Abstract
Viral hemorrhagic fevers caused by the arenaviruses Lassa virus in Africa and Machupo, Guanarito, Junin, and Sabia virus in South America are among the most devastating emerging human diseases with fatality rates of 15–35% and a limited antiviral therapeutic repertoire available. Here we used high throughput screening of synthetic combinatorial small molecule libraries to identify inhibitors of arenavirus infection using pseudotyped virion particles bearing the glycoproteins (GPs) of highly pathogenic arenaviruses. Our screening efforts resulted in the discovery of a series of novel small molecule inhibitors of viral entry that are highly active against both Old World and New World hemorrhagic arenaviruses. We observed potent inhibition of infection of human and primate cells with live hemorrhagic arenaviruses (IC50 = 500–800 nm). Investigations of the mechanism of action revealed that the candidate compounds efficiently block pH-dependent fusion by the arenavirus GPs (IC50 of 200–350 nm). Although our lead compounds were potent against phylogenetically distant arenaviruses, they did not show activity against other enveloped viruses with class I viral fusion proteins, indicating specificity for arenavirus GP-mediated membrane fusion.
Several arenaviruses, including the Old World virus Lassa virus (LASV)3 and the New World arenaviruses Junin (JUNV), Guanarito (GTOV), and Machupo (MACV), cause severe viral hemorrhagic fevers in humans and represent a serious public health problem (1). LASV is estimated to infect several hundred thousand individuals yearly in endemic regions of West Africa, resulting in significant mortality and high morbidity (2). There is no licensed vaccine available, and therapeutic options are restricted, resulting in 15–30% mortality in hospitalized Lassa fever patients. The New World arenavirus JUNV causes Argentine hemorrhagic fever, a severe illness with hemorrhagic and neurological manifestations and a case fatality rate of 15–35% (3). The related MACV and GTOV are the causative agents of severe viral hemorrhagic fevers in Bolivia and Venezuela, respectively (4). Because of their high mortality and the limited therapeutic repertoire available, hemorrhagic arenaviruses have been classified as Category A pathogens by the Centers for Disease Control and Prevention (5). Apart from the severe humanitarian burden in endemic regions, increased international air traffic has also led to the importation of arenaviral viral hemorrhagic fever cases into metropolitan areas around the globe (6, 7).
A hallmark of fatal arenavirus viral hemorrhagic fever cases is marked immunosuppression of the host and consequent uncontrolled fatal infection (1). Those who survive develop a vigorous anti-viral immune response during the second week of disease, control the infection, and ultimately clear the virus. A highly predictive factor for disease outcome is the extent of viremia, indicating a close competition between viral spread and replication and the immune system of the patient (2). Drugs targeting viral entry will slow viral spread and replication, providing the immune system of the patient a window of opportunity to develop anti-viral immune responses.
A notable difference between LASV and the pathogenic New World arenaviruses is their use of distinct primary cellular receptors, with LASV employing α-dystroglycan (α-DG) (8) and JUNV, MACV, GTOV, and Sabia virus using transferrin receptor 1 (TfR1) (9). Receptor binding and entry of arenaviruses are mediated by the viral envelope glycoprotein (GP). Arenavirus GP is synthesized as a single polypeptide that undergoes post-translational processing to yield the mature virion glycoproteins GP1 and GP2. GP1 is involved in receptor binding (10), whereas GP2 is similar to the fusion active portions of other enveloped viruses including retroviruses, paramyxoviruses, and filoviruses (11).
Our present study applied a novel cell-based high throughput screening assay of synthetic small molecule libraries to identify inhibitors of arenavirus infection using arenavirus GP as a target. Our screening efforts resulted in the discovery of a series of novel small inhibitors of viral entry that are highly effective against both Old World and New World hemorrhagic arenaviruses.
EXPERIMENTAL PROCEDURES
Chemical Libraries—The combinatorial chemical libraries were generated by parallel synthesis utilizing solution phase synthetic techniques as described (12–19). The multistep synthesis of such chemical libraries employing liquid-liquid and liquid-solid extractions to remove unreacted starting materials, reagents, and reagent byproducts provided the purified final products (>95% pure) irrespective of reaction efficiency. Implementation was in formats for the parallel synthesis of individual pure compounds (100–1000 member libraries, individual compounds) and modest sized libraries composed of small mixtures (1,000 membered libraries, 4–10 compounds/mixture), an approach compatible with our screening objective.
