Abstract
The IκB kinase (IKK)-related kinases, IKKε and TBK1, participate in the induction of type I interferons (IFNs) during viral infections. Deregulated activation of IKKε and TBK1 also contributes to the abnormal cell survival and transformation. However, how these kinases are negatively regulated remains unclear. We show here that the tumor suppressor CYLD has an essential role in preventing aberrant activation of IKKε/TBK1. CYLD deficiency causes constitutive activation of IKKε/TBK1, which is associated with hyper-induction of IFNs in virus-infected cells. We further show that CYLD targets a cytoplasmic RNA sensor, RIG-I, and inhibits the ubiquitination of this IKKε/TBK1 stimulator. Consistent with the requirement of ubiquitination in RIG-I function, CYLD potently inhibits RIG-I-mediated activation of the IFN-β promoter. These findings establish CYLD as a key negative regulator of IKKε/TBK1 and suggest a role for CYLD in the control of RIG-I ubiquitination.
Innate immunity serves as the first line of host defense against microbial infections. A central step in antiviral innate immunity involves the production of type I interferons (IFNs),3 which is triggered when host pattern recognition receptors (PRRs) detect viral products (1, 2). Two recently identified cytoplasmic PRRs, retinoic acid-induced gene I (RIG-I) and melanoma differentiation-associated gene 5, play critical roles in mediating antiviral innate immune responses (3). These PRRs signal for IFN production through common downstream signaling pathways that involve activation of two homologous protein kinases, IKKε and TBK1, as well as the typical IκB kinase (IKK) (4–9).
IKKε (also named IKKi) and TBK1 (also named T2K and NAK) are known as IKK-related kinases because of their structural homology to IKK (10–14), a central component of the NF-κB signaling pathway (15, 16). Unlike the typical IKK, which phosphorylates NF-κB inhibitors (IκBs) and mediates NF-κB activation, IKKε and TBK1 phosphorylate IRF3 and IRF7 to trigger their dimerization and nuclear translocation, thereby mediating induction of IFN gene expression (9, 16). In addition, emerging evidence suggests that IKKε and TBK1 also regulate cell survival and oncogenesis via an IFN-independent mechanism (17–20). However, how IKKε/TBK1 is negatively regulated is currently not well understood.
An emerging mechanism that regulates signal transduction in various biological processes is protein ubiquitination (21). Analogous to protein phosphorylation, which is reversibly controlled by protein kinases and phosphatases, protein ubiquitination is a reversible event mediated by the counteractive actions of ubiquitin-conjugating enzymes and deubiquitinating enzymes (DUBs) (22). Although ubiquitination is traditionally known as a process that mediates protein degradation by the proteasome, it is now evident that specific ubiquitin chains facilitate protein/protein interactions that lead to the activation of signaling molecules (23). Notably, ubiquitination of RIG-I, a PRR that recognizes double-stranded and 5′-phosphorylated single-stranded viral RNAs (3), triggers its function in the activation of IKKε/TBK1 (24). This activation event is mediated by the ubiquitin ligase TRIM25 (24), a member of the tripartite motif (TRIM) protein family (25). The counteracting DUB(s) involved in negative regulation of RIG-I is unclear.
In this study, we show that CYLD, a recently identified DUB (26–28), physically interacts with RIG-I and negatively regulates RIG-I ubiquitination. CYLD deficiency causes constitutive activation of IKKε and TBK1 along with heightened induction of IFNs by viral infection. Our findings establish CYLD as an important innate immune regulator. Given the oncogenic action of IKKε and TBK1, these findings also have implications on how CYLD suppresses tumorigenesis.
MATERIALS AND METHODS
Mice—Cyld knock-out mice were generated as described (29). Heterozygous (Cyld+/–) mice (in C57BL6/DBA-mixed genetic background) were intercrossed to generate Cyld–/– and Cyld+/+ littermates, and genotyping was performed by PCR using tail DNA (30). All mice were housed in specific pathogen-free cages and monitored periodically (every 3 months) for the lack of common pathogens. Animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine and the University of Texas MD Anderson Cancer Center.
Plasmids, Antibodies, and Cytokines—pcDNA-HA-based expression vectors encoding CYLD, a catalytically inactive CYLD mutant (amino acids 1–932), TRAF3, and ubiquitin were described previously (31–33). GST-IRF3 (amino acids 380–427) was a gift from John Hiscott (5). GST-IκBα (amino acids 1–54 of IκBα) and pCMV4-IκBαSS/AA were described previously (33, 38). FLAG-tagged RIG-I and a RIG-I truncation mutant containing the N-terminal CARD domains, RIG-I(2CARD), were provided by Chris Basler; FLAG-tagged MAVS was provided by Kui Li, and IFNβ-luc (luciferase driven by IFN-β promoter) was provided by Dimitris Thanos.
