Abstract
Dehydroepiandrosterone (DHEA) is an abundant circulating prohormone in humans, with a variety of reported actions on central and peripheral tissues. Despite its abundance, the functions of DHEA are relatively unknown because common animal models (laboratory rats and mice) have very low DHEA levels in the blood. Over the past decade, we have obtained considerable evidence from avian studies demonstrating that (1) DHEA is an important circulating prohormone in songbirds and (2) the enzyme 3β-hydroxysteroid dehydrogenase/isomerase (3β-HSD), responsible for converting DHEA into a more active androgen, is expressed at high levels in the songbird brain. Here, we first review biochemical and molecular studies demonstrating the widespread activity and expression of 3β-HSD in the adult and developing songbird brain. Studies examining neural 3β-HSD activity show effects of sex, stress, and season that are region-specific. Second, we review studies showing seasonal and stress-related changes in circulating DHEA in captive and wild songbird species. Third, we describe evidence that DHEA treatment can stimulate song behavior and the growth of neural circuits controlling song behavior. Importantly, brain 3β-HSD and aromatase can work in concert to locally metabolize DHEA into active androgens and estrogens, which are critical for controlling behavior and robust adult neuroplasticity in songbirds. DHEA is likely secreted by the avian gonads and/or adrenals, as is the case in humans, but DHEA may also be synthesized de novo in the songbird brain from cholesterol or other precursors. Irrespective of its source, DHEA seems to be an important neurohormone in songbirds, and 3β-HSD is a key enzyme in the songbird brain.
Keywords: 3beta-HSD, adrenal, aggression, aromatase, brain, DHEA, estrogen, neurosteroid, season, song, sparrow, stress, testosterone, zebra finch
1. Introduction
1.1. Hormone Action on Brain
Sex steroid hormones are critical for the development and function of the nervous system in all vertebrate species. Traditionally, the brain and spinal cord have been considered to be recipients of sex steroids produced by the gonads. This idea is based on a large body of evidence, starting with castration experiments in birds by Arnold Berthold in 1849. About 30 years ago, this idea was slightly modified when cytochrome P450 aromatase, or CYP19, was found in the brain (Naftolin et al., 1971,1975). Aromatase converts testosterone (T) into estradiol-17β(E2) (Fig. 1a), and this metabolism of T represents the final stages of T action. The same is true of brain 5α-reductase, which converts T to 5α-dihydrotestosterone (5α-DHT; Celotti et al., 1992). Although these developments no longer portray the brain as a passive recipient of gonadal steroids, they still assume that the brain can only metabolize T that is produced elsewhere.
Figure 1.
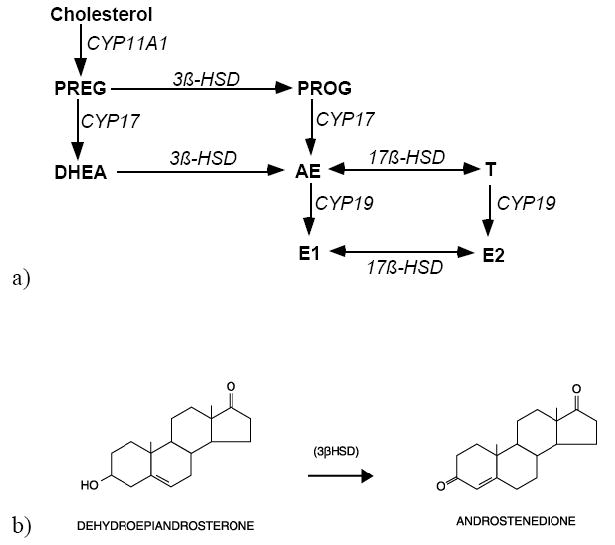
Simplified diagrams of sex steroid synthesis. a) Steroids are in bold; enzymes are in italics. Steroids: pregnenolone=PREG; progesterone=PROG; dehydroepiandrosterone=DHEA; androstenedione=AE; testosterone=T; estrone=E1; estradiol=E2. Enzymes: cytochrome P450 side-chain cleavage=CYP11A1; cytochrome P450 17α-hydroxylase/C17,20 lyase=CYP17; 3β-hydroxysteroid dehydrogenase/isomerase=3β-HSD; 17β-hydroxysteroid dehydrogenase=17β-HSD; aromatase=CYP19; b) 3β-HSD catalyzes the oxidation and isomerization of DHEA into AE.
Dehydroepiandrosterone (DHEA; Fig. 1b) is an abundant circulating hormone in humans and, together with DHEA-S, constitutes the most abundant steroid hormone secreted by the human adrenal cortex (Migeon et al., 1957; Odell and Parker, 1984). DHEA has gained increased attention, and a vast array of effects of DHEA or DHEA-S have been documented on many tissues either by acting directly (Baulieu, 1997) or by serving as important prohormones (Labrie et al., 2005). DHEA is also situated in a critical position in the biosynthetic pathway of androgens and estrogens (Fig. 1) that act by traditional intranuclear receptor systems (Mellon and Griffin, 2002; Payne and Hales, 2004). The enzyme 3β-hydroxysteroid dehydrogenase (3β–HSD) catalyzes the conversion of DHEA into androstenedione (AE) (Fig 1b). If acted upon by one isoform of 17β-HSD (Labrie et al., 1997), this AE can then be converted into T and then to potent metabolites of T, such as 5α-DHT and E2. Labrie and colleagues have coined the term “intracrinology” to describe the actions of 3β-HSD and downstream enzymes within target cells that utilize DHEA to synthesize active steroidal molecules (Labrie et al., 1995). In humans, the high concentration of DHEA(S) in blood and the conspicuous presence of steroid metabolizing enzymes in many peripheral tissues establishes the “intracrine” mechanism as a critical feature of human endocrine physiology.
DHEA has numerous actions on many tissues, including the prostate, mammary tissue, the immune system, bone and skin (Petrovsky, 2001; Labrie et al., 2005). More recently, attention has focused on DHEA actions on the brain. Some of these actions can be directly related to the capacity of DHEA and DHEA-S to modulate NMDA and GABA-A receptors (Compagnone and Mellon, 1998; Baulieu and Robel, 1998). However, DHEA has other reported functions that likely involve DHEA conversion into active androgens and/or estrogens. Prominent among these actions on brain is that DHEA and/or DHEA-S can influence developmental neurogenesis and neuronal survival and possibly protect the brain after neural injury in adults (Compagnone and Mellon, 1998; Kimonides et al., 1998; Cardounel et al., 1999; Marx et al., 2000; Li et al., 2001; Karishma and Herbert, 2002; Fiore et al., 2004). As we already conceive of circulating T functioning as a prohormone in the vertebrate brain (Naftolin et al., 1975; Balthazart, 1990; McEwen and Alves, 1999), it is a relatively small step to conceive of DHEA as functioning similarly. One impediment to achieving this conceptual breakthrough has been the absence of an appropriate animal model to explore this hypothesis. Outside of humans and some other primates, DHEA circulates at low levels in most species studied to date (Labrie et al., 2005).
