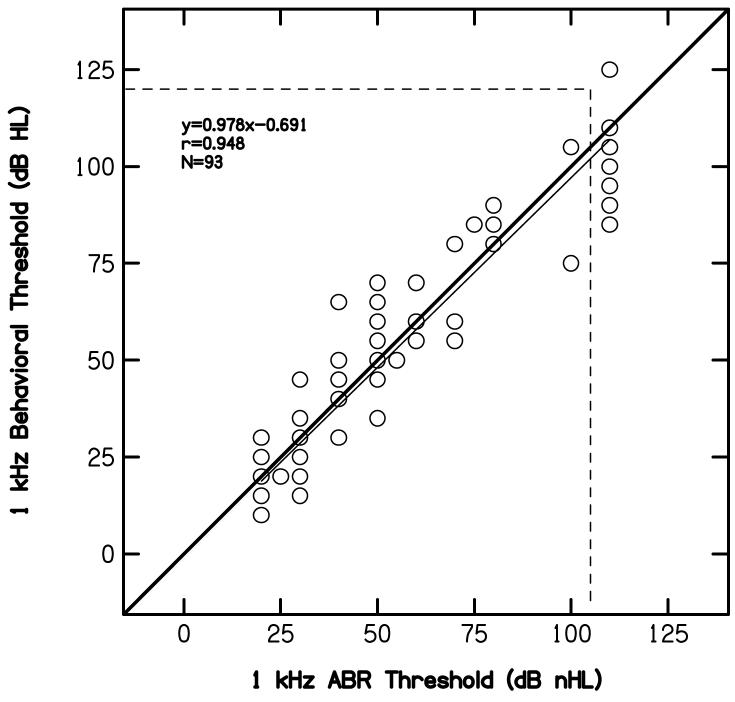
D. ABR versus behavioral thresholds at 1 kHz
Figure 5 displays behavioral thresholds at 1 kHz as a function of ABR thresholds for 1-kHz tonebursts, following the convention used in Figs. 3 and 4. In this case, nearly 90% of the variance in behavioral thresholds can be accounted for by the variance in ABR thresholds. In fact, this represents the highest correlation we observed between behavioral and ABR thresholds. Thus, a good approximation of hearing sensitivity for a mid frequency is possible, using this 1-kHz toneburst.
Figure 5.
Pure-tone behavioral thresholds at 1 kHz (dB HL) as a function of ABR thresholds (dB nHL) to a 1-kHz toneburst, following the conventions used in previous figures.
E. ABR versus behavioral thresholds at 2 and 4 kHz
Finally, a limited amount of ABR threshold data was available at 2 and 4 kHz. Those data are shown in Fig. 4, following the convention used previously. Because of the paucity of ABR data at these two frequencies, the data are combined in this figure and fit with a single regression line. In order to display the actual data from each frequency separately, behavioral and ABR threshold data at 2 kHz are represented as open circles, while the same data at 4 kHz are shown as filled circles. Even for this limited data set, there appears to be a reasonable correlation between the two threshold estimates. However, these data are viewed as supplemental in that they may not be providing additional information beyond what was provided by click-evoked ABR threshold measurements.