Antibodies for Tubulin (TU-02), IKKγ (FL419), TBK-1 (M-375), and TRAF3 (H122) were purchased from Santa Cruz Biotechnology. Anti-FLAG, anti-IKKε (anti-IKKi), and anti-HA-horseradish peroxidase (3F10) were from Sigma, eBioscience, and Roche Applied Science, respectively. Rabbit polyclonal antibodies for CYLD and RIG-I were described previously (33, 34). Anti-ubiquitin and anti-MAVS were provided by Drs. Vincent Chau and Zhijian Chen, respectively.
Cells and Transfection—Human embryonic kidney cell line 293T was cultured in Dulbecco's modified Eagle's media containing 5% fetal bovine serum. The cells were seeded in 6-well plates and transfected using Lipofectamine 2000 (Invitrogen). DCs were prepared by cultivating bone marrow cells in RPMI 1640 medium containing 10% fetal bovine serum and 20% of a granulocyte-macrophage colony-stimulating factor conditional medium (provided by Dr. Christopher Norbury). 50% of the culturing medium was replaced with fresh growth medium every 2 days, and cells were used for experiments between day 7 and day 9. To prepare CYLD+/+ and CYLD–/– MEFs, CYLD+/– mice were bred to produce CYLD–/– and CYLD+/+ control embryos. Cells were prepared from the embryos, cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, and immortalized using SV40 large T antigen.
Viruses and Infection—A VSV variant harboring a point mutation in the M gene (AV1) was provided by John Bell (35). AV1 is more potent than parental VSV in cytokine induction because of reduced activity of the mutant M in blocking nuclear export of host mRNA (35). Viral stock was prepared and titered by infecting baby hamster kidney cells.
For in vitro infection, DCs were washed with and resuspended in Hanks' balanced salt solution supplemented with 0.08% bovine serum albumin. The cells were incubated with the indicated doses of VSV strains for 30 min at 37 °C and then collected by centrifugation. Infected cells were cultured in DC growth medium for the indicated times. MEFs were seeded into 12-well plates (3.75 × 105 cells/well) and infected with VSV strains in serum-free medium for 1 h. The cells were washed once and cultured in growth medium.
ELISA and Real Time Quantitative Reverse Transcription-PCR—DCs and MEFs were infected with VSV AV1 as described above. At the indicated times of postinfection, the cell culture supernatants were collected and subjected to ELISA using a commercial IFN-α kit (PBL Biomedical Laboratories).
For analyzing RNA induction, total RNA was isolated from DCs and MEFs using TRIzol reagent (Invitrogen) and subjected to cDNA synthesis using RNase H-reverse transcriptase (Invitrogen) and oligo(dT) primers. Real time quantitative PCR was performed using iCycler Sequence Detection System (Bio-Rad) and Reverse Transcription Real Time™ SYBR green PCR master mix (Superarray). The expression of individual genes was calculated by a standard curve method and normalized to the expression of actin. The gene-specific primer sets (all for murine genes) were as follows: IFN-β, 5′-AGCTCCAAGAAAGGACGAACAT-3′ and 5′-GCCCTGTAGGTGAGGTTGATCT-3′; IFN-α, 5′-TGACCTCAAAGCCTGTGTGATG-3′ and 5′-AAGTATTTCCTCACAGCCAGCAG-3′; and actin, 5′-CGTGAAAAGATGACCCAGATCA-3′ and 5′-CACAGCCTGGATGGCTACGT-3′.
Luciferase Reporter Gene Assays—MEFs were seeded into 24-well plates (1 × 105 cells/well) and transfected using Lipofectamine 2000 (Invitrogen) with IFNβ-luc (300 ng) together with a control Renilla luciferase reporter driven by the constitutive thymidine kinase promoter (pRL-tk-luc, 5 ng). After 24 h of transfection, the cells were either mock-infected or infected with VSV AV1 for 16 h. Dual luciferase assays were performed according to the manufacturer's instruction (Promega, Madison, WI). In some experiments, the reporters were cotransfected with cDNA expression vectors, and luciferase assays were performed after 36 h. The IFNβ-specific luciferase activity was normalized on the basis of the Renilla luciferase activity.
IB, Coimmunoprecipitation, Protein Kinase Assay, and Ubiquitination Assay—Whole-cell lysates were prepared in a kinase cell lysis buffer supplemented with phosphatase inhibitors and subjected to IB, coimmunoprecipitation, and in vitro kinase assays as described previously (36). For ubiquitination assays, cells were lysed in kinase cell lysis buffer supplemented with 1 mm N-ethylmaleimide. RIG-I and MAVS were isolated by IP, and their ubiquitin-conjugated adducts were detected by IB using anti-ubiquitin.
