Abstract
The lion’s share of studies on regeneration in Plathelminthes (flatworms) has been so far carried out on a derived taxon of rhabditophorans, the freshwater planarians (Tricladida), and has shown this group’s outstanding regeneration capabilities in detail. Sharing a likely totipotent stem cell system, many other flatworm taxa are capable of regeneration as well. In this paper, we present the regeneration capacity of Macrostomum lignano, a representative of the Macrostomorpha, the basal-most taxon of rhabditophoran flatworms and one of the most basal extant bilaterian protostomes. Amputated or incised transversally, obliquely, and longitudinally at various cutting levels, M. lignano is able to regenerate the anterior-most body part (the rostrum) and any part posterior of the pharynx, but cannot regenerate a head. Repeated regeneration was observed for 29 successive amputations over a period of almost 12 months. Besides adults, also first-day hatchlings and older juveniles were shown to regenerate after transversal cutting. The minimum number of cells required for regeneration in adults (with a total of 25,000 cells) is 4,000, including 160 neoblasts. In hatchlings only 1,500 cells, including 50 neoblasts, are needed for regeneration. The life span of untreated M. lignano was determined to be about 10 months.
Keywords: Platyhelminthes, BrdU, Longevity, Ageing, Blastema
Introduction
Regeneration is a phenomenon found in members of most metazoan phyla but, until recently, remained a largely neglected field of developmental biology because the major zoological model organisms show little or no regeneration (Sánchez Alvarado 2000). In the Plathelminthes, the regeneration capacity of triclads has been most extensively investigated (see Brøndsted 1969; Saló and Bauñà 2002; Agata et al. 2003; Reddien and Sánchez Alvarado 2004, for reviews), although a number of other flatworm taxa have been studied as well.
Macrostomum lignano has been proposed as a basal flatworm model organism (Ladurner et al. 2005) to complement the work with the more derived triclad flatworms. The present study reports on the overall regeneration capacity of M. lignano. Transversal amputations and longitudinal incisions have been performed in anterior and in posterior body regions. Both adult and juvenile regeneration has been investigated, as well as the longevity of the species in laboratory conditions. This work aims to be a guide and reference for future studies concerned with regeneration in M. lignano, such as cell dynamics during regeneration and the properties of a tail plate regeneration blastema (Egger, personal observation; Egger et al. 2005).
New cytochemical and molecular techniques such as BrdU labeling (Ladurner et al. 2000; Newmark and Sánchez Alvarado 2000) and RNA interference (Orii et al. 2003; Reddien et al. 2005) have recently augmented the accessibility of flatworms for stem cell research and regeneration. With a likely totipotent stem cell system and a comparably simple bauplan, these organisms have the potential to be on the forefront to unravel the mechanisms of regeneration and aging.
Materials and methods
Animals
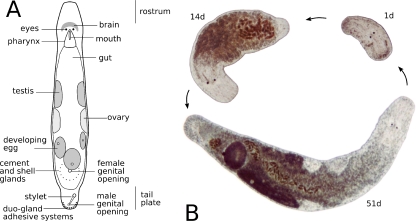
M. lignano, a “microturbellarian” flatworm about 1.5 mm long (Fig. 1a), was originally sampled from the sandy shores of the Northern Adriatic near Lignano, Italy (Ladurner et al. 2005). Laboratory cultures have been established since 1995. For specific culture conditions, see Egger and Ishida (2005) and literature therein.
Fig. 1.
a Schematic drawing of an adult specimen of M. lignano. The length of the animal is about 1.5 mm. b Regeneration cycle: from a pharynx-level amputee to a fully regenerated animal. Photographs were taken 1, 14, and 51 days after amputation from the same individual. All images in scale
Experimental animals, with the exception of juveniles, were 3 to 6 weeks old, counted from the day of hatching. To ensure a consistent age for these “standard animals”, eggs were either picked from culture dishes and left to develop in a separate dish, or all animals were removed from a dish, leaving only eggs. Embryonic development lasts for about 5 days and postembryonic development for about 2 weeks (Morris et al. 2004). New eggs and hatchlings were regularly removed from the dishes to prevent confusing younger animals with timed specimens. Amputations and incisions were performed on a slide with custom-broken and cleaned Gillette razor blades under a stereo microscope. Before amputation and also during regeneration, animals were fed ad libitum. Sometimes and especially during regeneration, 50 μg/ml of the antibiotics ampicillin, streptomycin, kanamycin, or neomycin (all of Sigma-Aldrich) were added to the culture medium. Animals from 24 different culture batches were used in 28 sets of experiments. There were altogether 378 specimens of M. lignano subjected to 734 amputations or incisions.