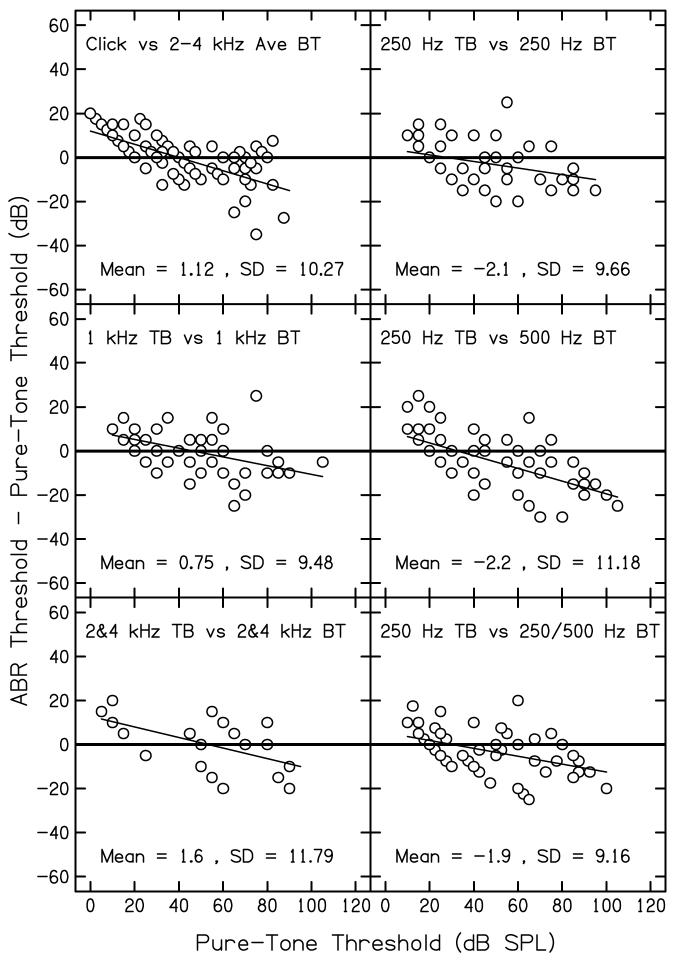
F. Differences between pure-tone thresholds and ABR thresholds
Figure 7 plots the difference between pure-tone audiometric thresholds and ABR thresholds as a function of the pure-tone threshold for the comparisons shown in the Figs. 3-6. Cases for which no response was observed were excluded from this analysis. Each panel shows results for a different comparison, as indicated in the figure. Also shown is the grand mean difference and standard deviation within each panel. The heavy solid lines drawn at zero serves as a reference; if there was perfect agreement between ABR and pure-tone thresholds, all data points would fall on these lines. Thus, these lines represents the same conditions as the heavy diagonal lines shown in Figs. 3-6. The thin solid lines represents linear regressions that were fit to these threshold differences.
Figure 7.
Differences between pure-tone audiometric thresholds (in dB HL) and ABR thresholds (measured in dB nHL) as a function of pure-tone audiometric threshold. Cases in which no response was measured are excluded. Each panel represents results for a different comparison, as indicated within each panel. Also shown within each panel is the grand mean and standard deviation of the differences.
Figure 6.
Pure-tone behavioral thresholds (dB HL) at either 1 or 2 kHz as a function of ABR thresholds (dB nHL) for either 2 or 4 kHz tonebursts, following the same conventions as used in previous figures. Open circles represent data for 2 kHz, while filled circles represent data for 4 kHz.
Although perfect agreement is never achieved, the differences between the two threshold estimates are less than 20 dB for the majority of cases. There are, however,cases in which larger differences are observed, although such cases are rare. Both positive and negative differences are observed, and, on average, the differences appear to be similar in the six panels. Thus, differences were not greater for clicks compared to tonebursts. In all six cases, there is a tendency for ABR thresholds to overestimate pure-tone thresholds when hearing is normal and to underestimate pure-tone thresholds when hearing loss exists.
G. Predicting pure-tone thresholds from ABR thresholds
The results summarized in Fig. 7 suggest that applying a single correction to measured ABR thresholds would not improve predictions of behavioral thresholds, because such an approach would tend to either improve the predictions in ears with normal hearing (negative correction) and increase the error in ears with hearing loss, or vice versa (positive correction). The best-fit regressions in Figs. 3-6, however, could be used in an effort to improve predictions. If these equations were used, they would have the effect of reducing the estimated pure-tone thresholds for low ABR thresholds and increasing it for high ABR thresholds, with the net effect of improving the accuracy of the predicted pure-tone thresholds for a wide range of thresholds.
