Summary
Structural changes of neurons in the brain during aging are complex and not well understood. Neurons have significant homeostatic control of essential brain functions, including synaptic excitability, gene expression, and metabolic regulation. Any deviations from the norm can have severe consequences as seen in aging and injury. In this review, we present some of the structural adaptations that neurons undergo throughout normal and pathological aging and discuss their effects on electrophysiological properties and cognition. During aging, it is evident that neurons undergo morphological changes such as a reduction in the complexity of dendrite arborization and dendritic length. Spine numbers are also decreased, and because spines are the major sites for excitatory synapses, changes in their numbers could reflect a change in synaptic densities. This idea has been supported by studies that demonstrate a decrease in the overall frequency of spontaneous glutamate receptor-mediated excitatory responses, as well as a decrease in the levels of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-d-aspartate receptor expression. Other properties such as γ-aminobutyric acid A receptor-mediated inhibitory responses and action potential firing rates are both significantly increased with age. These findings suggest that age-related neuronal dysfunction, which must underlie observed decline in cognitive function, probably involves a host of other subtle changes within the cortex that could include alterations in receptors, loss of dendrites, and spines and myelin dystrophy, as well as the alterations in synaptic transmission. Together these multiple alterations in the brain may constitute the substrate for age-related loss of cognitive function.
Keywords: Aging, Alzheimer’s disease, neuroscience, spatial complexity, electrophysiology, dendrites, spines
Introduction
Brain complexity is reflected in the intricacy of its structural makeup. The brain, however small, is not structurally simple. Cortical microcircuits have the ability to reorganize functionally in response to a variety of cues, both intrinsic and extrinsic. Although it is established that there is no overt loss of neurons during normal aging, other, more subtle, changes occur in individual neurons. These include shrinkage in soma size, loss or regression of dendrites and dendritic spines, alterations in neurotransmitter receptors, and changes in electrophysiological properties (Nakamura et al., 1985; Barnes, 1994; Jacobs et al., 2001; Hof et al., 2002; Duan et al., 2003; Chang et al., 2005). Whereas such morphological alterations have been well documented, little is known about the possible mechanisms underlying such changes. Dendrites, the primary substrate for neuronal information processing, are profusely branched and varied. The integrative characteristics of dendrites are determined by several factors, including their morphology, the spatiotemporal patterning of synaptic inputs, and the balance of inhibition and excitation. Thus, dendrites play a vital role in the functional properties of neuronal circuits and any structural changes can have profound and detrimental effects.
Morphological alterations during aging
Neuronal dendrites are instrumental in the formation and maintenance of neural networks, the regulation of synaptic plasticity and the integration of electrical inputs. Therefore, it is not surprising that there are many refined molecular cascades that are involved in the control of dendritic development and growth (Nguyen et al., 2004). In the cortex, the extent of a neuron’s dendritic arborization is an important determinant of the cell’s synaptic properties and affects how incoming information is integrated and processed. Dendritic shape and branching patterns vary among both neuronal classes and individual cells in each class (Samsonovich & Ascoli, 2006). Dendritic spines are specialized membrane compartments that protrude from the dendritic shaft of a neuron. Spines typically have a volume ranging from less than 0.01–0.8 µm3 and an average length of 0.5–2 µm (reviewed in Harris & Kater, 1994; Harris, 1999) and contain excitatory synapses. The linear density of spines on a mature neuron ranges between 1 and 10 spines per micrometer of dendritic length; however, spine density is not homogeneous throughout the dendritic tree, but increases at each order (Sorra & Harris, 2000). There is also a variation across cortical areas. Spine densities on basal dendrites in the prefrontal pole and orbitofrontal cortex are generally higher than in neurons of the primary visual and somatosensory cortices (Elston, 2000; Jacobs et al., 2001). It is estimated that the human brain contains more than 1013 dendritic spines (Nimchinsky et al., 2002).
Dendrite and dendritic spine changes during aging
It is known that cognitive abilities are impaired during normal aging. Once hypothesized to result from neuronal loss, cognitive decline is now known to be accompanied by subtle changes in neuronal morphology (Morrison & Hof, 2002). Many stereological studies have demonstrated minimal neuronal loss in cortical and hippocampal regions during normal aging (West et al., 1994, 2004; Morrison & Hof, 1997, 2002) suggesting that the age-related impairments that occur during normal aging and Alzheimer’s disease (AD) are due to distinct pathological processes. In the absence of neuronal degeneration, irregularities in dendritic arborization and in spine length or volume, distribution, number, or morphology can have detrimental effects. Many studies have demonstrated age-related regression in the dendritic arbors and the dendritic spines of pyramidal neurons located in the prefrontal, superior temporal and precentral cortices in humans (Scheibel et al., 1975; Nakamura et al., 1985; de Brabander et al., 1998) and in nonhuman primates (Peters et al., 1998). Dendritic shrinkage and spine loss have also been reported in aged dogs (Mervis, 1978). An early study by Cupp & Uemura (1980) examined Golgi-stained sections from the prefrontal region of young and old rhesus monkeys and concluded that entire branches or segments were lost from apical dendrites with aging. In addition, there was an approximate 25% loss of spines in that region in old animals (Cupp & Uemura, 1980; Uemura, 1980). Similar results have been obtained by Nakamura et al. (1985) who report a decline in the number of dendrites of pyramidal cells with age in the motor cortex. Results consistent with these have been demonstrated by many groups. Electron microscopic examination of areas 46 (Peters et al., 1998) and 17 (Peters et al., 2001) in rhesus monkeys showed a loss of branches from apical tufts of pyramidal cells accompanied by a loss of spines and a 40–55% reduction in the number of synapses. In a study by de Brabander et al. (1998), analysis of basal dendritic branching patterns of pyramidal cells in the human prefrontal cortex revealed a decrease in total dendritic length, total number of dendritic segments, and terminal dendritic length with age. Further analysis of total dendritic length, mean segment length, segment number, spine number, and spine density of pyramidal cells from areas 10 and 18 of human cortex found a 9–11% decrease in total dendritic length and a 50% decrease in spine density (Jacobs et al., 1997, 2001).
