Abstract
Internal initiation of translation is the process of beginning protein synthesis independent of the m7G cap structure at the 5′-end of an mRNA molecule. We have previously shown that the URE2 mRNA in the yeast Saccharomyces cerevisiae contains an internal ribosome entry site (IRES) whose activity is suppressed by eukaryotic initiation factor 2A (eIF2A; YGR054W). In this study, the minimal sequence required to efficiently direct internal initiation was determined using a system that abrogates cap-dependent scanning of the 40 S ribosomal subunit in both wild-type and eIF2A knock-out cells. Subsequently, secondary structural elements within the minimal sequence were determined by probing with RNases T1 and V1 and the small molecule diethylpyrocarbonate. It was found that the URE2 minimal IRES comprises a 104 nucleotide A-rich stem loop element encompassing the internal AUG codon. Interestingly, the internal AUG seems to be involved in base-pairing interactions that would theoretically hamper its ability to interact with incoming initiator tRNA molecules. Furthermore, none of the truncations used to identify the minimal IRES element were capable of abrogating the suppressive effect of eIF2A. Our data provide the first insight into the RNA structural requirements of the yeast translational machinery for cap-independent initiation of protein synthesis.
There are two modes of translation initiation: cap-dependent and cap-independent initiation of protein synthesis. Cap-dependent initiation relies on the presence of a 7-methyl guanosine moiety at the 5′ terminus of the mRNA. A protein complex, eIF4F, consisting of eukaryotic initiation factors 4E, 4G, and 4A recognizes this structure and recruits the 40 S ribosomal subunit. The 40 S subunit, in complex with associated factors, then scans the mRNA until it recognizes an AUG in the correct context for translation to begin (1). Unlike cap-dependent initiation, cap-independent or internal initiation does not require the presence of the m7G structure (1, 2). Instead, complex secondary structure elements, termed internal ribosome entry sites (IRESs),2 serve to recruit the ribosome for protein synthesis. Often proteins act in trans to either recruit the ribosome or reorganize the structure of the RNA for translation (2). Many cases of internal initiation have been documented in mammalian cells and viruses, but few have been described in the yeast Saccharomyces cerevisiae. Activity of viral IRES elements in yeast has not been obtained without perturbing general translation or using an in vitro system (3, 4). For the hepatitis C virus IRES, some sequence in addition to what has been defined as the minimal IRES seems to be required for activity in yeast (5). Gilbert et al. (6) recently reported the presence of several yeast IRES elements active under glucose starvation conditions. Several of these IRES elements depend on poly(A) stretches upstream of the internal AUG codon for activity. Furthermore, poly(A)-binding protein and one allele of eIF4G were implicated in the mechanism of internal initiation for these IRES elements. Although very insightful, this publication lacked a detailed structural characterization of any of the IRES elements identified.
Ure2p is a protein in yeast that is involved in nitrogen assimilation (7, 8). Two forms of Ure2p exist within the cell, and it has been shown that both originate from the same mRNA (9). The shorter version of the protein is an N-terminal truncation of the longer protein product. The longer form of the protein has been shown to contain an N-terminal prion-forming domain responsible for spontaneous aggregate formation. Interestingly, the short form of the protein results from internal initiation of translation (9). The IRES activity is evident in both rabbit reticulocyte lysate and translationally competent yeast lysates. Disappearance of the shorter form of the protein is observed upon mutation of the internal AUG to a non-start CTT codon. In an effort to define the functional relevance of eukaryotic initiation factor 2A, our laboratory investigated the role of this protein in internal initiation. As such, the activity of the URE2 IRES element is enhanced by ∼10-fold in the absence of the yeast homolog of eIF2A (10). This suppressive effect seems to result from trapping of the URE2 mRNA in 40 S and 80 S complexes as observed by gradient centrifugation, although the exact mechanism has not been determined. Understanding more of the details of this process will enable the use of genetic studies aimed at understanding internal initiation and the role of eIF2A in this process.
Considering the importance of RNA structures in cellular and viral internal initiation, the minimal number of nucleotides required for cap-independent initiation of protein synthesis was evaluated for the URE2 IRES. The minimal IRES element was determined using successive deletions of URE2 in combination with a galactose-inducible reporter harboring a stable stem loop structure that has been shown to block cap-dependent initiation of protein synthesis (11). Subsequently, secondary structure within the minimal URE2 IRES element was mapped using RNase and chemical probes. It is evident from these studies that the minimal URE2 IRES is relatively small and unstructured compared with most viral IRES elements. There also seems to be several cis-sequences that modulate levels of URE2 internal initiation. Assessing the activity of the URE2 IRES in both wild-type and eIF2A knock-out yeast strains did not reveal a region of the RNA that was necessary for the eIF2A-mediated effect. Taken together, we have determined the minimal URE2 IRES element to be a 104-nucleotide region encompassing the internal AUG, which is capable of forming a relatively unstable stem loop structure.
EXPERIMENTAL PROCEDURES
Yeast Strains and Culture Conditions—Yeast used for this study were of the BY4741 background (Research Genetics). For all experiments using yeast, wild-type BY4741 (MATa, his3-1, leu2-0, met15-0, ura3-0) and eukaryotic initiation factor 2A knock-out strains (MATa, his3-1, leu2-0, met15-0, ura3-0, ygr054::KanMX) were inoculated from fresh SD plates and grown overnight at 30 °C in 5 ml of SD medium (0.67% (w/v) yeast nitrogen base, 0.076 m (NH4)2 SO4, and 2% (w/v) agar) containing histidine, methionine, leucine, and 2% glucose. After 20-22 h of growth, yeast were spun down at 3000 rpm for 5 min and washed with 10 ml of sterile deionized water. Yeast were then resuspended in 5 ml of SD medium containing the aforementioned amino acids and 2% galactose for induction of mRNA expression. Yeast were incubated with galactose at 30 °C for 18-20 h before harvesting.
Yeast Plasmids and Cloning—The yeast plasmid p281-4, harboring a GAL1/10 promoter, a stable stem loop, and a β-galactosidase reporter gene, as described previously (11), was utilized for URE2 truncation analysis. Each truncation was amplified from pBIISK+(URE2) plasmid using PCR (Table 1) and subcloned into the XhoI and EcoRI sites of the p281-4 vector using standard cloning procedures. Approximately 400 ng of sequenced p281-4-URE2 plasmids was then transformed into both BY4741 and BY4741 ΔeIF2A yeast strains for β-galactosidase measurements using standard lithium acetate methods (12). For structure probing, NdeI and BamHI sites and the T7 promoter were added to URE2 during PCR and subcloned into the NdeI and BamHI sites of pUC19. For point mutants and deletions, mutations were generated using the pBIISK+(URE2) plasmid as a template in PCR with Pfu-turbo (Stratagene). PCR reactions were then digested for 1 h with DpnI and transformed directly into DH5α cells. Each clone was then sequenced for presence of the respective mutations. Following positive identification of mutant plasmids, mutant URE2 sequences were amplified and cloned into pUC19 as described above.
TABLE 1.