RESULTS
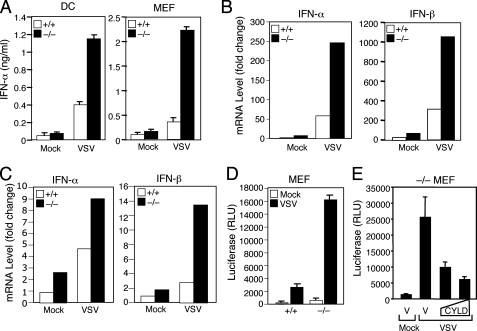
Hyperproduction of Type I IFNs by CYLD–/– Cells—To investigate the role of CYLD in regulating antiviral immune responses, we analyzed the effect of CYLD deficiency on cytokine production induced by vesicular stomatitis virus (VSV) using a strain (AV-1) known to induce strong production of cytokines (35). Infection of wild type dendritic cells (DCs) resulted in strong induction of IFN-α, as detected by ELISA (Fig. 1A). Interestingly, the level of IFN-α induction was markedly higher in CYLD–/– DCs (Fig. 1A), thus revealing a role for CYLD in negatively regulating virus-induced IFN production. Aberrant induction of IFN-α was also detected in CYLD–/– mouse embryonic fibroblasts (MEFs) (Fig. 1A). Furthermore, the negative role of CYLD in IFN regulation occurred at the level of RNA, because the CYLD deficiency caused a hyper-induction of IFN-α and IFN-β mRNAs by VSV (Fig. 1, B and C). To examine whether CYLD negatively regulates the transcription of IFN genes, we performed reporter gene assays using a luciferase reporter driven by the IFN-β promoter (IFNβ-luc). VSV infection induced IFNβ-luc expression in wild type MEFs (Fig. 1D). Moreover, a strikingly higher level of IFNβ-luc induction was detected in the CYLD–/– MEFs (Fig. 1D). This result was not because of variations in transfection, because the data were normalized based on expression of a constitutive Renilla luciferase. Furthermore, the VSV-hyperresponsive phenotype of CYLD–/– cells was reversed upon expression of exogenous CYLD (Fig. 1E). Thus, CYLD deficiency causes aberrant expression of type I IFN genes in virus-infected cells.
FIGURE 1.
Aberrant induction of type I IFN expression by VSV in CYLD–/– cells. A, bone marrow-derived DCs and MEFs prepared from CYLD+/+ and CYLD–/– mice were infected with VSV AV1 (m.o.i. 0.1 for DCs and 0.03 for MEFs) or treated under the same conditions in the absence of viruses (mock). Media were collected at 18 h post-infection and subjected to ELISA to detect IFN-α. Data are presented as means ± S.D. B and C, CYLD+/+ and CYLD–/– DCs (B) and MEFs (C) were infected as in A for 12 h, and RNA was isolated for real time PCR. RNA levels are presented as fold of induction over mock-infected CYLD+/+ cells. D, CYLD+/+ and CYLD–/– MEFs were transfected with IFNβ-luc and a control Renilla luciferase reporter, pRL-tk-luc. At 24 h post-transfection, the cells were either mock-infected or infected with VSV AV1 (m.o.i. 0.03) and subjected to dual luciferase assays after 16 h of infection. The IFNβ-specific luciferase activity was normalized on the basis of the control Renilla luciferase and presented as relative luciferase units (RLU). Data represent means of three independent experiments ± S.D. E, CYLD–/– MEFs were transfected with IFNβ-luc and pRL-tk-luc along with either pcDNA vector (V) or increasing doses (150 and 300 ng) of pcDNA-CYLD. The cells were infected with VSV AV1 and subjected to dual luciferase assays as described in D.
Loss of CYLD Causes Constitutive Activation of IKKε/TBK1—Because IKKε and TBK1 are critical signaling molecules in antiviral immune responses (4, 5), we next examined whether CYLD regulates the activity of these atypical IKKs. Infection of wild type DCs with VSV led to activation of IKKε (Fig. 2A, 1st panel, lanes 2 and 3). Remarkably, the CYLD deficiency caused constitutive activation of IKKε even in uninfected DCs (Fig. 2A, 1st panel, lane 4). The activity of IKKε in CYLD–/– cells was not further induced upon VSV infection (Fig. 2A, 1st panel, lanes 5 and 6). These results suggest that CYLD is critical for maintaining the inducible function of IKKε. As seen with IKKε, the other atypical IKK, TBK1, was constitutively activated in uninfected CYLD–/– cells (Fig. 2B). These data demonstrate an essential role for CYLD in preventing uncontrolled activation of IKKε and TBK1 and maintaining their ability to respond to viral infection.