Cells and Viruses—African green monkey kidney (VeroE6) cells, human lung epithelial (A549) cells, and human cervical epithelial (HeLa) cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum and supplemented with glutamine and penicillin/streptomycin. All of the infections of cells with live viruses LASV (Josiah), MACV (Carvallo), GTOV (VINH-9551), and JUNV (XJ-13) were performed in the BSL4 laboratory at the Special Pathogens Branch of the Centers for Disease Control and Prevention (Atlanta, GA). HeLa and VeroE6 cells were grown on gelatin-coated glass coverslips. Virus at a multiplicity of infection of 0.1–1.0 was preincubated with the indicated concentrations of drugs or solvent control for 45 min and then added to cell monolayers for 1 h. At 16–24 h after infection, the cells were fixed, and infection was assessed by immunofluorescence staining with mouse hyperimmune sera to Old World or New World arenaviruses as described (9). For infection with influenza A/WSN/33 influenza (WSN; H1N1), 200 plaque-forming units/well of virus were treated with the indicated drug concentrations as described above and added to monolayers of Madrin-Darby canine kidney cells in 96-well plates and incubated for 1 h at 37 °C to infect cells. After 12 h, infection was assessed by stained for influenza nucleoprotein using immune mouse serum. The cells were stained with a fluorescein isothiocyanate-conjugated anti-mouse IgG secondary antibody and visualized under a fluorescence microscope. Cells and cell clusters expressing NP were scored. For infection with the paramyxovirus Measles virus, infection was performed on VeroE6 cells using the measles-GFP virus as performed above for WSN. Infection was assessed by detection of GFP-expressing cells and cell clusters.
High Throughput Screening (HTS) of Combinatorial Chemical Libraries—Retroviral pseudotypes of LASV, JUNV, GTOV, and MACV virus as well as vesicular stomatitis virus (VSV) were generated and titers determined as described (20, 21). Pseudotypes (multiplicity of infection of 0.1) were preincubated with the compounds (concentration was 50 μm for individual compounds and 5 μm for individual compounds in mixtures of 4–10) for 45 min and then added to cell monolayers in 96-well plates in presence of drug. After 1 h, the inoculum was removed, and the cells were washed briefly and then incubated for 48 h. Infection was quantified using the Steady-Glo® high sensitivity luciferase reporter gene assay (Promega) in a Berthold® 96-well plate luminometer.
Cell Viability Assay—Cytotoxicity of candidate compounds was assessed using CellTiter-Glo® luminescent cell viability assay (Promega), which determines the number of viable cells in a culture by quantification of ATP. For the assay, 104 A549 cells were plated per well of a 96-well plate and cultured overnight, resulting in monolayers. The cells were treated with increasing concentrations of candidate compounds applied as in the pseudotype infection assay. After 48 h, CellTiter-Glo® reagent was added, the assay was performed according to the manufacturer's recommendations, and concentrations causing 50% cytotoxicity (CC50) were calculated.
GP-mediated Cell-Cell Fusion—A cell-cell fusion reporter assay (22) was used to characterize the ability of candidate compounds to block pH-dependent cell-cell fusion mediated by the GPs of LASV and JUNV. Briefly, Vero cells infected with vTF7–3 and expressing the envelope glycoprotein were co-cultured with reporter cells infected with vCB21R-lacZ, a recombinant vaccinia virus expressing β-galactosidase under the control of the T7 promoter. The reporter cells were obtained by incubating VeroE6 cells with vCB21R-lacZ at a multiplicity of infection of 2 and allowing the infection to proceed overnight in the presence of 100 μg/ml rifampicin. The GP-expressing cells and reporter cells were co-cultured in medium containing both araC and rifampicin for 5 h. Candidate drugs at the indicated concentrations were added for 45 min, and the cells were washed and then subjected to a 30-min pulse of neutral or acidic (pH 5.0) medium. β-Galactosidase expression is induced upon fusion of the effector and reporter cells and was detected in cell lysates after 5 h of continued cultivation at neutral pH using the chemiluminescent substrate GalactoLite Plus (Tropix). Cell-cell fusion was quantified using a Tropix TR717 microplate luminometer.
Solid Phase Virus Binding Assay—LASV (Josiah), produced and inactivated by γ-irradiation (23), was preincubated with the candidate compounds at 50 μm for 1 h and then added to α-DG immobilized in microtiter plates. After 2 h, the wells were washed and bound virus detected by enzyme-linked immunosorbent assay (24).
Flow Cytometry—A549 cells were incubated with the candidate compounds at 50 μm for 1 h at 37 °C, 5% CO2. The cells were then chilled on ice and detached with enzyme-free cell dissociation, and the cell surface expression of α-DG and TfR1 was assessed by flow cytometry (9, 20).