Primers used in this study
| Name | Descriptiona | Primer sequence |
|---|---|---|
| LR1 | URE2 290 rev into p281-4-3′ truncation | 5′-TTCCGGAATTCTGTGACTCATATCCGAAAATGC-3′ |
| LR2 | URE2 812 rev into p281-4-3′ truncation | 5′-TTCCGGAATTCTCAGCGCTTCTCTACGTTCAGC-3′ |
| LR3 | URE2 309 rev into p281-4-3′ truncation | 5′-TTCCGGAATTCTTGTAATTCTGGAATACTCCACGTG-3′ |
| LR4 | URE2 497 rev into p281-4-3′ truncation | 5′-TTCCGGAATTCTAACTCTTGCATTAGGGTTCAC-3′ |
| LR5 | URE2 fw into p281-4-3′ truncations | 5′-AAAAAACTCGAGAATAACAACGGCAACC-3′ |
| LR7 | URE2 98 fw into p281-4-5′ truncation | 5′-AAAAAACTCGAGGTAATATAAATTTTGAATTTTCAACAGG-3′ |
| LR8 | URE2 205 fw into p281-4-5′ truncation | 5′-AAAAAACTCGAGCAAAATAATGATAACGAGAATAATATCAAGAATACC-3′ |
| LR9 | URE2 246 fw into p281-4-5′ truncation | 5′-AAAAAACTCGAGCGACAACAACAACAGGCATTTTCG-3′ |
| LR10 | URE2 rev into p281-4-5′ truncations | 5′-TTCCGGAATTCTACCACGCAATGCCTTG-3′ |
| LR11 | RT-PCR LACZ fw | 5′-GCTGGCTGGAGTGCGATCTTCC-3′ |
| LR12 | RT-PCR LACZ rev | 5′-CCAGACGAAGCCGCCCTGTA-3′ |
| LR17 | RT-PCR PGK1 fw | 5′-CCTTCTTGAACGACTGTGTCGGTCC-3′ |
| LR18 | RT-PCR PGK1 rev | 5′-CATTGTCCAACCCTTGCCAGCC-3′ |
| LR27 | URE2 140 fw into p281-4-5′ truncation | 5′-AAAAAACTCGAGUAAUAACAAUAGCAGUAGUAAUAACAAUAAUGUUCAAAACA-3′ |
| LR28 | URE2 118 fw into p281-4-5′ truncation | 5′-AAAAAACTCGAGAAAACAAUAACAGCGGCCGCAA-3′ |
| LR29 | URE2 180 fw into p281-4-5′ truncation | 5′-AAAAAACTCGAGCAACAGGUGUAAAUAAUAAUAAUAAUAACAAUAGCAGUAGUAAU-3′ |
| LR31 | URE2 309 rev into pUC19 | 5′-TTAACGGGATCCTTGTAATTCTGGAATACTCCACGTG-3′ |
| LR34 | T7+URE2 98 fw into pUC19 | 5′-GGAATTCCATATGTAATACGACTCACTATAGGGAGGTAATATAAATTTTGAATTTTCAACAGG-3′ |
| LR35 | T7+URE2 205 fw into pUC19 | 5′-GGAATTCCATATGTAATACGACTCACTATAGGGAGCAAAATAATGATAACGAGAATAATATCAAGAATA CC-3′ |
| LR43 | URE2 A238G pBSKII rev mutagenesis | 5′-CTGTTGTTGTTGTCGATGTTGTTCTAAGGCATTCTTGA TATTATTCTCGTTATCATTATTTTGG-3′ |
| LR44 | URE2 A150U pBSKII fw mutagenesis | 5′-TTTTCAACAGGTGTAAATAATAATAATAATAACATTAGCAGTAGTAATAACAA TAATGTTCAAAACAATAACAGC-3′ |
| LR45 | URE2 A203C pBSKII fw mutagenesis | 5′-ATAACAGCGGCCGCAATGGTCGCCAAAATAATGATAACGAGAATAATATCAAGAATAC-3′ |
| LR46 | URE2 A203C pBSKII rev mutagenesis | 5′-GTATTCTTGATATTATTCTCGTTATCATTATTTTGGCGACCATTGCGGCCGCTGTTAT-3′ |
| LR47 | URE2 A238G pBSKII fw mutagenesis | 5′-GCCAAAATAATGATAACGAGAATAATATCAAGAATGCCTTGAGGCAACATCGACAACAACAA-3′ |
| LR79 | URE2 A150U pBSKII rev mutagenesis | 5′-GCTGTTATTGTTTTGAACATTATTGTTATTACTA CTGCTAATGTTATTATTATTATTATTTACACCTGTTGAAAA-3′ |
| LR109 | T7+URE2 205 A239G fw into pUC19 | 5′-GGAATTCCATATGTAATACGACTCACTATAGGGAGC AAAATAATGATAACGAGAATAATATCAAG-3′ |
| LR266 | URE2 fw into p281-4-Firefly | 5′-TCCTAGGATCCTAGGATCCTAGGATCTCCGCGGGCTCGAGAATAACAACGGCAACCAAGTGTCG-3′ |
| LR267 | URE2 309 rev into p281-4-Firefly | 5′-TCGTCACGACGTTGTAAAACGACGGGATCCCCGGGAATTCTTGTAATTCTGGAATACT CCACGTGACTCATATC-3′ |
| LR268 | URE2 497 rev into p281-4-Firefly | 5′-TCGTCACGACGTTGTAAAACGACGGGATCCCCGGGAATTCTAA CTCTTGCATTAGGGTTGACAGACACA-3′ |
| LR269 | URE2 812 rev into p281-4-Firefly | 5′-TCGTCACGACGTTGTAAAACGACGGGATCCCCGGGAA TTCTCAGCGCTTCTCTACGTTCAGCCA-3′ |
| LR278 | URE2 Full length rev into p281-4-Firefly | 5′-TCGTCACGACGTTGTAAAACGACGGGATCCCCGGGAATTCTACCACGCAATGCCTTG-3′ |
| LR271 | Firefly Luciferase rev into p281-4-Firefly | 5′-CTTACGCGAAATACGGGCAGACATGGCCTGCCCGGTTATTAT TACAATTTGGACTTTCCGCCCTTC-3′ |
| LR279 | Firefly Luciferase fw into p281-4-Firefly | 5′-CCCGTCGTTTTACAACGTCGTGACGAAGACGCCAAAAACATAAAGAAAGGC-3′ |
fw, forward; rev, reverse.
For p281-4-Firefly constructs, URE2 and firefly luciferase were amplified from pBIISK+(URE2) and pGL2-Basic plasmids, respectively, and gel-purified. Homologous ends were added to the primers for both the URE2 and luciferase reactions (Table 1). The p281-4 plasmid was digested with XhoI and BssHII, which cuts within the β-galactosidase coding region, and gel-purified. Approximately 60 ng of the firefly luciferase and URE2 truncation PCR products were then transformed with 36 ng of digested p281-4 plasmid into BY4741 yeast. Plasmids were recovered from the yeast and sequenced prior to conducting luciferase assays.
Reporter Assays—Spectrophotometric β-galactosidase assays were performed as described previously with the following modifications (9): two colonies were selected from each transformation and assayed in duplicate. In addition, optical densities at 420 and 600 nm were analyzed in flat, clear bottom 96-well plates (Corning) using a Spectramax M2 Plate Reader (Molecular Devices). These experiments were repeated at least three times.
Preparation of yeast cell lysates for firefly luciferase measurements was performed as described previously (13). Briefly, 1.5-ml culture was washed in phosphate-buffered saline and resuspended in 150 μl of reporter lysis buffer (Promega, Madison, WI). 50- to 100-μl glass beads were added to each sample and vortexed three times for 1 min. Cell debris were removed, and 20 μl of the supernatant was utilized for activity measurements. Two colonies were selected from each transformation and assayed in duplicate. Firefly luciferase activity was measured using an Lmax luminometer (Molecular Devices).
RT-PCR Analysis—Total RNA was isolated after induction with galactose for each construct using the MasterPure yeast RNA purification kit (Epicenter) and quantified by UV spectrophotometry. Subsequently, RNA was analyzed using primers that amplified a region of the mRNA encompassing a 1500-nucleotide region of LACZ with PGK1 as an internal control. RT-PCR was conducted using a one-step RT-PCR protocol in which an enzyme mix of Superscript II RT and Platinum Taq Polymerase (Invitrogen) were mixed to a final concentration of 12.5 units/μl and 2.5 units/μl, respectively, in dilution buffer (20 mm Tris-HCl, pH 7.5, 1 mm dithiothreitol, 0.01% Nonidet P-40, 0.1 mm EDTA, 0.1 m NaCl, and 50% glycerol). The reactions were then assembled in 2× reaction buffer (50 mm Tris-HCl, pH 8.35, 1 mm MgCl2, 60 mm KCl, and 0.002 mm dithiothreitol), 1 μl of enzyme mix, 50 pmol of each primer, and 100 ng of total RNA. Each RNA isolation was analyzed in triplicate with primers targeting LACZ and once with PGK1 primers (Table 1). Reactions containing LACZ or PGK1 primers were run for 25 or 20 cycles, respectively. After amplification, gel electrophoresis was conducted with 1% agarose gels to resolve the products and stained for 15 min in the presence of ethidium bromide followed by two washes in deionized water for 5 min. Data were quantified using ImageQuaNT 5.0. These experiments were repeated at least twice.
Structure Probing—RNA was in vitro transcribed using T7 RNA polymerase in the presence of 1 mm CTP, UTP, and ATP, 0.5 mm GTP, and 1.32 mm guanosine in a final volume of 100 μl for 1.5 h at 37 °C. The reaction was then phenol:chloroform-extracted and run through a Sephadex G-50 column to desalt the sample. RNA was precipitated with ethanol and resuspended in 11 μl of RNase-free H2O. T4 polynucleotide kinase was then used in the presence of 7000 Ci/mmol [γ-32P]ATP to end label the RNA in a final volume of 20μl for 45 min at 37 °C. 30μl of RNase-free H2O was then added to each reaction, and the reactions were extracted with phenol:chloroform. Extracted samples were desalted with a Sephadex G-50 column and run on a 7 m urea 6% 19:1 acrylamide:bisacrylamide gel. Bands corresponding to the correct molecular weight of RNA were then excised from the gel and eluted overnight in 520μl of ATE buffer (300 mm NaCH3CO2, pH 5.4, 10 mm Tris-HCl, pH 7.5, and 1 mm EDTA, pH 8.0) at 4 °C. 450 μl of ATE buffer was phenol:chloroform extracted, and the RNA was precipitated with ethanol in dry ice for 1 h. The precipitate was pelleted at 4 °C at 13,000 rpm for 15 min. The pellet was dissolved in 100 μl of ATE buffer and precipitated again in dry ice for 1 h followed by another spin at 4 °C at 13,000 rpm for 15 min. The pellet was then dried and dissolved in 50 μl of nuclease-free H2O. Radioactivity was then measured by scintillation spectroscopy, and RNA concentrations were determined using a spectrophotometer.