FIGURE 2.
Constitutive activation of IKKε and TBK1 in CYLD–/– DCs. A, DCs derived from the bone marrow of CYLD+/+ and CYLD–/– mice were either mock-infected or infected with VSV AV1 for 2 h at the indicated m.o.i. IKKε was isolated by IP using anti-IKKε and subjected to in vitro kinase assays using GST-IRF-3 as substrate (1st panel). The typical IKK complex was isolated by IP using anti-IKKβ and subjected to kinase assays using GST-IκBα as substrate (3rd panel). Cell lysates were subjected to IB to examine the expression of the indicated proteins (2nd, 4th, 5th, and 6th panels). B, CYLD+/+ and CYLD–/– DCs were lysed without mock infection and subjected to IKKε and TBK1 kinase assays using GST-IRF-3 as substrate and IB assays. C, CYLD+/+ and CYLD–/– MEFs were transfected with IFNβ-luc and pRL-tk-luc along with either pCMV4-IκBαSS/AA (+) or vector control (–). The cells were infected with VSV AV1 and subjected to dual luciferase assays as described in Fig. 1D.
Virus infection also activates the typical IKK complex, which contributes to the induction of various cytokines, although the IKK/NF-κB pathway is largely dispensable for IFN gene induction by VSV (37). We thus examined whether CYLD regulates the activity of the typical IKK in DCs. Following VSV infection, the typical IKK complex was isolated by IP using the IKKβ antibody and subjected to kinase assays using IκBα as substrate. As expected, VSV infection caused a dose-dependent induction of IKK activity in wild type DCs (Fig. 2A, 3rd panel, lanes 2 and 3). The CYLD deficiency enhanced both the basal and VSV-induced activity of IKK, although the effect was weaker than that on the activation of IKKε (Fig. 2A, 3rd panel, lanes 4–6). To examine the contribution of the typical IKK/NF-κB pathway to the hyper-induction of IFN in CYLD–/– cells, we transfected the CYLD–/– MEFs with a degradation-resistant IκBα mutant lacking its phosphorylation sites (IκBαSS/AA, see Ref. 38). Expression of IκBαSS/AA in both CYLD+/+ and CYLD–/– MEFs only partially inhibited the VSV-induced IFNβ-luc expression (Fig. 2C), a finding that is consistent with the unimportant role of NF-κB in VSV-induced IFN gene expression (37). Thus, CYLD plays a crucial role in preventing uncontrolled activation of IKKε/TBK1 and aberrant production of type I IFNs.
CYLD Inhibits the Ubiquitination and Signaling Function of RIG-I—Ubiquitination plays an important role in antiviral immune responses. In particular, ubiquitination controls the activity of the cytoplasmic RNA sensor RIG-I (24). To understand how CYLD regulates IKKε/TBK1, we examined the role of CYLD in regulating the ubiquitination of RIG-I. Consistent with a recent report, RIG-I became ubiquitinated when expressed in 293 cells (Fig. 3A, lane 2). Furthermore, a constitutively active form of RIG-I containing its N-terminal 2 CARD domains, RIG-I(2CARD), was even more robustly ubiquitinated (Fig. 3B, lane 2; note the lower expression level of 2CARD than the wild type RIG-I). Importantly, the ubiquitination of both RIG-I and RIG-I(2CARD) was efficiently inhibited by CYLD (Fig. 3, A and B, lane 3). This action of CYLD required its DUB activity, because a catalytically inactive CYLD mutant (amino acids 1–932) failed to inhibit RIG-I ubiquitination (Fig. 3, A and B, lane 4). To assess the physiological relevance of these findings, we examined the ubiquitination of RIG-I in wild type and CYLD–/– DCs. A low level of RIG-I ubiquitination was detected in wild type DCs (Fig. 3C, lane 1). In contrast, a notable accumulation of the ubiquitinated RIG-I was detected in the CYLD–/– DCs (Fig. 3C, lane 2). As seen with the constitutive IKKε activation, the constitutive RIG-I ubiquitination in CYLD–/– cells was not further enhanced upon VSV infection, although VSV induced the RIG-I ubiquitination in CYLD+/+ cells (supplemental Fig. 1). Parallel assays did not detect appreciable ubiquitination of the RIG-I-binding protein, MAVS, in wild type or CYLD–/– cells (Fig. 3C, lanes 3 and 4). CYLD deficiency also did not affect the ubiquitination of RelA (Fig. 3C, lanes 5 and 6). Thus, we propose that CYLD is a DUB that prevents uncontrolled ubiquitination of RIG-I.
FIGURE 3.
