Abstract
Two experiments evaluated the source(s) of emergent differential sample behavior in pigeons. Initially, pigeons learned two-sample, two-alternative symbolic matching in which different patterns of sample responding were required to produce the comparisons. Afterwards, two other samples nominally identical to the comparisons were added to the matching task. On new-sample trials, completion of either sample–response requirement produced comparison alternatives which were either the same as or different from the alternatives on the familiar-sample trials. Differential responding to the new samples developed only when the comparisons were the same as the familiar samples. The results are consistent with acquired sample equivalence and adventitious reinforcement accounts of emergent sample behavior and are inconsistent with bidirectional transfer (symmetry) between the response patterns explicitly required to the originally trained (familiar) samples and the subsequently reinforced comparisons.
Keywords: emergent sample behavior, differential sample responding, bidirectional transfer, symmetry, acquired equivalence, adventitious reinforcement, pigeons, pecking
Derived stimulus control refers to the finding that when behavior is explicitly brought under control of certain stimuli via differential reinforcement, other stimuli then exert control over that behavior despite no differential reinforcement history with respect to it (Barnes & Keenan, 1993; Saunders, Williams, & Spradlin, 1996). The phenomena of acquired and stimulus equivalence both illustrate this effect (e.g., Bovet & Vauclair, 1998; Hall, Mitchell, Graham, & Lavis, 2003; Kastak, Schusterman, & Kastak, 2001; Sidman, Wynne, Maguire, & Barnes, 1989; Spradlin, Cotter, & Baxley, 1973; Urcuioli, Zentall, Jackson-Smith, & Steirn, 1989; Wasserman, DeVolder, & Coppage, 1992).
In acquired equivalence, stimuli that have occasioned a common reinforced response or been associated with a common but distinctive reinforcer can then be shown to be interchangeable for one another in new contexts (Goldiamond, 1962; Urcuioli, 2006a; see also Honey & Hall, 1989). For example, after learning to match two samples to a common reinforced comparison and, later, one of those samples to a new comparison stimulus, pigeons will immediately match the remaining sample to the new comparison (e.g., Urcuioli, Zentall, & DeMarse, 1995; Wasserman, et al., 1992). In stimulus equivalence (Saunders, Drake, & Spradlin, 1999; Sidman, Kirk, & Willson-Morris, 1985; Sidman & Tailby, 1982; see also Sidman, 1992), acquisition of an arbitrary conditional discrimination such as symbolic matching-to-sample (MTS) often yields the ability, in humans at least, to match each conditional or sample stimulus to itself (reflexivity) and to “reverse” the trained sample–comparison relations by matching each former comparison stimulus to its former sample stimulus (symmetry). Unlike acquired equivalence, nonhuman animals typically do not exhibit the emergent effects that define stimulus equivalence when training and testing involves two-alternative MTS (e.g., Lionello-DeNolf & Urcuioli, 2002; although see García & Benjumea, 2006, and Schusterman & Kastak, 1993). Nevertheless, they regularly exhibit other types of emergent behavior that are the hallmarks of derived stimulus control.
A paradigmatically unusual and interesting example of this was reported by Manabe, Kawashima, and Staddon (1995, Experiment 3) who, interestingly enough, appealed to symmetry to explain their results. Using budgerigars, Manabe et al. required their birds to make a high-frequency call to one color sample and a low-frequency call to an alternative color sample to produce the comparison stimuli (geometric forms projected onto the comparison response panels) in two-alternative MTS. A single peck to the defined “correct” comparison was then reinforced. After the budgerigars achieved high levels of accuracy on both the high- versus low-call sample discrimination and the conditional (comparison–choice) discrimination, Manabe et al. added two new (geometric form) samples to the existing color–form matching task. These new samples were nominally identical to, and were matched to, the familiar form comparisons. On the form-sample trials, however, the budgerigars could make either a high- or a low-frequency call to obtain those comparisons.
Despite these nondifferential vocal-call contingencies, the budgerigars nonetheless exhibited differential vocal calling to the form samples as they learned to match accurately with them. Specifically, they “…transferred the call signaling the to-be-reinforced form in the color-to-form MTS task to the form in the identity form-to-form MTS task.” (Manabe et al., 1995, p. 132). In other words, if the budgerigars were explicitly required to make a high-frequency call to the color sample that occasioned a particular form-comparison choice, they eventually made a high call to that form stimulus when it appeared as one of the new samples in form sample – form comparison matching.
Manabe et al. (1995) proposed a stimulus equivalence account to explain this emergent differential sample behavior (see also Saunders & Williams, 1998; Sidman, 1994, 2000). Specifically, they argued that initial color–form MTS training yielded a bidirectional association between the vocal calls required to the color samples and the subsequently reinforced form (comparison) stimuli. In other words, the explicitly reinforced [high call–form] and [low call–form] relations in color–form MTS were symmetrical. Consequently, when the forms later appeared as sample stimuli in form–form MTS, they eventually occasioned the high- versus low-frequency calls that preceded them in original training. This explanation, if substantiated, would be newsworthy given the difficulties of demonstrating symmetry in nonhuman animals (e.g., D’Amato, Salmon, Loukas, & Tomie, 1985; Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Sidman et al., 1982; but see Frank & Wasserman, 2005). It was a major reason for the independent test of this account reported here.
We were also skeptical of the symmetry explanation in view of previous research from this laboratory (Urcuioli et al., 2002) showing that emergent differential sample behavior in pigeons does not require bidirectional associations of the sort postulated by Manabe et al. (1995). For instance, Urcuioli et al. (2002, Experiment 2) initially trained pigeons on two-sample MTS in which they had to complete a differential-reinforcement-of-low-rates-of responding (DRL) requirement to one sample and a fixed-ratio (FR) requirement to the other in order to obtain the comparisons. Later, two new samples matched to the same comparison alternatives were added to the task. As in the Manabe et al. procedure, pigeons could complete either the DRL or the FR requirement to these samples to obtain the comparisons. Importantly, the added samples were nominally different from the comparison alternatives, thus removing any possibility that responding to these samples could reflect symmetry between the required DRL versus FR patterns and the reinforced comparisons on the other matching trials. Nevertheless, differential (DRL vs. FR) responding emerged to the new samples in 6 of 12 pigeons (see also Urcuioli et al. 2002, Experiment 3 for similar results using different sample-peck locations).
Obviously, other mechanisms besides symmetry will produce such emergent behavior. One possibility is that the added samples became functionally equivalent to the originally trained samples via the common reinforced choices they occasioned (Urcuioli, 2006a; cf. Goldiamond, 1962). In fact, the pigeons exhibiting emergent differential sample behavior responded to each new sample in a manner very similar to that required to the sample occasioning the same reinforced choice. [This was also true, by the way, for Manabe et al.’s (1995) budgerigars.] Although acquired equivalence is usually demonstrated by reinforcing a new behavior to one sample in a presumed class and later observing that same behavior to another sample in the same class (e.g., Urcuioli et al., 1989; Wasserman et al., 1992), our data suggest that behavior already conditioned to a sample will emerge to another sample that later becomes functionally equivalent to it.
Adventitious reinforcement is a second possibility (Saunders & Williams, 1998). According to this account, the reinforced relation in original training between each (required) sample–response pattern and the corresponding comparison choice is reproduced on the new-sample trials because pigeons are likely to emit one of the two previously trained response patterns when confronted with a new sample. Furthermore, if these response patterns had become cues for comparison selection in original training, then pigeons ought to make the same choices following those patterns on the new-sample trials. If those choices are reinforced, so too are the differential response patterns preceding them. In short, adventitious reinforcement can yield emergent differential sample behavior if (a) both explicitly trained response patterns occur to the new samples soon after their introduction, and (b) each pattern cues a particular reinforced comparison choice. The former was confirmed by other analyses reported by Urcuioli et al. (2002, see their Table 2). The latter is supported by independent data showing that differential sample responding is a potent cue for matching by pigeons (e.g., Urcuioli & Honig, 1980; Urcuioli, Lionello-DeNolf, Michalek, & Vasconcelos, 2006).
