Abstract
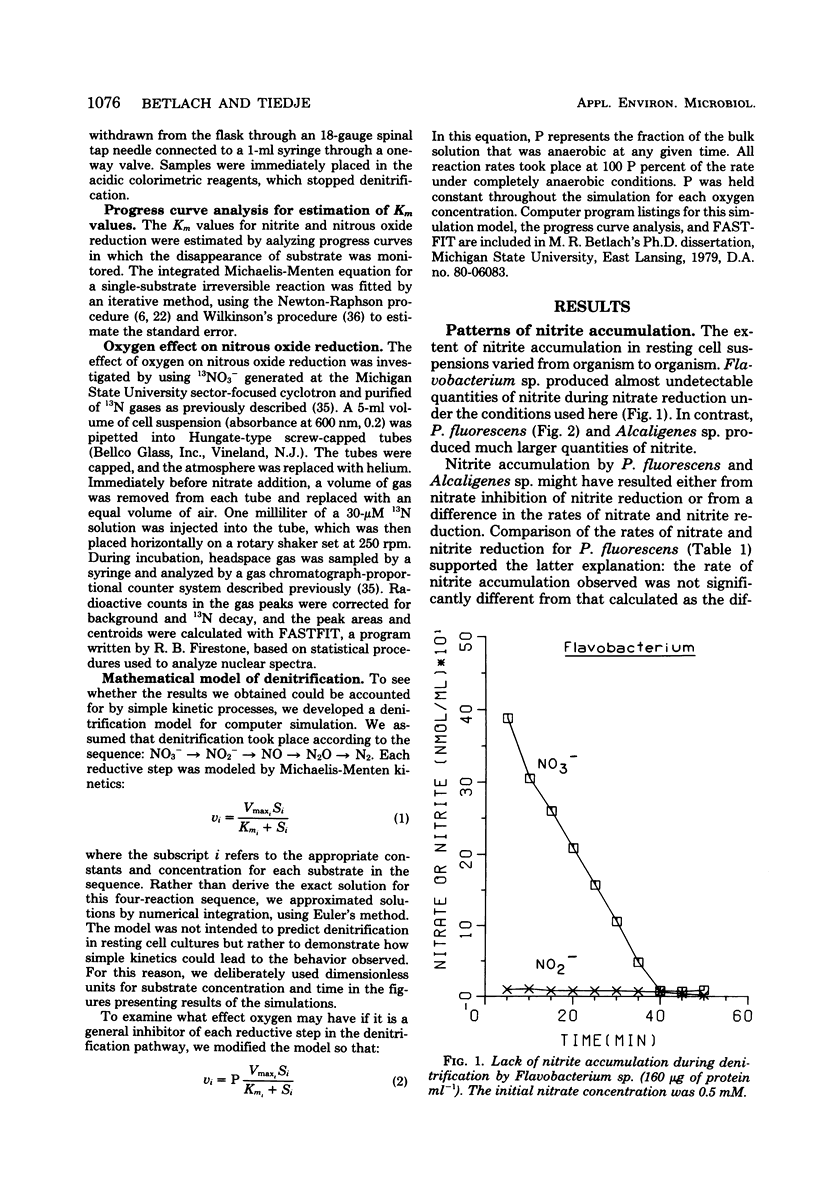
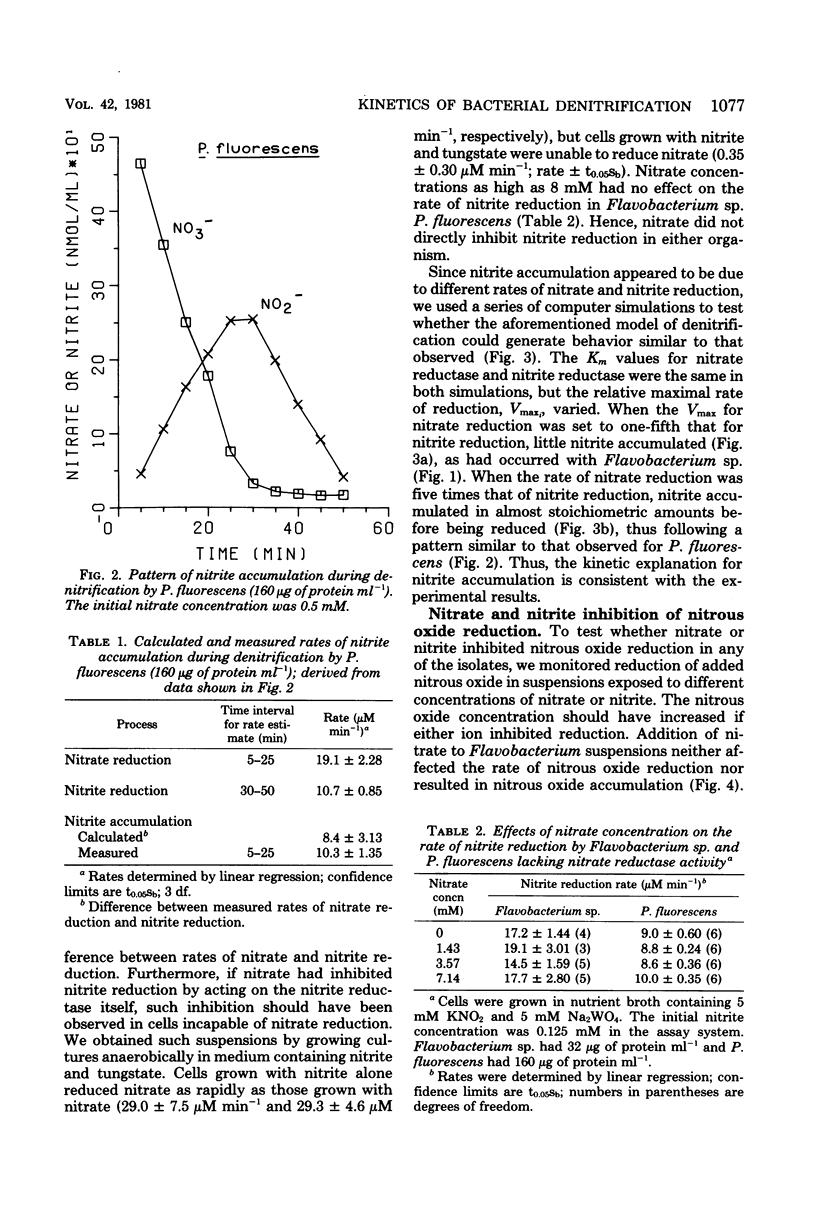
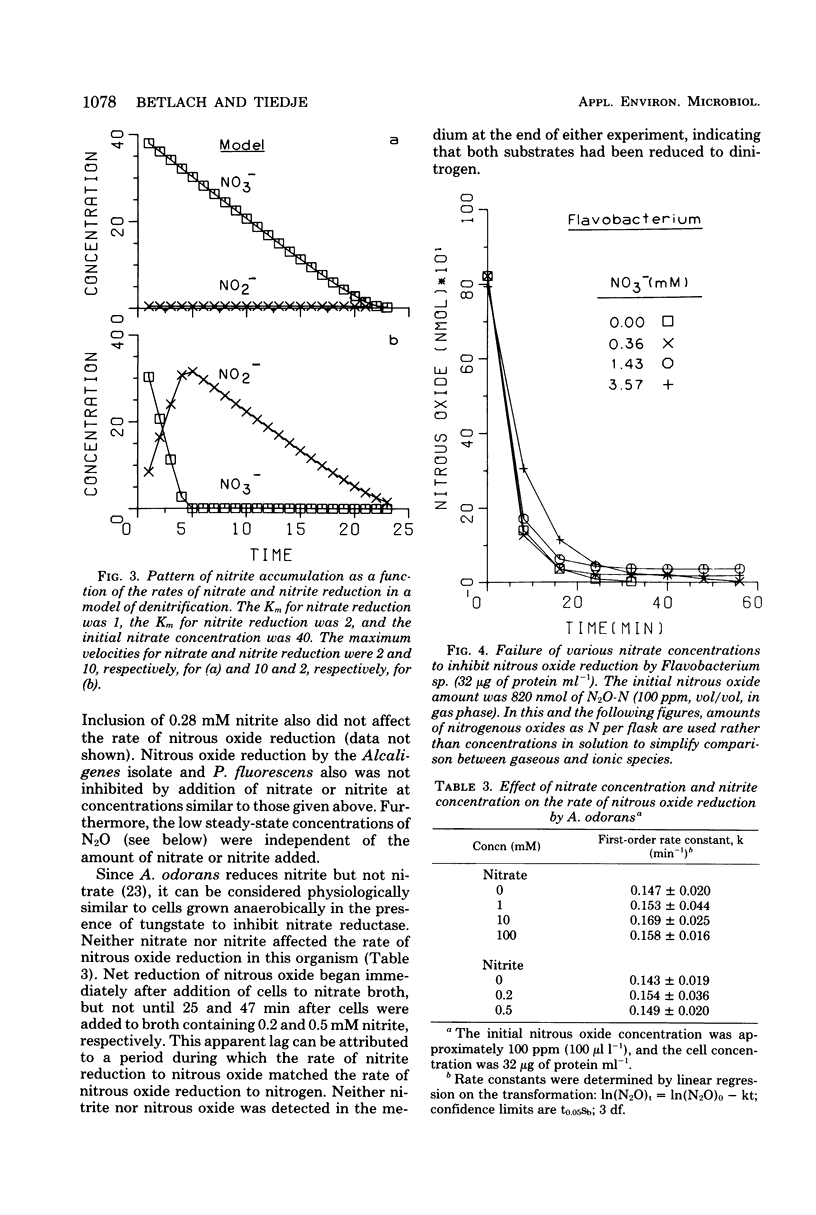
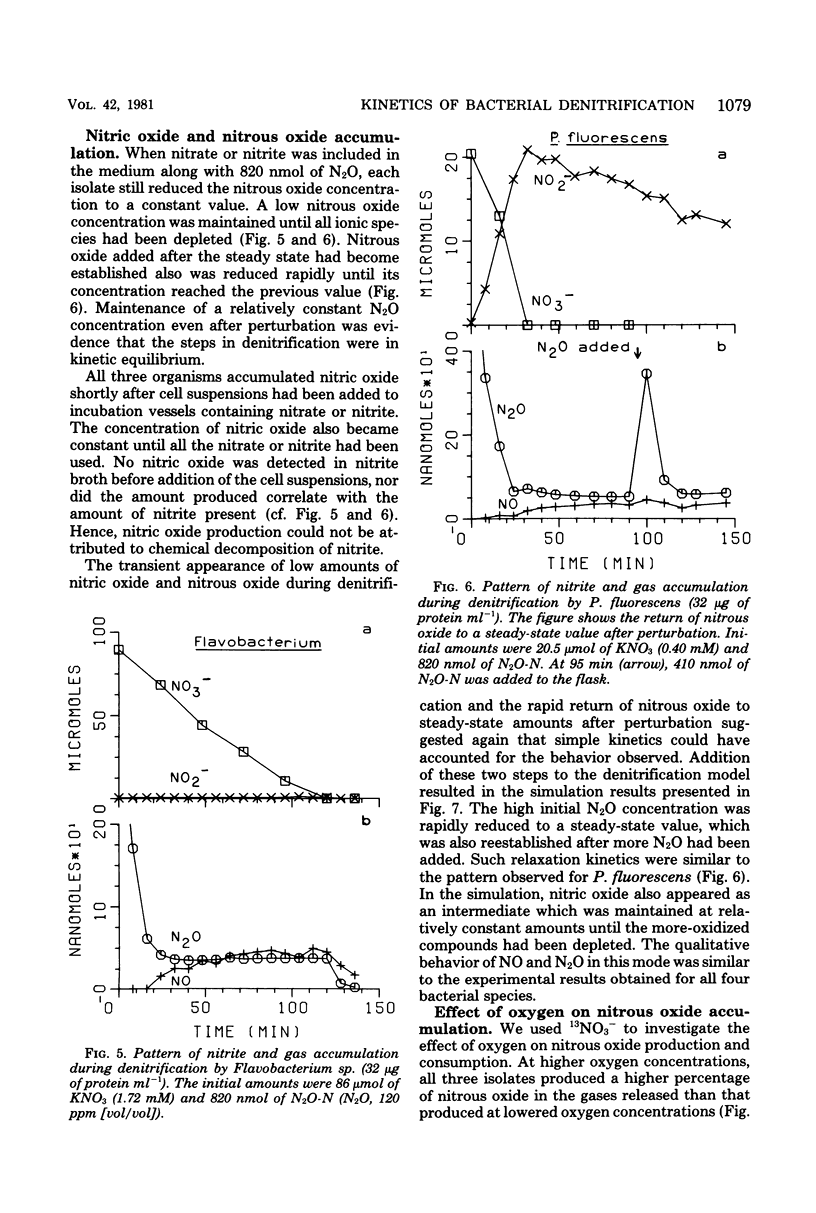
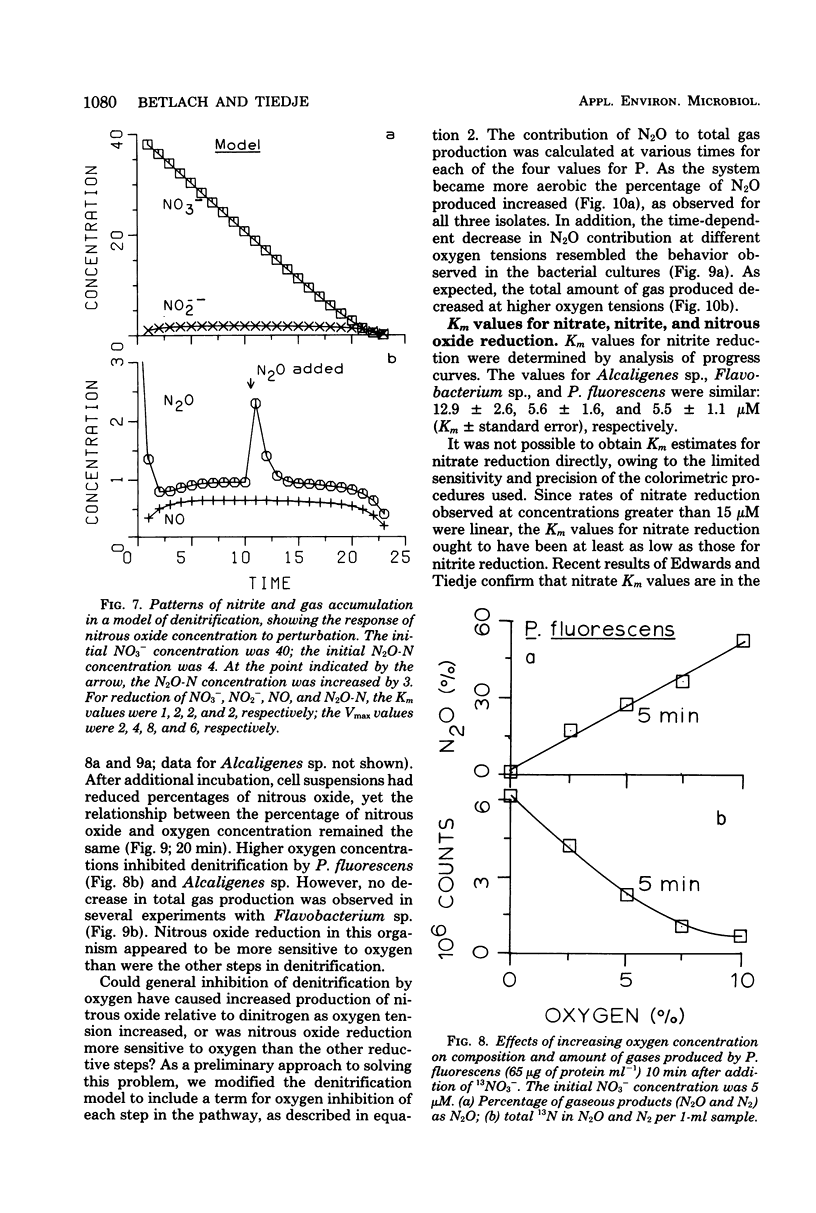
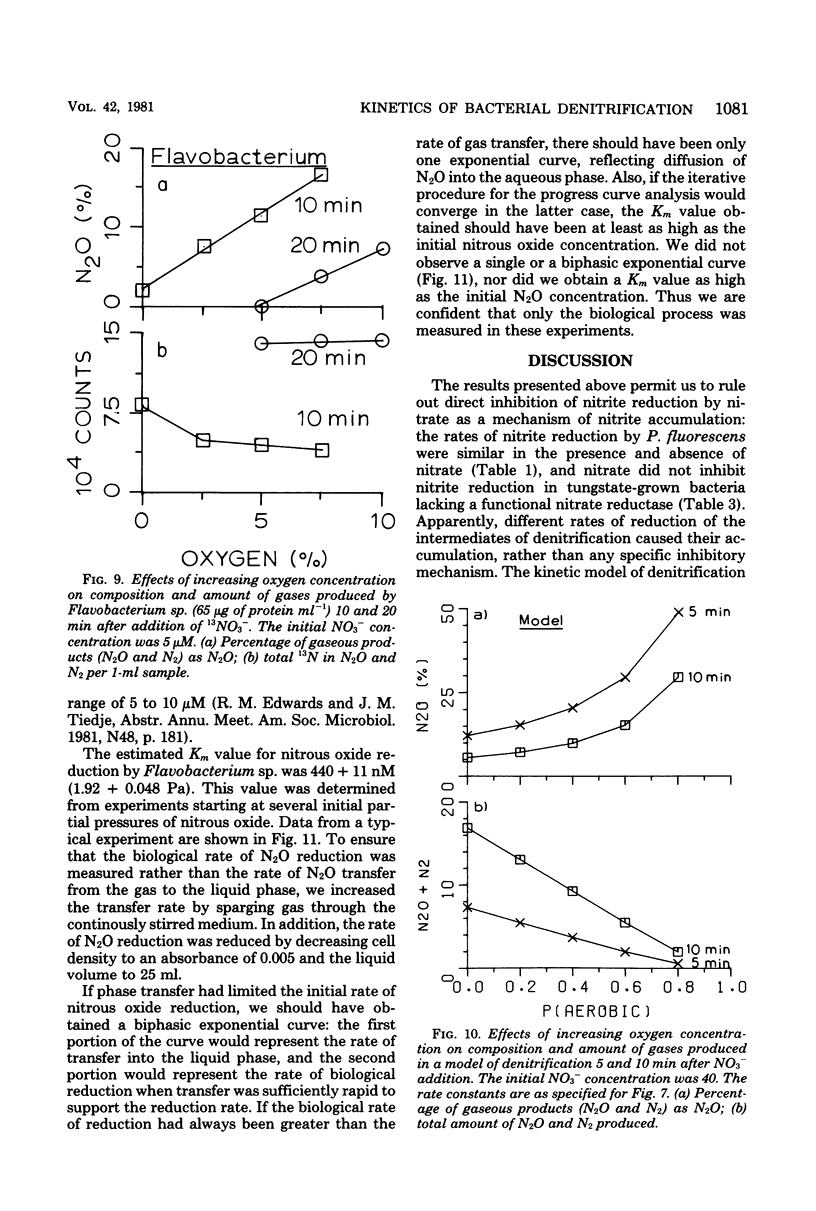
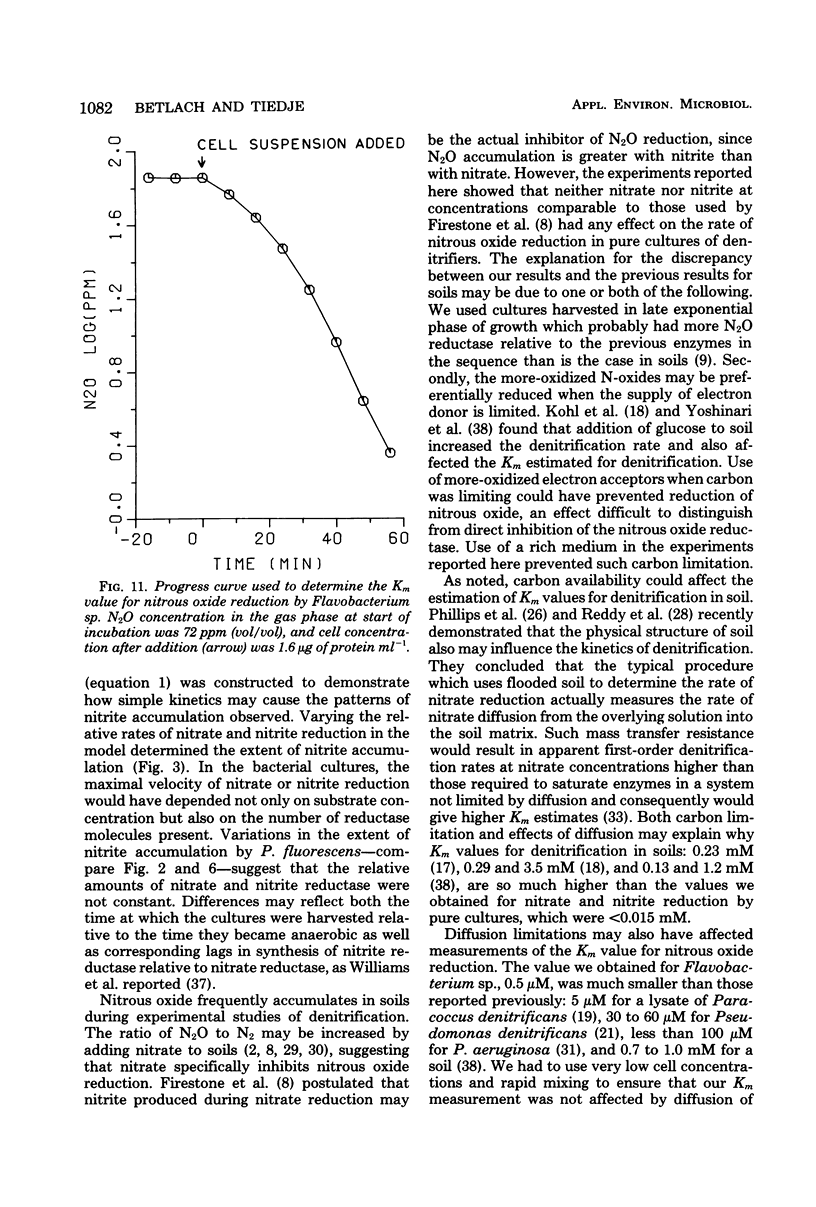
The kinetics of denitrification and the causes of nitrite and nitrous oxide accumulation were examined in resting cell suspensions of three denitrifiers. An Alcaligenes species and a Pseudomonas fluorescens isolate characteristically accumulated nitrite when reducing nitrate; a Flavobacterium isolate did not. Nitrate did not inhibit nitrite reduction in cultures grown with tungstate to prevent formation of an active nitrate reductase; rather, accumulation of nitrite seemed to depend on the relative rates of nitrate and nitrite