RESULTS
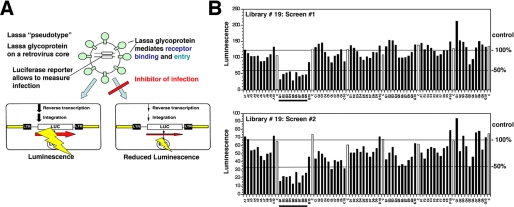
High Throughput Screening of Combinatorial Chemical Libraries for Inhibitors of LASV Entry—In our search for arenavirus entry inhibitors, we initially focused on LASV, which is the most prevalent human pathogen among the arenaviruses. Because LASV is a BSL4 pathogen, work with the virus is restricted. To perform HTS under BSL2 conditions, we generated recombinant retroviruses containing the GP of LASV in their envelope by pseudotyping (Fig. 1A). Because the GP of arenaviruses is necessary and sufficient for receptor binding and entry, recombinant retroviruses pseudotyped with arenavirus GPs represent powerful tools to screen for viral entry inhibitors. The chemical libraries were generated by the parallel synthesis of individual compounds or small mixtures in a format compatible with our screening objective. Candidate compounds derived from these libraries were synthesized using established chemical methods, are chemically stable, and generally have potential drug-like qualities (12–19). HTS of >80,000 compounds, including iminodiacetic acid- and pyrrolidine-based peptidomimetics as well as heterocyclic compounds, resulted in identification of 157 compounds or mixtures with an IC50 of <50 μm. The hits tended to appear in clusters corresponding to groups of structurally related compounds (Fig. 1B). Compound concentrations causing 50% cytotoxicity (CC50) was determined, and candidates with a selectivity index (CC50/IC50) of >10 were selected.
FIGURE 1.
HTS of combinatorial chemical libraries for inhibitors of LASV entry. A, for HTS of combinatorial chemical libraries, recombinant retroviruses containing a luciferase reporter in their genome were pseudotyped with the envelope GP of LASV. Because the GP of arenaviruses is necessary and sufficient for viral entry, these pseudotypes allow HTS for entry inhibitors using the luciferase reporter for rapid and reliable readout of infection. Inhibition of LASV pseudotype infection is detected as a reduced luciferase signal. B, example of HTS of a combinatorial chemical library. LASV pseudotypes were preincubated with compounds (concentration was 50 μm for individual compounds and 5 μm for individual compounds in mixtures of 4–10) for 45 min and then added to monolayers of HeLa cells in 96-well plates. After 1 h, the inoculum was removed, and the cells were washed and incubated for 48 h. Infection was quantified using luciferase reporter gene assay. The data shown are two independent experiments screening the same library. Luminescence is expressed as fold increase over background. A1–A10, B1–B10, C1–C10, D1–D10, E1–E10, F1–F10, and G1–G10 (black bars) represent mixtures of seven different compounds each. The samples labeled C (white bars) correspond to solvent only controls. 100 and 50% of mean control values are indicated as horizontal lines. The mixtures B1–B9 result in >50% inhibition of LASV pseudotype infection in both screens (underlined).
To assess target specificity, the 125 selected candidates were subjected to a counter screen using retroviral pseudotypes containing the GP of the rhabdovirus VSV. VSV GP recognizes distinct cellular receptors (8) and differs structurally from arenavirus GPs, belonging to a different class of viral fusion active proteins (25). Thus, inhibition of VSV pseudotype infection indicated unwanted off target effects. Because both arenaviruses and VSV enter cells by endocytosis followed by pH-dependent membrane fusion in endosomes (26, 27), our counter screen also allowed exclusion of drug effects that resulted in perturbation of membrane trafficking or depletion of endosomal proton gradients. Based on this counter screen, 32 single compounds and 53 compound mixtures were identified that specifically blocked LASV GP-mediated infection and had no effect on infection with VSV pseudotypes.
Nine of the 32 specific single compounds were selected by criteria of potency, chemical tractability, and drug-like properties. These candidates showed dose-dependent inhibition of LASV pseudotype infection at low micromolar IC50 values with IC50 < 1 μm for compounds 16G8, 17C8, and 17C9 (Fig. 2, A and B, and supplemental Fig. S1). Moreover, these candidates showed similar activity and specificity in several human and primate cell lines, indicating independence of the anti-viral activity from the cell type (Fig. 2C).
FIGURE 2.
Candidate inhibitors for LASV entry from combinatorial chemical libraries. A, selected candidate single compounds obtained by HTS of combinatorial chemical libraries utilizing retroviral pseudotypes of LASV. B, IC50 for the compounds in A determined based on the dose-response characteristic for the neutralization of LASV pseudotype infection in permissive human A549 lung epithelial cells (supplemental Fig. S1). C, activity of candidate compounds against LASV pseudotypes in different cell lines. Pseudotypes of LASV or VSV were incubated with candidate compounds prior to infection of human epithelial cell lines HeLa and A549 and the primate fibroblast VeroE6. Infection was determined after 48 h by luciferase assay with luminescence expressed as fold increase over background (n = 3 + S.D.).