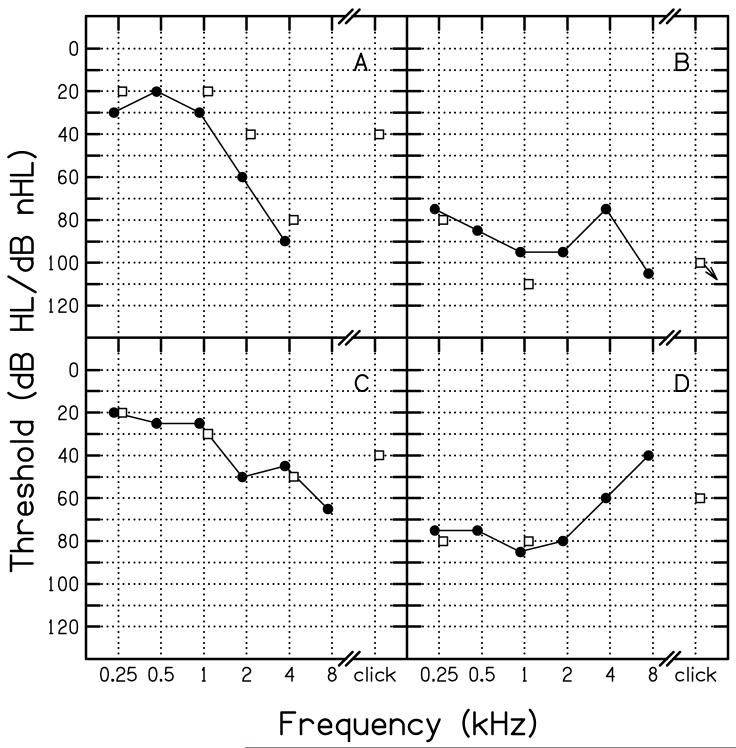
H. Case studies
Figure 8 provides four case examples in which ABR threshold estimates are compared to subsequently obtained pure-tone thresholds. These cases were chosen, not because they were representative examples, but rather to provide examples of the range of results that were observed in individual ears. Panel A shows results for the worst-case outlier (35 dB underestimation of pure-tone thresholds), which occurred when the click-evoked ABR threshold was used to predict the average pure-tone threshold at 2 and 4 kHz in a case of steep high-frequency hearing loss. In this case, behavioral thresholds at 2 and 4 kHz were 60 and 90 dB HL, respectively, resulting in an average threshold of 75 dB. At 1 kHz, the pure-tone threshold was 30 dB HL, and it was even lower at lower frequencies. The click-evoked ABR threshold, however, was 40 dB nHL. Thus, it understimated the average threhsolds at 2 and 4 kHz by 35 dB. Interestingly, the 2-kHz toneburst ABR threshold (40 dB nHL) underestimated the pure-tone threshold at the same frequency by 20 dB and the 4-kHz ABR threshold (80 dB nHL) underestimated the pure-tone threshold by 10 dB. Thus, errors in pure-tone threshold estimation remained when tonebursts were used, although the size of the error was less. All of these data suggest that the evoked-potential response came from lower-frequency regions of better hearing. While the extent of the error was less for toneburst stimuli, compared to clicks, these results, in total, suggests that it was spread of energy in the stimulus that elicited the ABR at lower levels relative to pure-tone thresholds.
Figure 8.
Case examples in which click-evoked and toneburst evoked ABR thresholds are compared to the pure-tone audiogram. Open squares represent ABR thresholds; filled circles represent audiometric thresholds. Thresholds refer to dB HL (re: ANSI, 1996) for pure-tone audiometry and dB nHL (locally determined) for ABR measurements.
Panel B compares ABR and pure-tone thresholds for a patient with a severe, relatively flat hearing loss. In this case, ABR thresholds overestimated the amount of hearing loss by between 5 and 15 dB. Thus, this result goes in the opposite direction of the case shown in Panel A, suggesting that some deviations in both cases may be a result of underlying variability in these measurements. While it is often assumed that the source of error is in the ABR measurements, it may be the case that the behavioral measurements are characterized by variability as well, especially in the patient population in the present study. If this alternative assumption is correct, then it would be a mistake to attribute all of the errors to the ABR measurements.
Panel C shows results for a patient with a gradually sloping moderate-to-severe hearing loss. In this case, there is good agreement between ABR and behavioral thresholds. This was true for comparisons between ABR toneburst thresholds and pure-tone thresholds and when ABR click-evoked thresholds were compared to average pure-tone thresholds at 2 and 4 kHz, although the agreement was less in this case.
Panel D compares the two threshold estimates in a patient with a severe rising audiometric configuration. At the two frequencies for which ABR toneburst thresholds were measured, the agreement is excellent. The ABR click-evoked threshold appears to be related to the threshold at 4 kHz, but underestimates the threshold at 2 kHz.