Prior to structure probing, RNA solutions were heated to 90 °C and quick cooled on ice. Labeled RNA (2 × 105 cpm) was then placed in reaction buffer (10 mm HEPES-KOH, pH 7.5, 100 mm KCl) without and with magnesium chloride (2.5 mm MgCl2) and allowed to fold at 30 °C for 10 min prior to adding RNases. After folding, 2-μl RNase dilutions of T1 (Ambion 1 unit/μl), and V1 (Pierce 911 units/ml or Ambion 100 units/ml) were added to the reaction, and the mixture was allowed to digest for exactly 1 min. Reactions were stopped with 3 μl of 5 mg/ml aurin tricarboxylic acid. In parallel, no RNase controls were conducted using 2 μl of RNase-free H2O instead of RNases. 1 μl of 0.5 m EDTA, pH 8.0, was added to each sample followed by 19 μl of 2× colorless urea loading dye. 7 μl of each reaction was then loaded onto 7 m urea 6% 19:1 acrylamide: bisacrylamide sequencing gels to resolve fragments. Data were then compared with RNA structures predicted with the MFOLD server (frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna-form1-2.3.cgi) at 30 °C, and areas of the structure that did not agree with the data were broken apart (14, 15).
Diethylpyrocarbonate (DEPC) probing was performed as described above but with the following modifications. Instead of adding RNases, 2 μl of DEPC (Acros Organics 97%) was added to reactions only in the presence of magnesium and mixed with a vortex mixer. In parallel, no DEPC reactions were conducted with RNase-free H2O. After a 10-min incubation at 30 °C, 1 μl of 10 mg/ml yeast tRNA was added to the reactions. RNA was then phenol:chloroform-extracted after adding 1 μl of 0.5 m EDTA, pH 8.0, and precipitated. RNA was then dissolved in 20 μl of aniline acetate solution (10 μl of aniline (Sigma 99%), 83 μl of RNase-free H2O, and 6 μl of glacial acetic acid). Reactions were incubated at 60 °C in the dark for 15 min followed by ethanol precipitation. Pellets were dissolved in 10 μl of 1× colorless urea loading dye, and 3 μl was utilized for gel electrophoresis. Each structure probing experiment was repeated at least twice.
RESULTS
The Coding Region 3′ of the Internal AUG Contains Multiple Regulatory Sequences—Upon identification of the first yeast IRES element, we sought to determine the minimal sequence within URE2 that maintains the ability to promote internal initiation. This was done using a plasmid that contains a GAL1/10 promoter and a stable hairpin upstream of LACZ (Fig. 1A). By inserting URE2 truncations in-frame between the stable stem loop and the LACZ coding region, the only protein produced results from internal initiation, as shown previously (9, 10). Thus, the use of in-frame truncations allowed us to elucidate the minimal IRES sequence by measuring β-galactosidase activity in yeast lysates. In parallel, the eIF2A-mediated suppression of internal initiation was examined by assaying each truncation in wild-type and ΔeIF2A yeast. Since a small number of other yeast IRES elements have been identified, and this is one of the few examples of an IRES residing within a coding region of a protein, there were not many common motifs or minimal IRES lengths that could be used for comparison. For that reason, we began by making truncations of regions 3′ to the internal AUG.
FIGURE 1.
URE2 truncations indicate enhancer and inhibitory sequences downstream of the internal AUG codon. A, β-galactosidase assays using the p281-4 reporter construct, which harbors a stable stem loop designed to inhibit cap-dependent scanning of the 40 S ribosomal subunit, were used to determine the importance of the deleted URE2 regions. Left, nucleotide numbers and schematic representations of the different URE2 mRNA truncations. Right, activity measurements of the respective URE2 mRNA truncations in both BY4741 (wild-type; gray bars) and BY4684 (ΔeIF2A; hatched bars) yeast cells. Activity measurements for each truncation containing a CTT mutation in place of the internal AUG are shown for wild-type (black bars) and ΔeIF2A cells (dotted bars). B, a new p281-4 construct (designated p281-4-Firefly) in which the LACZ gene from p281-4 was replaced by the firefly luciferase gene was created by homologous recombination. Luciferase activity was then measured for each 3′ truncation in wild-type and ΔeIF2A cells. β-Galactosidase (wild-type; gray bars and ΔeIF2A; black bars) and luciferase (wild-type; striped bars; ΔeIF2A, grid pattern) activity are plotted as percent of full-length URE2 measured in ΔeIF2A cells.
The results obtained revealed that there may be several elements that are capable of modulating internal initiation of URE2 in cis. For example, a putative enhancer element exists between nucleotides 812 and 1061 of the URE2 coding region. This enhancer sequence was identified because deletion of the region between 812 and 1061 results in low levels of β-galactosidase activity (Fig. 1A; compare 7-1061 to 7-812). Another potential cis element exists between nucleotides 309 and 497, which is capable of inhibiting URE2 IRES-mediated expression (Fig. 1A).
Because each truncation resulted in a different β-galactosidase fusion protein, it was possible that differences in the specific activity of β-galactosidase were responsible for the variations in activity observed (Fig. 1A). Therefore, we attempted to use Western blotting to determine the relative levels of the fusion proteins. As has been observed by others, we were unable to sensitively and reliably detect β-galactosidase fusion proteins from yeast cell extracts (16). We then attempted to generate constructs in which the β-galactosidase coding region was replaced with firefly luciferase for each 3′ URE2 truncation. The rationale for producing these constructs was that if the Ure2p fragment was affecting the specific activity of β-galactosidase, then it should not affect the specific activity of luciferase in the same way due to the structural differences between the two reporter proteins. When the luciferase activity obtained for each 3′ truncation was compared with the β-galactosidase activity, it was evident that there were no differences that could be attributed to changes in the specific activity of β-galactosidase (Fig. 1B). The luciferase activity for each truncation mirrored the activity obtained for each β-galactosidase measurement, suggesting that cis-acting sequences are responsible for modulating levels of internal initiation on the URE2 IRES.
Because no truncation resulted in activity in ΔeIF2A cells that was equal to the levels observed in wild-type cells, it was clear that there were no regions 3′ of the initiating AUG that were responsible for the eIF2A-mediated repression. Based on the data presented above showing that activity was maintained when the coding region was truncated at nucleotide 309, we will henceforth consider this the 3′ boundary of the URE2 IRES element.
For each truncation construct used in this study, the internal AUG was mutated to CTT to determine the possible contribution of initiation at alternative AUG codons to the observed activity. In each case, mutation of the internal AUG to CTT resulted in basal levels of internal initiation, suggesting that the contribution from other AUG codons is minimal (Fig. 1A). Although too little activity was observed to accurately assess the influence of eIF2A on expression from constructs containing the CTT mutation, most constructs displayed slightly greater activity in the eIF2A knock-out strain.
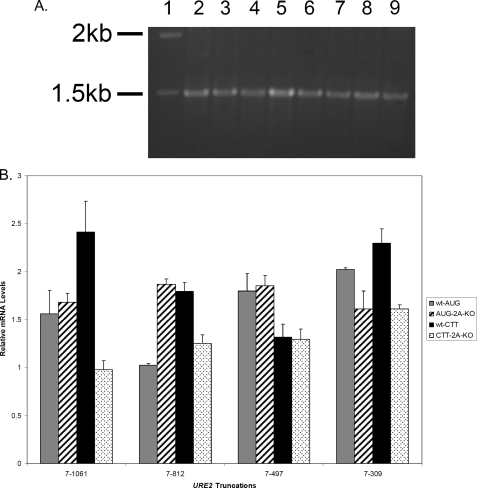
To evaluate the possibility that gross changes in RNA abundance resulted in changes in β-galactosidase activity, levels of LACZ reporter mRNA in each cell type were examined by RT-PCR. This was done using primers directed against the LACZ region that result in a 1500-bp fragment (Fig. 2A). These experiments were performed for each mRNA in both cell types and with the CTT mutants of each truncation. Small changes in RNA abundance may account for changes in β-galactosidase activity; therefore, we normalized each sample to levels of endogenous PGK1 mRNA (Fig. 2B). From these analyses, it is clear that the levels of LACZ mRNA do not change in such a way that can account for the observed levels of activity. In AUG-containing constructs, levels of mRNA are roughly equal in all cases examined (Fig. 2B). The only exception is for the RNA isolated from wild-type cells containing the 7-812 URE2 truncation, but this construct showed low levels of β-galactosidase activity in both the wild-type and ΔeIF2A cells (Fig. 2A).