Observation of living specimens
Regenerates and control animals were anesthetized in a 2:1 mixture of 7.14% MgCl2·6H2O and artificial seawater (Pfannkuche and Thiel 1988), transferred in a small drop onto a slide and slightly squeezed under a coverslip (Westheide and Purschke 1988). A majority of regenerates was subjected to squeeze preparations within 1 day after amputation to precisely determine and document the cutting level. At cutting levels within or close to the pharynx, the distance between eyes and posterior end of the regenerate was measured using the photographs and compared to the average pharynx length to determine the exact cutting level. There were 741 single-squeeze preparations made to allow for a total of 4,393 photographs to document the development of live specimens.
The specimens were observed with interference contrast (Nomarski) microscopy, using a Reichert-Jung Polyvar, a Leica DM5000, a Zeiss Axiovert 135, or a Leitz Diaplan microscope. Live animals were transferred back to their culture dishes after observation, so the same specimen could be observed at different time intervals (Fig. 1b). The maximum number a single specimen was subjected to squeeze preparations was 11 times, over a period of 6 weeks.
BrdU labeling
For BrdU (5-bromo-2′-deoxyuridine) labeling, living animals were soaked for 30 min in a 5 mM solution of BrdU (Sigma-Aldrich, see, for a review, Ladurner et al. 2000). Animals were then anesthetized in 7.14% MgCl2·6H2O for 5–10 min, fixed in 4% formaldehyde (FA) in phosphate-buffered saline (PBS) for 1 h and washed in PBS with 0.1% Triton (PBS–T) for 10 min. The specimens were incubated in 0.1 μg/ml Protease XIV (Sigma-Aldrich) at 37°C until the epidermis appeared slightly jagged, and they were then treated with 0.1 M HCl (on ice) for 10 min. The animals were subsequently incubated in 2 M HCl at 37°C for 1 h, rinsed in PBS, washed in BSA–T (PBS–T with 1% Albumin fraction V, Merck) for 30 min and incubated in primary mouse anti-BrdU antibody (1:600 in BSA–T, Roche) over night at 4°C. On the next day, animals were rinsed in PBS and then incubated in secondary goat anti-mouse antibody (fluorescein isothiocyanate-conjugated, 1:150 in BSA–T, DAKO) for 1 h at RT. After the last antibody incubation, the animals were washed in PBS and mounted in Vectashield (Vector). For the documentation of stained whole mounts, a confocal Zeiss LSM 510 was used.
Maceration
Up to 1-day-old hatchlings were washed in calcium- and magnesium-free medium (CMF) for 30 min. There were 18–20 animals grouped for each experiment. Each group was macerated in 50 μl of CMF–trypsin solution (1% trypsin) for 1 h. A 50 μl 1:1:6.5 glycerol/glacial acetic acid/water solution with 1:500 Hoechst 33342 were added to each tube and with the contents carefully pipetted up and down for 15–30 min. The number of cells was counted with a hemocytometer (Bürker), using aliquots of 1 μl.
Images
Photographs were taken with digital color cameras of the fabricates Pixera Penguin 600CL or 150CL, The Imaging Source DFK 41F02 or a Sony DFW-X700.
For picture editing and drawing schemes, the free programs GIMP (http://www.gimp.org) up to version 2.2.10 and Inkscape (http://www.inkscape.org) up to version 0.43 were used.
Results
The experimental animals, except animals for longevity studies, were subjected to transversal or oblique amputation, or to incision at various cutting levels (Figs. 2, 3, 4, 5, and 6). Transversal and oblique amputation resulted in an anterior piece (posterior regenerate) and a posterior piece (anterior regenerate). After closure of the wound by flattening of the surrounding epidermis cells, a regeneration blastema was formed in the course of successful regeneration.
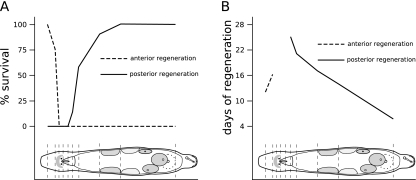
Fig. 2.