More recent investigations in nonhuman primates have also examined the state of neurons during aging. Retrograde tract tracing of cortical pyramidal neurons filled with the dye lucifer yellow revealed age-related changes in the complexity of the apical dendrites in old compared to young monkeys. A minor difference in the length of dendritic segments in old animals was observed; however, this outcome was not statistically significant. There was significant loss of dendritic spines along all levels of dendrites analyzed. The total number of spines decreased by 28–37% in the basal and apical dendrites of aged animals compared to young animals, while spine densities per micrometer of dendrite decreased by approximately 23% (Page et al., 2002). Duan et al. (2003) extended these findings and found regressive dendritic changes in apical dendrites in aged macaque monkeys compared to young animals. Sholl analysis of apical dendritic arbors revealed a reduction in the number of dendrites extending to 140 µm and 180 µm from the neuronal somata. Furthermore, significant age-related decreases in dendritic length and segment numbers were observed at the second branch order for apical dendrites. There was also an age-related decrease in spine number and density in both apical and basal arbors. Spine loss on apical dendrites was estimated at approximately 43% and occurred mainly on proximal dendrites, whereas basal dendritic loss was estimated at 27% and occurred primarily on distal branches. Overall, there was a 25% reduction in both apical and basal dendrites in aged animals compared to controls (Fig. 1) (Duan et al., 2003). Senescence-accelerated mice, which are established models of aging (Takeda et al., 1991), display a gradual retraction of apical dendrites with relative preservation of overall complexity (Shimada et al., 2006). There was a 45% decrease in total apical dendrite length and a decline in stem thickness. Furthermore, there was a 55% total reduction in spine density and synaptic loss in aged animals compared to young ones (Shimada et al., 2003, 2006). In contrast, no age-related changes were observed in basal dendrites. Immunohistochemical analysis of microtubule-associated protein 2, a dendritic marker, revealed a reduction in the immunoreactivity of microtubule-associated protein 2 in the anterior and posterior cortex of aged senescence-accelerated mouse prone 10 mice indicating that dendritic retraction was not limited to layers 2/3 pyramidal cells, but rather affects other layers as well (Shimada et al., 2006). The remarkable morphological alterations and loss of dendritic spines in cortical pyramidal cells, as evidenced by many detailed studies, may underlie the first signs of cognitive decline in learning and memory performance seen in normal aging.
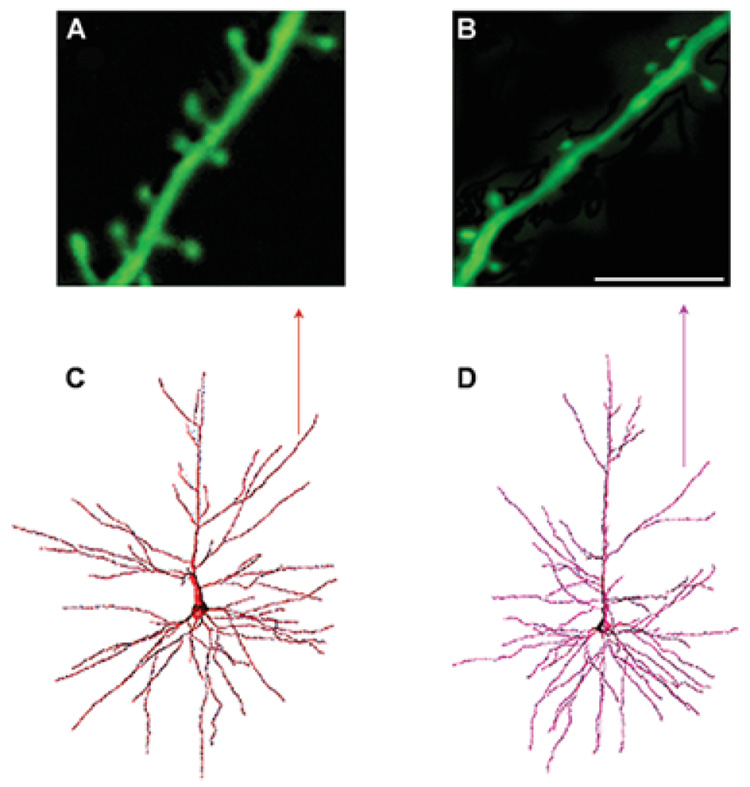
Fig. 1. Spine densities on neocortical pyramidal neurons from young and aged rhesus monkeys.
Panels A and B show confocal laser scanning images of apical dendritic segments in a young (A) and aged (B) rhesus monkey (scale bar = 8 µm). Note the increased spine density in the young monkey compared to the old monkey. Panels C and D show examples of a retrogradely traced neuron, filled with Lucifer Yellow, and reconstructed in 3-dimensions using NeuroZoom and NeuroGL software applications. The neuron in (C) is from a young animal and the neuron in (D) is from an aged animal. The arrow points to the dendritic segments analyzed in A and B. (Adapted from Duan et al., 2003).
Neurochemical changes
There is a strong link between dendritic changes and the post-synaptic effects of neurotransmitters. Neuronal alterations that occur during aging have a profound effect on the distribution of neurofilament proteins and impact important parameters of the cholinergic, serotonergic, dopaminergic, and glutamatergic systems. Changes to these systems render neurons vulnerable to impaired transmission. The resulting inability of neurons to function effectively leads to disruptions in corticocortical signaling pathways, such as those connecting the superior temporal cortex and the prefrontal cortex (Vickers et al., 1994; Gazzaley et al., 1996; Morrison & Hof, 1997; Hof et al., 2002). Several studies have demonstrated an age-related shift in the expression of neurofilament protein (Hof et al., 1990; Vickers et al., 1993, 1994). The increase of neurofilament protein in neurons is thought to make these neurons more prone to the formation of neurofibrillary tangles (NFT), a pathological hallmark of AD, and ultimately leads to neuro-degeneration and dementia. The expression and distribution of neurotransmitter receptors are also affected during aging. In particular, it has been shown that the number of neurons expressing certain ionotropic glutamate receptors (Glu R) and N-methyl-d-aspartate receptor (NMDA R) subunits is significantly reduced during aging (Gazzaley et al., 1996; Mishizen et al., 2001; Hof et al., 2002). Quantitative analysis of the distributions of Glu R2 and NMDA R1 in long and short corticocortical connections in young and old macaque and patas monkeys revealed a down-regulation of the expression of both receptors with aging. Glu R2 expression was decreased to a greater extent in the prefrontal cortex compared to other areas, such as the temporal cortex, whereas significant reductions in NMDA R1 occurred primarily in the long cortico-cortical projections from the superior temporal cortex (Hof et al., 2002).