FIGURE 2.
Abundance of mRNA is not responsible for the different levels of β-galactosidase activity observed in functional assays of 3′ truncations. RT-PCR analysis of levels of reporter mRNAs was conducted in both wild-type and ΔeIF2A knock-out cells using primers directed against the LACZ region of the mRNA. A, 1% agarose gel showing the results of RT-PCR analysis of AUG constructs in each cell type. The molecular weight standard (lane 1), full-length URE2 (lanes 2 and 3), URE2 nucleotides 7-812 (lanes 4 and 5), URE2 nucleotides 7-497 (lanes 6 and 7), and URE2 nucleotides 7-309 (lanes 8 and 9) are shown. Lanes 2, 4, 6, and 8 represent levels of mRNAs in wild-type cells, whereas lanes 3, 5, 7, and 9 represent levels of mRNA in eIF2A knock-out cells. B, bar graph showing levels of reporter mRNA relative to PGK1 mRNA for each truncation (with AUG and CTT at the position of the internal codon) in both cell types. Gray bars represent AUG-containing truncations in wild-type cells, hatched bars represent AUG-containing constructs in ΔeIF2A cells, black bars represent CTT-containing constructs in wild-type cells, and dotted bars represent relative mRNA levels of CTT-containing constructs in ΔeIF2A cells.
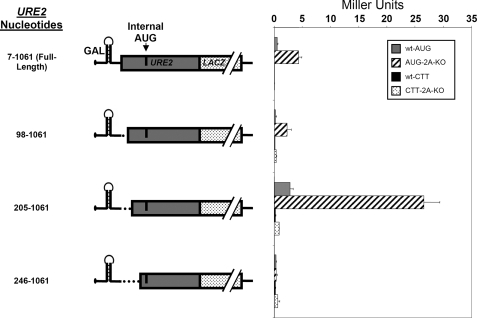
Upstream Sequences Are Capable of Modulating IRES-mediated Expression of Ure2p—To assess the 5′ border of the URE2 minimal IRES element, we made truncations upstream of the internal AUG codon similar to those made to determine the 3′ boundary in Fig. 1. As such, it became clear that an inhibitory element is located between nucleotides 98 and 205 (henceforth termed “inhibited IRES”; Fig. 3). Deletion of this inhibitory element results in an increase in internal initiation to levels ∼5-fold higher than the full-length URE2 coding region. Interestingly, none of the truncations resulted in a loss of eIF2A-mediated suppression, suggesting that the inhibitory effect is not due to direct binding of eIF2A to the mRNA in regions other than that corresponding to the minimal IRES element (Fig. 3). Another interesting feature is that truncations past nucleotide 205 resulted in a complete loss of internal initiation (Fig. 3: truncation at nucleotide 246). Therefore, from this point forward we will refer to nucleotide 205 as the 5′ boundary of the URE2 IRES.
FIGURE 3.
URE2 truncations indicate an inhibitory sequence upstream of the internal AUG codon. The p281-4 reporter, described above, was used to determine the importance of 5′ URE2 deletions. Left, nucleotide numbers and schematic representations of different URE2 truncations. Right, activity measurements of the respective URE2 truncations in both BY4741 (wild-type; gray bars) and BY4684 (ΔeIF2A; hatched bars) yeast cells. Activity measurements for each truncation containing a CTT mutation in place of the internal AUG are shown for wild-type (black bars) and ΔeIF2A cells (dotted bars).
As with the 3′ truncations, in each construct, the internal AUG was mutated to a CTT to exclude the possibility of alternative AUG codons acting as initiation codons. In each case where this mutation was made, levels of internal initiation dropped to basal levels. Therefore, the levels of internal initiation observed in Fig. 3 are due to authentic initiation events at the AUG located between nucleotides 280 and 282.
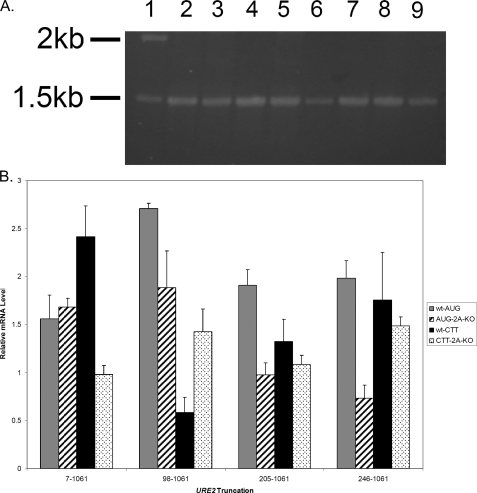
RNA analysis for each truncation was conducted as described above. Levels of mRNA in each construct are roughly equal (Fig. 4A), which suggests that changes in mRNA levels are not responsible for the changes in activity observed in experiments presented in Fig. 3. When the levels of reporter mRNA are normalized to a PGK1 control, it is clear that the RNA abundance is within 2-fold for all the AUG-containing constructs, and there is no correlation between those changes and changes in reporter activity (Fig. 4B). Furthermore, those that have higher levels of mRNA are in constructs that do not yield much reporter activity. Interestingly, in the 205-1061 URE2 truncation transformed into ΔeIF2A, levels of mRNA are the lowest among all the 5′ truncations, but yet the levels of activity are higher (Fig. 4B). This suggests a higher efficiency of translation initiation with this mRNA as compared with the other 5′ truncations.
FIGURE 4.
Abundance of mRNA is not responsible for the different levels of β-galactosidase activity observed in functional assays of 5′ truncations. RT-PCR analysis of levels of reporter mRNAs was conducted as described under “Experimental Procedures.” A, 1% agarose gel showing the results of RT-PCR analysis of AUG constructs in each cell type. The molecular weight standard (lane 1), full-length URE2 (lanes 2 and 3), URE2 nucleotides 98-1061 (lanes 4 and 5), URE2 nucleotides 205-1061 (lanes 6 and 7), and URE2 nucleotides 246-1061 (lanes 8 and 9) are shown. Lanes 2, 4, 6, and 8 represent levels of mRNAs in wild-type cells, whereas lanes 3, 5, 7, and 9 represent levels of mRNA in eIF2A knock-out cells. B, bar graph showing levels of reporter mRNA relative to PGK1 mRNA for each truncation (with AUG and CTT at the position of the internal codon) in both cell types. Gray bars represent AUG-containing truncations in wild-type cells, thatched bars represent AUG-containing constructs in ΔeIF2A cells, black bars represent CTT-containing constructs in wild-type cells, and dotted bars represent relative mRNA levels of CTT-containing constructs in ΔeIF2A cells.
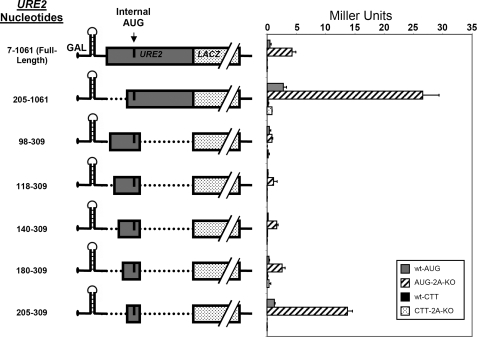
Internal Initiation on mRNAs with Combined Truncations Reflect Changes Observed with Single Truncations—Important 5′ truncations were combined with the 3′ truncation at nucleotide 309 to affirm that the minimal IRES could be isolated from the rest of the URE2 coding sequence without abrogating internal initiation. In other words, we sought to determine whether or not the putative minimal sequence could act independent of enhancer or inhibitory sequences located up- or downstream of nucleotides 205 or 309, respectively. It was evident that the levels of internal initiation observed in constructs containing truncations on both sides of the initiating AUG were representative of the levels observed when single truncations were analyzed. For example, the inhibitory element between 98 and 205 maintained the inhibitory effect when it was in the context of double truncations (Fig. 5; 98-309 truncation as compared with the 205-309 truncation). Additionally, losing the enhancer element located between nucleotides 812 and 1061 resulted in a partial loss of activity in the minimal IRES (Fig. 5; compare 205-1061 truncation with 205-309 truncation).
FIGURE 5.
The URE2 minimal IRES element is located between nucleotides 205 and 309, and the upstream inhibitory element can be defined to a 25-nucleotide region upstream of the minimal IRES. The p281-4 reporter, described above, was used to determine the importance of 5′ URE2 deletions. Left, nucleotide numbers and schematic representations of different URE2 truncations. Right, activity measurements of the respective URE2 truncations in both BY4741 (wild-type; gray bars) and BY4684 (ΔeIF2A; hatched bars) yeast cells. Activity measurements for each truncation containing a CTT mutation in place of the internal AUG are shown for wild-type (black bars) and ΔeIF2A cells (dotted bars).