Over the past 10 years, DHEA has been found to be an important circulating hormone in some bird species (Soma and Wingfield, 2001; Hau et al., 2004; Soma et al., 2004). Importantly, DHEA also has been shown to exert significant effects on the avian brain, activating behaviors and stimulating growth of some adult neural circuits (Soma et al., 2002). We have evidence that that DHEA influences the avian brain as a prohormone. First, we have found that the enzyme 3β-HSD is expressed and active in the songbird brain at surprisingly high levels. Together with aromatase and 5α-reductase, 3β-HSD can convert DHEA into active androgens and estrogens. Second, treatments with E2 replicate some of the actions of DHEA. In the following sections, we review general properties of DHEA in vertebrates and then 3β-HSD physiology and biochemistry in songbirds.
1.2. Dehydroepiandrosterone (DHEA) and its behavioral and neural effects
In humans, plasma levels of DHEA and DHEAS, together referred to as DHEA(S), are high during fetal life, low after birth, rise again at 6-10 years (“adrenarche”), peak during young adulthood, and decline dramatically thereafter (Rainey et al., 2002). High circulating DHEA(S) levels in children have been associated with aggressive behavior, specifically conduct disorder and oppositional defiant disorder (van Goozen et al., 1998; van Goozen et al., 2000). Prepubertal boys with conduct disorder have elevated plasma DHEAS but normal T levels (T levels are low prior to puberty in controls and boys with conduct disorder). Moreover, DHEAS levels are significantly correlated with the intensity of aggression. As discussed below, avian studies also indicate a role for DHEA in aggressive behavior (Soma, 2006; Demas et al, 2007).
In addition to their behavioral effects, DHEA and DHEAS have neuroprotective and neurotrophic effects in a variety of animal models (Bastianetto et al., 1999; Wolf and Kirschbaum, 1999; Schumacher et al., 2002). The mechanism for this DHEA action on the brain is not fully understood. The classic intracellular steroid receptors (e.g., androgen receptor) bind DHEA with very low affinity, and there is no conclusive evidence for an intracellular steroid receptor that is DHEA-specific (Mellon and Griffin, 2002). Nevertheless, many effects of DHEA on the nervous system, including neuroprotection, may depend on its conversion to sex steroids. For example, DHEA can protect the hippocampus from excitatory amino acid toxicity, but the effect can be blocked by administration of an aromatase inhibitor (Veiga et al., 2003). Estrogen is already known to be neuroprotective in many animal models (Wise, 2002; Garcia-Segura et al., 2003). We infer from these studies that 3β-HSD and aromatase may function together in the mammalian brain to confer neuroprotective functions to DHEA.
1.3. 3β-HSD in brain
3β-HSD is a membrane-bound enzyme responsible for the oxidation and the isomerization of the inactive Δ5-3β-hydroxy steroids, pregnenolone and DHEA, into active Δ4-keto steroids, progesterone and androstenedione (AE) respectively (Payne and Hales, 2004). Humans have two isoforms of 3β-HSD, whereas mice have six. Some of these isoforms catalyze the forward reaction described above, whereas others function as 3-ketosteroid reductases and likely inactivate some steroidal molecules. Isoforms that catalyze the conversion of DHEA to AE are dependent on the presence of nicotinamide adenine dinucleotide (NAD+) as a cofactor. It is this NAD-dependent reaction that we have identified and studied in the songbird brain, and it is the reaction (or isoform) that will be our focus for the remainder of this review.
3β-HSD is critical for the synthesis of mineralocorticoids, glucocorticoids, androgens and estrogens in the classic steroidogenic tissues, such as gonads, adrenals, and placenta. It is also expressed in other tissues, including mammary tissue, skin, heart, fat, kidney, liver and the brain (Payne and Hales, 2004; Oh et al., 1998; Bumke-Vogt et al., 2002; Labrie et al, 2005; Mensah-Nyagan, 1994). In brain, 3β-HSD activity and/or mRNA have been reported in neurons, astrocytes and oligodendrocytes in vitro (Zwain and Yen, 1999a, b). Perhaps the most widely recognized reaction catalyzed by 3β-HSD involves the formation of progesterone from pregnenolone. Progesterone is known to influence neuronal myelination (Schumacher et al., 2001) and metabolites of progesterone can bind to GABAA receptors, thereby potentiating neuronal hyperpolarization (Majewska, 1992). Consequently, most studies of neural 3β-HSD have focused on its role in progesterone synthesis (Mensah-Nyagan et al., 1998; Coirini et al., 2002; Coirini et al., 2003b; Coirini et al., 2003a; Tsutsui et al., 2003; Soma et al., 2005). The metabolism of DHEA by neural 3β-HSD has received much less attention. We hypothesize that DHEA is an important substrate for brain 3β-HSD, providing a mechanism for the local synthesis of neurally active androgens and estrogens in the songbird brain
1.4. Songbirds as models for neuroendocrine research
Songbird research is well-established in the behavioral neuroscience community (Konishi et al., 1989; Wingfield, 2005; Goodson et al., 2005). Singing is a learned behavior, as is human speech, and song is used in reproductive and aggressive contexts to attract mates and repel intruders, respectively. Importantly, singing is a natural behavior with clear functions and biological relevance. Numerous studies have examined the hormonal and neural bases of song production (Schlinger and Brenowitz, 2002). Singing is controlled by a network of discrete, steroid-sensitive brain regions (song nuclei; see Fig 2b). Androgen receptors are expressed in several song nuclei, and estrogen receptors are expressed in the HVC of some species. Interestingly, there is dramatic and natural neuroplasticity in the adult songbird brain, including the growth and shrinkage of entire song nuclei across the seasons and robust adult neurogenesis in much of the forebrain (Tramontin and Brenowitz, 2000). Adult neurogenesis in a homeothermic vertebrate was first conclusively demonstrated in songbirds, eventually leading to similar discoveries in rodents and humans (Nottebohm, 1996). In addition, adult neural progenitors were first identified as fully differentiated glia in songbirds (Alvarez-Buylla et al., 1990), again leading to similar discoveries in mammals (Alvarez-Buylla et al., 2001). Other key studies in songbirds have shown high levels of aromatase in the telencephalon, including the region surrounding HVC, and clarified the functions and regulation of brain aromatase (Schlinger, 1997; Saldanha et al., 2000). These discoveries and the first demonstration of gross sex differences in the vertebrate brain (Nottebohm and Arnold, 1976) are just some of the important contributions of this system to the study of behavior and neurobiology.
Figure 2.