DISCUSSION
The purpose of this study was to re-evaluate the accuracy with which click-evoked and toneburst-evoked ABR thresholds predict pure-tone behavioral thresholds. Much of this work is not new and, to a certain extent, the results are similar to what has been reported in some previous studies. What is new to the present work is the inclusion of ABR data for a specially-constructed 250-Hz toneburst that was used to estimate low-frequency pure-tone sensitivity. There are three main observations from the data reported in this paper:
Click-evoked ABR thresholds are correlated with the pure-tone behavioral thresholds averaged at 2 and 4 kHz, although prediction errors occur.
Toneburst thresholds correlate with pure-tone thresholds at the same frequency.
A specially-constructed 250-Hz toneburst can be used to measure ABR thresholds that correlate with low-frequency, pure-tone thresholds.
In total, these data suggest that ABR threshold measurements can be used to predict pure-tone thresholds with sufficient accuracy to initiate a program of habilitation for patients who are unable to provide reliable behavioral responses to sound.
There has been debate over the years as to whether click-evoked responses can be used to estimate the magnitude of hearing loss at any frequency. While some studies have suggested that a reasonable prediction can be made (Jerger and Mauldin, 1978; Gorga et al., 1985; van der Drift, 1987), others have argued that the relationship is too variable to provide such information, even for the frequencies for which the correlations between ABR and behavioral thresholds are highest (Stapells et al., 1994). The present results support findings that suggest a correlation between the two threshold estimates, at least when click-evoked ABR thresholds are compared to average pure-tone thresholds at 2 and 4 kHz. In fact, the correlation observed for this comparison in the present study (0.94) exceeds those reported by us previously for the same conditions (0.81) (Gorga et al., 1985). There are several hardware differences between the two studies, with the use of insert earphones in the present study representing a potentially important improvement. Perhaps the use of these earphones reduced the incidence of ear-canal collapse in younger patients, thus resulting in better agreement between ABR and behavioral thresholds. It may be important to note that the data reviewed here came from consecutive, unselected patients, with the only inclusion criteria being that both electrophysiological and behavioral thresholds had to be available, and that the status of the middle ear was the same at the time of both tests. There were other patients for whom ABR data were available but who were not included in the analyses because (1) they did not return to our hospital for follow-up testing, (2) their developmental level precluded the reliable measurement of behavioral thresholds, (3) middle-ear status was different on the days on which the ABR and behavioral tests were performed, or (4) test results were consistent with a diagnosis of AN. Perhaps these factors introduced a bias into the results summarized here, but it is unclear how the bias would influence the outcome.
It is expected that the greatest disagreement between click-evoked ABR thresholds and behavioral thresholds in the higher frequencies will occur when there is a steeply sloping high-frequency hearing loss. High-frequency hearing loss is common among adults, presumably due to the effects of noise exposure and aging. Compared to adults, however, high-frequency heairng loss is less common among children (Pittman and Stelmachowicz, 2003). One explanation for the generally good agreement between click-evoked thresholds and the average thresholds at 2 and 4 kHz observed in the present study may be due to the bias introduced by restricting the sample to children, for whom hearing-loss configurations are more variable.
Furthermore, toneburst thresholds at 1, 2 and 4 kHz correlate with pure-tone thresholds at the same frequencies, which also is not a new observation. The correlations suggest that predictions of behavioral threshold are possible based on electrophysiologic thresholds when toneburst stimuli are used to measure ABR thresholds.
The results described here with the specially constructed 250-Hz toneburst are encouraging. In a study concerned with the effects of stimulus phase (polarity) on ABR waveform morphology and latencies, ABRs were recorded in normal-hearing subjects, using Blackman-windowed, single-cycle sinusoids at octave frequencies from 250 Hz to 4 kHz (Gorga et al., 1991). The earlier study was focused on the interactions between stimulus phase and frequency, and included measurements only from subjects with normal hearing. However, the observation of robust responses from single-phase, Blackman-windowed, single-cycle 250-Hz toneburst led to the inclusion of this stimulus as part of our evoked-potential assessments.