In an effort to more precisely define the inhibitory element between nucleotides 98 and 205, further 5′ truncations were produced in combination with the 3′ IRES boundary at nucleotide 309. This analysis shows that the inhibitory effect can be reduced to a 25-nucleotide region between nucleotide 180 and 205. This element can be considered a bona fide regulatory sequence, because the Ure2p region of the fusion protein did not change in the constructs that led to the discovery of this element. The small size of this region in combination with secondary structure prediction data (see below) suggest that either a protein may bind to this region or a small structural element may interact with the minimal IRES to evoke an inhibitory effect. As with single truncations that suggest no region of the RNA is responsible for the eIF2A-mediated suppression, the increased activity of the minimal IRES in ΔeIF2A cells as compared with wild-type cells is not changed (Fig. 5).
When the AUG initiation codon is mutated to a CTT codon in constructs containing combined truncations, there is complete loss of the ability to internally initiate translation. This suggests that there is no change in cis sequences at the junction between the URE2 and LACZ that promotes initiation at alterative AUG codons. RNA analysis of these constructs supports the conclusion that gross changes in RNA abundance are not responsible for changes in internal initiation, because there are no substantial changes in mRNA levels for AUG-containing constructs that would explain the trend of activity observed in functional assays (Fig. 6A). This result was expected because the β-galactosidase activity for combined truncations mimicked the trends observed with single truncations, and the RNA analysis for single truncations indicated that RNA abundance was not responsible for the different levels of activity (Figs. 2 and 4). Normalizing the levels of LACZ-containing mRNA to levels of PGK1 mRNA supported the idea that the changes in steady-state RNA levels are not responsible for changes in reporter activity (Fig. 6B). At most there are 3-fold differences in RNA levels, but there was no correlation between mRNA abundance and relative activity between constructs. In fact, several samples showed higher levels of mRNA in AUG-containing constructs (such as the 118-309 URE2 truncation in wild-type and ΔeIF2A cells), but most of these constructs showed little or no activity in functional assays. In contrast, the clones containing the 205-309 URE2 truncation, which we define as the minimal IRES element, have levels of mRNA comparable with mRNA levels expressed from the construct containing nucleotides 7-1061 of URE2. This illustrates that the 205-309 and 7-1061 URE2 constructs can be compared directly for the ability to direct internal initiation.
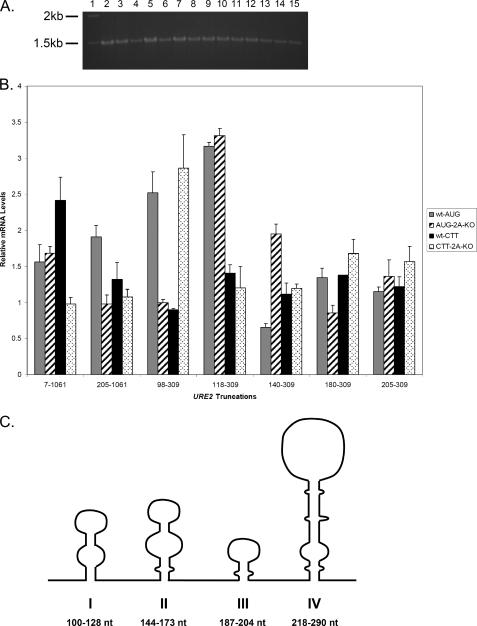
FIGURE 6.
Abundance of mRNA is not responsible for levels of β-galactosidase observed in functional assays of combined truncations. RT-PCR analysis of levels of reporter mRNAs was conducted as described under “Experimental Procedures.” A, 1% agarose gel showing the results of RT-PCR analysis of AUG constructs in each cell type. The molecular weight standard (lane 1), full-length URE2 (lanes 2 and 3), URE2 nucleotides 205-1061 (lanes 4 and 5), URE2 nucleotides 98-309 (lanes 6 and 7), URE2 nucleotides 118-309 (lanes 8 and 9), URE2 nucleotides 140-309 (lanes 10 and 11), URE2 nucleotides 180-309 (lanes 12 and 13), and URE2 nucleotides 205-309 (lanes 14 and 15) are shown. Lanes 2, 4, 6, 8, 10, 12, and 14 represent levels of mRNAs in wild-type cells, whereas lanes 3, 5, 7, 9, 11, 13, and 15 represent levels of mRNA in eIF2A knock-out cells. B, bar graph showing levels of reporter mRNA relative to PGK1 for each truncation (with AUG and CTT at the position of the internal codon) in both cell types. Gray bars represent AUG-containing truncations in wild-type cells, thatched bars represent AUG-containing constructs in ΔeIF2A cells, black bars represent CTT-containing constructs in wild-type cells, and dotted bars represent relative mRNA levels of CTT-containing constructs in ΔeIF2A cells. C, schematic representation of the inhibited IRES secondary structure as depicted by the MFOLD server. Each Roman numeral corresponds to the stem loop depicted directly above it.
Secondary structure predictions of the inhibited IRES element using the MFOLD server indicate that four stem loop structures can form within this region of the RNA (Fig. 6C) (14, 15). We have labeled each stem I-IV in consecutive order, with stem I being at the most 5′-end of the RNA. Stem loop IV corresponds to the region defined as the minimal IRES, which is capable of efficiently promoting internal initiation.
Structure Probing Analysis of the URE2 Minimal IRES Reveals a Stem Loop Structure That Contains the Initiator AUG—After defining the minimal sequence responsible for internal initiation, it was important to understand what structural elements exist within that region. Therefore, we tested the reliability of the secondary structure prediction generated with MFOLD (Fig. 6C). RNase T1, RNase V1, and DEPC were used to analyze the structure within the URE2 IRES RNA. RNase T1 is a small enzyme (∼13 kDa) with specificity for single stranded guanosine residues. RNase V1 is another small enzyme (∼10 kDa) capable of cleaving nucleotides participating in hydrogen-bonding interactions, and can less efficiently react with stacked single stranded nucleotides. DEPC is a small molecule probe (∼300 Da) that modifies the N7 position of single-stranded adenosine residues (17). DEPC is a very specific probe that is used because of its small size, which typically results in higher resolution in the mapping of single-stranded regions. Considering the URE2 IRES element is ∼43% adenosine residues, DEPC was a useful tool in elucidating regions with single-stranded character.
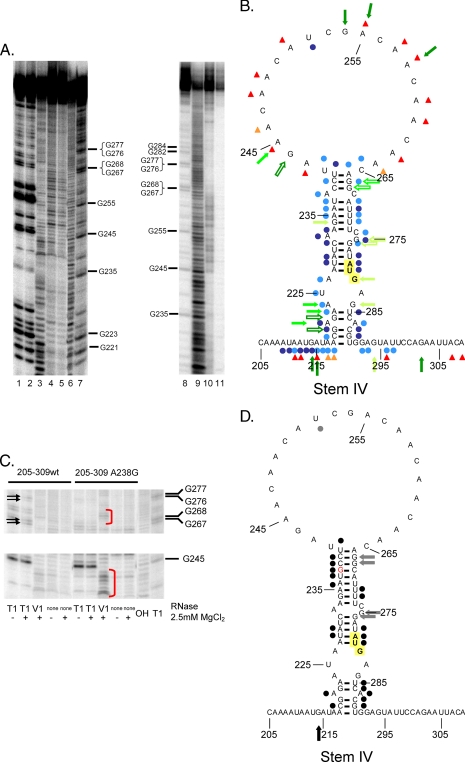
After comparing the structure probing data to structure predictions obtained using the MFOLD server, it was clear that the minimal IRES element folds into the predicted stem loop structure (stem IV), and the AUG is involved in base-pairing interactions (Fig. 7, A and B). Another interesting feature of the IRES is that there is a relatively large apical loop that would be predicted to destabilize stem IV (Fig. 7B) (18).
FIGURE 7.
The minimal IRES element contains a stem loop structure that directs internal initiation. A, 6% acrylamide/7 m urea gel analysis of RNase T1 (lanes 1 and 2), RNase V1 (lane 3), and DEPC-mediated strand scission analysis (lane 10). No RNase (lanes 4 and 5) and no DEPC reactions (lane 11) are shown. Lanes 2, 3, 5, 10, and 11 contain reactions conducted in the presence of 2.5 mm MgCl2. Additionally, base hydrolysis (lanes 6 and 9) and RNase T1 ladders (lanes 7 and 8) are shown. B, a composite structure that highlights the location of cleavages resulting from different probes is shown. Green arrows signify locations of RNase T1 cleavages, blue dots signify locations of RNase V1 cleavages, and red triangles show locations of DEPC-mediated cleavages. Lower efficiency cleavages are denoted by light-colored shapes. Outlined shapes show locations of Mg2+-dependent changes in cleavage efficiency. The internal AUG codon is transposed on a yellow background. C, parallel comparison of minimal IRES RNase probing and an A238G point mutant within the minimal IRES (stem IV). Arrows illustrate that RNase T1 cleavage efficiency at nucleotides 267, 268, 276, and 277 are higher in the wild-type minimal IRES sequence, whereas red brackets illustrate increased RNase V1 cleavages at nucleotides 237-243 in the A238G mutant of the minimal IRES. D, a composite structure that highlights the differences in cleavage efficiency for RNases T1 (arrows) and RNase V1 (dots) between the wild-type and A238G mutant form of the URE2 minimal IRES. Black shapes illustrate increased cleavage in the mutant as compared with the wild-type, while gray shapes illustrate decreased cleavages in the mutant as compared with the wild-type. There were no obvious differences between the wild-type and mutant with DEPC probing. The internal AUG is highlighted in yellow, and the mutated nucleotide is denoted in red.