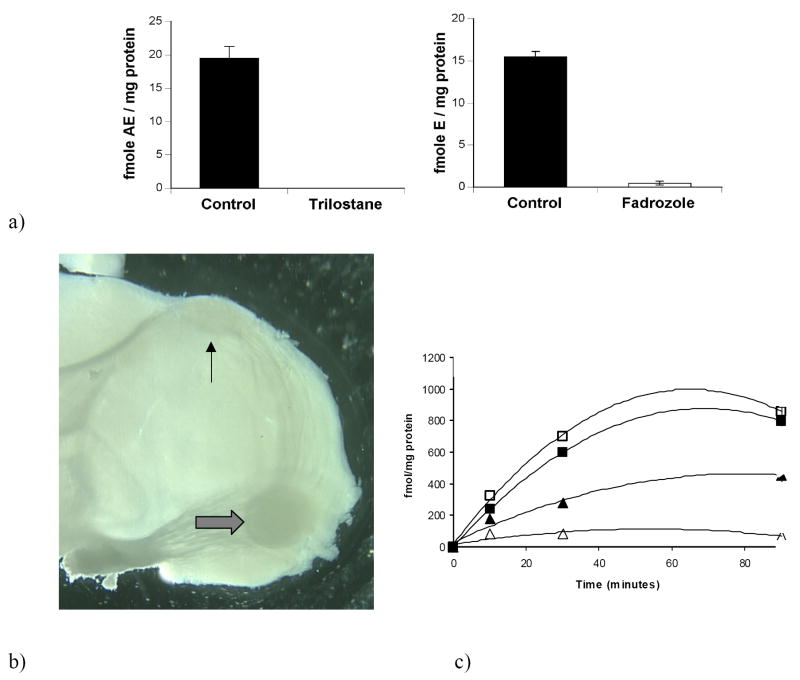
a) Conversion of 3H-DHEA into 3H-AE (left) and 3H-estrogens (right) by homogenates of the adult zebra finch telencephalon. The 3β-HSD inhibitor trilostane abolished 3H-AE production (left); the aromatase inhibitor Fadrozole decreased 3H-estrogen production by 97% (right; from Soma et al., 2004); b) Caudal view of a 300μm thick parasagittal slice of an adult male zebra finch telencephalon containing two song system motor nuclei: robust nucleus of the arcopallium (RA, thick arrow) and HVC (thin arrow). Note the tract that runs from HVC to RA; c) Incubation of male telencephalic slices with 200nM 3H-DHEA for 10, 30, and 90 mins in the presence or absence of the 3βHSD inhibitor trilostane. Tritiated estrogens and 5α-androstanedione (5α-A) were isolated and quantified: ▲, estrogens; Δ, estrogens + trilo; ■, 5αA; □, 5αA + trilo (from Tam and Schlinger, 2007).
Many researchers studying DHEA actions agree that progress has been severely hampered by the lack of small animal models e.g. (Allolio and Arlt, 2002). Non-human primates, like humans, have high levels of plasma DHEA(S) (Sapolsky et al., 1993), but primate studies can be very difficult logistically. Traditional small animal models, such as rats and mice, have very low or non-detectable levels of plasma DHEA(S) and no adrenal DHEA production (Baulieu and Robel, 1996). Importantly, almost all rat and mouse studies looking at the effects of DHEA treatment have used DHEA doses far above the physiological range of these species, making the results difficult to interpret (Thijssen and Nieuwenhuyse, 1999). In contrast, several lines of evidence indicate that songbirds and other birds are good small animal models in which to investigate the actions and mechanisms of DHEA in the nervous system (Tsutsui and Yamazaki, 1995; Migues et al., 2002).
1.5. Songbird hormones and behavior in the field
Many songbird species are seasonal breeders (Wingfield and Farner, 1993). In seasonally breeding birds, the gonads grow prior to the breeding season and regress after the termination of breeding. During the spring (breeding season), the gonads are active, and plasma sex steroid levels are high. During the autumn and winter (non-breeding season), the gonads are regressed, and circulating sex steroids are generally low or non-detectable. Singing and territorial behavior, which are sex steroid-dependent, are often elevated during the breeding season and reduced or absent during the non-breeding season (Schlinger and Brenowitz, 2002). However, there are also many avian species in which singing and territorial aggression are robustly expressed in the non-breeding season, when circulating sex steroids are low (see below).
Free-living songbirds are often more aggressive and typically have higher circulating hormone levels than captive songbirds (Wingfield and Farner, 1993). In addition, testing conditions in the laboratory can have large unexpected effects on behavior, particularly when animals are forced to interact in constrained spaces. For these reasons, field studies can be an important complement to laboratory studies when studying behavior and neuroendocrinology.
2. 3β-HSD in the songbird brain
2.1. 3β-HSD activity in songbird brain dissociated cell cultures
3β-HSD activity was first identified in the avian brain using primary cell cultures of the developing zebra finch telencephalon (Vanson et al, 1996). Previously, similar cultures prepared from zebra finches (1-5 days posthatching) and grown for 7 to 30 days in vitro, were found to contain a mixture of neurons and glia. When they were incubated with 3H-AE, considerable amounts of 3H-estrogens, as well as 3H-5α- and 3H-5β-reduced metabolites of 3H-AE were formed (Schlinger et al, 1994; Wade et al., 1995). Upon exposure to 3H-DHEA, not only was 3H-AE detected in the culture medium, but the products 3H-AE metabolism were also identified, including 3H-estrogens (Vanson et al, 1996). Product identities were confirmed after biochemical and chromotagraphy separation followed by recrystallization procedures. 3H-Estrogens formed from 3H-DHEA were substantially reduced or eliminated by the inclusion of a radioinert AE “cold trap” that substantially reduces further metabolism of newly formed 3H-AE. The inclusion of an effective aromatase inhibitor also blocked the formation of 3H-estrogens from 3H-DHEA. These data clearly showed that 3β-HSD was active in these cultures and functioned coordinately with other steroid metabolic enzymes like 5α-reductase and aromatase, to create potent androgens and estrogens. We also isolated 3H-progesterone from the culture medium after cells were exposed to 3H-pregenenolone (Vanson et al, 1996). At the time, we knew little about pregnenolone availability to the songbird brain, so we did not examine this pathway further. Nevertheless, this result provided additional confirmation of 3β-HSD activity in the cell cultures and suggests 3β-HSD in the songbird brain may naturally metabolize pregnenolone in addition to DHEA.
These studies confirmed 3β-HSD activity in dissociated cell cultures, but it remains critical to measure 3β-HSD in vivo. This issue was particularly important because we had identified aromatase in astrocytes cultured from the songbird brain (Schlinger et al., 1994) and these cells might only express aromatase in vivo after neural injury (Peterson et al., 2001). These results raised the concern that steroid metabolism in dissociated cell cultures might not reflect steroid metabolism in the uninjured brain. Subsequent experiments using brain tissue that was not cultured confirmed 3β-HSD activity in songbird brain (see below)
2.2. 3β-HSD activity in songbird brain homogenates
There is strong evidence that DHEA is converted in vitro to androgens and estrogens by songbird brain homogenates (Soma et al 2004; London et al, 2006). In these studies, brain tissue was homogenized and then incubated with 3H-DHEA. The 3β-HSD cofactor, NAD+, was provided to brain homogenates. In some studies, a “cold trap” of radioinert AE was used during incubations. The AE cold trap prevents subsequent metabolism of formed 3H-AE. After the incubation, steroids are extracted and then separated using thin layer chromatography (TLC) or high performance liquid chromatography (HPLC).
Adult and developing zebra finches demonstrate high 3β-HSD activity in brain homogenates (Soma et al, 2004; London et al, 2007). In adult zebra finches, brain homogenates metabolize 3H-DHEA to 3H-AE. In the absence of an AE cold trap, the formed 3H-AE is subsequently aromatized to 3H-estrone (small amounts of 3H-estradiol were also detected). Trilostane, a specific 3β-HSD inhibitor, was added to the incubation, and trilostane abolished the metabolism of 3H-DHEA to 3H-AE and 3H-estrogens (Fig 2a). Fadrozole, an aromatase inhibitor, reduced the formation of 3H-estrogens but not 3H-AE. Lastly, tritiated products were recrystallized (3 times) to constant specific activity to confirm product identity. Recently, we have confirmed and extended these results using HPLC coupled to flow scintillation detection (Pradhan and Soma, 2006). Interestingly, recent studies indicate that brain 3β-HSD activity is much higher in supernatants (following 1000g centrifugation to pellet whole cells and cell nuclei), compared to whole homogenates (as in Coirini et al., 2003b; Soma et al., 2005).