Electrophysiological changes during aging
Age-related alterations in synaptic transmission in the primate prefrontal cortex
The structural changes that occur in neurons with age are likely to impact the electrophysiological properties of neurons. Indeed, Luebke et al. (2004) have demonstrated a significant decrease in excitatory synaptic transmission in the monkey prefrontal cortex, manifested as a significantly reduced frequency of spontaneous excitatory post-synaptic currents (PSC), which represent the post synaptic cells’ response to both action potential-dependent and action potential-independent release of glutamate from pre synaptic nerve terminals (Fig. 2). Perhaps, the most plausible mechanism by which the frequency of spontaneous excitatory PSCs is significantly reduced is through a reduction of post-synaptic substrate. Such a reduction has been demonstrated by studies (described above) that have shown both a decrease in dendritic spines, the major post synaptic substrate for glutamatergic inputs (Jacobs et al., 1997; Page et al., 2002; Duan et al., 2003) and a decrease in glutamate receptors (Hof et al., 2002) in the primate neocortex with age. A second potential mechanism is an age-related decrease in the frequency of action potential firing in pre-synaptic glutamatergic neurons of the prefrontal cortex resulting in decreased glutamate release and hence decreased synaptic response frequency. This explanation is made less compelling by data that demonstrate no change in resting membrane potential and an increase in action potential firing rates in layer 2/3 neurons of the aged monkey prefrontal cortex (Chang et al., 2005). Nevertheless, a significant decrease in excitability and release of glutamate from neurons located in other layers or brain areas and forming synapses on layer 2/3 pyramidal cells cannot be ruled out.
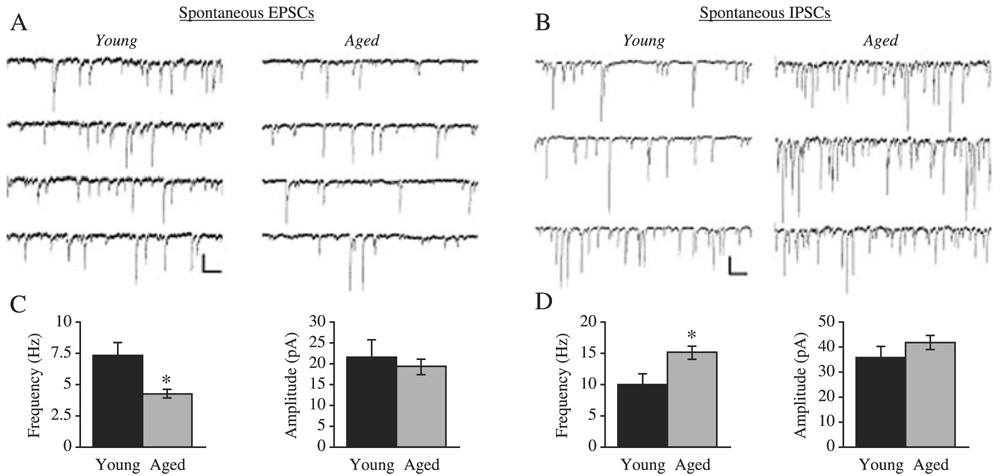
Fig. 2. Decreased frequency of excitatory and increased frequency of inhibitory PSCs in layers 2/3 pyramidal cells from aged monkeys.
(A) Representative traces of spontaneous excitatory PSCs obtained from cells from young (left) and aged (right) monkeys. (B) Representative traces of spontaneous inhibitory PSCs obtained from cells from young (left) and aged (right) monkeys. (C) Bar graphs showing the mean spontaneous EPSC frequency and amplitude values for young vs aged pyramidal cells. (D) Bar graphs showing the mean spontaneous EPSC frequency and amplitude values for young vs aged pyramidal cells. A and B Scale bar: 50 pA, 200 ms.
While the overall level of excitatory input to pyramidal cells in the primate prefrontal cortex (PFC) is reduced with age, several lines of evidence indicate that the post-synaptic non-NMDA glutamate receptors, although reported to be present in lower numbers (Hof et al., 2002), may be functionally intact. This is indicated by observations that the amplitude distribution of miniature excitatory PSCs did not differ in cells from the two age groups (Luebke et al., 2004). In addition, the kinetics of the post synaptic currents reflect the distribution or composition of receptor subunits and significant alterations in the subunits might be expected to result in alterations in the kinetics of PSCs (Geiger et al., 1995; Angulo et al., 1997; Swanson et al., 1997). The finding that the rise and decay times of the currents did not differ also suggests that glutamatergic non-NMDA receptors are not significantly functionally altered in layer 2/3 pyramidal cells of the primate with age (Luebke et al., 2004).
In contrast to the findings on excitatory transmission, investigations in aged monkey prefrontal cortex revealed a significant increase in the level of inhibitory synaptic input to layer 2/3 pyramidal cells, which was manifested as a significant increase in the frequency of spontaneous inhibitory PSCs (Luebke et al., 2004). Perhaps, the most straightforward explanation for the increase in spontaneous inhibitory PSC frequency is an increase in the action potential-dependent release of γ-aminobutyric acid (GABA) from pre synaptic interneurons. Future studies of GABAergic interneurons are required to determine whether this is a plausible explanation for the finding of increased inhibition of layer 2/3 pyramidal cells. The amplitude and kinetics of inhibitory miniature PSCs did not differ in cells from the two age groups, indicating that GABAA receptor function per se is not likely significantly altered with age in layer 2/3 pyramidal cells of the monkey prefrontal cortex.