The present experiments, then, were designed to evaluate the three proposed mechanisms for emergent sample behavior with a special focus on the bidirectional transfer (symmetry) account. Although Urcuioli et al. (2002) showed that symmetry is not necessary to produce emergent differential sample behavior, their results do not rule out the possibility that it could be sufficient. In other words, if the MTS contingencies preclude adventitious reinforcement and acquired equivalence, will differential responding emerge to new sample stimuli nominally identical to the comparisons that appear following other samples to which pigeons must respond differentially? We also tested the implication from the adventitious reinforcement and acquired equivalence accounts that differential sample behavior should emerge on new-sample trials independently of the particular sample–comparison relations reinforced on those trials providing that the comparisons are identical to those appearing on familiar-sample trials.
Experiment 1
The three groups of pigeons in this experiment were eventually trained on MTS with two line orientations and two hues as sample stimuli. Training was conducted in two stages: first, with only the line-orientation samples and afterwards, with both line-orientation and hue samples. During the initial stage, all pigeons matched vertical- and horizontal-line samples to red and green comparisons, respectively, that they obtained by completing a DRL 3-s schedule to one line sample and a FR 20 schedule to the other (analogous to the high vs. low vocal calls in Manabe et al., 1995). During the second stage of training (see Table 1), these matching trials were supplemented by additional trials with red and green samples, the same stimuli that continued to appear as comparisons following the line samples. On the hue-sample trials, completing either the DRL 3-s or the FR 20 requirement (denoted as drl/fr in Table 1) produced the comparisons. For two groups (SID and SODD), the hue comparisons that appeared were the same as those appearing on the line-sample trials (hence, the “S” in the group designations). “ID” and “ODD” designate whether the hue-sample matching contingencies were identity or oddity. For the other group (DFRN), the comparison alternatives on the hue-sample trials, a solid white field and a circular white annulus, were different from those on the line-sample trials.
Table 1.
Line- and Hue-Sample Matching Contingencies for Each Group in Experiment 1.
| Group | ||
| SID | SODD | DFRN |
| V - DRL 3 s → R+ | V - DRL 3 s → R+ | V - DRL 3 s → R+ |
| H - FR 20 → G+ | H - FR 20 → G+ | H - FR 20 → G+ |
| R - drl/fr → R+ | R - drl/fr → G+ | R - drl/fr → W+ |
| G - drl/fr → G+ | G - drl/fr → R+ | G - drl/fr → C+ |
Note. V = vertical lines, H = horizontal lines, R = red, G = green, W = white, C = circle, DRL and drl = differential-reinforcement-of-low-rates-of-responding, FR and fr = fixed-ratio, (+) = reinforced comparison. Sample stimuli and sample–response schedules appear to the left of the arrows; reinforced comparison choices appear to the right of the arrows. Counterbalancing of the DRL 3 s and FR 20 sample-response schedules and the nonreinforced comparisons are not shown. Underlining of “drl” and “fr” indicate the sample-response pattern that should emerge assuming bidirectional transfer. Italics indicate what pattern should emerge via adventitious reinforcement or acquired sample equivalence. SID = same comparison alternatives on all trials with identity matching contingences for red and green samples. SODD = same comparison alternatives on all trials with oddity contingencies for red and green samples. DFRN = different comparison alternatives following vertical and horizontal samples than following red and green samples.
The hue-sample trials permitted us to test predictions regarding emergent differential sample behavior derived from the three explanatory accounts. For instance, if the relations between the explicitly required DRL and FR sample-response patterns and the reinforced red and green choices, respectively, on line-sample trials are symmetrical (Manabe et al., 1995), then a DRL-like pattern of responding to the red sample and a FR-like pattern of responding to the green sample should emerge in all three groups (as underlined in Table 1). The only qualification is that for Group SODD, this prediction must assume that any additional influence of adventitious reinforcement or acquired sample equivalence (see below) does not mask or negate bidirectional transfer. In any event, the sample-response patterns observed in Group DFRN will be the most telling in regards to symmetry because the different comparisons appearing on their hue- and line-sample trials preclude any possibility that the other two mechanisms could affect sample responding.
By contrast, if the underlying mechanism is adventitious reinforcement (Saunders & Williams, 1998) or acquired sample equivalence (Urcuioli et al., 2002; Urcuioli, 2006a), Group DFRN should not exhibit emergent differential sample behavior to the red and green samples for the reason mentioned above. Groups SID and SODD, however, should do so because their hue samples are followed by the same comparisons as those following the line samples.
The latter two accounts also predict that Groups SID and SODD will differ in how differential responding will segregate to the red and green samples. For Group SID, the predicted response pattern to the red sample should correspond to that explicitly required to the vertical sample (e.g., a slow, spaced responding characteristic of DRL schedules, as italicized in Table 1) and the response pattern to the green sample should correspond to the pattern explicitly required to the horizontal sample (e.g., the rapid, uninterrupted responding characteristic of FR schedules, as italicized in Table 1). For Group SODD, just the opposite is predicted: Pigeons should exhibit an FR-like pattern to the red sample and a DRL-like pattern to the green sample (see italicized “drl” and “fr” in Table 1). The rationale is as follows.
The many-to-one sample-comparison relations for Group SID (viz., vertical and red samples both occasion a reinforced red-comparison choice, and horizontal and green samples both occasion a reinforced green-comparison choice) should yield an acquired equivalence between the vertical and red samples and between the horizontal and green samples (Urcuioli et al., 1989; 1995). Consequently, DRL responding already conditioned to the vertical sample should begin to appear to the added red sample, and FR responding already conditioned to the horizontal sample should begin to appear to the added green sample. The corresponding many-to-one relations for Group SODD, however, should produce acquired equivalence between their vertical and green samples and between their horizontal and red samples. For this group, then, DRL responding already conditioned to vertical should develop to the added green sample, and FR responding already conditioned to horizontal should develop to the added red sample—the opposite of that predicted for Group SID.
According to adventitious reinforcement, if pigeons obtain the comparison alternatives on a hue-sample trial by completing the DRL requirement, they should select the comparison they previously learned to choose after DRL responding. Likewise, if they obtain the comparison alternatives on a hue-sample trial by completing the FR requirement, they should select the comparison they previously learned to choose after FR responding. Differential reinforcement of these choices early in training should then promote the development of the DRL pattern to the red sample and the FR pattern to the green sample in Group SID, and vice versa in Group SODD (cf. Saunders & Williams, 1998; Urcuioli et al., 2002; cf. italicized “drl” and “fr” in Table 1).
Method
Subjects
Eighteen White Carneau pigeons (Columba livia) obtained from the Palmetto Pigeon Plant (Sumter, SC) participated in this experiment. Six were experimentally naïve. The remaining 12 had previous experience in a study (or studies) unrelated to the present experiment. The 18 pigeons were randomly divided into three groups with the constraints that each group contain 2 experimentally naïve pigeons and roughly equal numbers of experienced pigeons with the same prior history. All were housed individually in stainless-steel, wire-mesh cages in a colony room on a 14-hr:10-hr light-dark cycle with lights on at 07:00. Grit and water were freely available in the home cages. Prior to the experiment, each pigeon was deprived to 80% of its free-feeding body weight and maintained at that level throughout the experiment by restricting its daily feeding to the experimental sessions. Supplemental feedings were given in the home cage only if a pigeon did not obtain a sufficient amount of food in a session to maintain its 80% weight and on the 1 day per week the experiment was not run.
Apparatus
Two BRS/LVE (Laurel, MD) three-key pigeon chambers (Model PIP-016 panels inside Model SEC-002 enclosures) were used. The pigeons’ compartment in each chamber measured 30.5 × 36.8 × 34.3 cm and could be illuminated by a GE #1829 house light attached to the top center region of the panel. The house light was covered by a metal housing with an opening that directed light toward the ceiling. Each panel contained three 2.5-cm-diameter response keys horizontally aligned in a row 25.4 cm above the grid floor and approximately 8.3 cm apart, center-to-center. A 12-stimulus inline projector (BRS/LVE Model IC-901-IDD) was mounted behind each key. The center projector could display red and green homogeneous fields, three white vertical lines and three white horizontal lines on black backgrounds, and a solid inverted white triangle on black background (BRS/LVE Pattern No. 692). Each side-key projector could display red, green, and white homogeneous fields, and a small, open white circle on a black background (BRS/LVE Pattern No. 696). The food magazine was mounted behind a 5.8 × 5.8 cm opening approximately 11 cm between the center key and the grid floor. When the magazine was raised to provide access to Purina Pigeon ProGrains, it was illuminated by an ESB-28 light bulb attached to the back of the metal housing that covered the magazine opening. Continuously running blower fans attached to the chambers provided ventilation and masking noise. A single IBM-compatible 386 computer collected data and controlled all experimental events via a custom-built interface.