While the previous paper described phase effects, the present paper reports clinical results obtained with this specially-constructed, low-frequency stimulus. The results shown in Figs. 4, 6 and 7 indicate that this stimulus can be used to provide a reasonable estimate of low-frequency sensitivity. The fact that the present data were collected mainly in children further supports the use of this stimulus for the purposes of estimating low-frequency sensitivity in young patients.
Although the slopes of the functions relating pure-tone thresholds to ABR thresholds were close to one, there were deviations from unity slope, because errors occurred in one direction for ears with normal hearing, but in the opposite direction for ears with severe hearing loss. The linear equations provided in Figs. 3-6, however, could be used to improve predictions of pure-tone thresholds from ABR thresholds. The use of these equations would have the effect of lowering the predicted pure-tone threshold when ABR thresholds were low, increasing the predicted pure-tone threshold when ABR thresholds were high, and leaving threshold predictions unadjusted when ABR thresholds fell in the intermediate range. Thus, using these equations to improve predictions might be more accurate than applying a single correction, regardless of ABR threshold.
One concern when ABR thresholds are used to predict pure-tone thresholds relates to the differences in the reference equivalent sound pressure level (RESPL) for the two different measures. National standards exist defining the RESPL for pure-tone audiometry. The RESPL for pure tones, described in ANSI S3.26 (1996) were developed for average adult subjects, and thus, may be less representative of the RESPL for infants and children, especially when insert earphones are used (Voss and Herrmann, 2005). Still, pure-tone thresholds have the advantage that they are referenced to a single, national standard.
A different situation exists for the stimuli typically used during ABR measurement. While concerns related to the appropriateness of using reference levels developed on adult subjects with young patients still exist, the reference equivalent threshold level for clicks has not been described in an ANSI standard. (Notably, an international standard has been generated that provides recommended levels for clicks). Despite this limitation, 100-μs clicks have been in routine clinical use for many years, and there is a general consensus that 0 dB nHL occurs at pSPLs of 30-37 dB. There is less consensus regarding the temporal characteristics, windowing function, and RESPL for toneburst stimuli used during ABR measurements. A rationale for choosing the temporal characteristics and windowing function for the stimuli used in the present paper was described above, but these may not represent an ideal set of conditions. While the approach we took for setting the RESPL for ABR stimuli has been described previously (Gorga et al., 1993), this approach may be questioned because it was based on measurements in subjects with normal hearing, for whom the effects of temporal integration are larger than those observed in subjects with hearing loss (e.g., Wright, 1968; Gengel and Watson, 1971). There is no evidence of temporal integration during ABR measurements (e.g., Gorga et al., 1984), which is to be expected, since ABRs represent onset responses to the earliest portions of the stimulus. Thus, one threshold measurement (pure-tone behavioral threshold) is affected by temporal integration, while the other (ABR threshold) is not, and the effects of temporal integration differ for normal and impaired ears. As a result, one might predict that the agreement between ABR and behavioral thresholds would depend on whether hearing was normal or impaired. Data summarized in Fig. 7 provide support for this view. However, the correlations and threshold differences observed in the present data suggest that reasonable approximations of behavioral thresholds for a wide range of frequencies are possible from evoked-potential thresholds, providing practical validation of the present set of stimulus conditions, despite the theoretical concerns described above. There was a tendency for ABR thresholds to overestimate pure-tone thresholds in ears with normal or near-normal hearing, but to underestimate pure-tone thresholds in ears with hearing loss. These observations are not new, as there are reports in the literature of ABR threshold being more elevated with respect to pure-tone, behavioral threshold in subjects with normal hearing than in subjects with hearing loss (e.g., Johnson and Brown, in press; Stapells et al., 1990).
As stated above, part of this effect could be due to the differential influences of stimulus duration on behavioral thresholds in normal and impaired ears, and the lack of a temporal-integration effect in ABR thresholds for both normal and impaired ears. The clinical protocol also might have resulted in the overestimation of pure-tone thresholds in subjects with normal hearing. Recall that the data described in this paper were derived from clinical evaluations, when time efficiency is a concern. If an ABR was observed at 20 dB nHL for any stimulus, measurements were not made at lower levels on the assumption that responses at 20 dB nHL would be consistent with normal hearing. Consistent with the clinical view that test efficiency was important, knowing that the threshold was 5, 10 or 15 dB nHL would not add information that would alter clinical intervention. Once a response was observed at 20 dB nHL, no further measurements were made for that stimulus, and, instead, efforts were switched to another stimulus. This approach resulted in an overestimation of pure-tone thresholds, based on ABR measurements, in every case for which the pure-tone threshold was less than 20 dB HL. However, these errors had little or no clinical consequence, because the ABR thresholds would still result in a classification of normal hearing.