Action potential rates are increased with age
Single and multiple unit recordings of pyramidal cells in the prefrontal cortex of awake, behaving monkeys have shown that these cells dramatically increase (or decrease) firing frequencies during different epochs of a working memory task. Because the sustained firing pattern of prefrontal neurons represents a precise encoding of information during the execution of memory tasks, age-related alterations in these firing patterns could plausibly result in perturbed cognitive performance. Leventhal and co-workers have demonstrated that visual cortical neurons in the anesthetized aged monkey exhibit significantly increased spontaneous action potential (AP) firing rates in vivo, that are associated with degradation of stimulus selectivity (Schmolesky et al., 2000; Leventhal et al., 2003). Recently, Chang et al. (2005) have demonstrated a significant increase in AP firing rates in layers 2/3 prefrontal cortical pyramidal cells in aged compared to young monkeys. Interestingly, in the aged group of animals, firing rate correlated with performance on working memory tasks in a U-shaped manner, with monkeys exhibiting very low and very high firing rates performing poorly and those exhibiting intermediate firing rates (which were significantly higher than seen in young subjects) performing well. These findings are consistent with the idea that the optimal firing rate in aged monkeys is shifted to higher frequencies. Such a shift may plausibly be a compensatory response to increased AP conduction failure (Rosene et al., 2003) secondary to the extensive myelin dystrophy seen in the primate prefrontal cortex with aging (reviewed in Peters, 2002). Thus, in the aged prefrontal cortex, higher rates of firing may be required to maintain functions encoded by lower rates in the young prefrontal cortex.
Quantitative measures of spatial complexity in aging
The spatial complexity of branching patterns in neuronal dendritic arbors has traditionally been measured in two dimensions, using Sholl analysis (Sholl, 1953). Estimates of spatial complexity have also been derived from fractal analysis, both in two dimensions (Smith et al., 1989; Jelinek & Elston, 2001) and in three dimensions (Caserta et al., 1995; Henry et al., 2002). Rather than being a local property of individual branches, in these studies ’complexity’ was quantified by a power law scaling exponent that described the global rate of increase or decrease of branch numbers with distance from the soma, over a large region of the tree. Recent theoretical work has extended these scaling analyses by separately measuring the contributions of branching and tapering to the three-dimensional dendritic mass distribution, and relating these measures to the electrotonic structure of the neuron (Rothnie et al., 2006). How dendritic mass is distributed with respect to any particular point on the tree is the major factor in determining electrotonic structure, and hence electrical function in neurons (Rall, 1959, 1964; Clements & Redman, 1989; Zador et al., 1995).
A recent theoretical study by Rothnie et al. (2006) measured spatial complexity in the dendritic trees of two distinct types of layers 2/3 neocortical pyramidal neurons from rhesus monkeys: long corticocortical projection neurons from superior temporal cortex to prefrontal area 46, and local projection neurons that contribute to local circuits within area 46. Global spatial complexity was quantified by three power law scaling exponents describing rates of change of dendritic mass (dM), branching (dN) and branch diameter, or taper (dT), with distance from the soma. Two distinct scaling subregions within which scaling behavior was constant, and could be described by a single value of dM, dN, or dT were found in both apical and basal trees. These scaling regions were robust, being present in both long and local projection neurons, and exhibited a remarkable homeostatic pattern of mass distribution such that branching and tapering rates were inversely related in a fine balance that maintained a constant spatial gradient of slowly decreasing mass with distance from the soma, over most of the tree. In proximal scaling regions (Region I, Fig. 3), where the branching rate (dN) was high, tapering (dT) was also high, effectively canceling out any mass increase due to branching. In medial regions (Region II, Fig. 3) where the branching rate was close to 0, tapering was also close to 0, maintaining the same uniform mass distribution across both scaling regions of the tree. This pattern was interpreted as a form of global mass homeostasis in which the conserved quantity was the spatial gradient of dendritic mass with distance from the soma (Rothnie et al., 2006).
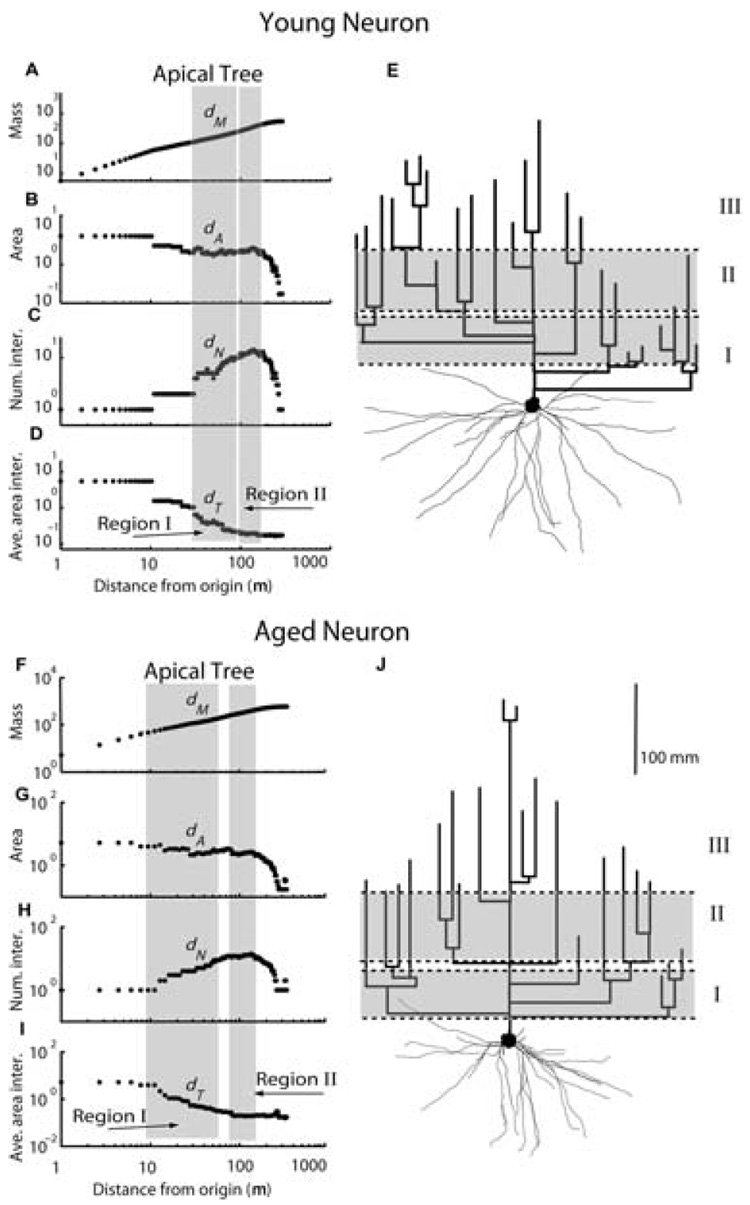
Fig. 3. Scaling regions (gray shaded bands) for a typical young (top panel) and an aged (bottom panel) layer 2/3 pyramidal cell projecting from STC to area 46.