Whereas some of the data seemed to clearly support the structure predicted from MFOLD (i.e. DEPC and T1), we were not as confident that the RNase V1 data supported that prediction because of the low intensity of V1 cleavage. Therefore, we made a single point mutation (A238G) in the minimal IRES element (stem IV). The rationale for making this mutation was that, if the MFOLD prediction was correct, the mutation would stabilize the helix in a predictable way that would allow us to more accurately assign cleavage events in the wild-type situation. If the MFOLD prediction was not correct, the mutation would be expected to disrupt or alter the structure that was forming and presumably change the digestion pattern that we observe in an unpredictable way. After probing the mutant minimal IRES element, we determined that the digestion pattern was changing in a way that indicated the MFOLD prediction was correct. For example, RNase V1 was able to cleave much more efficiently between nucleotides 236 and 241, whereas RNase T1 was able to cleave less efficiently at guanines 267-268 and 276-277 in stem IV (Fig. 7C). Consistent with the idea that the IRES was stabilized in a predictable way, guanine residue 214 was more efficiently cleaved by RNase T1, which suggests there were less options available to the enzyme in the mutant situation as compared with the wild-type IRES (Fig. 7D). Another feature that is consistent with the formation of the stem is that DEPC probing did not seem to change upon stabilization of stem IV with the A238G mutation (Fig. 7D). Furthermore, we did not detect the presence of tertiary interactions in these experiments. One region of the minimal IRES (nucleotides 209-217) was efficiently cleaved by RNase V1 (Fig. 7, A and B). However, sequence alignment of this region with other regions within the minimal IRES element did not predict the formation of base pairing indicative of tertiary interactions (data not shown). As mentioned above, it is known that RNase V1 can cleave nucleotides in stacked single-stranded regions. Due to the high frequency of adenosines in this region, RNase V1 may be reacting with stacked residues.
The Inhibitory Element between Nucleotides 98 and 205 Does Not Change the Structure of the Minimal IRES Element—We have shown that the region directly upstream of the minimal IRES element is capable of repressing the URE2 IRES activity. Therefore, we addressed the question of whether or not there was a change in the structure of the minimal IRES element (stem IV) in the presence of stems I-III that might lead to the inhibitory effect. To examine this possibility, we probed the structure of the inhibited IRES in the same way that we did for the minimal IRES described above. Interestingly, the structure of the minimal element did not change in the presence of the upstream inhibitory sequence (Fig. 8, A and B). Considering our data that further define the inhibitory effect to the region between nucleotides 180 and 205, it is interesting to note that stem loop III exists within this region of the RNA and may suppress IRES activity (Fig. 8B).
FIGURE 8.
The inhibited IRES element does not significantly differ in secondary structure in the region of the minimal IRES element. A, 6% acrylamide/7 m urea gel analysis of base hydrolysis (lanes 2 and 9) and RNase T1 ladders (lanes 1 and 8) are shown. RNase T1 (lanes 6 and 7), RNase V1 (lane 5), and DEPC-mediated strand scission analysis (lane 10). No RNase (lanes 3 and 4) and no DEPC reactions (lane 11) are shown. Lanes 3, 5, 6, 10, and 11 contain reactions conducted in the presence of 2.5 mm MgCl2. B, a composite structure that highlights the location of cleavages resulting from different probes is shown. Color and shape identities corresponding to intensity and probes are as depicted in Fig. 7B. The internal AUG codon is highlighted in yellow. C, parallel comparison of inhibited IRES RNase probing and the A148U/A202C/A238G triple mutant. Arrows illustrate increased RNase T1 cleavage efficiency in the wild type as compared with the mutant, which occur at nucleotides 153 and 156 and at nucleotide 255 in stem IV that corresponds to the minimal IRES region. Red brackets illustrate increased RNase V1 cleavages at nucleotides 147-151 in the triple mutant of the inhibited IRES. D, a composite structure that highlights the differences in cleavage efficiency for between the wild-type and A148U/A202C/A238G triple mutant form of the URE2-inhibited IRES. Shapes coincide with the labeling depicted in Fig. 7D, except that triangles are added to denote changes in DEPC modification in the mutant as compared with the wild type. Shape colors are as depicted in Fig. 7D where black indicates increased cleavage efficiency and gray indicates decreased cleavage efficiency in the mutant relative to the wild type. The internal AUG is highlighted in yellow, and mutated nucleotides are shown in red letters.
As with the minimal IRES element, we decided to investigate the validity of the structures by determining if introduction of point mutations would change the structure in a predictable way. Therefore, we introduced three point mutations (A148U in stem II, A202C in stem III, and A238G in stem IV) in the inhibited IRES. These mutations were selected to stabilize each of the stems predicted to form by MFOLD, with the exception of the most 5′ stem. The mutation in stem IV enabled RNase V1 to cleave more efficiently in the region of the minimal IRES element between nucleotides 230-240 (Fig. 8C). Concordantly, RNase T1 was able to cleave less efficiently at guanosine residues 267-268 and 275-276, which is the same result observed with the isolated minimal IRES element (stem IV). These data help to support the structure of the minimal IRES, because the enzymes responded in the same way with the inhibited IRES as they did with the minimal IRES element. Unlike the situation with the isolated minimal IRES element, a change in DEPC scission at several nucleotides was observed in the triple mutant of the inhibited IRES. Specifically, several nucleotides in the region of stem IV were cleaved less efficiently in the mutant as compared with the wild type (Fig. 8D).
There are also a number of nucleotides in stem loop II that were cleaved less efficiently with DEPC in the mutant (Fig. 8C). These data were very useful in elucidating the structure of the region located between nucleotides 100-128 and 144-173 (corresponding to the regions of stem I and II). In the wild-type inhibited IRES, the data suggest these regions are flexible because they are able to be modified with DEPC and cleaved by RNase V1 (Fig. 8B). However, in the mutant inhibited IRES, they more exclusively form the stem loops predicted with MFOLD. In contrast, the region corresponding to stem III, which has been shown to be responsible for the inhibitory effect, is capable of forming the predicted helical structure. Although introduction of a stabilizing mutation in stem III is evident by structure probing (Fig. 8D), it did not change the pattern of nuclease digestion in the region of stem III like it did with stem II. This suggests that stem loop III is forming in this region of the RNA in the wild-type inhibited IRES, although the importance of this structure has not been further tested. We were unable to find evidence of tertiary interactions with this region of the RNA that may have been responsible for the inhibitory effect. The lack of significant change in secondary structure in stem IV in the presence of the inhibitory region and the lack of efficient cleavages by V1 in the loops of stems III and IV provides confidence that there are no tertiary interactions between the inhibitory region and the minimal IRES detectable in these structure probing analyses.