CYLD physically interacts with RIG-I and inhibits the ubiquitination and signaling function of RIG-I. A, ubiquitination of transfected RIG-I. 293 cells were transfected with (+) or without (–) FLAG-tagged RIG-I either in the absence (–) or presence of wild type (Wt) CYLD or a catalytically inactive CYLD mutant (Mut, CYLD residues 1–932). All cells were also transfected with HA-tagged ubiquitin (Ub). RIG-I was isolated by IP using anti-FLAG, and the ubiquitin-conjugated RIG-I was detected by IB using anti-HA (upper panel). The level of RIG-I expression was monitored by IB (lower panel). B, ubiquitination of RIG-I mutant. 293 cells were transfected as in A except for the replacement of wild type RIG-I with a RIG-I truncation mutant encoding its N-terminal 2 CARD domains (2CARD). The ubiquitination and expression of RIG-I(2CARD) were analyzed as in A. C, ubiquitination of endogenous RIG-I. Endogenous RIG-I and MAVS were isolated by IP from lysates of CYLD+/+ and CYLD–/– DCs, and the ubiquitinated RIG-I and MAVS were detected by IB using anti-ubiquitin (upper panel). As a control, parallel ubiquitination of RelA was analyzed using MG132-treated DCs. Cell lysates were subjected to IB to monitor the expression of RIG-I, MAVS, and RelA. D, CYLD+/+ and CYLD–/– MEFs were transfected with (+) or without (–) the indicated expression vectors along with IFNβ-luc and the control reporter pRL-tk-luc. After 36 h, cell lysates were subjected to dual luciferase assays. The IFNβ-specific luciferase activity was normalized on the basis of the control Renilla luciferase and presented as in Fig. 1D. E, 293 cells were transfected with HA-CYLD along with an empty pcDNA vector, FLAG-RIG-I, FLAG-2CARD, FLAG-MAVS, or HA-TRAF3. The expressed proteins were isolated by IP using anti-FLAG (for RIG-I, 2CARD, and MAVS) or anti-TRAF3 (for TRAF3), and the co-precipitated CYLD was analyzed by IB using anti-HA (top panel). The membranes were reprobed with anti-FLAG or anti-HA to detect the expression of RIG-I and 2CARD (left, middle panel), MAVS (right, 2nd panel), and TRAF3 (right, 3rd panel). The cell lysates were subjected to direct IB to analyze CYLD expression (bottom panel).
To directly examine whether CYLD regulates the signaling function of RIG-I, we performed reporter gene assays. As reported previously, the constitutively active RIG-I(2CARD) induced the expression of IFN-βluc reporter in wild type MEFs (Fig. 3D). Consistent with the biochemical studies described above, the signaling function of RIG-I(2CARD) was greatly potentiated in CYLD–/– MEFs (Fig. 3D). Moreover, restoration of CYLD expression in these mutant cells led to efficient suppression of the RIG-I(2CARD) signaling function (Fig. 3D). These results suggest that CYLD negatively regulates the ubiquitination and signaling function of RIG-I, which provides an insight into the mechanism of IKKε/TBK1 regulation by CYLD.
CYLD Physically Interacts with RIG-I—To determine the mechanism by which CYLD regulates RIG-I function, we examined the potential physical association between RIG-I and CYLD. When coexpressed in 293 cells, CYLD indeed formed a complex with wild type FLAG-tagged RIG-I, which was precipitated by the FLAG antibody (Fig. 3E, lane 2). This molecular interaction was specific, because the FLAG antibody did not precipitate CYLD in cells lacking FLAG-RIG-I. The N-terminal CARD domain of RIG-I, forming the catalytically active region, appeared to be the binding site for CYLD, because RIG-I(2CARD) was sufficient for CYLD binding (Fig. 3E, lane 3). In fact, this RIG-I mutant exhibited strikingly higher CYLD binding activity than the wild type RIG-I (Fig. 3E), which may suggest that CYLD preferentially binds to activated RIG-I. We also examined the molecular interaction of CYLD with other known components of the RIG-I signaling complex, including MAVS and TRAF3 (39–41). Like the RIG-I(2CARD), MAVS strongly interacted with CYLD, whereas TRAF3 only exhibited a weak CYLD binding activity (Fig. 3E, lanes 4 and 5; supplemental Fig. 2). Thus, RIG-I appears to be a primary target of CYLD in the antiviral signaling pathway.
DISCUSSION
The results presented in this paper establish CYLD as a novel regulator of antiviral innate immune responses. CYLD prevents uncontrolled activation of IKKε and TBK1, thereby ensuring normal induction of IFNs during a viral infection. In CYLD-deficient DCs, IKKε and TBK1 are already in their active state. The CYLD deficiency only weakly enhanced the activation of IKKβ. Our data suggest that CYLD inhibits the ubiquitination and signaling function of RIG-I, which is known to mediate virus-induced activation of IKKε/TBK1 (3, 9).