Scaling exponents have been fitted as the slopes of log-log plots of accumulated mass (dM, A,F), cross-sectional area (dA, B,G) branch numbers (dN, C,H), and average area of branch intersections (dT, D,I), with distance from the soma. E,J: Two-dimensional (2D) projection of 3D reconstructed young and aged neurons, in which the basal trees are drawn in anatomical space, whereas the apical trees are drawn in dendrogram space (see Rothnie et al., 2006 for methodological details). Proximal (I), medial (II) scaling regions, and a distal (III) nonscaling or die-off region, are indicated on the dendrogram representation of apical trees.
The effect of aging on this homeostatic pattern of mass distribution was examined in a recent morphometric study of young and aged layer 2/3 long and local projection neurons (Kabaso et al., 2003, 2004, 2006). Despite significant age-related changes in specific indices of branching and tapering, these changes remained compensatory, such that the existences of two scaling regions, and the spatial gradient of mass distribution across them, were unchanged with age in either long or local projection neurons. In local projection neurons, aging did not alter specific indices of mass distribution (dM), branching (dN), or tapering (dT) rates in either proximal or medial scaling regions. Scaling exponents in long projection neurons, in contrast, were significantly altered with age. In apical trees, the mass scaling exponent dM was significantly larger in the proximal scaling region and tended to be larger in the medial region, indicating a consistently faster rate of mass accumulation with distance from the soma. Separate analysis of branching and tapering rates showed that this altered mass distribution was due not to increase branching rates, but to reduced tapering across both regions. In basal trees, scaling exponents were unchanged with age in the proximal region, but the branching exponent, dN, was significantly lower with age in the medial region, reflecting a significantly faster rate of branch die-off with age. The combined effects of these changes to dendritic diameters and lengths can alter the passive electrotonic structure of aged neurons sufficiently that, unless compensated by changes in densities of active conductances and synaptic input, the baseline excitability of these neurons will be altered with age (Kabaso, 2004, 2006; Chang et al., 2005). Interestingly, the more rapid branch die-off in the medial regions of basal trees with age tended to be compensated by a positive taper, or slight ’flaring’ of individual branches in the aged neurons, such that the mass distribution again remained relatively uniform across proximal and medial scaling regions. An analogous form of mass homeostasis in which the conserved quantity was total dendritic size, was reportedly recently by Samsonovich and Ascoli in a morphometric meta-analysis of cortical pyramidal neurons (Samsonovich & Ascoli, 2006). These authors demonstrated that fluctuations in dendritic size in one portion of a neuron are systematically counterbalanced by the remaining dendrites in the same cell, in a pattern of mass homeostasis that was robust among different brain regions, cell types, and experimental conditions. Maintenance of a form of homeostasis related to spatial gradients of mass with age, despite changes in overall dendritic size and branching patterns, might reflect an intrinsic cellular control mechanism that is conserved with aging.
Alzheimer’s disease and dendritic complexity
Alzheimer’s disease (AD) is a progressive neurodegenerative disease of the central nervous system and is present in approximately 80% of all dementia cases in the elderly (Terry, 2006). Pathologically, AD selectively damages brain regions and neural circuits and is distinguished by the presence of dystrophic neurites, amyloid beta (Aβ) plaques and NFTs. Recent stereologic studies have shown that neuronal death is limited in normal aging, whereas in AD, there is considerable neuronal loss. The neuronal degeneration observed in AD is reflected by significant neuron and synapse loss in specific brain regions (West et al., 1994, 2004; Morrison & Hof, 1997, 2002; Price et al., 2001; Hof et al., 2003; Hof & Morrison, 2004). The circuits that are most vulnerable to degeneration are the perforant path, which connects the entorhinal cortex with the hippocampus, and the long cortico-cortical projections that link association cortices such as inferior temporal cortex and prefrontal cortex (Morrison & Hof, 2002). During normal aging, plaques can be found in the neocortical, hippocampal, and entorhinal regions of cognitively normal elderly people. In addition, NFTs, although rare in the aging brain, are commonly found in the medial temporal areas after 50 years of age. The presence of these few plaques and NFTs does not seem to have a significant effect on cognition (Price et al., 1991; Arriagada et al., 1992; Kazee & Johnson, 1998; Goldman et al., 2001). In AD, however, the robust number of plaques and NFTs has significant detrimental effects on neuronal morphology and synapses. Unlike in normal aging when neurons become smaller, neuronal loss is extensive in the neocortical and entorhinal regions of the AD brain, reaching roughly 30% (Terry et al., 1981). This loss is accompanied by an approximate 45% decline in neocortical synapses (Terry et al., 1991). Neuronal loss in the hippocampus is also evident, with an average cell loss of 68% in the CA1 region of AD patients compared to aged–matched controls (West et al., 1994). Studies from our laboratory have demonstrated that neurons in AD undergo morphological alterations. Data from three control and three demented cases, with clinical dementia rating (CDR) scores of 0 and 3, respectively, showed a large reduction in spine density with only minor changes in spine length and volume. Electron microscopy studies investigating synaptic loss and its relation to the stage of AD found that there was significant synaptic loss in the brains of patients with early onset AD compared to mild cognitively impaired and nondemented individuals. The loss of synapses also correlated with the Mini-mental state score and other cognitive tests. While there was a reduction in synapse numbers in mild cognitively impaired subjects than in nondemented subjects, it was not significant (Scheff et al., 2006). Other groups have corroborated these data and found a significant reduction in spine density as well as a decrease in overall dendritic area in AD patients when compared to age-matched controls (Ferrer et al., 1990; Einstein et al., 1994; Moolman et al., 2004). Knowles et al. (1999) examined the effect of amyloid plaques on neuronal processes in humans by measuring mean curvature, length, and width distribution of dendritic projections. After examining over 5000 dendrites from ten AD cases and five controls, it was found that dendrites were extremely dysmorphic in AD, with an increased curvature and curvilinear length. This was true for dendrites that traversed plaques as well as those that were in close proximity to plaques (Knowles et al., 1999).