reduction. Each isolate rapidly reduced nitrous oxide even when nitrate or nitrite had been included in the incubation mixture. Nitrate also did not inhibit nitrous oxide reduction in Alcaligenes odorans, an organism incapable of nitrate reduction. Thus, added nitrate or nitrite does not always cause nitrous oxide accumulation, as has often been reported for denitrifying soils. All strains produced small amounts of nitric oxide during denitrification in a pattern suggesting that nitric oxide was also under kinetic control similar to that of nitrite and nitrous oxide. Apparent Km values for nitrate and nitrite reduction were 15 μM or less for each isolate. The Km value for nitrous oxide reduction by Flavobacterium sp. was 0.5 μM. Numerical solutions to a mathematical model of denitrification based on Michaelis-Menten kinetics showed that differences in reduction rates of the nitrogenous compounds were sufficient to account for the observed patterns of nitrite, nitric oxide, and nitrous oxide accumulation. Addition of oxygen inhibited gas production from 13NO3− by Alcaligenes sp. and P. fluorescens, but it did not reduce gas production by Flavobacterium sp. However, all three isolates produced higher ratios of nitrous oxide to dinitrogen as the oxygen tension increased. Inclusion of oxygen in the model as a nonspecific inhibitor of each step in denitrification resulted in decreased gas production but increased ratios of nitrous oxide to dinitrogen, as observed experimentally. The simplicity of this kinetic model of denitrification and its ability to unify disparate observations should make the model a useful guide in research on the physiology of denitrifier