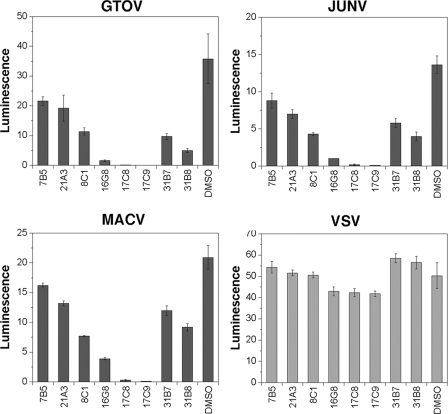
Broad Activity of Candidate Compounds against Human Pathogenic Arenaviruses—The South American hemorrhagic fever (HF) viruses JUNV, GTOV, and MACV are genetically and structurally distinct from the Old World arenavirus LASV and show different receptor usage. Despite these differences, most compounds identified by screening with LASV pseudotypes showed significant activity against pseudotypes of the South American HF viruses (Fig. 3). In particular, compounds 16G8, 17C8, and 17C9 were potent against the South American HF viruses, indicating broad activity against the major human pathogenic arenaviruses.
FIGURE 3.
Activity of candidate compounds against pseudotypes of the South American HF viruses. Retroviral pseudotypes of the South American HF viruses GTOV, JUNV, and MACV, as well as VSV pseudotypes were pretreated with candidate compounds at 20 μm prior to infection of A549 cells and infection determined by luciferase assay as in Fig. 1B. Luminescence is expressed as fold increase over background (n = 3 + S.D.). Note the marked reduction in infection with pseudotypes of the South American HF viruses after exposure to candidate compounds 17C8 and 17C9.
The most potent lead compounds 8C1, 16G8, and 17C8 were then tested against LASV, JUNV, and MACV in BSL4 facilities at the Centers for Disease Control and Prevention. The results revealed activity profiles of the compounds similar to those observed with the corresponding retroviral pseudotypes (Fig. 4). Compound 8C1 exhibited greater activity against LASV than the South American HF viruses, and compound 17C8 showed the most potent activity against all viruses.
FIGURE 4.
Activity of compounds against live arenaviruses. A, infectious LASV was pretreated with candidate compounds 8C1 (20 μm), 17G8 (10 μm), and 17C8 (10 μm) or solvent control (Me2SO) and added to permissive VeroE6 cells. After 1 h of infection, the cells were washed and incubated for 16 h. The cells were fixed, and infection was detected by immunofluorescence detection of viral antigens as described under “Experimental Procedures.” B, blocking of infection of VeroE6 cells with LASV, JUNV, and MACV with candidate drugs as in A. The bars represent average numbers of infected cells in four independent visual fields. One representative example of several independent infections is shown. DMSO, dimethyl sulfoxide.
Candidate Compounds Block pH-dependent Membrane Fusion Mediated by Arenavirus GPs—Arenavirus entry involves binding to cell surface receptors, endocytosis, and delivery to the endosome followed by pH-dependent membrane fusion. Our candidate compounds may interfere with any of these steps or a combination thereof. To distinguish between these possibilities, we addressed the impact of the candidate compounds on the virus-receptor interaction. The compounds had no effect on LASV binding to α-DG as assessed in a solid phase receptor binding assay (data not shown). Further, incubation of cells with >20-fold IC50 of the compounds did not significantly affect cell surface expression of the known cellular receptors for LASV and the South American HF viruses, α-DG and TfR1, respectively (data not shown), excluding down-regulation of the cellular receptor as a mechanism of inhibition. Next, we investigated drug effects on endocytotic pathways implicated in arenavirus entry, clathrin-mediated endocytosis proposed for JUNV (28, 29) and cholesterol-dependent endocytotic pathways implicated in LASV entry (28). Using well established cell biological assays for clathrin-mediated and cholesterol-dependent lipid raft-mediated endocytosis utilizing transferrin and cholera toxin B as markers, we found no inhibitory effect of our compounds at 20-fold IC50 (supplemental Fig. S2). Together, our data suggest that perturbation of virus-receptor binding or subsequent endocytotic uptake by our candidate compounds is unlikely but that they rather target a step beyond attachment and internalization.
Upon internalization by endocytosis, arenaviruses are delivered to endosomes, where the viruses enter the cytoplasm by pH-induced GP-mediated fusion of the viral and endosomal membranes (26, 30). To determine the effect of the compounds 8C1 and 17C8 on arenavirus GP-mediated membrane fusion, we used a cellular fusion reporter assay (22). Briefly, permissive cells expressing arenavirus GPs and T7 RNA polymerase were co-cultured with cells expressing β-galactosidase under control of the T7 promoter for 5 h. Candidate drugs were added for 45 min, and the cells were washed and then exposed to a 30-min pulse of neutral or low pH (5.0) medium. Cell-cell fusion mediated by the arenavirus GP upon acidification resulted in expression of the β-galactosidase reporter measured by chemiluminescence. As shown in Fig. 5, compound 8C1 efficiently blocked LASV GP-mediated cell-cell fusion in a dose-dependent manner and displayed only weak inhibition of JUNV GP, consistent with its activity with the respective viruses (Fig. 4). In contrast, but similarly consistent with the results obtained with virus infection, 17C8 efficiently blocked cell-cell fusion mediated by both viral GPs. The potent activity of our candidate compounds in the cell-based GP fusion assay (IC50 = 200–350 nm) indicates that inhibition of pH-dependent membrane fusion is a major mechanism of their anti-viral activity.