Survival rate of anterior and posterior regeneration in M. lignano at different amputation levels. a Percentage of fully regenerated amputees at different cutting levels. Note that in the area between brain and most of the pharynx, neither anterior nor posterior regeneration leads to fully regenerated animals. b Days an amputated animal needs for full regeneration. The farther the cut is away from brain or pharynx, the quicker regeneration is completed. The time values for anterior regeneration are less accurate than for posterior regeneration
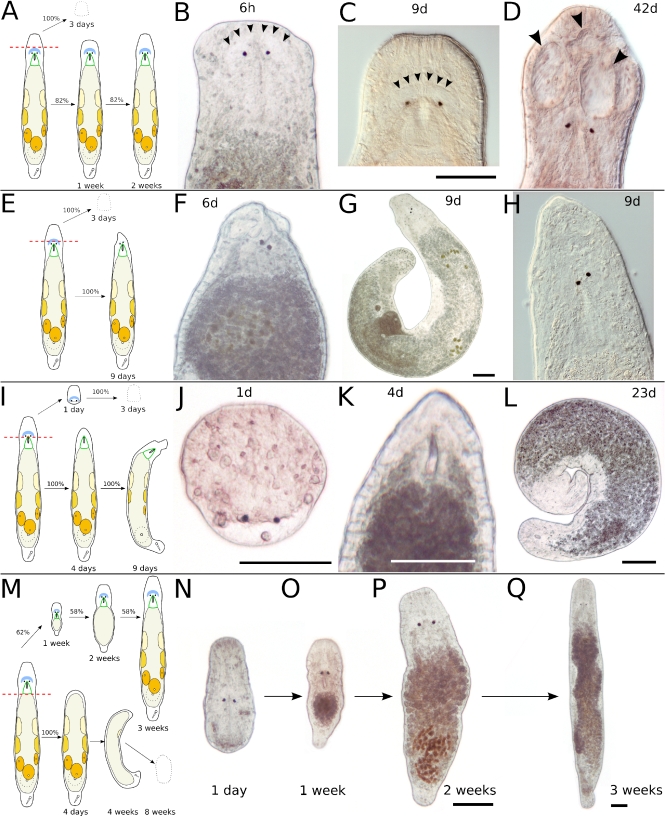
Fig. 3.
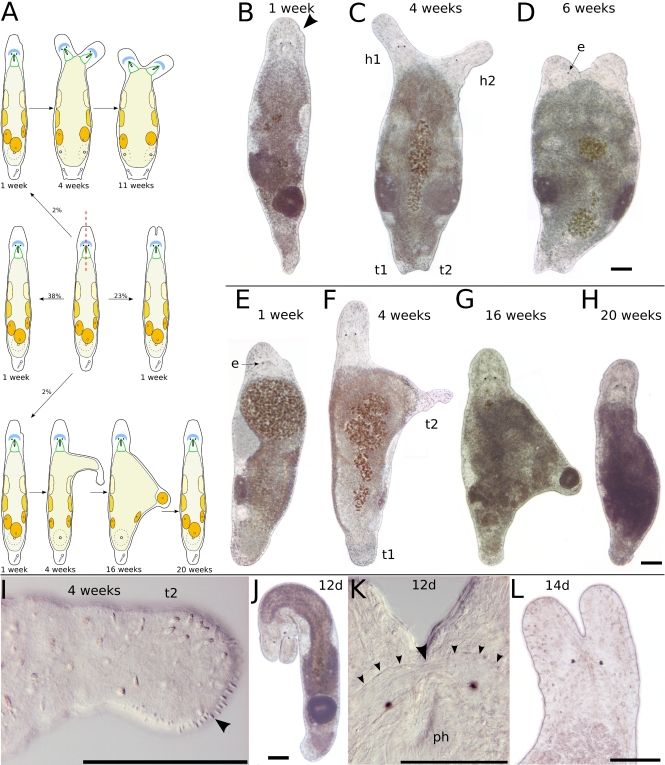
Transversal amputation in the rostrum (a–d), in the brain (e–h), behind the eyes (i–l), and at the end of the pharynx (m–q). a Schematic drawing of amputation in the rostrum. The anterior piece perishes within about 3 days; the posterior piece regenerates in about 2 weeks. b Six hours after amputation, the brain is intact. Arrowheads mark the anterior border of the neuropil. c Same specimen as in b, 9 days after amputation. The rostrum is not yet at full length. Arrowheads mark the anterior border of the neuropil. d Different specimen, 42 days after amputation. The rostrum is at full length but developed grooves (arrowheads). e Schematic drawing of amputation in the brain. The anterior piece perishes in about 3 days; the posterior piece fails a complete regeneration. f Six days after amputation. g Different specimen, 9 days after amputation. h Detail of specimen in g. Note loose rostrum and skewed eyes (f–h). i Schematic drawing of amputation directly behind the eyes. The anterior piece perishes in about 3 days; the posterior piece does not regenerate eyes and a proper rostrum. j Anterior piece 1 day after amputation. The wound is closed, but no blastema is formed. The space behind the eyes is acellular and fluid-filled. k Posterior piece of same specimen 4 days after amputation. Note the blastema-like tissue anterior of the pharynx, which does not regenerate eyes and brain. l Same specimen, 23 days after amputation. The animal is curled up, with an incompletely regenerated rostrum. Gonads are still present. m Schematic drawing of amputation at the end of the pharynx. The anterior piece regenerates to a complete animal in 58% of cases. n–q Same specimen at different time intervals after amputation. All scale bars are 100 μm. Same scale bar in b–d, f, h, and n–p
Fig. 4.