response to environmental effectors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackmer A. M., Bremner J. M., Schmidt E. L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980 Dec;40(6):1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag J. M., Kurek E. J. Nitrite and nitrous oxide accumulation during denitrification in the presence of pesticide derivatives. Appl Environ Microbiol. 1980 Apr;39(4):845–849. doi: 10.1128/aem.39.4.845-849.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C. C., Bryan B. A. Denitrification. Annu Rev Microbiol. 1976;30:241–262. doi: 10.1146/annurev.mi.30.100176.001325. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Morrison J. F. The analysis of progress curves for enzyme-catalysed reactions by non-linear regression. Biochim Biophys Acta. 1977 Apr 12;481(2):297–312. doi: 10.1016/0005-2744(77)90264-9. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitric oxide as an intermediate in denitrification: evidence from nitrogen-13 isotope exchange. Biochem Biophys Res Commun. 1979 Nov 14;91(1):10–16. doi: 10.1016/0006-291x(79)90575-8. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Tiedje J. M. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl Environ Microbiol. 1979 Oct;38(4):673–679. doi: 10.1128/aem.38.4.673-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble T. N., Betlach M. R., Tiedje J. M. Numerically dominant denitrifying bacteria from world soils. Appl Environ Microbiol. 1977 Apr;33(4):926–939. doi: 10.1128/aem.33.4.926-939.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- Lam Y., Nicholas D. J. Aerobic and anaerobic respiration in Micrococcus denitrificans. Biochim Biophys Acta. 1969 Apr 8;172(3):450–461. doi: 10.1016/0005-2728(69)90141-8. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Mori T. Studies on denitrification. IX. Nitrous oxide, its production and reduction to nitrogen. J Biochem. 1968 Dec;64(6):863–871. doi: 10.1093/oxfordjournals.jbchem.a128968. [DOI] [PubMed] [Google Scholar]
- Nimmo I. A., Atkins G. L. A comparison of two methods for fitting the integrated Michaelis-Menten equation. Biochem J. 1974 Sep;141(3):913–914. doi: 10.1042/bj1410913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Riley P. S. Suppression by nitrate of enzymatic reduction of nitric oxide. Proc Soc Exp Biol Med. 1969 Oct;132(1):258–260. doi: 10.3181/00379727-132-34192. [DOI] [PubMed] [Google Scholar]
- Pichinoty F., Véron M., Mandel M., Durand M., Job C., Garcia J. L. Etude physiologique et taxonomique du genre Alcaligenes: A. denitrificans, A. odorans et A. faecalis. Can J Microbiol. 1978 Jun;24(6):743–753. doi: 10.1139/m78-123. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sperl G. T., DeMoss J. A. In vitro incorporation of molybdate into demolybdoproteins in Escherichia coli. J Bacteriol. 1979 Feb;137(2):719–726. doi: 10.1128/jb.137.2.719-726.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Hollocher T. C. Nitrogen 15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977 Jan 10;252(1):212–218. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Rowe J. J., Romero P., Eagon R. G. Denitrifying Pseudomonas aeruginosa: some parameters of growth and active transport. Appl Environ Microbiol. 1978 Aug;36(2):257–263. doi: 10.1128/aem.36.2.257-263.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