FIGURE 5.
Compounds block pH-dependent membrane fusion. The candidate compounds 8C1 and 17C8 were tested for their ability to block cell-cell fusion mediated by the GPs of LASV and JUNV. The candidate compound 8C1 inhibited LASV GP-mediated fusion in a dose-dependent manner but was weak against JUNV GP-mediated fusion. However, 17C8 inhibited fusion mediated by both viral GPs. Fraction cell fusion is defined as the ratio of chemiluminescence between the indicated concentration of compound and an untreated cell control. The data represent two independent experiments per GP and drug or solvent control. DMSO, dimethyl sulfoxide.
The Lead Compounds 16G8 and 17C8 Show Broad Activity against Arenaviruses but not Other RNA Viruses Containing Class I Fusion Proteins—Although the pathogenic Clade B New World arenaviruses JUNV, MACV, GTOV, and Sabia virus utilize TfR1 as a receptor (9), the nonpathogenic Clade B virus Tacaribe virus (TACV) and the recently emerged North American arenavirus Whitewater Arroyo virus (WWAV) infect cells independent of TfR1 and α-DG (31, 32), indicating a complex pattern of receptor use within the New World arenaviruses. To test the activity of our lead compounds 16G8 and 17C8 against TACV and WWAV, we generated retroviral pseudotypes using the GPs derived from these viruses as recently described (31, 32). Similar to our findings with pseudotypes of LASV, JUNV, MACV, and GTOV, the compounds 16G8 and 17C8 efficiently blocked infection of cells with WWAV and TACV pseudotypes (Fig. 6), indicating a remarkably broad activity within the arenavirus family independent of receptor use.
FIGURE 6.
Selective activity of compounds against arenaviruses. Retroviral pseudotypes of TACV, WWA, Ebola virus, and Amphotropic retrovirus, as well as recombinant measles virus expressing GFP and WSN influenza virus were incubated with the indicated concentrations of compounds and added to VeroE6 cells or Madrin-Darby canine kidney cells for influenza. Pseudotype infection was assessed after 48 h by luciferase reporter assay. To determine infection with recombinant measles virus, GFP-expressing cells were scored, and WSN infection was assessed by immunofluorescence staining for viral NP (for details see “Experimental Procedures”). Luminescence signals are given as fold increase over background (n = 3 + S.D.). For the quantification of measles virus and influenza infection, the total number of infected cell clusters was counted per well (n = 3 + S.D.).
Because recent molecular modeling and biochemical studies revealed similarities between arenavirus GP2 and class I viral fusion proteins of other enveloped viruses including retroviruses, orthomyxoviruses, paramyxoviruses, and filoviruses (11), we addressed the activity of our lead compounds 16G8 and 17C8 against recombinant Amphotropic murine retrovirus, retroviral pseudotypes bearing the GP of the filovirus Ebola virus Zaire, a recombinant paramyxovirus measles virus (Edmonston strain) containing a GFP reporter gene, and the orthomyxovirus influenza virus (strain WSN). In contrast to efficient blocking of entry of arenaviruses, our lead compounds had no significant effect on any of the other viruses tested (Fig. 6), indicating specific inhibition of arenavirus GP-mediated membrane fusion.
DISCUSSION
In the present study, we used HTS of combinatorial small molecule libraries to identify inhibitors of arenavirus infection. Our screening efforts resulted in the discovery of a series of novel small inhibitors of viral entry that are remarkably effective against both Old World and New World hemorrhagic arenaviruses. Inhibition of virus infection is potent (IC50 = 500–800 nm), and our initial studies pinpoint blockage of pH-dependent fusion by the arenaviral GPs as a mechanism of antiviral activity (IC50 of 200–350 nm). Our most potent lead compounds show broad anti-viral activity within the arenavirus family and efficiently block entry of the major human pathogenic arenaviruses.