Wild adult song sparrows also have high 3β-HSD activity in brain homogenates. Song sparrow brain homogenates convert 3H-DHEA to 3H-AE and 3H-estrogens, with highest levels of 3β-HSD activity in the diencephalon and telencephalon (unpublished results). Similar to the above studies in zebra finches, trilostane reduced 3H-AE production, and fadrozole reduced 3H-estrogen production.
2.3. 3β-HSD activity in songbird brain slices
3β-HSD activity can also be measured in freshly prepared slices of the adult and developing zebra finch brain that contain no added co-factors (Fig 2c; Tam and Schlinger, 2007). Because these slices represent a relatively intact condition, with cells not disturbed or artificially mixed by homogenization, we believe they reflect the activity most likely to be found in vivo. For example, requisite cofactors must be co-localized with enzymes to drive reactions in cells within the slices. In addition, for sequential reactions to occur, the enzymes catalyzing steroid-metabolism must be in cells that are sufficiently close to each other to be captured together within the slice. Under these conditions, we can readily detect conversion of DHEA to estrogens as well as 5α- and 5β-reduced androgens. The activities of 3β-HSD isoforms that catalyze the forward reaction (3H-DHEA to 3H-AE) are dependent on the presence of nicotinamide adenine dinucleotide (NAD+) as a cofactor (Payne and Hales, 2004). Our results indicate that the hydroxysteroid dehydrogenase/isomerase isoform of 3β-HSD occurs in brain and that NAD+ is present in brain cells that express 3β-HSD. Aromatase, on the other hand, utilizes NADPH as an electron donor to catalyze the formation of an aromatic ring on the androgenic substrate (Payne and Hales, 2004). Because tritiated substrates are acted upon by both 3β-HSD and aromatase in these slices, these results suggest that either the same cells express both enzymes and co-factors, or the enzymes are present in separate cells that are spatially co-localized within the slice.
2.4. Regulation of songbird brain 3β-HSD activity
The regulation of brain 3β-HSD in vertebrates has received little attention. This is a critical gap in our knowledge, because identifying the environmental and endocrine factors that regulate the metabolism of DHEA will provide important clues to its neural functions.
In adult zebra finches, baseline brain 3β-HSD activity is higher in females than males (Soma et al., 2004), and this is the first report of a sex difference in brain 3β-HSD. Interestingly, acute stress (10 min restraint) rapidly decreases 3β-HSD activity in females but not males. Thus, in stressed zebra finches, the sex difference is reversed, and males have higher 3β-HSD activity than females (Soma et al., 2004). This is the first report of rapid regulation of 3β-HSD activity in any tissue. In breeding male song sparrows, stress also rapidly affects 3β-HSD activity. However, in male song sparrows, acute stress increases brain 3β-HSD activity (unpublished results).
In wild song sparrows under natural conditions, there are seasonal changes in brain 3β-HSD activity. Male song sparrows are interesting in that they are aggressive year-round, except briefly during molt (annual replacement of feathers) after breeding (Wingfield and Hahn, 1994; Soma and Wingfield, 1999). In particular, song sparrows are aggressive during the non-breeding season (autumn and winter), when plasma T, AE, and E2 levels are non-detectable. Nonetheless, plasma DHEA levels are detectable during the non-breeding season (see below). We examined brain 3β-HSD activity in free-living male song sparrows caught during the breeding season, molt, and non-breeding season. Interestingly, brain 3β-HSD activity was upregulated during the non-breeding season, when aggression is high and plasma T levels are low.
Ongoing studies are examining endocrine factors that regulate 3β-HSD activity under controlled conditions in vitro. Such studies have been useful in understanding the regulation of 3β-HSD in gonads and adrenals, and should also prove useful for studying brain 3β-HSD. For example, endogenous steroids can regulate their synthesis via local feedback loops (Gower and Cooke, 1983). Since E2 is a potent downstream product of DHEA metabolism, we studied the in vitro effects of E2 on 3β-HSD activity in zebra finch brain homogenates and supernatants (Pradhan and Soma, 2006). We found a dose-dependent inhibitory effect of E2 on 3β-HSD activity. These effects of E2 occurred within only 10 min, indicating a rapid effect of E2 on 3β-HSD activity. Moreover, these rapid E2 effects were present in supernatants that lack intact cells and cell nuclei, which also argues against a genomic effect of E2. Interestingly, E2 was more effective at inhibiting 3β-HSD in females than males, similar to the effects of acute stress (Soma et al., 2004). In the future, songbird studies should examine the in vitro effects of GABA, endozepines, and Neuropeptide Y, as in previous frog studies (Mensah-Nyagan et al., 1999).
Taken together, these studies of songbirds indicate that brain 3β-HSD can be regulated on a short timescale (minutes) and longer timescale (days to weeks). Rapid regulation could involve changes in the phosphorylation of 3β-HSD, similar to aromatase (Balthazart et al., 2001) and P450c17 (Zhang et al., 1995). Long-term regulation might involve changes in gene transcription and translation (as in Soma et al., 2005).
2.5. Molecular biology of songbird 3β-HSD
Using primers based on chicken 3β-HSD sequence, we amplified 3β-HSD cDNA from several adult and developing zebra finch brain regions (London et al., 2006). Based on these PCR products, we generated zebra finch specific subclones and used these as templates for probes used to screen zebra finch cDNA libraries, for Northern blots and for in situ hybridization experiments. Full-length zebra finch 3β-HSD cDNAs were then isolated from both a testicular and a whole brain cDNA libraries. The nucleotide sequences of these cDNAs were 96% identical and likely coded for the same mRNA. They contained an open reading frame of 1134 bps and a predicted amino acid sequence of 377 amino acids, similar to 3β-HSD of other species. The clones shared 86% nucleotide sequence similarity with the chicken, the only other avian 3β-HSD sequence available.
Neural 3β-HSD expression was confirmed by Northern blot analyses. Five transcripts were identified in the ovary and testes, with at least two of these transcripts (1.6 and 3.0 kb) visible in brain. Mulitple 3β-HSD transcripts are present in mammalian tissues (Bain et al., 1991; Zhao et al., 1991; Guennoun et al., 1995) and these correspond with multiple protein isoforms (Payne and Hales, 2004). We have no evidence for more than one 3β-HSD protein in the zebra finch.
In situ hybridization showed, as expected, 3β-HSD expression over testicular interstitial cells and ovarian follicular cells in tissues from both adult and developing zebra finches (Freking et al., 2000; London et al., 2006; London and Schlinger, 2007). In brain, 3β-HSD hybridization was also present in juvenile (Fig 3) and adult birds of both sexes. At posthatch day 20 and in adults, hybridization patterns were similar with low to moderate levels of expression seen in specific nuclei in the telencephalon, diencephalon, midbrain and hindbrain (London et al., 2006). Hybridization was conspicuous in the hippocampus, the lateral striatum, and nucleus taeniae (homologue for the mammalian medial amygdala), the preoptic area (POA), and ventromedial nucleus (VMN). Many of these areas also express aromatase (Shen et al., 1995; Saldanha et al., 2000) but we do not know if these enzymes are expressed in the same, or in different cells. In addition, several nuclei of the avian song control system expressed 3β-HSD including HVC, RA, lMAN and area X. Nucleus HVC does not itself express aromatase, but is innervated by aromatase-positive fibers (Saldanha et al., 2000) and synapses (Peterson et al., 2005). It is possible that cells expressing 3β-HSD are innervated by aromatase-positive synapses and in this way can produce estrogens from DHEA.