It has been suggested that the amyloid precursor protein (APP) and APP cleavage products play a crucial role in neuro-protection, interneuronal connections, and synaptic plasticity (Seabrook et al., 1999) and appear to be located in neuronal structures, including synaptic compartments (Schubert et al., 1991; Shigematsu et al., 1992). In AD, the accumulation of Aβ, in particular the oligomeric forms of Aβ, mediate neurotoxic effects including interrupting synaptic transmission, synaptic plasticity, and disrupting neuronal connectivity (Wang et al. 2002; Raymond et al., 2003). Alterations in neuronal morphology and trajectory may cause disruption in neuronal signaling and function and are thought to ultimately contribute to the neuronal loss observed in AD (Knowles et al., 1999). Many studies using transgenic mice containing varying forms of the mutant human APP have investigated the morphological effects of Aβ on neurons. While many of these transgenic mice do not exhibit neuronal loss, a few of them (APP23 and APP751/PS-1M146L) exhibit significant age-related neuronal loss in brain regions containing Aβ aggregates (Calhoun et al., 1998; Schmitz et al., 2004). Moreover, neuronal loss was evident in regions that were distant from plaques suggesting a putative role for intracellular Aβ or soluble Aβ in neurodegeneration (Schmitz et al., 2004). This phenomenon of neuronal degeneration in the absence of Aβ plaques has also been observed in the presenilin-1 mutant mice, PS1L286V, and PS1H163R, where accelerated neuronal loss was demonstrated in the frontal cortex and hippocampus. Interestingly, these animals showed signs of intracellular Aβ in the aforementioned brain regions (Chui et al., 1999). In the context of extracellular Aβ aggregates, neurons that are in close proximity to (10–20 µm) or passing through Aβ plaques undergo spine loss and shaft atrophy and develop axonal varicosities (Le et al., 2001; Tsai et al., 2004). There is also increased curvature of dendritic processes, a decrease in dendritic density and abruptly terminated dendrite endings. Segments farther than 20 µm from Aβ plaques appeared unaffected (Knowles et al., 1998, 1999; Tsai et al., 2004). In APP/PS1 and J20 mice, swollen bulbous, dystrophic neurites were seen along with a 36% reduction in the number of spines (Moolman et al., 2004). Other studies in Tg2576, PDAPP, PDGF-APP, and APPV717F mice also demonstrated a reduction in spine density in neurons in the CA1 region and dentate gyrus of the hippocampus and in the somatosensory cortex (Games et al., 1995; Hsia et al., 1999; Mucke & Masliah, 2000; Lanz et al., 2003; Wu et al., 2004; Jacobsen et al., 2006). Interestingly, while changes in spine density occurred in the presence of Aβ aggregates, in some cases, the reduction in spine density was independent of the presence of fibrillar Aβ plaques and increased with age and the incidence of pathology. A more extensive study by Rutten et al. (2005) in APP751/PS-1M146L mice supported previous studies indicating a reduction in synaptic boutons. They also reported that transgenic animals had larger pre-synaptic boutons while in close proximity to Aβ plaques as well as a decrease in volume compared to wild-type controls (Rutten et al., 2005).
A recent study, Alpar et al. (2006a) examined more global morphological changes in the Tg2576 mouse model. Pyramidal cells in layers 2/3 of the primary somatosensory cortex were analyzed in three dimensions. No changes were observed in basal dendritic arbors; however, many unambiguous changes were found in the apical dendrites. Total length, surface area, and volume of basal dendrites were the same between Tg2576 mice and controls. There were also no variations in the branching patterns of basal dendrites and no change in the number of dendritic endings or in dendritic density. In contrast, apical arbors were shorter in length and less branched than controls and exhibited an increase in diameter in the proximal dendritic segments and a reduction in the distal; however, the total surface area and total volume did not change significantly. Spine density was reduced in both apical and basal arbors along the entire course of the dendrite (Alpar et al., 2006a). It is unclear if the actions of mutated human APP (hAPP) or the elevated Aβ levels contribute to the morphological aberrations observed. Studies in APP null mice showed a reduction in dendritic length and branching (Perez et al., 1997; Seabrook et al., 1999). Studies in mice expressing nonmutated hAPP (Lamb et al., 1993) found no overall difference in dendritic length. However, there was a significant increase in the total surface area and volume of basal dendrites in transgenic mice compared to controls (Alpar et al., 2006b). Whereas there was a shortening of third and fourth order branching, the average diameter of second, third, and fourth order segments was larger in hAPP mice than controls.
The mechanism of tau-mediated neuronal death in AD remains elusive. The abnormal phosphorylation of tau is considered one of the earliest signs of neuronal degeneration and precedes NFT formation and plaque aggregation. Few studies have investigated the effect of NFTs and tau on dendrites and dendritic spines. In a recent report where organotypic hippocampal slice cultures infected with Sindbis virus containing an EGFP-tau construct were assessed, it was found that while there was a loss of individual neurons over time, spine density and spine morphology were essentially the same as controls (Shahani et al., 2006). Preliminary studies in our laboratory have examined the effect of tau in a transgenic mouse model that expresses all six isoforms of human tau, but not mutant tau (Andorfer et al., 2003). These mice exhibit tau pathology in neurons of the neocortex and hippocampus in patterns similar to that occurring in NFTs of human brain and exhibited overt neuronal loss (Andorfer et al., 2003, 2005). Examination of dendritic arbors demonstrated a reduction in the total number of spines (~35%), as well as a decrease in spine density that became more severe with age. In humans, it has been demonstrated that dendrites that are immunopositive for Alz50, an antibody that recognizes NFTs and neuropil threads, are highly altered, with less than 5% of these dendrites being straight (Knowles et al., 1999). Le et al. (2001) found that there was a significant disruption in neuronal microarchitecture in cells that were positive for phosphorylated tau, and reported similar findings of an increase in the curvature of the dendrite, as well as an increase in length. Taken together, the changes that neuronal projections undergo in AD may account for the cognitive decline observed. Exposure to APP or proteolytic cleavage products and hyperphosphorylation of tau causes distinct remodeling of pyramidal neurons, which may severely impact synapse propagation ultimately resulting in neuronal death.