Transversal amputation in or behind the pharynx and at the tail plate (a–e), between testes and ovaries (f–i), and at the tail plate (h–m). Oblique amputation between the eyes (n–q). a Schematic drawing of amputation in or behind the pharynx and at the tail plate. Only the centerpiece is observed, which will regenerate a tail plate with duo-gland adhesive systems and often also a stylet, in the absence of a brain. b Seven days after amputation, a tail plate with developing stylet (arrowhead) has regenerated. c Another specimen, 22 days after amputation. A tail plate and a stylet (arrowhead) have regenerated, but the stylet is small and misplaced. d Stylet, detail of b. e Stylet, detail of c. f Schematic drawing of amputation between testes and ovaries. Only the anterior piece is observed. After 4 days, a small stylet has appeared in the tail plate, and the testes resume spermatogenesis. g Four days after amputation. h Left testis, detail of g. Note the round spermatids in the center of the testis (arrowheads). i Tail plate, detail of g. Arrowhead points at small stylet. j Schematic drawing of amputation at the tail plate. The posterior piece will die within 8 days; the anterior piece will regenerate within 6–10 days. k Fully regenerated animal 10 days after amputation. l Detail of the tail plate of another specimen after 10 days of regeneration. About 140 duo-gland adhesive systems at the rim of the tail plate. Arrowhead points at male genital opening. m Same animal as in l, different focal plane. fsv false seminal vesicle, st stylet, sv seminal vesicle. n Schematic drawing of oblique amputation between the eyes. The anterior piece perishes in about 3 days; the posterior piece does not regenerate the missing eye. o Amputated specimen 7 h after amputation. p Same specimen as in o, 17 days after amputation. Note that the gonads are fully developed at the side of the missing eye. q Detail of p. All scale bars are 100 μm, except 10 μm in e. Sub-panels d–e and h–i are of the same scale
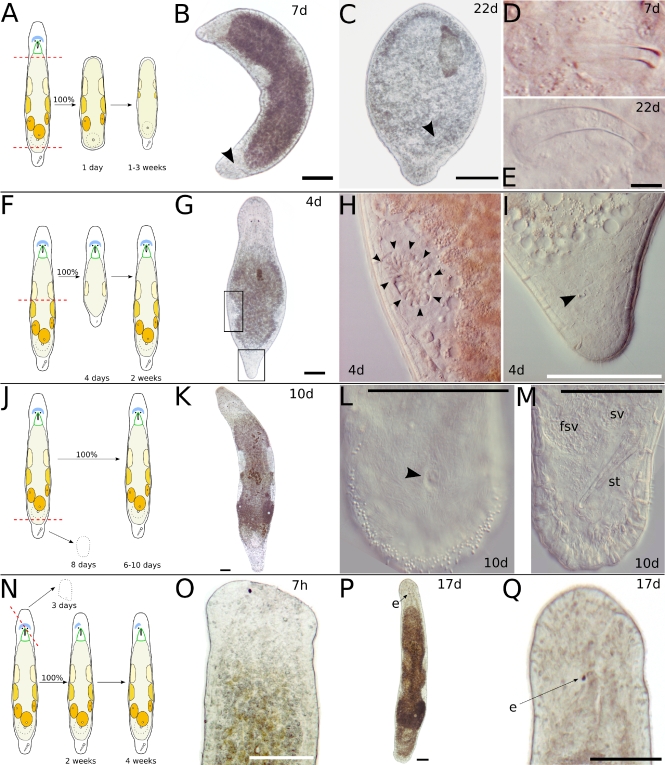
Fig. 5.