Procedure
The experiment consisted of three phases: preliminary training, line-sample matching, and line- and hue-sample matching.
Preliminary Training
The experimentally naïve pigeons were first trained to eat quickly and reliably from a periodically raised and illuminated food magazine, after which their key peck response was shaped by the method of successive approximations to a white triangle on the center key. All pigeons were then trained over the course of four 60-trial sessions to peck vertical and horizontal lines on the center key, red and green hues on the center and side keys, and the homogeneous white and the circle stimuli on the side keys. A single peck to the stimulus appearing on each trial was immediately reinforced by access to food, the duration of which was adjusted daily (as needed) for each pigeon to maintain its 80% body weight. Successive trials were separated by a 10-s intertrial interval (ITI).
Next, all pigeons received 10–16 sessions during which they learned to respond differentially to vertical and horizontal lines on the center key. A DRL 3-s schedule was in effect for pecking one line stimulus, and an FR schedule was in effect for the other line stimulus, counterbalanced within each group. The FR value was gradually raised across sessions from an initial value of 2 to a terminal value of 20, with a minimum of 2 sessions at the terminal value. Successive trials in each 60-trial session were separated by a 10-s ITI with the house light off during the first 9 s. The house light came on for the last 1 s of the ITI and remained on until the end of the reinforcement cycle. Reinforcement durations varied from 2–6 s across sessions for each bird in a manner that maintained its 80% body weight as closely as possible.
Line-Sample Matching
After completing preliminary training, all pigeons learned to match vertical- and horizontal-line samples on the center key to red and green comparisons, respectively, on the adjacent side keys. Obtaining the comparisons required completion of a DRL 3-s sample-response requirement for one line sample and a FR 20 requirement for the other. The requirements for each pigeon matched those during its preliminary training.
Each 100-trial MTS session contained equal numbers of the four possible trial types (two samples × two left-right configurations of the comparisons) which occurred in random order with the constraint that no single trial type occur more than three times in a row. The sample stimulus on each trial went off as the comparison alternatives appeared, after which a single peck to either comparison turned both off and produced either food reinforcement or a timeout. For all pigeons, pecking the red comparison after the vertical sample and pecking the green comparison after the horizontal sample was reinforced. Pecking the alternative comparison, green after vertical or red after horizontal, immediately turned off the house light for a period equal to the reinforcement duration for that session. A 10-s ITI, the first 9 s of which was spent in darkness, separated successive trials. The house light came on for the last 1 s of the ITI and remained on until the end of the reinforcement cycle or until an incorrect comparison choice. Reinforcement durations for each pigeon again varied between 2–6 s across sessions.
Pigeons were trained on line-sample MTS for a minimum of 10 sessions and until matching accuracy was 90% correct or higher for 5 of 6 consecutive sessions.
Line- and Hue-Sample Matching
Next, two new samples (red and green) were introduced into the matching task and, for one group, a new set of comparison alternatives (white and circle) as well. Table 1 shows the matching contingencies for each group.
Each 96-trial session was divided equally among the two familiar samples, the vertical and horizontal lines, and the two new samples, red and green. On vertical- and horizontal-sample trials, the sample-response and choice contingencies were identical to those in effect during each pigeon’s line-sample task. On red- and green-sample trials, spacing two successive key pecks 3 s or more apart (drl) or pecking 20 times (fr), whichever occurred first, produced the comparisons.
For Groups SID and SODD, the comparisons following the red and green samples were the same as those following the vertical and horizontal samples—namely, red and green hues. For Group SID, pecking the red comparison after the red sample and the green comparison after the green sample was reinforced (i.e., identity contingencies were in effect). For Group SODD, pecking the green comparison after the red sample and the green comparison after the red sample was reinforced (i.e., oddity contingencies were in effect). For Group DFRN, the comparison alternatives on the hue- and line-sample trials differed: white and circle versus red and green, respectively. For this group, pecking the white comparison after the red sample and the circle comparison after the green sample was reinforced.
The eight possible trial types (four samples × two left-right configurations of the comparisons) were presented equally often and in random order in each MTS session with the constraint that none occur more than three times in a row. Each pigeon was trained on its respective task for 30 sessions. All other procedural details were the same as those for line-sample matching.
For all statistical analyses, Type I error rate was set at .05 on a per-decision basis using the tabled F values reported by Rodger (1975).
Results
By the last two preliminary training sessions, all pigeons exhibited a slow, spaced pattern of responding on the DRL 3-s schedule and rapid, uninterrupted responding on the FR 20 schedule. These patterns continued throughout line-sample MTS and were evident in the interresponse times (IRTs) for pecking these samples. For example, for the last two MTS sessions and averaged across all pigeons, 68.5% of the IRTs for the DRL line sample exceeded 1500 ms1 (range: 44.0–90.8%) versus only 0.8% of the IRTs for the FR line sample (range: 0.1–3.6%). Indeed, 93.1% of the FR IRTs were less than 500 ms (range: 61.1–99.2%). Matching with the line samples was acquired quickly and did not differ significantly between groups. Groups SID, SODD, and DFRN needed an average of 6.3, 5.3, and 5.3 sessions, respectively, to reach 90% or better accuracy, F(2, 15) = 0.33. Accuracies over the last five MTS sessions were comparable as well: 96.0%, 96.8%, and 96.4% correct, respectively, F(2, 15) = 0.38.
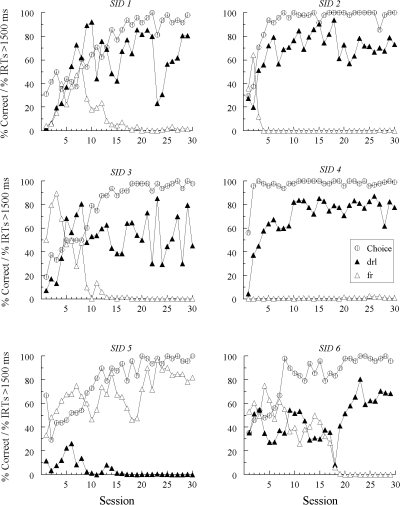
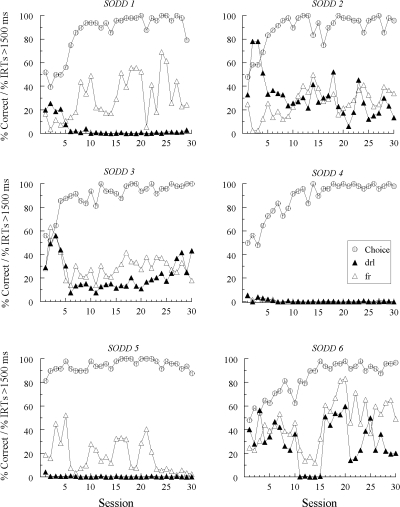
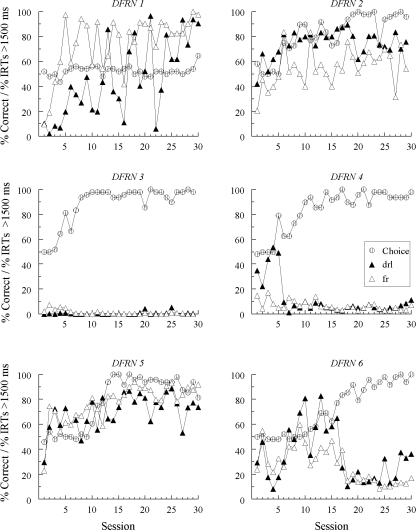
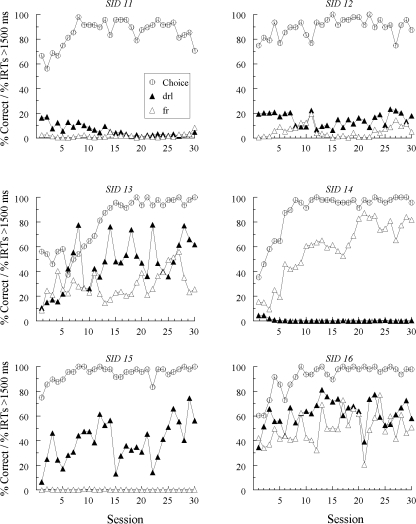
Figures 1, 2, and 3 show percent correct comparison selections (“Choice”) over the 30 sessions in which the new (red and green) samples were added to the matching task. Also shown are the percentages of IRTs greater than 1500 ms for each new sample (note that the figure legend denotes the response patterns expected on the basis of symmetry). The choice functions show that every pigeon except one (DFRN 1) learned to match accurately with the red and green hue samples by the end of training. Hue-sample acquisition was more rapid for Groups SID and SODD than for Group DFRN (10.2 and 8.3 sessions versus 17.7 sessions, respectively, F(2, 15) = 3.98), a difference that was not unexpected given that the former groups had different new-sample comparison alternatives (red and green) than the latter group (white and circle). Line-sample accuracies remained uniformly high (not shown).