Figure 3.
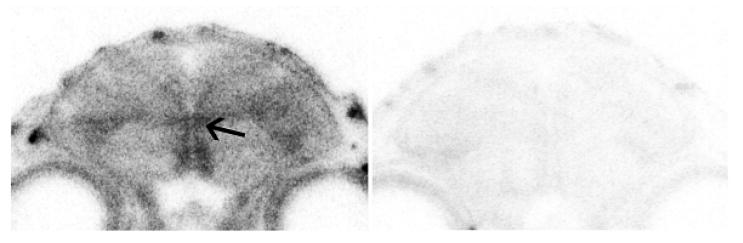
In situ hybridization for 3β-HSD mRNA in the anterior part of the brain of a juvenile male zebra finch (posthatch day 5). Expression is observed along the lateral ventricle (arrows point to intermediate portion of right hemisphere lateral ventricle) with antisense (left) but not sense (right) hybridizations (from London et al., 2007).
Notably, at posthatch days 1 and 5, ages when the brain is growing markedly and the neural song system is beginning to form, hybridization was most conspicuous along the lateral margins of the lateral ventricles (Fig 3; London and Schlinger, 2007). It is the lateral ventricles that contain the proliferative layers of the developing avian telencephalon. The subventricular zone contains active neural stem cells and the cell bodies of radial glia (Goldman et al., 1996). Mitotic activity is high at P1-P5 (DeWulf and Bottjer, 2002, 2005) so 3β-HSD is positioned to metabolize or synthesize steroids that could assist with appropriate proliferation or with the differentiation, migration or survival of newly born cells.
2.6. 3β-HSD as part of a neurosteroid synthetic pathway in songbirds
It is important to recognize that the songbird brain expresses several other enzymes and transporters in the steroidogenic pathway giving it the capacity to synthesize steroids de novo (Schlinger et al., 2001). Steroids synthesized entirely within the nervous system have been called “neurosteroids” (Baulieu, 1991). We have evidence for the mRNA expression of StAR (steroidogenic acute regulatory protein), CYP11A1 (side-chain cleavage enzyme), CYP17 (17α-hydroxylase enzyme) and aromatase, as described above (Shen et al., 1995; London et al., 2003, 2006; London and Schlinger, 2007). Expression of these factors is seen in brains of developing and adult zebra finches of both sexes. In several brain regions, these factors exhibit spatial overlap with each other and with 3β-HSD. Consequently, the zebra finch brain likely has the capacity to synthesize steroids de novo with 3β-HSD as a component of a more complex neurosteroidogenic environment. Studies are underway to determine the extent to which these enzymes and transporters control local neural steroid concentrations.
3. Dehydroepiandrosterone (DHEA) in songbirds
3.1. Circulating DHEA levels
As mentioned above, in some songbirds, such as the male song sparrow, singing and territorial aggression are robustly displayed during the non-breeding season, even though plasma sex steroid levels are non-detectable in winter (Soma and Wingfield, 1999). Moreover, castration does not decrease aggressive behavior in non-breeding song sparrows (Wingfield, 1994). In contrast, inhibition of aromatase does decrease non-breeding aggression, indicating a role for estrogens (Soma et al., 1999, 2000, 2003). Because T and AE (aromatizable androgens) are not detectable in the circulation of non-breeding song sparrows, adrenal DHEA might provide an indirect source of sex steroids for the brain in winter.
In song sparrows, plasma DHEA is indeed detectable and several times higher than plasma T during the non-breeding season (Fig 4a; Soma and Wingfield, 2001; Newman and Soma, 2006). DHEA concentrations are high in the adrenals and regressed testes of non-breeding birds, suggesting that both adrenals and testes may secrete DHEA at this time of year (Soma and Wingfield, 2001). However, neither acute restraint stress nor GnRH injections increased plasma DHEA concentrations, leaving the question of DHEA regulation unresolved. Interestingly, seasonal changes in plasma DHEA in song sparrows correlate with seasonal changes in male aggressive behavior (Soma et al., 2001), with lowest levels of both at molt.
Figure 4.
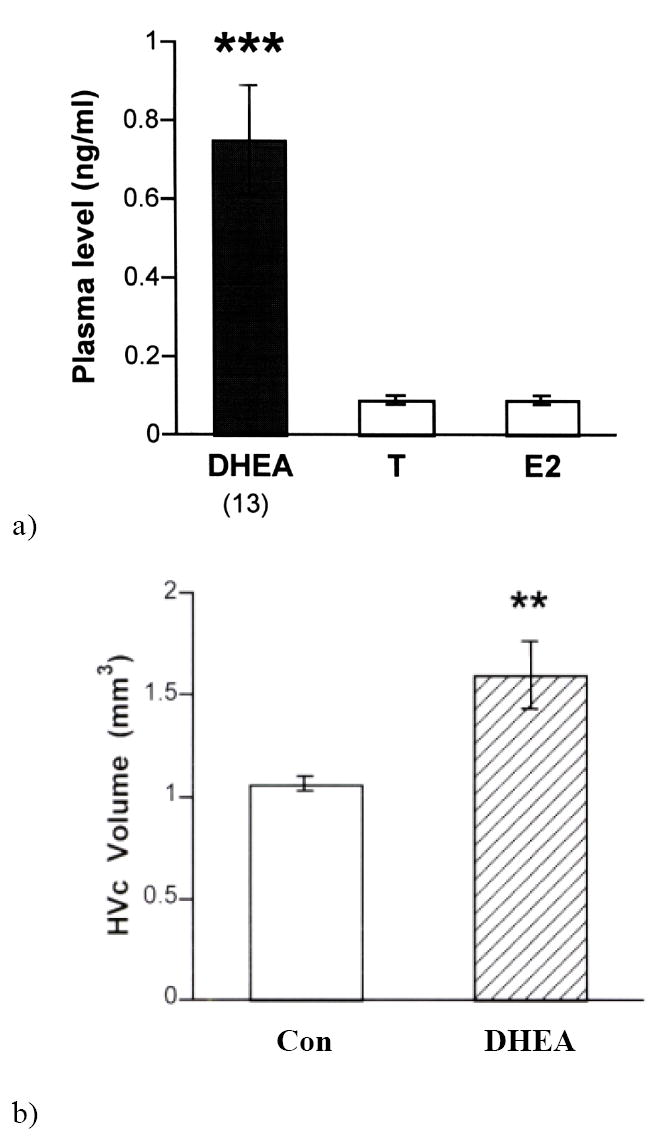
a) Plasma dehydroepiandrosterone (DHEA) levels in wild adult male song sparrows in the non-breeding season (autumn and winter). Plasma levels of DHEA were significantly higher than plasma testosterone (T) and estradiol (E2) levels. Plasma concentrations of T and E2 were non-detectable (< 0.1 ng/ml). From Soma et al. (2001); b) Effect of DHEA treatment on adult song system neuroanatomy. Compared to controls (CON), DHEA treatment significantly increases the volume of HVC. Overall telencephalon volume is not affected by DHEA treatment **p<0.01. (from Soma et al., 2002).