Longitudinal incision in the head. a Schematic drawing of possible results after longitudinal incision in the head. b One week after incision, only a small bump at the incision level (arrowhead) remained. c Four weeks after incision, a second head (h2) with pharynx but only a single eye appeared. The animal gained breadth and also had a second tail plate (t2) with stylet and a second female genital opening. d Six weeks after incision, the animal had lost two of its three eyes and gained more breadth. b–d show the same specimen. e Another specimen 1 week after incision. The animal had lost its eye on the side of the incision. f Four weeks after incision, the animal had regenerated its eye and built a tail-plate-like structure at the side. g Sixteen weeks after incision, the tail-plate-like extension had lost its duo-gland adhesive systems and harbored a small egg in its tip. h Twenty weeks after incision, the animal had completely reorganized its tail-plate-like protrusion and was of usual shape. i Detail of f, showing the tip of the tail-plate-like structure. Arrowhead points at duo-gland adhesive systems. e–i show the same specimen. j Specimen with deep cleft dividing the brain, 12 days after incision. k Healed brain 12 days after incision. Arrowhead points at the regrown commissure in the neuropil. l Divided rostrum 2 weeks after incision. All scale bars 100 μm. Sub-panels b–d and e–h are of the same scale. e eye, h1 original head, h2 new head, t1 original tail, t2 new tail
Fig. 6.
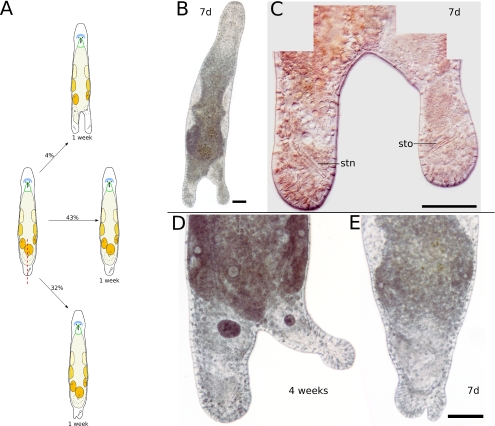
Longitudinal incision in the tail plate. a Schematic drawing of possible results after longitudinal incision in the tail plate. b One week after incision, the tail plate has duplicated. c Detail of b, showing a second stylet (stn) in the new tail plate, mirroring the stylet (sto) in the old tail plate. d Specimen with bifurcated tail plate 4 weeks after incision. The new tail is probably too small to build a second stylet. e Specimen with slightly bifurcated tail plate 7 days after incision. All scale bars are 100 μm. Sub-panels d and e are of the same scale
An overview of the number of amputated or incised specimens, the survival rates, and the regeneration times for all studied cutting levels is given in Table 1. In Fig. 2a, the percentage of successful regeneration at various cutting levels is illustrated with a head-frequency-like graph. Figure 2b shows the rate of regeneration at the same cutting levels. Animals lost during manipulation were not taken into account for calculating survival rates.
Table 1.