Fig 1.
The percentages of correct choice responses (Choice) and the percentages of sample–response IRTs > 1500 ms with the new (red and green) samples during four-sample matching acquisition for each Group SID pigeon in Experiment 1. IRT data are labeled “drl” and “fr” to reflect the response patterns predicted to emerge to the hue samples based on bidirectional transfer (symmetry) between the explicitly required DRL and FR patterns to the line samples and subsequently reinforced hue-comparison choices.
Fig 2.
The percentages of correct choice responses (Choice) and the percentages of sample-response IRTs > 1500 ms with the new (red and green) samples during four-sample matching acquisition for each Group SODD pigeon in Experiment 1. IRT data are labeled “drl” and “fr” to reflect the response patterns predicted to emerge to the hue samples based on bidirectional transfer (symmetry) between the explicitly required DRL and FR patterns to the line samples and subsequently reinforced hue-comparison choices.
Fig 3.
The percentages of correct choice responses (Choice) and the percentages of sample-response IRTs > 1500 ms with the new (red and green) samples during four-sample matching acquisition for each Group DFRN pigeon in Experiment 1. IRT data are labeled “drl” and “fr” to reflect the response patterns predicted to emerge to the hue samples based on bidirectional transfer (symmetry) between the explicitly required DRL and FR patterns to the line samples and subsequently reinforced hue-comparison choices.
Of greater interest are the patterns of responding to the new samples, shown by the filled- and open-triangle functions in Figures 1, 2, and 3. For these IRT data, the new samples are labeled “drl” or “fr” to indicate the response patterns expected to emerge to the red and green samples based on bidirectional transfer (symmetry). To reiterate, given that selecting the red comparison was reinforced after completing the DRL schedule on the line-sample trials, symmetry predicts that DRL patterning should emerge to the red sample. Likewise, given that selecting the green comparison was reinforced after completing the FR schedule on the line-sample trials, FR patterning should emerge to the green sample (see Table 1).
As the separation in the IRT functions show, differential responding to the hue samples eventually emerged for every Group SID pigeon. In Group SODD, 4 birds (SODD 1, SODD 3, SODD 5, and SODD 6) also responded differentially to red and green for an extended number of sessions sometime during their training, although in less substantial fashion and in a different manner than for Group SID (see below). Finally, and in contrast to the other two groups, most pigeons in Group DFRN showed either no signs of emergent differential sample responding (e.g., DFRN 3 and DFRN 4), or a pattern of responding that was highly erratic. The exception was DFRN 2, which tended to show a higher percentage of IRTs > 1500 ms on red-sample (“drl”) trials than on green-sample (“fr”) trials.
Closer examination of the observed emergent differential sample responding shows that all pigeons except one in Group SID pecked in a DRL-like fashion (viz., with a relatively high percentage of IRTs > 1500 ms) to the hue sample nominally identical to the reinforced comparison that followed the required DRL pattern on the line-sample trials and, likewise, in a FR-like fashion (viz., with a relative low percentage of IRTs > 1500 ms) to the hue sample nominally identical to the reinforced comparison that followed the required FR pattern on the line-sample trials. The exception was SID 5 whose emergent sample behavior was precisely the opposite: It pecked relatively rapidly to the hue sample with the ostensibly bidirectional DRL association and relatively slowly to the hue sample with the ostensibly bidirectional FR association.
By contrast, the Group SODD pigeons exhibiting emergent differential sample behavior mostly pecked in a DRL-like fashion to the hue sample nominally identical to the reinforced comparison that followed the required FR pattern on the line-sample trials and in a mostly FR-like fashion to the hue sample nominally identical to the reinforced comparison that followed the required DRL pattern on the line-sample trials. Note that the filled-symbol (drl) functions in Figure 2 are generally below the open-symbol (fr) functions for SODD 1, SODD 3, SODD 5 and SODD 6.
It is important to note that the between-group differences in emergent sample behavior cannot be traced to corresponding differences in line-sample responding. For example, the percentages of IRTs > 1500 ms on the explicitly required DRL and FR schedules over the last 15 sessions were 68.3% and 0.4%, respectively, in Group SID, 68.4% and 0.5% in Group SODD, and 71.4% and 1.5% in Group DFRN. ANOVA on these data showed no overall between-group difference, F(2, 14) = 0.33, and no Group × Trial Type interaction, F(2, 14) = 0.17.
Discussion
The results of Experiment 1 do not support a bidirectional transfer (symmetry) account of emergent sample behavior in pigeons (cf. Manabe et al., 1995). The finding that there was very little, if any, evidence of emergent differential sample responding in Group DFRN indicates that the reinforced relations between the required DRL versus FR patterns and the red versus green reinforced comparisons were not symmetrical. In other words, despite reinforced pecking to red after completion of a DRL schedule on familiar-sample trials, these pigeons did not develop a slow, spaced pattern of responding just to red when it later appeared as a sample stimulus. Likewise, despite reinforced pecking just to green after completion of a FR schedule on familiar-sample trials, these pigeons did not develop a rapid, uninterrupted pattern of responding to green when it later appeared as a sample stimulus.
Furthermore, the symmetry account is directly contradicted by the results from Group SODD. Although some pigeons in this group showed emergent differential sample behavior, the way in which differential responding segregated to the red and green samples was exactly the opposite of what symmetry would predict (cf. Table 1 and Figure 2).
In view of what we know about the functional stimuli in pigeons’ two-alternative MTS, these results are not surprising. After all, postulating symmetry between the explicitly reinforced DRL and FR patterns and the subsequently reinforced red and green comparisons assumes that red is red, and green is green, no matter where they appear (viz., on a side key or on a center key). But this assumption is unjustified. For example, Lionello and Urcuioli (1998) demonstrated that the same nominal stimulus is functionally different for pigeons when it appears in different locations (see also Urcuioli, 2006b; 2007a). They showed that pigeons trained to high levels of matching accuracy could not maintain those levels when the sample stimuli, which had routinely appeared on the center key, appeared on the left or right side keys. Indeed, accuracies often fell to chance levels on the latter trials. Thus, red on the center key (where it typically appears as a sample) is not the same stimulus as red on a side key (where it typically appears as a comparison), and vice versa. This perspective also helps to make sense of the many reported failures to find symmetry in animals trained and tested in two-alternative MTS (e.g., D’Amato et al., 1985; Hogan & Zentall, 1977; Lipkens, Kop, & Matthijs, 1988; although see Lionello-DeNolf & Urcuioli, 2002).
The emergent sample behavior observed in Groups SID and SODD and its absence in Group DFRN, however, are consistent with each of the two alternative accounts. Considering acquired equivalence first, the common reinforced comparison choice following the vertical and red samples, and the horizontal and green samples in Group SID should have made those common-choice samples functionally equivalent. Likewise, the vertical and green samples, and horizontal and red samples for Group SODD should have become functionally equivalent for the same reason (see Table 1). Acquired equivalence should then promote the emergence of the same pattern of responding to each hue sample that was explicitly conditioned to each line sample that occasioned the same reinforced choice, as was observed in both Group SID and Group SODD.