Concluding comments
Over the past several years, it has become evident that the anatomical and functional organization of the human brain is dynamic and changes in response to many stimuli. In humans, age-related synapse loss, neuronal loss, and cognitive decline, are commonly seen in neurodegenerative disorders such as AD. However, age-associated cognitive impairment without neuronal loss has also been reported in many species and is likely mediated by dendritic and synaptic alterations. Otherwise, intact circuits in the brain are vulnerable in normal aging as a result of ultrastructural morphologic modifications in dendrites and reduced spine densities and are reflected in compromised synaptic communication. Thus, unlike AD where cognitive decline can be attributed to neuronal loss, age-related cognitive deficits appear to evolve from cellular changes that lead to the disruption of cortical connections. Although many studies have begun to elucidate the mechanistic relationships between synaptic integrity and age-associated cognitive impairment, there is still much that remains unknown. Understanding these mechanisms will help determine when and how dendritic changes contribute to the brain’s capacity for memory, perception, and action, and will aid in the development of new therapeutic avenues to prevent or cure cognitive impairment.
Acknowledgments
We thank W.G.M. Janssen, B. Wicinski, A. Rodriguez, and D.B. Ehlenberger, and other members of the Hof, Morrison, Wearne, and Luebke laboratories who have been involved in our studies of brain aging for their support. This work was supported by National Institutes of Health grants AG00001, AG02219, AG05138, MH71818, and DC05669.
References
- Alpar A, Ueberham U, Bruckner MK, Arendt T, Gartner U. The expression of wild-type human amyloid precursor protein affects the dendritic phenotype of neocortical pyramidal neurons in transgenic mice. Int. J. Dev. Neurosci. 2006a;24:133–140. doi: 10.1016/j.ijdevneu.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Alpar A, Ueberham U, Bruckner MK, Seeger G, Arendt T, Gartner U. Different dendrite and dendritic spine alterations in basal and apical arbors in mutant human amyloid precursor protein transgenic mice. Brain Res. 2006b;1099:189–198. doi: 10.1016/j.brainres.2006.04.109. [DOI] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J. Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Lambolez B, Audinat E, Hestrin S, Rossier J. Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells. J. Neurosci. 1997;17:6685–6696. doi: 10.1523/JNEUROSCI.17-17-06685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippo-campal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J. Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- Caserta F, Eldred WD, Fernandez E, Hausman RE, Stanford LR, Bulderev SV, Schwarzer S, Stanley HE. Determination of fractal dimension of physiologically characterized neurons in two and three dimensions. J. Neurosci. Methods. 1995;56:133–144. doi: 10.1016/0165-0270(94)00115-w. [DOI] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb. Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat. Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- Clements JD, Redman SJ. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J. Physiol. 1989;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp. Neurol. 1980;69:143–163. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb. Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Einstein G, Buranosky R, Crain BJ. Dendritic pathology of granule cells in Alzheimer’s disease is unrelated to neuritic plaques. J. Neurosci. 1994;14:5077–5088. doi: 10.1523/JNEUROSCI.14-08-05077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Guionnet N, Cruz-Sanchez F, Tunon T. Neuronal alterations in patients with dementia: a Golgi study on biopsy samples. Neurosci. Lett. 1990;114:11–16. doi: 10.1016/0304-3940(90)90420-e. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Borthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Siegel SJ, Kordower JH, Mufson EJ, Morrison JH. Circuit-specific alterations of N-methyl-d-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc. Natl Acad. Sci. USA. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Goldman WP, Price JL, Storandt M, Grant EA, McKeel DW, Jr, Rubin EH, Morris JC. Absence of cognitive impairment or decline in preclinical Alzheimer’s disease. Neurology. 2001;56:361–367. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Henry BI, Hof PR, Rothnie P, Wearne SL. Fractal analysis of aggregates of non-uniformly sized particles: an application to macaque monkey cortical pyramidal neurons. New Jersey: World Scientific Publishing Co, Pty. Ltd.; 2002. [Google Scholar]
- Hof PR, Bussire T, Gold G, Kovari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH. Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2003;62:55–67. doi: 10.1093/jnen/62.1.55. [DOI] [PubMed] [Google Scholar]
- Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease. I. Superior frontal and inferior temporal cortex. J. Comp. Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, Morrison JH. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 2002;928:175–186. doi: 10.1016/s0006-8993(01)03345-5. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl Acad. Sci. USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J. Comp. Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cereb. Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek H, Elston G. Pyramidal neurones in macaque visual cortex: interareal phenotypic variation of dendritic branching patterns. Fractals. 2001;9:287–295. [Google Scholar]
- Kabaso DM, Henry BI, Hof PR, Wearne SL. Effects on action potential backpropagation of age-related changes in dendritic branching and spine densities of neocortical pyramidal neurons in macaque monkey. Soc. Neurosci. Abstr. 2003;29:810.8. [Google Scholar]
- Kabaso DM, Luebke JI, Henry BI, Hof PR, Wearne SL. Morphologic changes in dendritic structure and spine densities may account for age-related increases in action potential firing rates. Soc. Neurosci. Abstr. 2004;30:638.18. [Google Scholar]
- Kabaso DM, Nilson J, Luebke JI, Hof PR, Wearne SL. Electrotonic analysis of morphologic contributions to increased excitability with aging in neurons of the prefrontal cortex of monkeys. Soc. Neurosci. Abstr. 2006;32:237.10. [Google Scholar]
- Kazee AM, Johnson EM. Alzheimer’s disease pathology in non-demented elderly. J. Alzheimers Dis. 1998;1:81–89. doi: 10.3233/jad-1998-1202. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Gomez-Isla T, Hyman BT. Abeta associated neuropil changes: correlation with neuronal loss and dementia. J. Neuropathol. Exp. Neurol. 1998;57:1122–1130. doi: 10.1097/00005072-199812000-00003. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb BT, Sisodia SS, Lawler AM, Slunt HH, Kitt CA, Kearns WG, Pearson PL, Price DL, Gearhart JD. Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice. Nat. Genet. 1993;5:22–30. doi: 10.1038/ng0993-22. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 2003;13:246–253. doi: 10.1016/s0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- Le R, Cruz L, Urbanc B, Knowles RB, Hsiao-Ashe K, Duff K, Irizarry MC, Stanley HE, Hyman BT. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J. Neuropathol. Exp. Neurol. 2001;60:753–758. doi: 10.1093/jnen/60.8.753. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Luebke R, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Mervis R. Structural alterations in neurons of aged canine neocortex: a Golgi study. Exp. Neurol. 1978;62:417–432. doi: 10.1016/0014-4886(78)90065-1. [DOI] [PubMed] [Google Scholar]