In recent years, entry inhibitors have emerged as a new class of anti-viral drugs, with the human immunodeficiency virus fusion-blocking peptide enfuvirtide being the first available fusion inhibitor applied for clinical treatment of a viral infection in humans (33). Several nonpeptidic entry inhibitors against human immunodeficiency virus and paramyxoviruses are currently in development (33–36). Using a novel approach combining HTS of combinatorial chemical libraries with a pseudotype-based cellular assay of viral entry, we have discovered two classes of small molecule lead compounds that are potent inhibitors of infection with human pathogenic arenaviruses in cell culture. The most potent of these compounds, 16G8 and 17C8, emerged from a sublibrary that has attractive drug-like characteristics. Examination of the entire library that contains 16G8, 17C8, and 17C9 (Fig. 7) reveals a well defined structure-activity relationship profile within the screen as well as clustering of the observed hits. Such consistent trends are representative of a specific interaction between an inhibitor and its protein-based biological target, as demonstrated in earlier studies using combinatorial libraries as sources of inhibitors of protein-protein interactions (12, 18).
FIGURE 7.
Structure-activity relationships of candidate compounds. A, the structures of libraries 16 and 17. The library was synthesized and screened as single compounds containing single R1 (A1–A3), R2 (B1–B5), and R3 (C1–C10) substitutions. B, example of HTS of chemical libraries 16 and 17. LASV pseudotypes were preincubated with compounds (concentration, 50 μm) for 45 min and then added to monolayers of HeLa cells in 96-well plates. Infection was determined as in Fig. 1. Luminescence is expressed as fold increase over background. The samples labeled Me2SO (white bars) correspond to solvent only controls. The hits 16G8, 17C8, and 17C9 are indicated. Analysis of the results indicates that the key elements required for activity include the closely related 2-indole (C7), 2-benzofuran (C8), or 2-benzothiophene (C9) aryl substituent (R3) attached to the piperazinone core. These bicyclic aryl substituents proved more potent than the corresponding 2-pyrrole, 2-furan, 2-thiophene, or phenyl aryl substituents defining a clear pattern of activity. Similarly, although not as widely explored as R3 in the initial library, the most potent activity was observed with a phenethyl C6 substituent at position R1 (PhCH2CH2- > PhCH2-> CH2CH3), and the nature of the aryl substitutions pattern at N2 (R2) significantly modulate this activity.
Although one of our lead compounds, 8C1, has specificity for LASV entry, our most potent leads, 16G8 and 17C8, showed high activity against both LASV and the more distantly related South American hemorrhagic fever viruses JUNV, MACV, and GTOV, as well as TACV and the recently emerged North American arenavirus WWAV. Considering the genetic distance between the envelope GPs of Old World and New World arenaviruses and their differences in structure, receptor use, and mechanisms of cell entry, this broad inhibition within the arenavirus family is remarkable.
Our initial studies on the mechanism of action utilizing a cell-cell fusion assay indicate that the candidate compounds act at the level of GP-mediated membrane fusion with IC50 = 200–350 nm (Fig. 5). Because compounds were washed out prior to the low pH pulse in our assay system, the efficient blocking of GP-mediated membrane fusion suggests that the compounds remain bound to the viral GP.
The current structural model of the arenavirus GP is a trimeric complex with the transmembrane subunit GP2 containing N- and C-heptad repeat sequences that are predicted to fold into a six-helix bundle post-fusion conformation (11). The presence of these structures in GP2 has recently resulted in the classification of arenavirus GPs among the viral class I fusion proteins (11). Considering the overall similarity between the fusion cores of class I fusion proteins (37), we tested the activity of our most potent and broadly active leads against a series of prototypic RNA viruses bearing class I fusion proteins. In contrast to the potent neutralization of all arenaviruses tested, our drugs were inactive against amphotropic murine retrovirus, the filovirus Ebola, the paramyxovirus measles virus, and the orthomyxovirus influenza. The broad activity against arenaviruses concomitant with the lack of inhibition of other viral class I fusion proteins suggests the presence of conserved molecular structures and/or interactions in the fusion active GP complex of arenaviruses absent from other related viral GPs. Indeed, recent studies revealed unique mechanistic features of arenavirus GP-mediated membrane fusion. In contrast to other viral GPs, the arenavirus GP precursor contains a stable signal peptide of unusual length that is retained as an essential component of the mature GP complex (38–41) and is crucial for the pH-dependent fusion activity of arenavirus GPs (22, 42, 43). The specificity of our compounds for arenavirus GP-mediated membrane fusion may allow the use of these molecules as molecular probes to dissect such unusual features of arenavirus fusion.
For drug development, the chemical properties of our initial leads make them amenable to optimization of their potency, solubility, and bioavailability. Small molecule entry inhibitors have a number of properties that allow their application in endemic areas in the developing world. Their chemical stability minimizes logistic problems of transport and storage in the tropical and subtropical climates. The possibility of oral administration is further compatible with a limited public health infrastructure. Based on the broad activity of our lead compounds within the arenavirus family, they are likely active against different genetic variants of a given arenavirus species. This is of great importance because human pathogenic arenaviruses show remarkable genetic variability between isolates from different geographic regions (44).