Such neuroendocrine mechanisms may be present in many other avian species. For example, many birds that breed in the tropics defend territories year-round and have very low levels of circulating sex steroids throughout the year (Goymann et al., 2004). One such species is the spotted antbird. In this species, both sexes sing and aggressively defend territories year-round (Hau et al., 2000). Spotted antbirds have basal plasma T and E2 levels, even during the breeding season, except for transient increases during territorial encounters (Hau et al., 2000). Combined treatment with an aromatase inhibitor (ATD) and an androgen receptor antagonist (flutamide) decreased male aggressive vocalizations in the breeding season, even though plasma T was non-detectable in control subjects. In the non-breeding season, male and female spotted antbirds show high levels of territorial aggression towards same-sex intruders (Hau et al., 2004). Relative to plasma concentrations of T and E2 (low or non-detectable), plasma concentrations of DHEA are elevated in males and females. In males, plasma DHEA levels are positively correlated with aggressive vocalizations and/or the duration of territorial intrusions (Hau et al., 2004). Other avian species that display aggression during the non-breeding season may use similar mechanisms (Spinney et al., 2006).
3.2. Behavioral and neuroanatomical effects of DHEA treatment
The effects of DHEA treatment on adult songbirds have been examined. Like breeding birds, non-breeding song sparrows defend territories and use songs in that context. These birds were given a physiological dose of DHEA for two weeks (Soma et al., 2002). DHEA treatment increased territorial singing behavior but not other typical territorial behaviors. This is the first demonstration of DHEA effects on male-male aggression in any animal. DHEA treatment also had marked effects on the adult brain (Fig 4b) and increased the size of a forebrain song nucleus (HVC) by ~50% (to maximal spring size). This is one of the largest reported effects of DHEA on the adult brain. The effects of DHEA treatment on neuroanatomy are similar to the effects of T or E2 treatment (Soma et al. 2004), suggesting that DHEA stimulates growth of nucleus HVC after metabolism by 3β-HSD and aromatase. Importantly, unlike T treatment, DHEA treatment does not increase growth of a peripheral secondary sex character used during reproduction (the cloacal protuberance) or suppress immune function (Soma et al., 2002; Owen-Ashley et al., 2004). Thus, exogenous DHEA has pronouned effects on behavior and the central nervous system, with reduced effects on peripheral steroid targets.
The effects of DHEA treatment on developing songbirds have also been examined. In zebra finches, we have detected circulating DHEA during the first week post-hatch. Developing female zebra finches (1 to 3 days post-hatch) were implanted subcutaneously with either DHEA or control pellets. Subjects were sacrificed during adulthood (100 days post-hatch) for neuroanatomical measurements. Volumes of song nuclei and cell sizes and neuronal density were determined. DHEA-treated females had a greater density of HVC neurons and tended to have larger HVC neurons (unpublished data). Although modest, these effects suggest developmental roles for DHEA in songbirds.
4. Conclusions
Models of steroid action have become increasingly complex as we have come to appreciate the steroid metabolic and synthetic properties of the brain (Fig. 5). Following the identification of aromatase and 5α-reductase in the male brain as well as masculine neural estrogen and androgen actions, we now consider circulating T as a prohormone. In songbirds, brain 3β-HSD and aromatase can function together to convert DHEA into active androgens and estrogens, and some neural actions of DHEA can be mimicked by E2. We conclude that circulating DHEA, like T, functions as a prohormone that is activated in the brain by appropriate steroid metabolic reactions (Fig. 5).
Figure 5.
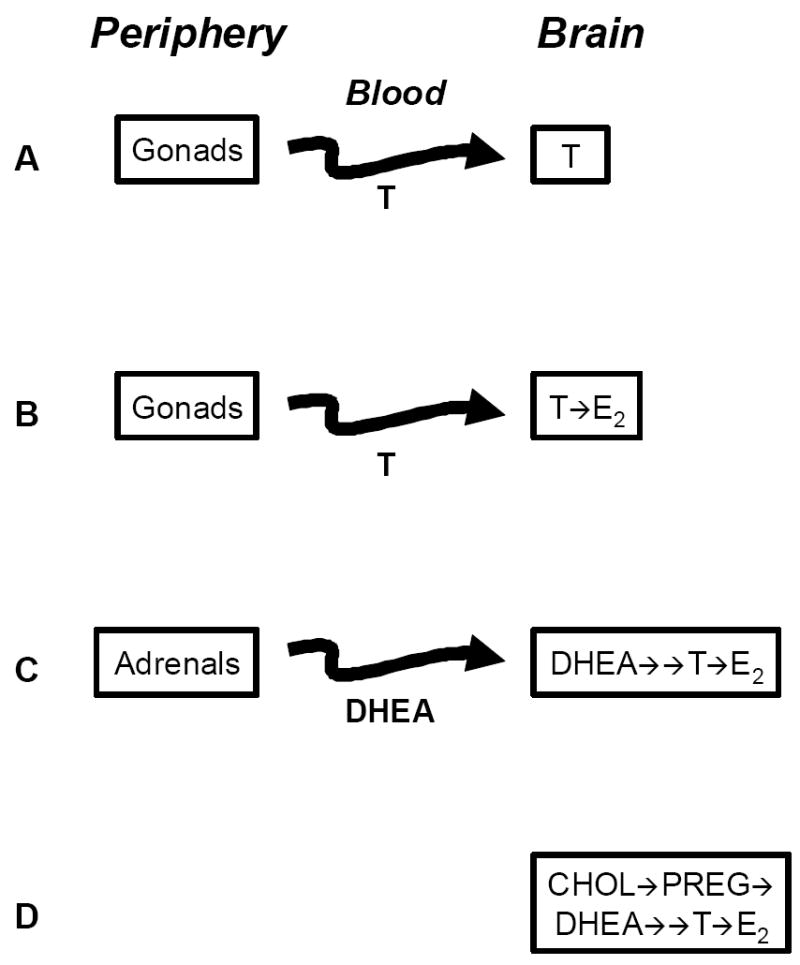
Changing concepts of sex steroid actions on the vertebrate brain. A) Brain as a simple target of gonadal testosterone (T); B) T as a prohormone: the brain expresses aromatase to metabolize T into estradiol (E2); C) DHEA as a prohormone secreted by the adrenals: the brain expresses 3β-HSD and aromatase to metabolize DHEA into active androgens and estrogens; D) the brain expresses all steroidogenic enzymes and transporters needed to convert cholesterol (Chol) into pregnenolone (Preg), DHEA, and active androgens (T) and estrogens (E2) (From Soma 2006).
Because of its position within the larger steroidogenic pathway, DHEA provides several advantages as a steroidal signaling molecule. DHEA may affect the brain but have little impact on other steroid target tissues that lack 3β-HSD. As a consequence, DHEA can function as an important prohormone during the non-breeding season to stimulate aggressive behavior without inappropriately stimulating peripheral reproductive tissues. This appears to be a critical function of DHEA in some species that aggressively defend territories during non-breeding seasons (Wingfield et al., 2001).
Many questions about the physiology of DHEA and the role of brain 3β-HSD remain unanswered. For example, regulation of DHEA metabolism by 3β-HSD in the avian brain remains largely unknown. Studies in amphibians provide critical insights into the regulation of 3β-HSD activity by GABA and endozepines, which are important regulators of aggression and anxiety (Mensah-Nyagan et al., 2001). In addition, little is known about the sites of synthesis and regulation of DHEA in songbirds, and these remain critical issues for future studies. Direct neural application of trilostane, the 3β-HSD inhibitor, to freely behaving birds should yield important insights as well.