Overview of the fate of amputated and incised animals
| Cutting level | Number of specimens | Fully regenerated (%) | Days until full regeneration | Days until death, if no full regeneration | |
|---|---|---|---|---|---|
| Transversal amputation | |||||
| Anterior regeneration | Just anterior of brain | 11 | 81.82 | About 2 weeks | |
| Just anterior of eyes | 5 | 0 | |||
| Anterior half of pharynx | 7 | 0 | More than 9 weeks | ||
| Middle of pharynx to gonads | 114 | 0 | More than 9 weeks | ||
| Tail plate | 13 | 0 | Up to 8 days | ||
| Posterior regeneration | Posterior end of rostrum | 12 | 0 | Up to 3 days | |
| Posterior of eyes | 13 | 0 | Up to 3 days | ||
| Mid of pharynx | 15 | 0 | Up to 6 days | ||
| Middle to end of pharynx | 35 | 14.29 | About 25 days | Up to 16 days | |
| End of pharynx, a little bit of gut | 50 | 58 | About 3 weeks | Up to 14 days most within 7 days | |
| Possibly anterior tip of testes | 11 | 90.91 | About 17 days | 2 days | |
| Mid of gonads | 5 | 100 | About 2 weeks | ||
| Tail plate | 32 | 100 | 6–10 days | ||
| Juveniles oblique amputation | |||||
| Two-sided regeneration | Posterior of eyes and at tail plate | 6 | 0 | More than 3 weeks | |
| In rostrum and at gonad level | 1 | 100 | About 4 weeks | ||
| Repeated regeneration | Between testes and tail plate | 20 | 100 | Cut 29 times during 12 months | |
| Posterior regeneration | Half of body to tail plate | 24 | 0.63 | 2–4 weeks | |
| Anterior regeneration | Between eyes | 9 | 0 | ||
| Posterior regeneration | Between eyes | 9 | 0 | Up to 3 days | |
| Longitudinal incision | |||||
| Anterior regeneration | Cut symmetrically | 19 | 36.84 | About 1 week | |
| Cut asymmetrically | 28 | 39.29 | About 1 week | ||
| Posterior regeneration | Cut symmetrically | 13 | 53.85 | About 1 week | |
| Cut asymmetrically | 15 | 40 | About 1 week | ||
Transversal cutting level
Only either the anterior or the posterior regenerate of transversally cut animals could regenerate a complete animal. If cut in the area between the brain and the anterior half of the pharynx, both anterior and posterior regenerates were unable to regenerate a complete animal (Fig. 3e–l, n=28 for posterior regeneration, n=12 for anterior regeneration).
Anterior regeneration
The crescent-shaped brain, located just in front of the eyes, can be used as the reference point for successful anterior regeneration. If the brain was not damaged by amputation, the rostrum as the anterior-most body part was fully regenerated within about 2 weeks (n=9, Fig. 3a–d). About 20% of rostrum regenerates developed distinct grooves in the rostrum (n=2, Fig. 3d).
Animals that were amputated at a level very close to or in the brain developed a short and loose rostrum or lost one or more eyes post-amputation, or had the eyes arranged at a skewed angle to each other (n=5, Fig. 3e–h).
No complete anterior regeneration took place in animals amputated at any level posterior of the eyes (n=134). At cutting levels just posterior of the eyes (n=7, Fig. 3i–l) and at more posterior cutting levels (n=127, Fig. 3m), no amputee was able to regenerate eyes, brain, and a full-sized rostrum. However, in large amputees, a blastema-like structure was formed at the anterior end of the anterior regenerates (Fig. 3i,k) but did not lead to full regeneration.
The pharynx only recovered from amputation if just the anterior tip of the pharynx was removed. If the amputation level was mid-pharynx or posterior of the pharynx, the pharynx was not regenerated in anterior regenerates (Fig. 3m).
In brain-damaged and even more so in brainless regenerating animals, there was a tendency to curl up and to swim in circles (Fig. 3g,i,l,m). Movement was slower than in control animals and seemingly undirected. Eyeless regenerates did not show light avoiding behavior that is common in control animals. Brain- and eyeless regenerates were never observed to copulate with each other, even if the male copulatory organ (stylet) was present and the seminal vesicle filled with sperm. Eggs could still be produced and laid, generating viable offspring. Over the course of several weeks, gonads and stylet were reduced, but much quicker in regenerates lacking a pharynx and, therefore, unable to feed. The loss of brain and eyes did not necessarily lead to early death of anterior regenerates. Two specimens, one with pharynx and one without pharynx, survived for more than 9 weeks. Even without brain, the pharynx was capable of food intake, and diatoms were observed in the gut several weeks after amputation (data not shown). This was in remarkable contrast to observations on triclads: at least, in some species, brainless individuals cannot feed (Hyman 1951).
Anterior regenerates from animals cut at the tail plate level (n=13, Fig. 4j) did not form discernible regenerative structures and perished at the latest after 8 days. The movement of these regenerates was very slow, but a basic attachment behavior with their duo-gland adhesive systems persisted.
Posterior regeneration
All posterior regenerates cut anterior to the middle of the pharynx died (n=40, Fig. 3a,e, and i), after moving around rapidly for a couple of days. Posterior regenerates cut just behind the eyes or in the anterior half of the pharynx managed to close the wound with a fluid-filled gap between old tissue and epidermis (Fig. 3j) but did not form a blastema.