A recent study suggests that ubiquitination plays an important role in IKKε/TBK1 activation by RIG-I (24). During viral infection, RIG-I is transiently ubiquitinated via the specific action of an E3 ubiquitin ligase, TRIM25, and this molecular event appears to serve as a trigger for initiating an antiviral signaling pathway leading to activation of IKKε/TBK1 and induction of type I IFNs. Our current work demonstrates that CYLD functions as a DUB that negatively regulates RIG-I ubiquitination. CYLD physically interacts with RIG-I and inhibits the ubiquitination of RIG-I in both transfected cells and under endogenous conditions. Remarkably, loss of CYLD in DCs causes accumulation of ubiquitinated RIG-I. This finding implies that RIG-I may be undergoing constant ubiquitination and deubiquitination, with deubiquitination being a dominant event. In support of the previous report (24), we have shown that the accumulation of ubiquitinated RIG-I in CYLD–/– cells is associated with constitutive activation of IKKε and TBK1.
It is currently unclear whether CYLD regulates the ubiquitination of any other signaling molecules in the IKKε/TBK1 signaling pathway. We found that CYLD strongly interacts with MAVS, although we were unable to detect significant MAVS ubiquitination in either wild type or CYLD-deficient cells. CYLD also weakly interacted with TRAF3, an adaptor protein that mediates activation of IKKε/TBK1 downstream of RIG-I and TLRs (40, 41). However, the ubiquitination of TRAF3 appears to be regulated by a recently identified ovarian tumor domain-containing DUB, DUBA (42). Nevertheless, our data suggest that CYLD has a nonredundant role in maintaining the inducible nature of the IKKε/TBK1 signaling pathway.
We have shown that the constitutive activation of IKKε/TBK1 in CYLD-deficient DCs and MEFs greatly promotes VSV-induced expression of type I IFN genes. However, under uninfected conditions, the CYLD-deficient cells only produce a low level of type I IFNs. This result suggests that additional signaling events, induced by VSV, contribute to the productive expression of IFNs. We have obtained evidence that the typical IKK/NF-κB only has a minor role in VSV-induced IFNβ promoter activity, a finding that is consistent with a recent report (37). It remains to be examined whether the CYLD deficiency promotes aberrant production of IFNs by nonviral insults. This is an intriguing question, because aberrant production of IFNs is associated with autoimmune disorders (43–45). The constitutive activation of IKKε/TBK1 may also contribute to other pathological processes. In this regard, recent studies have identified both IKKε and TBK1 as oncoprotein kinases, whose deregulation promotes cell survival and transformation independent of IFNs (17–20). Because CYLD is a tumor suppressor, our findings that CYLD controls the activity of IKKe/TBK1 have implications on the tumor-suppressing mechanism of CYLD.
Supplementary Material
Acknowledgments
We thank John Bell, Vincent Chau, Zhijian Chen, John Hiscott, Kui Li, Christopher Norbury, and Dimitris Thanos for reagents.
This work was supported, in whole or in part, by National Institutes of Health Grants AI064639, AI057555, and CA94922. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: IFN, interferon; PRR, pattern recognition receptor; RIG-I, retinoic acid induced gene I; IKK, IκB kinase; DUB, deubiquitinating enzyme; TRIM, tripartite motif; DC, dendritic cell; VSV, vesicular stomatitis virus; MEF, embryonic fibroblast; ELISA, enzyme-linked immunosorbent assay; m.o.i., multiplicity of infection; HA, hemagglutinin; IP, immunoprecipitation; IB, immunoblot; MAVS, mitochondrial antiviral signaling protein.