Adventitious reinforcement, too, can explain the emergence of differential sample behavior in Groups SID and SODD and its absence in Group DFRN, and the specific emergent patterns observed in the former two groups. In short, after learning during line-sample MTS training to peck the red comparison after a DRL pattern and the green comparison after an FR pattern, emitting these patterns on the hue-sample trials should cue their previously reinforced comparison choices. The differential reinforcement for these choices on the red- and green-sample trials would then promote development of a DRL pattern to the red sample and an FR pattern to the green sample in Group SID, and vice versa in Group SODD (see Table 1; cf. Saunders & Williams, 1998; Urcuioli et al., 2002), as was observed.
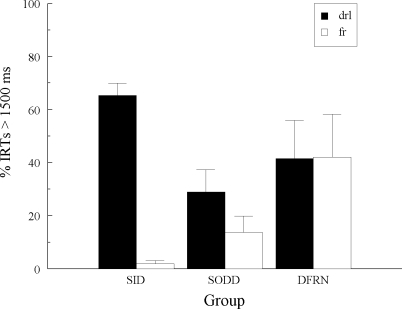
Figure 4 summarizes the hue-sample IRTs by showing the average percentages of IRTs >1500 ms over the last 15 training sessions as a function of the response pattern predicted from acquired equivalence and adventitious reinforcement in Groups SID and SODD (see italicized “drl” and “fr” in Table 1)2. For Group DFRN, the predicted patterns are those based on symmetry given that the alternative accounts do not apply for this group. Figure 4 underscores the finding of no emergent differential sample responding in Group DFRN and the clear evidence for such responding in Groups SID and SODD. The emergent effect is also more substantial in Group SID than in Group SODD, corroborating the individual-subject data (see Figures 1 and 2). ANOVAs on the IRT data from each group showed that the percentages of IRTs > 1500 ms were significantly higher on “drl” than on “fr” trials in Group SID, F(1, 4) = 268.53, and in Group SODD, F(1, 5) = 7.12, but not in Group DFRN , F(1, 5) = 0.01. A separate, two-way ANOVA on the data from Groups SID and SODD showed the expected, significant Group × “drl/fr” interaction, F(1, 9) = 56.72.
Fig 4.
The average percentage of hue-sample IRTs > 1500 ms (+ 1 SEM) for each group in Experiment 1 in which the predicted emergent sample behavior would be like that observed on a DRL schedule (“drl”) or on an FR schedule (“fr”) assuming acquired equivalence or adventitious reinforcement (Groups SID and SODD) or bidirectional transfer (Group DFRN).
The disparities between the latter groups in the number of pigeons exhibiting an emergent effect (more in Group SID) and in the size of the effect (larger in Group SID) suggest that something else may have affected performances. For instance, perhaps the stronger effect in Group SID arose because bidirectional transfer (symmetry) supplemented the influences of acquired equivalence and/or adventitious reinforcement. Likewise, perhaps the weaker effect in Group SODD reflects the opposing influence of bidirectional transfer vis-à-vis the latter mechanisms. Thus, even though there are good reasons to reject symmetry as the sole source of pigeons’ emergent differential sample behavior, it might make a relatively weak contribution to that behavior in its ability to add to or detract from the influence of acquired equivalence and/or adventitious reinforcement.
Experiment 2
The purpose of Experiment 2 was twofold. First, we wanted to evaluate the possibility that the absence of emergent differential sample behavior in Group DFRN in Experiment 1 simply reflected the fact that these pigeons would not have shown the effect under any conditions. After all, even when the comparisons are the same on all matching trials, not all pigeons show emergent differential sample behavior (see Figures 1 and 2; also see Urcuioli et al., 2002, Figures 2 and 3). In this experiment, then, the Group DFRN pigeons were retrained with the same comparisons on line- and hue-sample trials. If selection factors were responsible for their results in Experiment 1, these pigeons should again show no signs of emergent differential sample behavior. On the other hand, if their prior results were the consequence of presenting different comparisons following the different samples, then they should eventually respond differentially to the added hue samples in this experiment.
Second, we wanted to determine if the weaker emergent effect in Group SODD than in Group SID in Experiment 1 was due to the oddity versus identity contingencies, respectively, on their hue-sample trials. To find out, we retrained the former Group SODD birds with identity contingencies and the former Group SID birds with oddity contingencies. If contingencies are important, oddity should again yield weaker emergent sample behavior effects than identity.
Method
Subjects and Apparatus
The pigeons from Experiment 1 were used. Those previously assigned to Group DFRN were assigned to hue-sample identity contingencies in this experiment (see Group SID in Table 2), as were the pigeons previously assigned to Group SODD. The Group SID pigeons from Experiment 1 were assigned to hue-sample oddity contingencies (see Group SODD in Table 2).
Table 2.
Line- and Hue-Sample Matching Contingencies for Each Group in Experiment 2.
| Group | |
| SID | SODD |
| V — FR 20 → B+ | V — FR 20 → B+ |
| H — DRL 3 s → Y+ | H — DRL 3 s → Y+ |
| B — drl/fr → B+ | B — drl/fr → Y+ |
| Y — drl/fr → Y+ | Y — drl/fr → B+ |
Note. V = vertical lines, H = horizontal lines, B = blue, Y = yellow, DRL and drl = differential-reinforcement-of-low-rates-of-responding schedules, FR and fr = fixed-ratio schedule, (+) = reinforced comparison. Sample stimuli and sample-response schedules appear to the left of the arrows; reinforced comparison choices appear to the right of the arrows. Counterbalancing of the DRL 3 s and FR 20 sample-response schedules and the nonreinforced comparisons are not shown. SID = same comparison alternatives on all trials with identity matching contingences for red and green samples. SODD = same comparison alternatives on all trials with oddity contingencies for red and green samples.
Procedure
Preliminary Training
The DRL and FR requirements for the vertical and horizontal stimuli were reversed for each pigeon vis-à-vis Experiment 1 in order to create matching tasks as different as possible relative to those on which pigeons were previously trained. The reversal was conducted off the matching baseline in a series of 60-trial successive discrimination sessions like those used during the final phase of preliminary training in Experiment 1. The DRL 3-s requirement for one line stimulus was in effect throughout the reversal sessions, whereas the FR parameter for the remaining stimulus was gradually raised from 5 to 20 over the 7–18 sessions, depending on individual performances. A minimum of 2 sessions was run with the FR 20 schedule. All other procedural details were identical to those in Experiment 1.
Line-Sample Matching
Next, pigeons learned to match vertical- and horizontal-line samples to blue and yellow comparisons, respectively, with each pigeon’s sample-response requirements identical to those for its preliminary training. All other details including the performance criteria were the same as those for line-sample MTS in Experiment 1.
Line- and Hue-Sample Matching
Following acquisition of line-sample MTS, 30 additional training sessions were given with two new samples (blue and yellow hues) added to the line-sample task. On blue- and yellow-sample trials, completing either the DRL 3-s or the FR 20 requirement, whichever occurred first, produced blue and yellow comparison alternatives. For Group SID (see Table 2), pecking the blue comparison on blue-sample trials and the yellow comparison on yellow-sample trials was reinforced (viz., identity contingencies were in effect). For Group SODD, pecking the yellow comparison on blue-sample trials and the blue comparison on yellow-sample trials was reinforced (viz., oddity contingencies were in effect.) All other details were identical to those for the corresponding task in Experiment 1.
Results and Discussion
All pigeons eventually behaved in a schedule-appropriate manner to the reversal of the DRL and FR schedules during preliminary training, and they continued to do so throughout subsequent line-sample MTS training. For example, for the last two matching sessions and averaged across all pigeons, 63.3% of the IRTs to DRL line sample were > 1500 ms (range: 44.6–89.3%) versus 0.9% for the FR line sample (range: 0.0–3.5%). Matching acquisition was comparable for the two groups: Groups SID and SODD reached 90% or better accuracy in an average of 4.5 and 4.7 sessions, respectively, F(1, 16) = 0.04. Terminal accuracy levels were also comparable: 97.4% and 97.5% correct, respectively, over the last five line-sample sessions, F(1, 16) = 0.01.