Only a small number of posteriorly regenerating animals survived amputation at a cutting level between the middle of the pharynx and its posterior end (n=35). The survival rate of posterior regenerates drastically increased at cutting levels just behind the pharynx (n=50, Fig. 3m–q), and raised to over 90% for cutting levels barely at the anterior tip of the testes (n=11). Any cutting level more posterior yields 100% posterior regeneration efficiency (n=37). All survivors could fully regenerate all amputated tissues.
Posterior regenerates cut just posterior of the pharynx were fully restored about 3 weeks after amputation (Fig. 3m–q). The first duo-gland adhesive systems were observed 3 days after cutting. One week after amputation, the animals had formed a gut and a small tail plate by halving their rostrum and pharynx size (Fig. 3o) and were similar in size and shape to a 1-day old hatchling. One week later, testes and stylet were clearly visible in most pharynx-level regenerates; the ovaries appeared after another 2–3 days. These values are roughly equivalent with the appearance of gonads in hatchlings. After the first week, two more weeks were required for full regeneration.
Five specimens were cut between testes and ovaries to test whether the testes are broken down in the course of the regeneration of tail plate and ovaries (Fig. 4f–i). At 30 h after amputation, sperm production in the testes had stopped and any remaining sperm present previous to the amputation had been reabsorbed. After 4 days, the persisting testes resumed spermatogenesis—observable by spermatids in the testes (Fig. 4h), and the first sign of the stylet concurrently appeared in the regenerating tail plate (Fig. 4i). At this time, the ovaries were not yet regenerated. The regeneration was completed about 2 weeks after amputation.
Tail plate regenerates required 6–10 days for the regeneration of the full set of amputated organs (n=32, Fig. 4j–m). After an initial burst of regeneration involving blastema formation, the tip of the regenerated stylet appeared 72 h after amputation, and a small number of sperm was found in the seminal vesicle 1 day later. The stylet was fully restored 5 days after amputation, constituting an already fully functional tail plate. At this time, 75.67±5.03 (n=3) duo-gland adhesive systems were regenerated at the tip of the tail plate. Control standard animals had an average of 129.85±17.06 (n=13) duo-gland adhesive systems. One to five more days were required for the regeneration of a complete set of duo-gland adhesive systems.
Functional gonad recovery
To test whether adult specimens are able to regenerate gonads de novo, 14 animals were selected that had been amputated at a level posterior of the pharynx, but anterior of the testes. In that way, all gonadal tissue was removed from the anterior pieces, which were left to regenerate. All posterior pieces were discarded. About 3 weeks after amputation, the fully regenerated amputees were put together in seven separate pairs to test whether the regenerated gonads were capable of producing fertile offspring. After 4 weeks, all pairs had spawned offspring. Some of these first-generation hatchlings were put together in separate dishes and, 2 weeks later, all dishes with first-generation hatchlings had spawned second-generation hatchlings, showing that amputees lacking any gonads were able to regenerate viable gonads and to produce fertile progeny.
Repeated regeneration
Twenty standard animals (about 3 weeks old) were subjected to repeated transversal amputations during the course of several months. The interval between successive amputations varied between 3 and 19 days. All posterior pieces were discarded. The cutting level was anywhere between gonad level and tail plate level. The animals were kept in single culture dishes to keep track of individuals.
One animal was lost due to a handling error after three amputations. All of the remaining specimens survived five amputations, and 12 animals survived ten amputations. After the 13th amputation, a large animal cut at about mid-body level developed six additional small tail plates (data not shown). The animal was very broad due to hypertrophic testes and, thus, had a comparably large wound surface. This specimen died after the 17th amputation; no more animals were lost to date. Six months or 16 amputations after the start of the experiment, the first specimen had lost an eye. Other animals followed, and after more than 8 months or 25 amputations after the start of the experiment, all animals were eyeless. Besides the loss of eyes, some specimens also developed epidermal grooves and outgrowths and hypertrophic testes in the course of time. At present and almost 12 months after the start of the experiment, ten animals have survived 29 amputations. All remaining animals are vividly swimming in their culture dishes. A decline of the regeneration rate could not be observed. The experiment is still being continued.
Regeneration of juveniles
Hatchlings up to 1 day old were transversally amputated at cutting levels just posterior of the pharynx to back at the tail plate (n=12). The posterior pieces were discarded. Amputees from cutting levels just behind the pharynx were not able to recover and died after 2 or 3 days. Eight specimens, including regenerates cut at about mid-body level, regenerated completely showing a typical blastema formation. The regenerates developed to full-grown adults within 2–4 weeks after amputation, depending on the level of amputation.