Acknowledgments
Supported by National Institutes of Health MH061994 to BAS. KKS is supported by grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Michael Smith Foundation for Health Research, and the Canada Foundation for Innovation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metabol. 2002;13:288–294. doi: 10.1016/s1043-2760(02)00617-3. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nature Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Bain PA, Yooo M, Clark T, Hammond SH, Payne AH. Multiple forms of mouse 3B-hydroxysteroid dehydrogenase/isomerase and differential expression in gonads, adrenal glands, liver and kidneys of both sexes. Proc Natl Acad Sci USA. 1991;88:8870–8874. doi: 10.1073/pnas.88.20.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J. Brain aromatization of testosterone regulates male reproductive behavior in birds. Prog Clin Biol Res. 1990;342:92–98. [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Ramassamy C, Poirier J, Quirion R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Mol Brain Res. 1999;66:35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Rec Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Nat Acad Sci USA. 1998;95:4089–4091. doi: 10.1073/pnas.95.8.4089. comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E-E. Neurosteroids: A function of the brain. In: Costa E, Paul SM, editors. Neurosteroids and Brain Function. New York: Thieme Medical Publishers; 1991. pp. 63–73. [Google Scholar]
- Baulieu E-E, Robel P. Dehydroepiandrosterone and dehydroepiandrosterone sulfate as neuroactive neurosteroids. J Endocrinol. 1996;150(Suppl):S221–239. [PubMed] [Google Scholar]
- Bumke-Vogt C, Bahr V, Diederich S, Herrmann SM, Aagnostopoulos I, Oelkers W, Quinkler M. Expression of progesterone receptor and progesterone-metabolizing enzymes in the female and male human kidney. J Endocrinol. 2002;178:349–364. doi: 10.1677/joe.0.1750349. [DOI] [PubMed] [Google Scholar]
- Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: mechanism of action. Proc Soc Exp Biol Med. 1999;222:145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Martini L. 5α-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocrinol. 1992;13:163–215. [PubMed] [Google Scholar]
- Coirini H, Gouezou M, Liere P, Delespierre B, Pianos A, Eychenne B, Schumacher M, Guennoun R. 3 beta-hydroxysteroid dehydrogenase expression in rat spinal cord. Neurosci. 2002;113:883–891. doi: 10.1016/s0306-4522(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Coirini H, Gouezou M, Delespierre B, Schumacher M, Guennoun R. 3 beta-hydroxy steroid dehydrogenase isomerase (3 beta-HSD) activity in the rat sciatic nerve: kinetic analysis and regulation by steroids. J Steroid Biochem Mol Bio. 2003a;85:89–94. doi: 10.1016/s0960-0760(03)00133-x. [DOI] [PubMed] [Google Scholar]
- Coirini H, Gouezou M, Delespierre B, Liere P, Pianos A, Eychenne B, Schumacher M, Guennoun R. Characterization and regulation of the 3 beta-hydroxysteroid dehydrogenase isomerase enzyme in the rat sciatic nerve. J Neurochem. 2003b;84:119–126. doi: 10.1046/j.1471-4159.2003.01512.x. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Nat Acad Sci USA. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Cooper MA, Albers HE, Soma KK. Novel mechanisms underlying neuroendocrine regulation of aggression: a synthesis of rodent, avian and primate studies. In: Blaustein JD, editor. Behavioral Neurochemistry and Neuroendocrinology. Vol. 21. 2007. Lajtha A (series ed) Handbook of Neurochemistry and Molecular Neurobiology, in press. [Google Scholar]
- DeWulf V, Bottjer SW. Age and sex differrences in mitotic activity within the zebra finch telencephalon. J Neurosci. 2002;22:4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone: a map of proliferative activity. J Comp Neurol. 2005;481:70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. doi: 10.1002/jnr.10821. [DOI] [PubMed] [Google Scholar]
- Freking F, Nazairians T, Schlinger BA. The expression of the sex steroid-sythesizing enzymes CYP11A1, 3-beta-HSD, CYP17, and CYP 19 in gonads and adrenals of adult and developing zebra finches. Gen Comp Endocrinol. 2000;119:140–151. doi: 10.1006/gcen.2000.7503. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobio. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996;30:505–520. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Saldanha CJ, Hahn TP, Soma KK. Recent advances in behavioral neuroendocrinology: Insights from studies on birds. Horm Behav. 2005;48:461–473. doi: 10.1016/j.yhbeh.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower DB, Cooke GM. Regulation of steroid-transforming enzymes by endogenous steroids. J Steroid Biochem. 1983;19:1527–1556. doi: 10.1016/0022-4731(83)91130-5. [DOI] [PubMed] [Google Scholar]
- Goymann W, Moore IT, Scheuerlein A, Hirschenhauser K, Grafen A, Wingfield JC. Testosterone in tropical birds: Effects of environmental and social factors. Am Nat. 2004;164:327–334. doi: 10.1086/422856. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouézou M, Lombès M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Soma KK, Wingfield JC. Testosterone and year round territorial aggression in a tropical bird. Gen Comp Endocrinol. 2000;117:20–33. doi: 10.1006/gcen.1999.7390. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Emlen S, Ricklefs R, Wingfield JC. Contributions of bird studies to biology. Science. 1989;246:465–472. doi: 10.1126/science.2683069. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Simard J, Van L-T, Labrie C. DHEA and peripheral androgen and estrogen formation: intracinology. Ann New York Acad Sci. 1995;774:16–28. doi: 10.1111/j.1749-6632.1995.tb17369.x. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin S-X, Simard J, Pelletier G. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, B A. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- Li H, Klein G, Sun P, Buchan AM. Dehydroepiandrosterone (DHEA) reduces neuronal injury in a rat model of global cerebral ischemia. Brain Res. 2001;888:263–266. doi: 10.1016/s0006-8993(00)03077-8. [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: A study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- London S, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinol. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London S, Schlinger BA. Steroidogenic Enzymes Along the Ventricular Proliferative Zone in the Developing Songbird Brain. J Comp Neurol. 2007;502:507–521. doi: 10.1002/cne.21335. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobio. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Marx CE, Jarskog LF, Lauder JM, Gilmore JH, Lieberman JA, Morrow AL. Neurosteroid modulation of embryonic neuronal survival in vitro following anoxia. Brain Res. 2000;871:104–112. doi: 10.1016/s0006-8993(00)02452-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Revs. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Anatomical and biochemical evidence for the synthesis of unconjugated and sulfated neurosteroids in amphibians. Brain Res Revs. 2001;37:13–24. doi: 10.1016/s0165-0173(01)00110-2. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- Mensah-Nyagan GA, Do-Rego JL, Beaujean D, Feuilloley M, Marcual A, Lange C, Pelletier G, Vaudry H. Biosynthesis of neuroandrogens in the frog brain. Trends Comp Endocrinol Neurobiol. 1998:400–402. doi: 10.1111/j.1749-6632.1998.tb10812.x. [DOI] [PubMed] [Google Scholar]
- Migeon CJ, Keller AR, Lawrence B, Shepart TH. Dehydroepiandrosterone and androsterone in human plasma: Effects of age and sex:day-to-day and diurnal variations. J Clin Endocr Metab. 1957;17:1051–1062. doi: 10.1210/jcem-17-9-1051. [DOI] [PubMed] [Google Scholar]
- Migues PV, Johnston ANB, Rose SPR. Dehydroepiandrosterone and uts sulphate enhance memory retention in day old chicks. Neurosci. 2002;109:243–251. doi: 10.1016/s0306-4522(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Newman AEM, Soma KK. Neural secretion of DHEA and corticosterone during acute stress? Soc Neurosci Absts Program No 269.16 2006 [Google Scholar]