There may be additional value in initiating ABR tests with click stimuli. The responses for these stimuli are typically the easiest to measure, probably because their rapid onsets result in the greatest neural synchrony. Thus, responses to these stimuli can be collected quickly. It is probably for this reason that clicks are often used in ABR-based UNHS programs2. They also provide guidance in evaluating responses to subsequent stimuli, for which the responses typically are not as well defined. In addition, the responses measured with these stimuli provide a useful screening for AN. Although this disorder is rare, it probably occurs in at least 1/100 infants and children with hearing loss, and may occur more frequently than that (Berlin et al., 2003). ABR tests and other evoked-potential tests (such as the ASSR) cannot be used to provide estimates of auditory sensitivity when AN exists. Click-evoked responses can be used to provide a quick and useful screening for AN, and thus, help to avoid diagnostic errors related to hearing sensitivity or, at the very least, avoid unnecessary testing. While it is possible that ABR waveforms elicited by high-frequency tonebursts might also be useful in identifying AN, we are not aware of published data that have demonstrated that this is the case.
Despite the generally good threshold agreements described above, an important bias might have been introduced as a result of the way these data were collected. Recall that the data summarized in this paper were extracted from the medical records of patients seen for clinical purposes. With few exceptions, the sequence of events was such that the ABR thresholds were measured first, while the behavioral data were collected during a subsequent visit. Because both the ABR measurements and behavioral hearing tests were performed as part of clinical evaluations, the audiologists performing behavioral audiological assessments had access to the ABR data. It is possible that this knowledge introduced a bias towards finding agreement between the two threshold estimates. In this retrospective study, there is no way of assessing whether such a bias was introduced; but, it is important to recognize that the clinician performing the behavioral audiometric assessment was not blinded to the ABR results. If this bias was present, then caution should be exercised in the interpretation of the present set of data.
ACKNOWLEDGEMENTS
This work was supported, in part, by grants from the NIH (NIDCD R01 DC2251 and T32 DC0013). We thank Susan Jerger and two anonymous reviewers for their helpful comments on an earlier version of this paper. We thank Hongyong Tan for her help with data analyses.
Footnotes
It is important to keep in mind the distinction between the notions of frequency specificity and place specificity. Frequency specificity has to do with the characteristics of the stimulus. Place specificity relates to the representation of that stimulus in the cochlea. While it is the case that one would need a frequency-specific stimulus if one wished to excite a relatively local place along the cochlea, it is not sufficient to assure a place-specific response, due to the limitations imposed by cochlear mechanics. There is a spread of excitation with level, even in ears with normal hearing. These limitations are made worse in the presence of cochlear hearing loss (especially losses involving the outer hair cells, which is the most common form of hearing loss). The frequency dependence of threshold sensitivity that is evident in normal ears is markedly reduced by cochlear damage (e.g., Liberman and Dodds, 1984). Combining eliciting stimuli with noise maskers may not result in improvements in place specificity in cases of outer hair cell damage because the effects of noise are also altered by hearing loss. See Gorga and Neely (2002) for a schematic description as to why this might be the case.
The use of clicks in ABR-based UNHS programs would be expected to have the same properties as when these stimuli are used in diagnostic procedures. One might predict, therefore, that clicks relate to high-frequency thresholds in screening paradigms in much the same way they relate to pure-tone thresholds in diagnostic evaluations.