The establishment of the viral GP as a promising drug target combined with the use of preselected libraries of chemically tractable compounds with potential drug-like properties in HTS allows for rapid and biosafe screening of a number of compounds with a high probability to isolate potent leads. Importantly, our screening approach does not require information about the structure of the viral GPs or cellular receptors involved and is applicable for the development of small molecule inhibitors to other enveloped viruses.
Supplementary Material
Acknowledgments
We thank Dr. Michael Buchmeier, Dr. Paula M. Cannon, Dr. Gary Nabel, Dr. Kevin P. Campbell, and Dr. Juan-Carlos de la Torre for valuable materials.
This work was supported, in whole or in part, by National Institutes of Health Grants AI55540, CA78045 (to D. L. B.), and 1U54 AI065359 from the United States Public Health Service. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S2.
Footnotes
The abbreviations used are: LASV, Lassa fever virus; JUNV, Junin virus; GTOV, Guanarito virus; MACV, Machupo virus; VSV, vesicular stomatitis virus; GP, glycoprotein; α-DG, α-dystroglycan; TfR1, transferrin receptor 1; HTS, high throughput screening; HF, hemorrhagic fever; TACV, Tacaribe virus; WWAV, Whitewater Arroyo virus.
References
- 1.Geisbert, T. W., and Jahrling, P. B. (2004) Nat. Med. 10 S110-121 [DOI] [PubMed] [Google Scholar]
- 2.McCormick, J. B., and Fisher-Hoch, S. P. (2002) Curr. Top Microbiol. Immunol. 262 75-109 [DOI] [PubMed] [Google Scholar]
- 3.Weissenbacher, M. C., Laguens, R. P., and Coto, C. E. (1987) Curr. Top Microbiol. Immunol. 134 79-116 [DOI] [PubMed] [Google Scholar]
- 4.Peters, C. J. (2002) Curr. Top Microbiol. Immunol. 262 65-74 [DOI] [PubMed] [Google Scholar]
- 5.Borio, L., Inglesby, T., Peters, C. J., Schmaljohn, A. L., Hughes, J. M., Jahrling, P. B., Ksiazek, T., Johnson, K. M., Meyerhoff, A., O'Toole, T., Ascher, M. S., Bartlett, J., Breman, J. G., Eitzen, E. M., Jr., Hamburg, M., Hauer, J., Henderson, D. A., Johnson, R. T., Kwik, G., Layton, M., Lillibridge, S., Nabel, G. J., Osterholm, M. T., Perl, T. M., Russell, P., and Tonat, K. (2002) Jama 287 2391-2405 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz, H., Kohler, B., Laue, T., Drosten, C., Veldkamp, P. J., Gunther, S., Emmerich, P., Geisen, H. P., Fleischer, K., Beersma, M. F., and Hoerauf, A. (2002) Microbes Infect. 4 43-50 [DOI] [PubMed] [Google Scholar]
- 7.Isaacson, M. (2001) Clin. Infect. Dis. 33 1707-1712 [DOI] [PubMed] [Google Scholar]
- 8.Cao, W., Henry, M. D., Borrow, P., Yamada, H., Elder, J. H., Ravkov, E. V., Nichol, S. T., Compans, R. W., Campbell, K. P., and Oldstone, M. B. (1998) Science 282 2079-2081 [DOI] [PubMed] [Google Scholar]
- 9.Radoshitzky, S. R., Abraham, J., Spiropoulou, C. F., Kuhn, J. H., Nguyen, D., Li, W., Nagel, J., Schmidt, P. J., Nunberg, J. H., Andrews, N. C., Farzan, M., and Choe, H. (2007) Nature 446 92-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrow, P., and Oldstone, M. B. (1992) J. Virol. 66 7270-7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eschli, B., Quirin, K., Wepf, A., Weber, J., Zinkernagel, R., and Hengartner, H. (2006) J. Virol. 80 5897-5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg, T., Cohen, S. B., Desharnais, J., Sonderegger, C., Maslyar, D. J., Goldberg, J., Boger, D. L., and Vogt, P. K. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 3830-3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boger, D. L., Desharnais, J., and Capps, K. (2003) Angew Chem. Int. Ed. Engl. 42 4138-4176 [DOI] [PubMed] [Google Scholar]
- 14.Boger, D. L., Goldberg, J., Jiang, W., Chai, W., Ducray, P., Lee, J. K., Ozer, R. S., and Andersson, C. M. (1998) Bioorg. Med. Chem. 6 1347-1378 [DOI] [PubMed] [Google Scholar]
- 15.Boger, D. L., Goldberg, J., Satoh, S., Ambroise, Y., Cohen, S. B., and Vogt, P. K. (2000) Helv. Chim. Acta 83 1825-1845 [Google Scholar]
- 16.Boger, D. L., Goldberg, J., Silletti, S., Kessler, T., and Cheresh, D. A. (2001) J. Am. Chem. Soc. 123 1280-1288 [DOI] [PubMed] [Google Scholar]