- Mishizen A, Ikonomovic M, Armstrong DM. Glutamate receptors in aging and Alzheimer’s disease. In: Hof PR, Mobbs CV, editors. Functional Neurobiology of Aging. San Diego, CA: Academic Press; 2001. pp. 283–314. [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J. Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog. Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Akiguchi I, Kameyama M, Mizuno N. Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. Acta Neuropathol. (Berl) 1985;65:281–284. doi: 10.1007/BF00687009. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Shu T, Sanada K, Lariviere RC, Tseng HC, Park SK, Julien JP, Tsai LH. A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat. Cell Biol. 2004;6:595–608. doi: 10.1038/ncb1139. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Page TL, Einstein M, Duan H, He Y, Flores T, Rolshud D, Erwin JM, Wearne SL, Morrison JH, Hof PR. Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci. Lett. 2002;317:37–41. doi: 10.1016/s0304-3940(01)02428-4. [DOI] [PubMed] [Google Scholar]
- Perez RG, Zheng H, Van der Ploeg LH, Koo EH. The beta-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. J. Neurosci. 1997;17:9407–9414. doi: 10.1523/JNEUROSCI.17-24-09407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J. Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cereb. Cortex. 2001;11:93–103. doi: 10.1093/cercor/11.2.93. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb. Cortex. 1998;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol. Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Rall W. Branching dendritic trees and motoneuron membrane resistivity. Exp. Neurol. 1959;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rall W. Theoretical Significance of Dendritic Trees for Input-Output Relations. Stanford, CA: Stanford University Press; 1964. [Google Scholar]
- Raymond CR, Ireland DR, Abraham WC. NMDA receptor regulation by amyloid-beta does not account for its inhibition of LTP in rat hippocampus. Brain Res. 2003;968:263–272. doi: 10.1016/s0006-8993(03)02269-8. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Luebke JI, Mangiamele LA, Sandell JH, Peters A. Anatomical and physiological properties of the corpus callosum in the aged rhesus monkey. Soc. Neurosci. Abstr. 2003;6:735.7. [Google Scholar]
- Rothnie P, Kabaso D, Hof PR, Henry BI, Wearne SL. Functionally relevant measures of spatial complexity in neuronal dendritic arbors. J. Theor. Biol. 2006;238:505–526. doi: 10.1016/j.jtbi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Rutten BP, Van der Kolk NM, Schafer S, van Zandvoort MA, Bayer TA, Steinbusch HW, Schmitz C. Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice. Am. J. Pathol. 2005;167:161–173. doi: 10.1016/S0002-9440(10)62963-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich AV, Ascoli GA. Morphological homeostasis in cortical dendrites. Proc. Natl Acad. Sci. USA. 2006;103:1569–1574. doi: 10.1073/pnas.0510057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp. Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rutten BP, Pielen A, Schafer S, Wirths O, Tremp G, Czech C, Blanchard V, Multhaup G, Rezaie P, Korr H, Steinbusch HW, Pradier L, Bayer TA. Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer’s disease. Am. J. Pathol. 2004;164:1495–1502. doi: 10.1016/S0002-9440(10)63235-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters CL, Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563:184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Shahani N, Subramaniam S, Wolf T, Tackenberg C, Brandt R. Tau aggregation and progressive neuronal degeneration in the absence of changes in spine density and morphology after targeted expression of Alzheimer’s disease-relevant tau constructs in organotypic hippocampal slices. J. Neurosci. 2006;26:6103–6114. doi: 10.1523/JNEUROSCI.4245-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu K, McGeer PL, McGeer EG. Localization of amyloid precursor protein in selective postsynaptic densities of rat cortical neurons. Brain Res. 1992;592:353–357. doi: 10.1016/0006-8993(92)91697-d. [DOI] [PubMed] [Google Scholar]
- Shimada A, Keino H, Satoh M, Kishikawa M, Hosokawa M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: a model of cerebral degeneration. Synapse. 2003;48:198–204. doi: 10.1002/syn.10209. [DOI] [PubMed] [Google Scholar]
- Shimada A, Tsuzuki M, Keino H, Satoh M, Chiba Y, Saitoh Y, Hosokawa M. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: a model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 2006;32:1–14. doi: 10.1111/j.1365-2990.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Smith TG, Jr, Marks WB, Lange GD, Sheriff WH, Jr, Neale EA. A fractal analysis of cell images. J. Neurosci. Methods. 1989;27:173–180. doi: 10.1016/0165-0270(89)90100-3. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J. Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J. Am. Geriatr. Soc. 1991;39:911–919. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- Terry RD. Alzheimer’s disease and the aging brain. J. Geriatr. Psychiatry Neurol. 2006;19:125–128. doi: 10.1177/0891988706291079. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann. Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: synaptic density. Exp. Neurol. 1980;69:164–172. doi: 10.1016/0014-4886(80)90151-x. [DOI] [PubMed] [Google Scholar]
- Vickers JC, Huntley GW, Edwards AM, Moran T, Rogers SW, Heinemann SF, Morrison JH. Quantitative localization of AMPA/kainate and kainate glutamate receptor subunit immunoreactivity in neurochemically identified subpopulations of neurons in the prefrontal cortex of the macaque monkey. J. Neurosci. 1993;13:2982–2992. doi: 10.1523/JNEUROSCI.13-07-02982.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers JC, Riederer BM, Marugg RA, Buée-Scherrer V, Buée L, Delacourte A, Morrison JH. Alterations in neurofilament protein immunoreactivity in human hippocampal neurons related to normal aging and Alzheimer’s disease. Neuroscience. 1994;62:1–13. doi: 10.1016/0306-4522(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol. Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chawla F, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Selective vulnerability of dentate granule cells prior to amyloid deposition in PDAPP mice: digital morphometric analyses. Proc. Natl Acad. Sci. USA. 2004;101:7141–7146. doi: 10.1073/pnas.0402147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador AM, Agmon-Snir H, Segev I. The morphoelectrotonic transform: a graphical approach to dendritic function. J. Neurosci. 1995;15:1669–1682. doi: 10.1523/JNEUROSCI.15-03-01669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]