- Nottebohm F. The King Solomon Lectures in Neuroethology. A white canary on Mount Acropolis. J Comp Physiol [A] 1996;179:149–156. doi: 10.1007/BF00222782. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Odell WD, Parker LN. Control of adrenal androgen production. Endocr Res. 1984;10:617–630. doi: 10.1080/07435808409036520. [DOI] [PubMed] [Google Scholar]
- Oh SH, Oh JM, Kim JJ, Choi MK, Park ST, Park OK, Chung YT. Arch Histol Cytol. 1998;61:297–303. doi: 10.1679/aohc.61.297. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Hasselquist D, Wingfield JC. Androgens and the immunocompetencehandicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am Nat. 2004;164:490–505. doi: 10.1086/423714. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Revs. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J Neuroendocrinol. 2001;13:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is Presynaptic and Sexually-Dimorphic in the Adult Zebra Finch Brain. Proc Roy Soc Lond B. 2005 doi: 10.1098/rspb.2005.3181. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N. Towards a unified model of neuroendocrine immune interaction. Immuno Cell Bio. 2001;79:350–357. doi: 10.1046/j.1440-1711.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Soma KK. Rapid estrogen regulation of steroid synthesis in brain. Soc Neurosci Absts Program No 269.17 2006 [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerek MJ, Kim Y-H, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific anitbody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Vogelma JH, ROretreich N, Altman J. Senescent decline in serum dehydroepiandrosterone-sulfate ceoncentrations in a population of wild baboons. J Gerontol. 1993;48:B196–200. doi: 10.1093/geronj/48.5.b196. [DOI] [PubMed] [Google Scholar]
- Schlinger BA. The activity and expression of aromatase in songbirds. Brain Res Bull. 1997;44:359–364. doi: 10.1016/s0361-9230(97)00215-3. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Amsterdam: Academic Press; 2002. pp. 799–839. [Google Scholar]
- Schlinger BA, Amur-Umarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and nonneuronal aromatase in primary cultures of developing zebra finch telencephalon. J Neurosci. 1994;14:7541–7552. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Mercier G, Desarnaud F, Lacor P, Benavides J, Ferzaz B, Robert F, Baulieu EE. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res Revs. 2001;37:343–359. doi: 10.1016/s0165-0173(01)00139-4. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Avellana-Adalid V, Evercooren ABV, Courtin F, El-Etr M, Gago N, Guennoun R, Le Goascogne C, Li WW, Pierre M, Robert F. Steroid synthesis and metabolism by glia: Trophic and protective effects. Glia. 2002:S4–S5. [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Opposite effects of stressful experience on memory formation in males versus females. Dialogues Clin Neurosci. 2002;4:139–147. doi: 10.31887/DCNS.2002.4.2/tshors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Pickett J, Wood G, Paczynski M. Acute stress persistently enhances estrogen levels in the female rat. Stress. 1999;3:163–171. doi: 10.3109/10253899909001120. [DOI] [PubMed] [Google Scholar]
- Soma KK. Testosterone and aggression: Berthold, birds, and beyond. J Neuroendocrinol. 2006;18:543–551. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma K, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999;115:442–453. doi: 10.1006/gcen.1999.7334. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Endocrinology of aggression in the nonbreeding season. In: Adams N, Slotow R, editors. Proc 22nd Intl Ornithol Congress. Durban, South Africa: Int’l Ornithologists Union; 1999. [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldhana CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Phys A. 2000;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: Seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wissman AM, Brenowitz EA, Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002;41:203–212. doi: 10.1006/hbeh.2001.1750. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Schlinger BA. DHEA Metabolism by 3b-HSD in Adult Zebra Finch Brain: Sex Difference and Rapid Effect of Stress. Endocrinol. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: Estrogen increases 3b-HSD mRNA and Activity in rat hypothalamus. Endocrinol. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis) Horm Behav. 2006;50:762–71. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Tam H, Schlinger BA. Activities of 3B-HSD and aromatase in slices of the adult and developing zebra finch brain. Gen Comp Endocrinol. 2007;150:26–33. doi: 10.1016/j.ygcen.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen JHH, Nieuwenhuyse H. DHEA: A Comprehensive Review. New York: Pathenon Publ Group; 1999. [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Yamazaki T. Avian neurosteroids. I. Pregnenolone biosynthesis in the quail brain. Brain Res. 1995;678:1–9. doi: 10.1016/0006-8993(95)00116-8. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Sakamoto H, Ukena K. A novel aspect of the cerebellum: biosynthesis of neurosteroids in the Purkinje cell. Cerebellum. 2003;2:215–222. doi: 10.1080/14734220310016169. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Ketteni PT, Thijssen JHH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biol Psychiatry. 1998;43:156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, van den Ban E, Matthys W, Cohen-Ketteni PT, Thijssen JHH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: A comparison with psychiatric and normal controls. J Am Acad Child Adolesc Psych. 2000;39:1446–1451. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- Vanson A, Arnold AP, Schlinger BA. 3B-Hydroxysteroid dehydrogenase/ Isomerase and Aromatase Activity in Primary Cultures of Developing Zebra Finch Telencephalon: Dehydroepiandrosterone as Substrate for Synthesis of Androstenedione and Estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J Neurobio. 2003;56:398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- Wade J, Schlinger BA, Arnold AP. Aromatase and 5 beta-reductase activity in cultures of developing zebra finch brain: an investigation of sex and regional differences. J Neurobio. 1995;27:240–251. doi: 10.1002/neu.480270210. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav. 1994;28:1–15. doi: 10.1006/hbeh.1994.1001. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Historical contributions of research on birds to behavioral neuroendocrinology. Horm Behav. 2005;48:395–402. doi: 10.1016/j.yhbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Farner DS. Endocrinology of Reproduction in wild species. In: Farner DS, K JR, Parkes KC, editors. Avian Biology. San Diego: Academic Press; 1993. pp. 163–327. [Google Scholar]
- Wingfield JC, Lynn SE, Soma KK. Avoiding the “costs” of testosterone: ecological bases of hormone-behavior interactions. Brain, Behav Evol. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estrogens and neuroprotection. Trends in Endocrinol Metabol. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the cetrnal nervous system: effects on cognition and emotion in animals and humans. Brain Res Revs. 1999;30:264–268. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17increases 17,20-lyase activity: implications for adrenarche and the polycysticovary syndrome. Proc Natl Acad Sci U S A. 1995;92:10619–23. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HF, Labrie C, Simard J, de Launoit Y, Trudel C, Martel C, Rhéaume E, Dupont E, Luu-The V, Pelletier G, et al. Characterization of rat 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase cDNAs and differential tissue-specific expression of the corresponding mRNAs in steroidogenic and peripheral tissues. J Biol Chem. 1991;266:583–593. [PubMed] [Google Scholar]
- Zwain IH, Yen SSC. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinol. 1999a;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SSC. Dehydroepiandrosterone: Biosynthesis and metabolism in the brain. Endocrinol. 1999b;140:880–887. doi: 10.1210/endo.140.2.6528. [DOI] [PubMed] [Google Scholar]