- 17.Cheng, S., Tarby, C. M., Comer, D. D., Williams, J. P., Caporale, L. H., Myers, P. L., and Boger, D. L. (1996) Bioorg. Med. Chem. 4 727-737 [DOI] [PubMed] [Google Scholar]
- 18.Goldberg, J., Jin, Q., Ambroise, Y., Satoh, S., Desharnais, J., Capps, K., and Boger, D. L. (2002) J. Am. Chem. Soc. 124 544-555 [DOI] [PubMed] [Google Scholar]
- 19.Silletti, S., Kessler, T., Goldberg, J., Boger, D. L., and Cheresh, D. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 119-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojek, J. M., Spiropoulou, C. F., Campbell, K. P., and Kunz, S. (2007) J. Virol. 81 5685-5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojek, J. M., Spiropoulou, C. F., and Kunz, S. (2006) Virology 349 476-491 [DOI] [PubMed] [Google Scholar]
- 22.York, J., and Nunberg, J. H. (2006) J. Virol. 80 7775-7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiropoulou, C. F., Kunz, S., Rollin, P. E., Campbell, K. P., and Oldstone, M. B. (2002) J. Virol. 76 5140-5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunz, S., Rojek, J., Perez, M., Spiropoulou, C., and MB, O. (2005) J. Virol. 79 5979-5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche, S., Rey, F. A., Gaudin, Y., and Bressanelli, S. (2007) Science 315 843-848 [DOI] [PubMed] [Google Scholar]
- 26.Borrow, P., and Oldstone, M. B. (1994) Virology 198 1-9 [DOI] [PubMed] [Google Scholar]
- 27.Rose, J. K., and Whitt, M. A. (2001) in Fields Virology (Fields, B. N., Knipe, D. L., and Howley, P. M., eds) pp. 1221-1244, Lippincott-Raven, Philadelphia
- 28.Rojek, J. M., Perez, M., and Kunz, S. (2008) J. Virol. 82 1505-1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, M. G., Cordo, S. M., and Candurra, N. A. (2007) J. Gen. Virol. 88 1776-1784 [DOI] [PubMed] [Google Scholar]
- 30.Castilla, V., Mersich, S. E., Candurra, N. A., and Damonte, E. B. (1994) Arch. Virol. 136 363-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flanagan, M. L., Oldenburg, J., Reignier, T., Holt, N., Hamilton, G. A., Martin, V. K., and Cannon, P. M. (2008) J. Virol. 82 938-948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reignier, T., Oldenburg, J., Flanagan, M. L., Hamilton, G. A., Martin, V. K., and Cannon, P. M. (2008) Virology 371 439-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Este, J. A., and Telenti, A. (2007) Lancet 370 81-88 [DOI] [PubMed] [Google Scholar]
- 34.Cianci, C., Langley, D. R., Dischino, D. D., Sun, Y., Yu, K. L., Stanley, A., Roach, J., Li, Z., Dalterio, R., Colonno, R., Meanwell, N. A., and Krystal, M. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 15046-15051. Epub 12004 Oct 15046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plemper, R. K., Erlandson, K. J., Lakdawala, A. S., Sun, A., Prussia, A., Boonsombat, J., Aki-Sener, E., Yalcin, I., Yildiz, I., Temiz-Arpaci, O., Tekiner, B., Liotta, D. C., Snyder, J. P., and Compans, R. W. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 5628-5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey, G., Rits-Volloch, S., Zhang, X. Q., Schooley, R. T., Chen, B., and Harrison, S. C. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 13938-13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissenhorn, W., Hinz, A., and Gaudin, Y. (2007) FEBS Lett. 581 2150-2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichler, R., Lenz, O., Strecker, T., Eickmann, M., Klenk, H. D., and Garten, W. (2003) EMBO Rep. 4 1084-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichler, R., Lenz, O., Strecker, T., and Garten, W. (2003) FEBS Lett. 538 203-206 [DOI] [PubMed] [Google Scholar]
- 40.Froeschke, M., Basler, M., Groettrup, M., and Dobberstein, B. (2003) J. Biol. Chem. 278 41914-41920 [DOI] [PubMed] [Google Scholar]
- 41.York, J., Romanowski, V., Lu, M., and Nunberg, J. H. (2004) J. Virol. 78 10783-10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.York, J., and Nunberg, J. H. (2007) J. Virol. 10 10 [Google Scholar]
- 43.York, J., and Nunberg, J. H. (2007) Virology 359 72-81 [DOI] [PubMed] [Google Scholar]
- 44.Clegg, J. C. (2002) Curr. Top Microbiol. Immunol. 262 1-24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.