REFERENCES
- American National Standards Institute (ANSI) Specifications for audiometers, S3.6. New York, NY: 1996. [Google Scholar]
- Berlin CI, Hood L, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony: Diagnosis and management. Men. Ret. Devel. Dis. Res. Rev. 2003;9:225–231. doi: 10.1002/mrdd.10084. [DOI] [PubMed] [Google Scholar]
- Don M, Eggermont JJ. Analysis of the click-evoked brainstem potentials using high-pass noise masking. J. Acoust. Soc. Am. 1978;63:1084–1092. doi: 10.1121/1.381816. [DOI] [PubMed] [Google Scholar]
- Don M, Eggermont JJ, Brackmann DE. Reconstruction of the audiogram using brain stem responses and high-pass noise masking. Ann. Otol. Rhinol, Laryngol. 1979;83(Suppl 57):1–20. doi: 10.1177/00034894790880s301. [DOI] [PubMed] [Google Scholar]
- Don M, Masuda A, Nelson R, Brackmann D. Successful detection of small acoustic tumors using the stacked derived-band auditory brainstem response amplitude. Am. J. Otol. 1997;18:608–621. [PubMed] [Google Scholar]
- Eggermont JJ, Don M. Analysis of the click-evoked brainstem potentials in humans using high-pass noise masking. II. Effects of click intensity. J. Acoust. Soc. Am. 1980;68:1671–1675. doi: 10.1121/1.385199. [DOI] [PubMed] [Google Scholar]
- Fowler CG. Effects of stimulus phase on the normal auditory brainstem response. J. Sp. Hear. Res. 1992;35:167–174. doi: 10.1044/jshr.3501.167. [DOI] [PubMed] [Google Scholar]
- Galambos R, Hecox KE. Clinical applications of the auditory brain stem response. Otolaryngol. Clin. North Am. 1978;11:709–722. [PubMed] [Google Scholar]
- Galambos R, Hicks GE, Wilson MJ. The auditory brain stem response reliably predicts hearing loss in graduates of a tertiary intensive care nursery. Ear Hear. 1984;5:254–260. doi: 10.1097/00003446-198407000-00011. [DOI] [PubMed] [Google Scholar]
- Gengel RW, Watson CS. Temporal integration: I. Clinical implications of a laboratory study. II. Additional data from hearing-impaired subjects. J. Speech Hear. Dis. 1971;36:213–224. doi: 10.1044/jshd.3602.213. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Beauchaine KA, Reiland JK, Worthington DW, Javel E. Effects of stimulus duration on ABR thresholds and on behavioral thresholds. J. Acoust. Soc. Am. 1984;76:616–619. doi: 10.1121/1.391158. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JK, Beaucahine KL. The effects of stimulus phase on the latency of the auditory brainstem response. J. Am. Acad. Audiol. 1991;2:1–6. [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KL, Bergman BM. A comparison of ABR thresholds and latencies elicited by air and bone conducted stimuli. Ear Hear. 1993;14:85–94. doi: 10.1097/00003446-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST. Some factors that may influence the accuracy of auditory brainstem response estimates of hearing loss. In: Seewald RC, Gravel JS, editors. A Sound Foundation Through Early Amplification 2001. Phonak AG; Chicago, IL: 2002. pp. 49–61. [Google Scholar]
- Gorga MP, Worthington DW, Reiland JK, Beauchaine KA, Goldgar DE. Some comparisons between auditory brainstem response thresholds, latencies and the pure-tone audiogram. Ear and Hearing. 1985;6:105–112. doi: 10.1097/00003446-198503000-00008. [DOI] [PubMed] [Google Scholar]
- Jerger J, Mauldin L. Prediction of ssensorineural level from the brainstem evoked response. Arch. Otolaryngol. 1978;104:456–461. doi: 10.1001/archotol.1978.00790080038010. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Brown CJ. Threshold prediction using the auditory steady-state response and the toneburst auditory brainstem response: A within-subject comparison. Ear and Hearing. doi: 10.1097/01.aud.0000188105.75872.a3. in press. [DOI] [PubMed] [Google Scholar]
- Kiang NY-S. Stimulus representation in the discharge patterns of auditory neurons. In: Tower DB, editor. The Nervous System. Vol. 3.Human Communication and Its Disorders. Raven Press; New York: 1975. pp. 81–96. [Google Scholar]