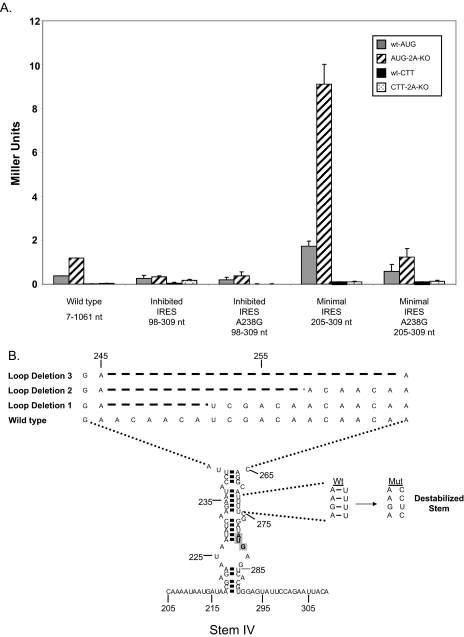
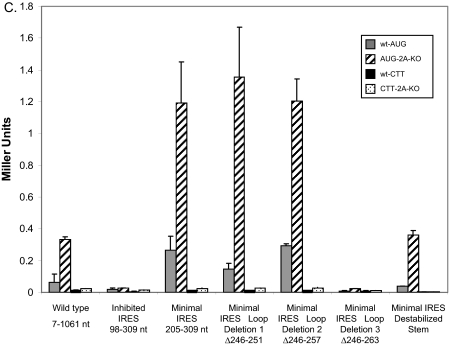
Mutations That Alter the Structure or Stability of the Stem Region Result in Reduced Levels of Internal Initiation—Because it was determined that the URE2 minimal IRES element (stem IV) can fold into a small stem loop structure, we were interested in knowing the role of the stability of the stem on internal initiation. Furthermore, we wanted to know the gross effect of different regions of the minimal IRES element on cap-independent translation. To address the question of stem stability the A238G mutation that was used in structure probing analyses was employed. The activity of this mutation in the monocistronic reporter construct described above (Fig. 1) showed a reduction in internal initiation by ∼5-fold (Fig. 9A). However, eIF2A-mediated repression was still present. To address the question of the role of different regions of the IRES element on internal initiation, several mutations and deletions in different regions that abrogated those structures were utilized. The two regions we focused on were the apical loop and the stem structure located directly below the large apical loop (Fig. 9B). For the apical loop, three consecutive deletions of six nucleotides were produced and assayed for β-galactosidase activity (Fig. 9B). Deleting either 6 or 12 nucleotides from the loop region did not change activity of the minimal IRES. However, deletion of 18 nucleotides from the loop caused a complete loss of activity. This loss is on the order of that observed when the internal AUG codon is mutated to CTT (Fig. 9C). Furthermore, destabilizing the double-stranded region by perturbation of 3-bp interactions resulted in a reduction in internal initiation by roughly 3-fold (Fig. 9C). In these cases, the suppressive effect of eIF2A was observed.
FIGURE 9.
Effects of apical loop deletions and reductions in stem stability on internal initiation on the URE2 IRES. A, the monocistronic β-galactosidase reporter plasmid, p281-4 described above, was used to assess the effect of the A238G mutation on the inhibited IRES element and the minimal IRES (stem IV). Bar patterns and shades correspond to the yeast strains and constructs described in Figs. 1, 2, 3, 4, 5 and 6. B, schematic representation of three loop deletions and three mutations (U271/272/274C) within the double-stranded region of stem IV. Dashed lines represent nucleotides that were deleted relative to wild-type within the loop region. The expected changes in base pairing potential upon introduction of three point mutations in the helix are shown to the right of the structure. The internal AUG codon is highlighted in gray. C, activity measurements for the apical loop deletion and mutations in stem IV shown in B using the p281-4 reporter construct. Bar patterns and shades correspond to yeast strains and constructs described above.
DISCUSSION
We have shown that the minimal IRES element for the URE2 IRES is located between nucleotides 205 and 309 using a reporter assay designed to minimize the effect of cap-dependent initiation of translation. In these experiments, several controls were utilized to affirm that changes in activity measurements were resulting from differences in translation initiation. First, the internal AUG was mutated to CTT for each of the constructs to confirm that activity was not resulting from initiation at alternative AUG codons. Second, mRNA abundance was measured using RT-PCR to be sure that gross changes in levels of mRNA were not the cause of changes in IRES-mediated expression. Finally, primers were designed in such a way that the codon at the junction between the URE2 sequence and the start of LACZ did not change between URE2 truncations, which eliminates the possibility of rare codons causing ribosome stalling and artificially low levels of β-galactosidase activity.
To date, IRES elements have been reported in cellular yeast mRNAs encoding Hap4p, Yap1p, the yeast homolog for TFIID (Taf145p), and one of the two alleles for eukaryotic initiation factor 4G (eIF4G encoded by TIF4631) (4, 13). Originally, the HAP4, YAP1, and TAF145 elements were identified based on the ability of translationally competent yeast extracts to conduct internal initiation on these mRNAs. Since the identification of these elements, they have all been shown to be active in living cells (13, 19, 20). Of the aforementioned list of yeast cellular IRES elements, only the TIF4631 IRES has been defined by deletion analysis, but no structural data exists for the minimal IRES element (13). We consider comparisons with the HAP4, YAP1, TAF145, and TIF4631 IRES elements difficult in light of evidence suggesting that each contains cryptic promoter activity that may have been responsible for the internal initiation observed in the original papers that described these IRES elements (21-23). However, it is possible to make comparisons between the URE2 IRES and IRES elements in other eukaryotic systems.
The URE2 IRES element is 104 nucleotides long, which is in the range of IRES element sizes previously observed. Some IRES elements have been shown to be as small as 22 nucleotides (24). There is also a report of an element that interacts with ribosomal RNA to recruit the ribosome which is only 9 nucleotides in length (25). On the other end of the spectrum, the minimal sequence for functionality of some IRES elements have been reported to be larger than 300 nucleotides. For example, viral IRES elements like hepatitis C virus and encephalomyocarditis virus are on the order of 340 and 430 nucleotides, respectively, and the c-myc cellular IRES is almost 400 nucleotides (26-28). In terms of stability, the URE2 IRES element has a ΔG of approximately -15 kcal/mol at 30 °C, which indicates it is much less stable than most viral and cellular IRES elements (27, 28). The low level of stability of the URE2 IRES may be an important feature of this IRES, because the AUG is buried, so stem IV may need to be melted for efficient initiation of translation.
At the start of these studies, there were no cellular examples of the format of the URE2 IRES, wherein the minimal sequence is located within the coding region of a protein. Interestingly, a cellular IRES that resembles the format of the URE2 IRES has been identified in the p53 mRNA, but it is unknown whether there are sequences up- or downstream of the internal AUG that are capable of modulating internal initiation (29). This IRES element has a much higher predicted stability as compared with the URE2 IRES (-92 versus -15 kcal/mol, respectively), and none of the predicted structures within this region resemble the URE2 IRES. Furthermore, the p53 IRES is regulated in a cell cycle-dependent manner. Although cell cycle-dependent regulation of the URE2 IRES cannot be excluded, it is more likely that this IRES is regulated in response to nutritional cues because of the role of Ure2p in nitrogen assimilation.
The importance of sequences within the coding region capable of modulating IRES activity is a novel and interesting feature of the URE2 IRES element. There have been reports of viral IRES elements in Giardiavirus and human immunodeficiency virus type 2 that utilize sequences downstream of the internal AUG to direct cap-independent initiation (30, 31). However, to our knowledge, there are no cellular IRES elements that utilize downstream elements to modulate levels of internal initiation. Usually downstream sequences are not examined, because the IRES element is located in the 5′-untranslated region of the mRNA. The fact that the URE2 IRES element resides in the coding region of full-length Ure2p mRNA triggered the investigation into the role of downstream sequences in controlling internal initiation. These experiments suggest that there may be downstream sequences that are capable of modulating internal initiation of many IRES elements. We were able to verify the possible contribution of these regions of RNA by replacing the β-galactosidase coding region with firefly luciferase. This allowed us to show that the different Ure2p fragments were not likely responsible for differentially altering the specific activity of β-galactosidase protein. Therefore, the observed changes in activity are reflective of cis-acting sequences within the URE2 mRNA. Given the magnitude of the effect imparted by the enhancer sequence located between nucleotides 812 and 1061 of the URE2 IRES, downstream regulatory elements could have very profound effects on the activity of other IRES elements.
Examination of the different truncations and mutations in both wild-type and eIF2A knock-out yeast did not reveal a region of the RNA that is responsible for the eIF2A-mediated effect. Interestingly, the invasive growth IRES elements described by Gilbert (6) are also responsive to eIF2A.3 By sequence comparison of URE2 with the invasive growth IRES elements we were unable to identify any common cis-sequence that may be responsible for the eIF2A-mediated effect. Together, this suggests that eIF2A acts on the pathway of internal initiation in a general manner rather than through specific cis-elements located within the mRNA. Additionally, it alludes to the possibility of other proteins that act on the pathway in response to environmental conditions to regulate internal initiation of specific subsets of mRNAs. This would allow for the cell to differentially regulate expression from IRES elements in response to different environmental stimuli. It would be of interest to investigate the role of proteins that bind the URE2 IRES in cap-independent translation.
The structural determination of the URE2 IRES element reveals some striking characteristics. The presence of base pairing interactions between nucleotides within the internal AUG suggests that the unwinding of this structure, at least in part, is an important step in internal initiation. If the structure were not at least partly unwound, the tRNA anticodon would not be able to recognize the start site because of its involvement in base pairing interactions. To our knowledge, this is a unique aspect of the URE2 IRES element. Probing experiments of Apaf-1, Baf-1, Cat-1, and c-myc IRES elements suggest that the internal AUG codons are not imbedded in higher order RNA structures (27, 32-34). However, in those experiments, the possibility of a buried initiation codon may not have been apparent, because the internal AUG was at the 3′ terminus of the synthetic RNA being probed. There may be sequence downstream of the initiating AUG codon that results in the formation of secondary structure around the internal AUG for the Apaf-1, Baf-1, Cat-1, and c-myc IRES elements. Interestingly, there were no obvious changes in the structure of the minimal IRES region when the sequence was probed in the presence of the inhibitory sequence immediately upstream. In fact, no tertiary interactions were detectable when the URE2 minimal IRES element was probed either in the presence or absence of the inhibitory sequence. The small stem loop region between nucleotides 180-205 may play a role in the inhibitory effect by interacting with a protein that is capable of rearranging the RNA into an inactive form of the IRES or by altering the positioning of the initiating AUG on the 40 S subunit (i.e. perhaps not directly to the P-site).