References
- 1.Perry, A. K., Chen, G., Zheng, D., Tang, H., and Cheng, G. (2005) Cell. Res. 15 407–422 [DOI] [PubMed] [Google Scholar]
- 2.Kawai, T., and Akira, S. (2006) Nat. Immunol. 7 131–137 [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama, M., and Fujita, T. (2007) J. Biol. Chem. 282 15315–15318 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald, K. A., McWhirter, S. M., Faia, K. L., Rowe, D. C., Latz, E., Golenbock, D. T., Coyle, A. J., Liao, S. M., and Maniatis, T. (2003) Nat. Immunol. 4 491–496 [DOI] [PubMed] [Google Scholar]
- 5.Sharma, S., tenOever, B. R., Grandvaux, N., Zhou, G. P., Lin, R., and Hiscott, J. (2003) Science 300 1148–1151 [DOI] [PubMed] [Google Scholar]
- 6.Hemmi, H., Takeuchi, O., Sato, S., Yamamoto, M., Kaisho, T., Sanjo, H., Kawai, T., Hoshino, K., Takeda, K., and Akira, S. (2004) J. Exp. Med. 199 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry, A. K., Chow, E. K., Goodnough, J. B., Yeh, W. C., and Cheng, G. (2004) J. Exp. Med. 199 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui, K., Kumagai, Y., Kato, H., Sato, S., Kawagoe, T., Uematsu, S., Takeuchi, O., and Akira, S. (2006) J. Immunol. 177 5785–5789 [DOI] [PubMed] [Google Scholar]
- 9.Hiscott, J., Nguyen, T. L., Arguello, M., Nakhaei, P., and Paz, S. (2006) Oncogene 25 6844–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada, T., Kawai, T., Takeda, K., Matsumoto, M., Inoue, J., Tatsumi, Y., Kanamaru, A., and Akira, S. (1999) Int. Immunol. 11 1357–1362 [DOI] [PubMed] [Google Scholar]
- 11.Peters, R. T., Liao, S. M., and Maniatis, T. (2000) Mol. Cell 5 513–522 [DOI] [PubMed] [Google Scholar]
- 12.Pomerantz, J. L., and Baltimore, D. (1999) EMBO J. 18 6694–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnard, M., Mirtsos, C., Suzuki, S., Graham, K., Juang, J., Ng, M., Itie, A., Wakeham, A., Shahinian, A., and Henzel, W. J. (2000) EMBO J. 19 4976–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tojima, Y., Fujimoto, A., Delhase, M., Chen, Y., Hatakeyama, S., Nakayama, K., Kaneko, Y., Nimura, Y., Motoyama, N., and Ikeda, K. (2000) Nature 404 778–782 [DOI] [PubMed] [Google Scholar]
- 15.Hayden, M. S., and Ghosh, S. (2004) Genes Dev. 18 2195–2224 [DOI] [PubMed] [Google Scholar]
- 16.Hacker, H., and Karin, M. (2006) Sci. STKE 357 re13. [DOI] [PubMed] [Google Scholar]
- 17.Eddy, S. F., Guo, S., Demicco, E. G., Romieu-Mourez, R., Landesman-Bollag, E., Seldin, D. C., and Sonenshein, G. E. (2005) Cancer Res. 65 11375–11383 [DOI] [PubMed] [Google Scholar]
- 18.Chien, Y., Kim, S., Bumeister, R., Loo, Y. M., Kwon, S. W., Johnson, C. L., Balakireva, M. G., Romeo, Y., Kopelovich, L., Gale, M. J., Yeaman, C., Camonis, J. H., Zhao, Y., and White, M. A. (2006) Cell 127 157–170 [DOI] [PubMed] [Google Scholar]
- 19.Boehm, J. S., Zhao, J. J., Yao, J., Kim, S. Y., Firestein, R., Dunn, I. F., Sjostrom, S. K., Garraway, L. A., Weremowicz, S., Richardson, A. L., Greulich, H., Stewart, C. J., Mulvey, L. A., Shen, R. R., Ambrogio, L., Hirozane-Kishikawa, T., Hill, D. E., Vidal, M., Meyerson, M., Grenier, J. K., Hinkle, G., Root, D. E., Roberts, T. M., Lander, E. S., Polyak, K., and Hahn, W. C. (2007) Cell 129 1065–1079 [DOI] [PubMed] [Google Scholar]
- 20.Agami, R. (2007) Cell 129 1043–1045 [DOI] [PubMed] [Google Scholar]
- 21.Haglund, K., and Dikic, I. (2005) EMBO J. 24 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijman, S. M., Luna-Vargas, M. P., Velds, A., Brummelkamp, T. R., Dirac, A. M., Sixma, T. K., and Bernards, R. (2005) Cell 123 773–786 [DOI] [PubMed] [Google Scholar]
- 23.Adhikari, A., Xu, M., and Chen, Z. J. (2007) Oncogene 26 3214–3226 [DOI] [PubMed] [Google Scholar]