- Kiang NY-S, Watanabe T, Thomas EC, Clark LF. Discharge Patterns of Single Fibers n the Cat’s Auditory Nerve. The M.I.T. Press; Cambridge, MA: 1965. M.I.T. Research Monograph No. 35. [Google Scholar]
- Kodera K, Yamane H, Yamada O, Suzuki JI. Brain stem response audiometry at speech frequencies. Audiol. 1977;16:469–479. doi: 10.3109/00206097709080018. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Munnerley GM, Greville KA, Purdy SC, Keith WJ. Frequency-specific auditory brainstem responses relationship to behavioural thresholds in cochlear-impaired adults. Audiology. 1991;30:25–32. doi: 10.3109/00206099109072867. [DOI] [PubMed] [Google Scholar]
- Picton TW, Ouellette J, Hamel G, Smith AD. Brainstem evoked potentials to tonepips in notched noise. J. Otolaryngol. 1979;8:289–314. [PubMed] [Google Scholar]
- Pittman AL, Stelmachowicz PG. Hearing loss in children and adults: Audiometric configuration, asymmetry, and progression. Ear Hear. 2003;24:198–205. doi: 10.1097/01.AUD.0000069226.22983.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy SC, Abbas PJ. ABR thresholds to tonebursts gated with Blackman and linear windows in adults with high-frequency sensorineural hearing loss. Ear Hear. 2002;23:358–368. doi: 10.1097/00003446-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Rose JE, Hind JE, Anderson DJ, Brugge JF. Some effects of stimulus intensity on response of auditroy-nerve fibers in the squirrel monkey. J. Neurophsyiol. 1971;34:685–699. doi: 10.1152/jn.1971.34.4.685. [DOI] [PubMed] [Google Scholar]
- Schulman-Galambos C, Galambos R. Brain stem evoked response audiometry in newborn hearing screening. Arch. Otoalryngol. 1979;105:86–90. doi: 10.1001/archotol.1979.00790140032006. [DOI] [PubMed] [Google Scholar]
- Stapells DR. Threshold estimation by the tone-evoked auditory brainstem response: a literature meta-analysis. J Speech Lang Pathol Audiol. 2000;24:74–83. [Google Scholar]
- Stapells DR, Picton TW, Durieux-Smith A, Edwards CG, Moran LM. Thresholds for short-latency auditory-evoked potentials to tones in notched noise in normal-hearing and hearing-impaired subjects. Audiol. 1990;29:262–274. doi: 10.3109/00206099009072857. [DOI] [PubMed] [Google Scholar]
- Stapells DR, Picton TW, Duireux-Smith A. Electrophysiologic measures of frequency-specific auditory function. In: Jacobson JT, editor. Principles and Applications in Auditory Evoked Potentials. Allyn and Bacon; Needham Heights, MA: 1994. pp. 251–283. [Google Scholar]
- Stapells DR, Gravel JS, Martin BM. Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear. 1995;16:361–371. doi: 10.1097/00003446-199508000-00003. [DOI] [PubMed] [Google Scholar]
- Stelmachowicz PG, Pittman AL, Hooever BM, Lewis DE, Moeller MP. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch. Otolaryngol. Head Neck Surg. 2004;130:556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hirai Y, Horiuchi K. Auditory brainstem responses to tone pips. Scand. Audiol. 1977;6:123–126. doi: 10.3109/01050397709043111. [DOI] [PubMed] [Google Scholar]
- Suzki JI, Kodera K, Kaga K. Auditory evoked brainstem response assessment in otolaryngology. Ann.NY Acad. Sci. 1982;388:487–500. doi: 10.1111/j.1749-6632.1982.tb50811.x. [DOI] [PubMed] [Google Scholar]
- Teas DC, Eldredge DH, Davis H. Cochlear responses to acoustic transients. An interpretation of whole nerve action potentials. J. Acoust. Soc. Am. 1962;34:1438–1459. [Google Scholar]
- Voss SE, Herrmann BS. Sound pressures generated by earphones: Adult versus infant ears; Paper presented at the 2005 Meeting of the American Auditory Society; 2005. [Google Scholar]
- Wright H. The effects of sensorineural hearing loss on threshold duration functions. J. Speech Hear. Res. 1968;11:842–852. doi: 10.1044/jshr.1104.842. [DOI] [PubMed] [Google Scholar]