Another feature of the minimal URE2 IRES is the presence of CAA repeats in the apical loop region of the minimal IRES. Phylogenetic data shows that the URE2 gene is well conserved among different Saccharomyces species. The variation that does exist is primarily in the wobble position of the amino acid codons suggesting the importance of the amino acid sequence of Ure2p. Variable wobble nucleotides are not structurally informative, because many changes do not drastically alter the base pairing potential of the IRES. The single instance of covariation occurs between nucleotide 237 and 270, which is a U:A in S. cerevisiae and switches between C:G and G:C depending on the species. Because phylogenetic data tell little about key nucleotides, the functional analysis of loop deletions was important for understanding the relevance of the CAA loop repeats in efficiency of internal initiation. We show that deletion of up to 12 nucleotides from the loop of stem IV does not result in a reduction in activity, suggesting the CAA repeats are not important for IRES activity. Furthermore, this implies that the mechanism for internal initiation is different from the invasive growth IRES elements that depend on A-rich stretches and poly(A)-binding protein for activity (6). However, point mutations were not tested to rule out the possibility of poly(A)-binding protein dependence. The result with the 18-nucleotide deletion raises the possibility that stabilization of the stem region and inability to expose the buried AUG for initiator tRNA recognition causes reductions in IRES-mediated initiation on URE2. It is known that the stability of a stem depends on the size of the loop. Optimal loop sizes have been reported to be between 4 and 7 nucleotides (18). Thus, we tentatively conclude that the key feature is more likely to be the size of the loop than the richness in adenosine nucleotides.
The functional results of the A238G mutation further implicate stem stability in IRES activity and suggest that the stem must be unwound for efficient initiation of translation. As such, we would hypothesize that the three mutations that destabilize stem IV might have resulted in an increase in activity. Instead, a decrease in activity relative to the wild-type sequence was observed suggesting that optimal stem stability has been selected naturally in yeast. The MFOLD stability prediction supports this hypothesis, because the predicted stabilities of the wild-type and triple stem mutants are -15.69 and -12.93 kcal/mol, respectively. However, the possibility that binding sites for proteins important for internal initiation are being altered in the presence of these mutations cannot be excluded. In future studies it will be important to distinguish between these possibilities with more point mutations and compensatory mutations.
In conclusion, the elucidation of the sequence and structure of the first yeast IRES element is an important step in fully understanding the mechanism of internal initiation in yeast. Understanding cellular IRES elements in yeast may allow for genetic approaches to elucidate the fine points of the mechanism of IRES-mediated translation. It will be of interest to conduct a more in-depth study of the functional relevance of the structure and different nucleotides within the minimal IRES element. Discerning the functional role of different nucleotides may allow for the identification of other cellular IRES elements using a comparative analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants GM-68079, GM-62853, and T32 GM-08056. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IRES, internal ribosome entry site; DEPC, diethylpyrocarbonate; RT, reverse transcription.
W. Gilbert, personal communication.
References
- 1.Merrick, W. C. (2004) Gene (Amst.) 332 1-11 [DOI] [PubMed] [Google Scholar]
- 2.Komar, A. A., and Hatzoglou, M. (2005) J. Biol. Chem. 280 23425-23428 [DOI] [PubMed] [Google Scholar]
- 3.Thompson, S. R., Gulyas, K. D., and Sarnow, P. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12972-12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iizuka, N., Najita, L., Franzusoff, A., and Sarnow, P. (1994) Mol. Cell Biol. 14 7322-7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld, A. B., and Racaniello, V. R. (2005) J. Virol. 79 10126-10137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert, W. V., Zhou, K., Butler, T. K., and Doudna, J. A. (2007) Science 317 1224-1227 [DOI] [PubMed] [Google Scholar]
- 7.Cooper, T. G. (2002) FEMS Microbiol. Rev. 26 223-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofman-Bang, J. (1999) Mol. Biotechnol. 12 35-73 [DOI] [PubMed] [Google Scholar]
- 9.Komar, A. A., Lesnik, T., Cullin, C., Merrick, W. C., Trachsel, H., and Altmann, M. (2003) EMBO J. 22 1199-1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komar, A. A., Gross, S. R., Barth-Baus, D., Strachan, R., Hensold, J. O., Goss Kinzy, T., and Merrick, W. C. (2005) J. Biol. Chem. 280 15601-15611 [DOI] [PubMed] [Google Scholar]
- 11.Altmann, M., Muller, P. P., Wittmer, B., Ruchti, F., Lanker, S., and Trachsel, H. (1993) EMBO J. 12 3997-4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito, H., Fukuda, Y., Murata, K., and Kimura, A. (1983) J. Bacteriol. 153 163-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou, W., Edelman, G. M., and Mauro, V. P. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 1531-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuker, M. (2003) Nucleic Acids Res. 31 3406-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews, D. H., Sabina, J., Zuker, M., and Turner, D. H. (1999) J. Mol. Biol. 288 911-940 [DOI] [PubMed] [Google Scholar]
- 16.Silver, P. A., Keegan, L. P., and Ptashne, M. (1984) Proc. Natl. Acad. Sci. U. S. A. 81 5951-5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehresmann, C., Baudin, F., Mougel, M., Romby, P., Ebel, J. P., and Ehresmann, B. (1987) Nucleic Acids Res. 15 9109-9128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groebe, D. R., and Uhlenbeck, O. C. (1988) Nucleic Acids Res. 16 11725-11735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raychaudhuri, S., Fontanes, V., Banerjee, R., Bernavichute, Y., and Dasgupta, A. (2006) Biochem. Biophys. Res. Commun. 350 788-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seino, A., Yanagida, Y., Aizawa, M., and Kobatake, E. (2005) Biochim. Biophys. Acta 1681 166-174 [DOI] [PubMed] [Google Scholar]
- 21.Hecht, K., Bailey, J. E., and Minas, W. (2002) FEMS Yeast. Res. 2 215-224 [DOI] [PubMed] [Google Scholar]
- 22.Verge, V., Vonlanthen, M., Masson, J. M., Trachsel, H., and Altmann, M. (2004) RNA (N. Y.) 10 277-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han, B., and Zhang, J. T. (2002) Mol. Cell Biol. 22 7372-7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chappell, S. A., and Mauro, V. P. (2003) J. Biol. Chem. 278 33793-33800 [DOI] [PubMed] [Google Scholar]
- 25.Chappell, S. A., Edelman, G. M., and Mauro, V. P. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 9590-9594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieft, J. S., Zhou, K., Jubin, R., Murray, M. G., Lau, J. Y., and Doudna, J. A. (1999) J. Mol. Biol. 292 513-529 [DOI] [PubMed] [Google Scholar]
- 27.Le Quesne, J. P., Stoneley, M., Fraser, G. A., and Willis, A. E. (2001) J. Mol. Biol. 310 111-126 [DOI] [PubMed] [Google Scholar]
- 28.Pilipenko, E. V., Blinov, V. M., Chernov, B. K., Dmitrieva, T. M., and Agol, V. I. (1989) Nucleic Acids Res. 17 5701-5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray, P. S., Grover, R., and Das, S. (2006) EMBO Rep. 7 404-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garlapati, S., and Wang, C. C. (2004) J. Biol. Chem. 279 3389-3397 [DOI] [PubMed] [Google Scholar]
- 31.Herbreteau, C. H., Weill, L., Decimo, D., Prevot, D., Darlix, J. L., Sargueil, B., and Ohlmann, T. (2005) Nat. Struct. Mol. Biol. 12 1001-1007 [DOI] [PubMed] [Google Scholar]
- 32.Pickering, B. M., Mitchell, S. A., Spriggs, K. A., Stoneley, M., and Willis, A. E. (2004) Mol. Cell Biol. 24 5595-5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaman, I., Fernandez, J., Liu, H., Caprara, M., Komar, A. A., Koromilas, A. E., Zhou, L., Snider, M. D., Scheuner, D., Kaufman, R. J., and Hatzoglou, M. (2003) Cell 113 519-531 [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, S. A., Spriggs, K. A., Coldwell, M. J., Jackson, R. J., and Willis, A. E. (2003) Mol. Cell 11 757-771 [DOI] [PubMed] [Google Scholar]