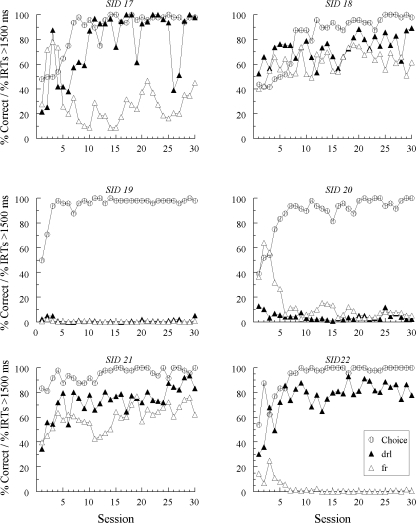
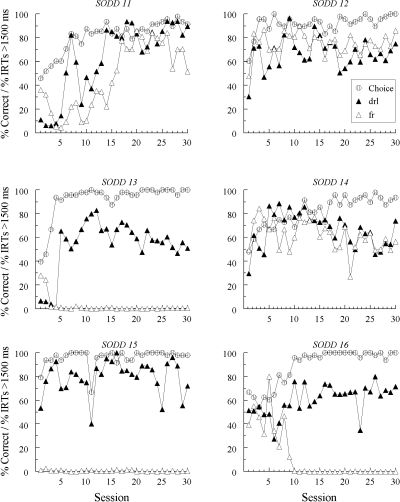
Figures 5, 6, and 7 plot each pigeon’s accuracy (“Choice”) with the added (blue and yellow) sample and the percentage of IRTs > 1500 ms to each hue sample over the 30 training sessions. The Group SID results are split with Figure 5 showing data from those pigeons previously assigned to Group SODD in Experiment 1 and Figure 6 showing data from those previously assigned to Group DFRN. In all three figures, the IRT results are labeled as “drl” or “fr” as a function of whether the hue sample occasioned the same reinforced choice as the line sample for which DRL or FR sample responding was explicitly required.
Fig 5.
The percentages of correct choice responses (Choice) and the percentages of sample-response IRTs > 1500 ms with the new (blue and yellow) samples during four-sample matching acquisition for each Group SID pigeon in Experiment 2 that was previously assigned to the SODD condition in Experiment 1. IRT data are labeled “drl” and “fr” as a function of whether each hue sample occasioned the same comparison choice as the line sample to which DRL or FR responding was required.
Fig 6.
The percentages of correct choice responses (Choice) and the percentages of sample-response IRTs > 1500 ms with the new (blue and yellow) samples during four-sample matching acquisition for each Group SID pigeon in Experiment 2 that was previously assigned to the DFRN condition in Experiment 1. IRT data are labeled “drl” and “fr” as a function of whether each hue sample occasioned the same comparison choice as the line sample to which DRL or FR responding was required.
Fig 7.
The percentages of correct choice responses (Choice) and the percentages of sample-response IRTs > 1500 ms with the new (blue and yellow) samples during four-sample matching acquisition for each Group SODD pigeon in Experiment 2 (previously assigned to the SID condition in Experiment 1). IRT data are labeled “drl” and “fr” as a function of whether each hue sample occasioned the same comparison choice as the line sample to which DRL or FR responding was required.
The choice functions show that all pigeons learned to match accurately with the blue and yellow samples. The SID and SODD conditions did not differ from one another in the average number of sessions to reach 90% or better accuracy with the hue samples: 7.7 and 10.0 sessions, respectively, F(1, 16) = 0.59. Likewise, matching acquisition in the SID condition did not differ as function of group assignment in Experiment 1: 8.8 versus 6.5 sessions, F(1, 10) = 1.28.
There was evidence of emergent differential sample responding in both conditions. Three of the 6 pigeons in the SID condition previously trained with oddity contingencies in Experiment 1 (SID 12, SID 13, and SID 15—see Figure 5) showed higher percentages of IRTs > 1500 ms to the hue sample occasioning the same reinforced choice as the line sample to which DRL responding was required than to the hue sample occasioning the same reinforced choice as the line sample to which FR 20 responding was required. One pigeon (SID 14) showed the opposite pattern; it was not the same pigeon exhibiting the reversal in Experiment 1. Likewise, 3 of the 6 pigeons in the SID condition previously assigned to Group DFRN in Experiment 1 (SID 17, SID 21, and SID 22—see Figure 6) showed a similar emergent differential sample behavior effect. Finally, 3 of the 6 pigeons in the SODD condition (SODD 13, SODD 15, and SODD 16—see Figure 7) also responded in a DRL-like fashion to the hue sample occasioning the same reinforced choice as the DRL line sample and in a FR-like fashion to the hue sample occasioning the same reinforced choice as the FR line sample. Pigeon SODD 11 responded in a DRL-like fashion to both hue samples, but did so more frequently to the hue sample occasioning the same reinforced choice as the DRL line sample.
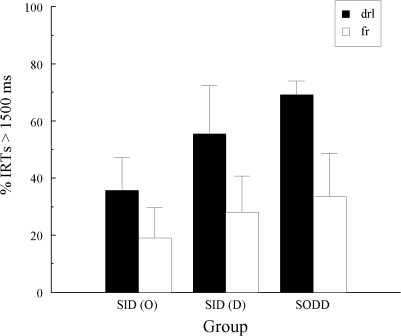
Figure 8 summarizes the average percentages of IRTs >1500 ms over the last 15 sessions as a function of whether each hue sample had a DRL or a FR association vis-à-vis the sample-response requirement for the line sample with which it shared a common reinforced choice.3 The results from the SID condition have been divided according to previous training in Experiment 1—oddity contingencies (O) or different comparisons (D). An overall ANOVA on these IRT data with group and schedule association (DRL versus FR) as factors showed a significant schedule-association effect, F(1, 14) = 12.16, but no group effect, F(2, 14) = 1.21, and no group × schedule-association interaction, F(2, 14) = 0.51. In short, independently of group assignment, there was a relatively high percentage of long IRTs to the hue sample that occasioned the same choice as the line sample to which pigeons had to complete a DRL schedule, and a relatively low percentage of long IRTs to the hue sample that occasioned the same choice as the line sample to which pigeons had to complete a FR schedule. Furthermore, the DRL versus FR IRT differences were comparable across groups.
Fig 8.
The average percentage of hue-sample IRTs > 1500 ms (+ 1 SEM) for each group in Experiment 2 in which the predicted emergent sample behavior would be like that observed on a DRL schedule (“drl”) or on a FR schedule (“fr”) assuming acquired equivalence or adventitious reinforcement. The Group SID data are divided according to whether these pigeons were trained with oddity (O) contingencies or with different (D) comparisons on the hue-sample trials in Experiment 1.
The results of this experiment are clear in regards to the two questions posed in the introduction. First, the absence of emergent differential sample behavior in Group DFRN in Experiment 1 (see Figure 3) was not due to “who these birds were” but, instead, to the use of different comparison alternatives on their new (hue) sample trials versus familiar (line) sample trials. With the same comparisons appearing on all matching trials, many of these pigeons now exhibited an emergent effect (see Figure 6).
Second, the difference in the size of the emergent effect between Groups SID and SODD in Experiment 1 (see Figure 4) was not reproduced here (see Figure 8): Group SODD showed just as strong an effect as Group SID. Apparently, the between-group difference observed in Experiment 1 was the result of random error, not to the nature of the sample–comparison contingencies (identity vs. oddity) on the new-sample trials. Consequently, the present data do not support the idea that bidirectional transfer had contributed to the emergent sample behavior observed in Groups SID and SODD in Experiment 1.
General Discussion
The results of the present experiments demonstrate that emergent DRL versus FR sample responding in pigeons’ MTS does not arise from bidirectional transfer between required DRL versus FR sample responding and the reinforced comparisons on other matching trials. If the latter relations were symmetrical, emergent differential sample behavior should have been clearly evident in Group DFRN, which was trained under ostensibly ideal conditions for detecting bidirectional transfer. For this group, training on line–hue MTS with DRL versus FR sample-response requirements for producing the hue comparisons was followed by training on a second, concurrent MTS task using the same hues as sample stimuli. Those hue samples were matched to different comparison stimuli than those in the line–hue task, so any bidirectional transfer could operate without contamination by, or competition from, adventitious reinforcement or acquired equivalence. The failure of emergent differential sample behavior to develop after 30 training sessions under these conditions indicates that symmetry, as postulated by Manabe et al. (1995), is not a viable explanation for such behavior. Furthermore, the absence of an emergent effect cannot be dismissed simply by arguing that none of the Group DFRN pigeons would have exhibited emergent behavior under any circumstance. To the contrary, Experiment 2 showed that when these same pigeons were trained with a common set of comparisons on line- and hue-sample trials, 3 of them developed a differential pattern of sample responding to the hue samples for which differential responding was not required.