- 24.Gack, M. U., Shin, Y. C., Joo, C. H., Urano, T., Liang, C., Sun, L., Takeuchi, O., Akira, S., Chen, Z., Inoue, S., and Jung, J. U. (2007) Nature 446 916–920 [DOI] [PubMed] [Google Scholar]
- 25.Nisole, S., Stoye, J. P., and Saib, A. (2005) Nat. Rev. Microbiol. 3 799–808 [DOI] [PubMed] [Google Scholar]
- 26.Brummelkamp, T. R., Nijman, S. M., Dirac, A. M., and Bernards, R. (2003) Nature 424 797–801 [DOI] [PubMed] [Google Scholar]
- 27.Kovalenko, A., Chable-Bessia, C., Cantarella, G., Israel, A., Wallach, D., and Courtois, G. (2003) Nature 424 801–805 [DOI] [PubMed] [Google Scholar]
- 28.Trompouki, E., Hatzivassiliou, E., Tsichritzis, T., Farmer, H., Ashworth, A., and Mosialos, G. (2003) Nature 424 793–796 [DOI] [PubMed] [Google Scholar]
- 29.Reiley, W. W., Zhang, M., Jin, W., Losiewicz, M., Donohue, K. B., Norbury, C. C., and Sun, S. C. (2006) Nat. Immunol. 7 411–417 [DOI] [PubMed] [Google Scholar]
- 30.Reiley, W. W., Jin, W., Lee, A. J., Wright, A., Wu, X., Tewalt, E. F., Leonard, T. O., Norbury, C. C., Fitzpatrick, L., Zhang, M., and Sun, S. C. (2007) J. Exp. Med. 204 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao, G., Cvijic, M. E., Fong, A., Harhaj, E. W., Uhlik, M. T., Waterfield, M., and Sun, S. C. (2001) EMBO J. 20 6805–6815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao, G., Zhang, M., Harhaj, E. W., and Sun, S. C. (2004) J. Biol. Chem. 279 26243–26250 [DOI] [PubMed] [Google Scholar]
- 33.Reiley, W., Zhang, M., and Sun, S.-C. (2004) J. Biol. Chem. 279 55161–55167 [DOI] [PubMed] [Google Scholar]
- 34.Imaizumi, T., Hatakeyama, M., Yamashita, K., Yoshida, H., Ishikawa, A., Taima, K., Satoh, K., Mori, F., and Wakabayashi, K. (2004) Endothelium 11 169–173 [DOI] [PubMed] [Google Scholar]
- 35.Stojdl, D. F., Lichty, B. D., tenOever, B. R., Paterson, J. M., Power, A. T., Knowles, S., Marius, R., Reynard, J., Poliquin, L., Atkins, H., Brown, E. G., Durbin, R. K., Durbin, J. E., Hiscott, J., and Bell, J. C. (2003) Cancer Cell 4 263–275 [DOI] [PubMed] [Google Scholar]
- 36.Uhlik, M., Good, L., Xiao, G., Harhaj, E. W., Zandi, E., Karin, M., and Sun, S.-C. (1998) J. Biol. Chem. 273 21132–21136 [DOI] [PubMed] [Google Scholar]
- 37.Zhao, T., Yang, L., Sun, Q., Arguello, M., Ballard, D. W., Hiscott, J., and Lin, R. (2007) Nat. Immunol. 8 592–600 [DOI] [PubMed] [Google Scholar]
- 38.Good, L., Maggirwar, S. B., Kealiher, A., Uhlik, M., and Sun, S.-C. (1996) Biochem. Biophys. Res. Commun. 223 123–128 [DOI] [PubMed] [Google Scholar]
- 39.Seth, R. B., Sun, L., and Chen, Z. J. (2006) Cell. Res. 16 141–147 [DOI] [PubMed] [Google Scholar]
- 40.Häcker, H., Redecke, V., Blagoev, B., Kratchmarova, I., Hsu, L. C., Wang, G. G., Kamps, M. P., Raz, E., Wagner, H., Häcker, G., Mann, M., and Karin, M. (2006) Nature 439 204–207 [DOI] [PubMed] [Google Scholar]
- 41.Oganesyan, G., Saha, S. K., Guo, B., He, J. Q., Shahangian, A., Zarnegar, B., Perry, A., and Cheng, G. (2006) Nature 439 208–211 [DOI] [PubMed] [Google Scholar]
- 42.Kayagaki, N., Phung, Q., Chan, S., Chaudhari, R., Quan, C., O'Rourke, K. M., Eby, M., Pietras, E., Cheng, G., Bazan, J. F., Zhang, Z., Arnott, D., and Dixit, V. M. (2007) Science 318 1628–1632 [DOI] [PubMed] [Google Scholar]
- 43.Crow, M. K. (2005) Curr. Rheumatol. Rep. 7 463–468 [DOI] [PubMed] [Google Scholar]
- 44.Nordmark, G., Alm, G. V., and Rönnblom, L. (2006) Nat. Clin. Pract. Rheumatol. 2 262–269 [DOI] [PubMed] [Google Scholar]
- 45.Baccala, R., Hoebe, K., Kono, D. H., Beutler, B., and Theofilopoulos, A. N. (2007) Nat. Med. 13 543–551 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.