The clear absence of any evidence of symmetry stands in stark contrast to recent demonstrations by Frank and Wasserman (2005) and García and Benjumea (2006) of this elusive, stimulus-equivalence effect in pigeons. As alluded to earlier, one reason for the discrepancy is that for symmetry to occur in the present paradigm, the functional stimuli must be the nominal stimuli themselves (e.g., red, green, etc.) independently of where they appear. But this is not the case in standard two-alternative MTS tasks. Interestingly, the Frank and Wasserman (2005) demonstration involved successive (go/no-go) matching in which samples and comparisons appeared singly and at only one location, thus avoiding the location problem inherent in two-alternative tasks. This procedural feature appears to be a necessary, although not a sufficient, condition for observing symmetry (Frank, 2007; Urcuioli, 2007b).
The García and Benjumea (2006) study required pigeons to peck five times to a left or a right key, each displaying the same stimulus, in order to obtain red and green comparisons on those same keys. Selecting one color (e.g., red) was reinforced following left sample pecking and the other (e.g., green) was reinforced following right sample pecking. Later, when tested with red on both keys or green on both keys, pigeons pecked more often to the key to which five pecks in training had preceded the reinforced choice of that color. Here, too, there was no change from training to testing in where red and green appeared (viz., always on a left or right key). Also, these authors argued that their symmetry effect depended on differential sample pecking (left vs. right) providing the cue for color comparison choices during training. In this respect, their procedure closely parallels the one used here, in which DRL and FR responding purportedly cued pigeons’ choices during initial line-sample MTS training. However, the two procedures differ from one another in whether the crucial stimuli shift locations from training to testing.
In any event, the emergent differential sample behavior observed in Groups SID and SODD, and the absence of such behavior in Group DFRN, are consistent with an explanation stating that such behavior originates from adventitious reinforcement and/or acquired sample equivalence. Both mechanisms require the same comparison alternatives on all matching trials, as was the case for Groups SID and SODD, and if this condition is met, both imply that the specific matching contingencies with the newly added samples should not matter. Indeed, considering both experiments together, the emergent effect appears to be as robust with new-sample oddity contingencies (Group SODD) as with new-sample identity contingencies (Group SID). These results complement our previous findings (Urcuioli et al., 2002, Experiments 2 and 3) showing emergent differential sample behavior with new-sample symbolic contingencies (albeit with comparisons identical to those appearing following the originally trained samples.)
Of course, not all birds in Groups SID and SODD responded differentially to the new (red and green) samples. But this was not unexpected given that with DRL and FR as the target sample behavior, Urcuioli et al. (2002, Experiment 2) found that only 6 of their 12 pigeons showed emergent differential sample responding and of the 6 that did, responding to the new samples for 4 pigeons became increasingly nondifferential with extended training. One reason for this may have to do with the fact that it is possible for different components of DRL and FR responding to blend together on the new-sample trials and still produce the comparison alternatives. For instance, a pigeon could peck 17 or 18 times in rapid succession (i.e., with short IRTs) to red or to green and then make the last two or three responses with relatively long IRTs. A second reason could be the lack of variation in response patterns to red and green early in training on the concurrent line- and hue-sample task. For instance, if pigeons pecked rapidly to each new sample on all (or virtually all) occasions, the comparisons would nonetheless appear after 20 pecks on these trials (see, for example, the data from SODD 4 in Figure 2 and from SID 19 in Figure 6). The same applies to a consistent DRL-like pattern to both new samples (see, for example, SODD 12 and SODD 14 in Figure 7). Under such conditions, reinforcement for the pigeon’s subsequent comparison choice cannot possibly produce across-sample segregation of two response patterns (cf. Saunders & Williams, 1998).
For 2 pigeons, SID 5 in Experiment 1 and SID 14 in Experiment 2, the segregation which did occur was the opposite of that predicted. Both pigeons pecked very rapidly (i.e., with short IRTs) to the hue sample that occasioned the same comparison choice as the line sample to which they had to space two successive responses 3 s apart, and relatively slowly (i.e., with long IRTs) to the hue sample that occasioned the same comparison choice as the line sample to which they had to peck 20 times. This peculiar result was not due to a programming or a recording error (we checked). Moreover, it was not accompanied by unstable choice accuracy on the hue-sample trials, something which might be expected assuming that the DRL and FR pattern required on the line-sample trials had cued comparison choice (Urcuioli & Honig, 1980). In other words, considering all matching trials together, DRL responding would “signal” one reinforced choice on line-sample trials but the opposite reinforced choice on hue-sample trials, and likewise for the FR pattern. A previous instance in which a pattern “reversal” was observed was accompanied by unstable new-sample accuracies (Urcuioli et al., 2002, Experiment 2; Bird F6).
We have no explanation, then, for the peculiar results of these “outliers” and hasten to add that their data contradict all three explanatory accounts we have discussed. Shortly after completing Experiment 2, we retrained each of these pigeons on the matching tasks in which they exhibited this reversed effect for further study. The reversed emergent sample-behavior patterns were recovered but with continued training, the differential response patterns for one pigeon (SID 5) deteriorated, eventually resulting in rapid pecking to both hue samples (i.e., a nondifferential FR pattern). With the remaining pigeon (SID 14), we tested the hypothesis that requiring DRL versus FR patterns to the line samples per se was sufficient for it to respond differentially to the hue samples. To do this, we replaced the hue comparisons on the hue-sample trials with comparisons different from those appearing on the line-sample trials, creating a MTS task like that for Group DFRN in Experiment 1 (see Table 1). This change caused deterioration in differential responding to the hue samples; in other words, SID 14 eventually responded nondifferentially to these samples. Unfortunately, when returned to baseline (viz., same comparisons on all trials), this pigeon did not show reemergence of the (reversed) DRL versus FR pattern. Obviously, some other poorly understood factor contributes to pigeons’ emergent differential sample behavior. Nevertheless, this uncertainty should not detract from the overall pattern of replicable results across experiments and what these results indicate about the origins of such behavior.
Another issue is how to distinguish empirically between adventitious reinforcement and acquired equivalence. Urcuioli et al. (2002) commented that both make exactly the same predictions for emergent sample behavior, so other means must be used to differentiate between them. We may have been too hasty in our earlier remarks. For instance, unlike the standard many-to-one procedure for generating acquired equivalence in pigeons in which all samples are trained concurrently from the outset of conditional discrimination training (e.g., Urcuioli et al., 1989; 1995; Urcuioli & Lionello-Denolf, 2001; Wasserman et al., 1992), the procedure used here and in Urcuioli et al. (2002) involved sequential training of the samples that eventually occasioned the same comparison choice (e.g., line samples first, with hue samples added later). If acquired sample equivalence (as judged by transfer of new comparison choices across those samples—cf. Urcuioli, 2006a) does not occur when many-to-one relations are trained sequentially, this would be evidence against this explanation of emergent sample behavior. With temporal and hedonic samples, sequential training does yield acquired sample equivalence (Grant & Kelly, 2001; Grant & Spetch, 1993). The issue, then, is whether the same holds for other kinds of conditional cues including those arising from explicitly trained differential sample behavior (e.g., Urcuioli et al. 2006).
Still, other data from this lab may help to distinguish between acquired equivalence and adventitious reinforcement. Specifically, Urcuioli et al. (2002, Experiment 1) found that pigeons did not exhibit emergent differential sample behavior when the added samples were on for a fixed duration (viz., the average time for a pigeon to complete the explicitly required DRL and FR requirements). But why should fixed-duration contingencies preclude emergent differential sample responding if its source is acquired sample equivalence? In other words, if the originally trained and new samples become functionally equivalent via the common reinforced choice they occasion and if the former also occasion differential sample responding, shouldn’t the latter do so as well? The fact that there were no signs of emergent differential sample behavior under these conditions seems to us to be problematic for an acquired equivalence account.
But even if there is uncertainty about a satisfactory empirical distinction between acquired equivalence and adventitious reinforcement, the present results are certainly clear that bidirectional transfer (symmetry) is not involved in the production of emergent differential sample responding by pigeons.
Acknowledgments
This research was supported by NIMH Grant MH 66915. The authors express their appreciation to Natalie Lucas, Maggie Sweeney, Sam Shukla, and Jeremy Uitto for assistance in conducting this research.
Footnotes
1500 ms was chosen as a “threshold” or “cutoff” value because it is one half of the 3-s DRL interval and captures very well the spacing of successive key pecks typical even of less-than-efficient responding on such a schedule. Furthermore, well-trained pigeons rarely space successive key pecks more than 500 or 750 ms apart on a FR 20 schedule, and the few IRTs in that range are often the result of periodic air pecks, off-key pecks, or a peck topography in which the displaced key does not fully return to its nondisplaced position (thus failing to record a subsequent peck).
The results omit SID 5 because its reversed pattern was seen in only 1 of the 18 pigeons here and in only 1 of 18 pigeons in Urcuioli et al. (2002, Experiments 2 and 3). The data for Groups SID and DFRN correspond to the “drl” and “fr” functions in Figures 1 and 3, respectively. The data for Group SODD, however, correspond to the opposite functions in Figure 2 because those functions, unlike the summary data shown here, were predicted from symmetry rather than from acquired equivalence or adventitious reinforcement.
References
- Barnes D, Keenan M. A transfer of functions through derived arbitrary and non-arbitrary stimulus relations. Journal of the Experimental Analysis of Behavior. 1993;59:61–81. doi: 10.1901/jeab.1993.59-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovet D, Vauclair J. Functional categorization of objects and of their pictures in baboons (Papio anubis). Learning and Motivation. 1998;29:309–322. [Google Scholar]
- D’Amato M.R, Salmon D.P, Loukas E, Tomie A. Symmetry and transitivity of conditional relations in monkeys (Cebus apella) and pigeons (Columba livia). Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A.J. 2007. An examination of the temporal and spatial stimulus control in emergent symmetry in pigeons. [Google Scholar]
- Frank A.J, Wasserman E.A. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–167. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A, Benjumea S. The emergence of symmetry in a conditional discrimination task using different responses as proprioceptive samples in pigeons. Journal of the Experimental Analysis of Behavior. 2006;86:65–80. doi: 10.1901/jeab.2006.67-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldiamond I. Perception. In: Bachrach A.J, editor. Experimental foundations of clinical psychology. NY: Basic Books; 1962. pp. 280–340. [Google Scholar]
- Grant D.S, Kelly R. Many-to-one matching with temporal and hedonic samples in pigeons. Learning and Motivation. 2001;32:477–498. [Google Scholar]
- Grant D.S, Spetch M.L. Analogical and nonanalogical coding of samples differing in duration in a choice-matching task with pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:15–25. [Google Scholar]
- Hall G.C, Mitchell C, Graham S, Lavis Y. Acquired equivalence and distinctiveness in human discrimination learning: Evidence for associative mediation. Journal of Experimental Psychology: General. 2003;132:266–276. doi: 10.1037/0096-3445.132.2.266. [DOI] [PubMed] [Google Scholar]
- Hogan D.E, Zentall T.R. Backward associations in the pigeon. American Journal of Psychology. 1977;90:3–15. [Google Scholar]
- Honey R.C, Hall G. The acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:338–346. [PubMed] [Google Scholar]
- Kastak C.R, Schusterman R.J, Kastak D. Equivalence classification by California sea lions using class-specific reinforcers. Journal of the Experimental Analysis of Behavior. 2001;76:131–158. doi: 10.1901/jeab.2001.76-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello K.M, Urcuioli P.J. Control by sample location in pigeons’ matching-to-sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M, Urcuioli P.J. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop P.F.M, Matthijs W. A test for symmetry and transitivity in the conditional discrimination performance of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe K, Kawashima T, Staddon J.E.R. Differential vocalization in budgerigars: Towards an experimental analysis of naming. Journal of the Experimental Analysis of Behavior. 1995;63:111–126. doi: 10.1901/jeab.1995.63-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger R.S. The number of non-zero, post hoc contrasts from ANOVA and error rate. I. British Journal of Mathematical and Statistical Psychology. 1975;28:71–78. [Google Scholar]
- Saunders R.R, Drake K.M, Spradlin J.E. Equivalence class establishment, expansion, and modification in preschool children. Journal of the Experimental Analysis of Behavior. 1999;71:195–214. doi: 10.1901/jeab.1999.71-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K.J, Williams D.C. Do parakeets exhibit derived stimulus control? Some thoughts on experimental control procedures. Journal of the Experimental Analysis of Behavior. 1998;70:321–324. doi: 10.1901/jeab.1998.70-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K.J, Williams D.C, Spradlin J.E. Derived stimulus control: Are there differences among procedures and processes? In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. NY: North-Holland; 1996. pp. 93–109. [Google Scholar]
- Schusterman R.J, Kastak D. A California sea lion (Zalophus californianus) is capable of forming equivalence relations. The Psychological Record. 1993;43:823–839. [Google Scholar]
- Sidman M. Equivalence relations: Some basic considerations. In: Hayes S.C, Hayes L.J, editors. Understanding verbal relations. Reno, NV: Context Press; 1992. pp. 15–27. [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston: Authors Cooperative; 1994. [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Kirk B, Willson-Morris M. Six-member stimulus classes generated by conditional-discrimination procedures. Journal of the Experimental Analysis of Behavior. 1985;43:21–42. doi: 10.1901/jeab.1985.43-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching to sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Wynne C.K, Maguire R.W, Barnes T. Functional classes and equivalence relations. Journal of the Experimental Analysis of Behavior. 1989;52:261–274. doi: 10.1901/jeab.1989.52-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradlin J.E, Cotter V.W, Baxley N. Establishing a conditional discrimination without direct training: A study of transfer with retarded adolescents. American Journal of Mental Deficiency. 1973;77:556–566. [PubMed] [Google Scholar]
- Urcuioli P.J. Responses and acquired equivalence classes. In: Wasserman E.A, Zentall T.R, editors. Comparative cognition: Experimental explorations of animal intelligence. NY: Oxford University Press; 2006a. pp. 405–421. [Google Scholar]
- Urcuioli P.J. When discrimination fails (or at least falters). Journal of Experimental Psychology: Animal Behavior Processes. 2006b;32:359–370. doi: 10.1037/0097-7403.32.4.359. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J. Sample and comparison location as factors in matching acquisition, transfer, and acquired equivalence. Learning & Behavior. 2007a;35:252–261. doi: 10.3758/bf03206431. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J. Symmetry, anti-symmetry, and a theory of pigeon equivalence-class formation. 2007b. Oct, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Honig W.K. Control of choice in conditional discriminations by sample-specific behaviors. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:251–277. [PubMed] [Google Scholar]
- Urcuioli P.J, Lionello-DeNolf K.M. Some tests of the anticipatory mediated generalization model of acquired sample equivalence in pigeons’ many-to-one matching. Animal Learning & Behavior. 2001;29:265–280. [Google Scholar]
- Urcuioli P.J, Lionello-DeNolf K.M, Michalek S, Vasconcelos M. Some tests of response membership in acquired equivalence classes. Journal of the Experimental Analysis of Behavior. 2006;86:81–107. doi: 10.1901/jeab.2006.52-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Pierce J.N, Lionello-DeNolf K.M, Friedrich A, Fetterman J.G, Green C. The development of emergent differential sample behavior in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:409–432. doi: 10.1901/jeab.2002.78-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Zentall T.R, DeMarse T. Transfer to derived sample-comparison relations by pigeons following many-to-one and one-to-many matching with identical training relations. Quarterly Journal of Experimental Psychology. 1995;48B:158–178. [Google Scholar]
- Urcuioli P.J, Zentall T.R, Jackson-Smith P, Steirn J.N. Evidence for common coding in many-to-one matching: Retention, intertrial interference, and transfer. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:264–273. [Google Scholar]
- Wasserman E.A, DeVolder C.J, Coppage D.J. Nonsimilarity-based conceptualization in pigeons. Psychological Science. 1992;3:374–379. [Google Scholar]