In another experiment, hatchlings up to about 6 days old were also shown to regenerate completely after transversal amputation (n=12).
Regeneration of centerpieces
Two transversal amputations, one in the anterior and one in the posterior region of an animal, created centerpieces with wounds at the anterior and posterior end (Fig. 4a–e).
In six specimens, the anterior cutting level was anywhere between the anterior tip of the pharynx and the ovaries, whereas in the posterior amputation, the tail plate was removed (Fig. 4a). All of these specimens regenerated a tail plate with duo-gland adhesive systems, but only 50% regenerated the stylet as well. A stylet could regenerate in the absence of the brain and also in the absence of the pharynx. Centerpieces with intact pharynx were able to feed, and those pieces with testes and regenerated stylets produced sperm that filled the seminal vesicles.
The centerpieces developed anteriorly an abortive blastema as described in the section “Anterior regeneration”.
Oblique cutting level
In transversal amputations, it was shown that eyes and brain do not regenerate. To test whether the regeneration of eyes is possible when only about half the brain and one eye are amputated, animals were cut in an oblique angle between the eyes (Fig. 4n–q).
Anterior regenerates (n=9) were observed up to 4 weeks after amputation and were never found to fully restore the brain tissue or to regenerate the missing eye (Fig. 4o–q). On the contrary, two thirds of the regenerates lost their second eye as well. The regenerated rostrum often showed grooves and shape deformations, just like in transversally cut animals amputated at the brain level.
Posterior regenerates (n=9) survived for , which corroborates the findings in transversally cut animals amputated just posterior to the eyes. The gonads on the side of the missing eye and brain part were not reduced (Fig. 4p).
Animals that were amputated obliquely at mid-body or at the tail plate level had a normal posterior regeneration (data not shown).
Longitudinal incision
Longitudinal incisions did not cut the animals in two halves. Both anterolongitudinal and posterolongitudinal incisions have been performed (Figs. 5, 6). In 38.3% of anteriorly incised animals (n=47) and in 42.86% of posteriorly incised animals (n=28), the wounds closed and no apparent trace of the insection could be observed after 1 week. Even brain and pharynx halves were able to grow together seamlessly (Fig. 5k). Oblique incisions at the side of the animal did not create aberrant phenotypes (n=2, data not shown).
Anterolongitudinal incision
In anteriorly incised specimens, almost one fourth (23.4%, n=11) developed a hump-like rostrum (Fig. 5l), with the cleft reaching through the brain in two cases (Fig. 5j). Brains cut in two halves by incision were able to reconnect the separated halves (Fig. 5k). Not all incisions were performed exactly along the median axis, so that 27.66% (n=13) of the anteriorly cut animals lost an eye and 8.51% (n=4) even lost both eyes. In 12.77% (n=6), only a small indentation in the rostral epidermis remained from the incision. Several humps (n=3) became less pronounced in the course of time. Although incised only anteriorly, two specimens showed an increased number (+50%) of duo-gland adhesive systems in the tail plate. The deepness of the incision ranged from rostrum-only to halfway through the animal. The probability of the formation of a hump increased with the size of the wound. In 38.3% (n=18), the animals showed no sign of the incision after 1 week. The behavior of the incised animals with intact eyes remained unchanged.
Two specimens exhibited each a unique response to anterior incisions. The first specimen was cut along the antero-posterior axis besides the eyes at a depth of about the pharynx base. One week later, only a small bump at the wound site remained from the incision (Fig. 5b). After 4 weeks, a second head had formed, complete with rostrum and a pharynx connected to the gut. The extra head only had one eye though. In addition, the tail plate was duplicated, with a second stylet and a seminal vesicle filled with sperm. A second female genital opening had also appeared. The breadth of the whole animal had increased significantly (Fig. 5c), even more so after 6 weeks, when the animal was more than twice as broad as a standard animal (Fig. 5d). At this time, the regenerate had lost two of its eyes and moved around sluggishly. The deterioration continued when the animal lost its last eye after 11 weeks and eventually died 13 weeks after the incision.
The second specimen which showed a special mode of regeneration was incised between the eyes, albeit not exactly at the center. One week later, it showed a bump and was missing the eye beneath the bump (Fig. 5e). Four weeks after the incision, however, it had regenerated the lost eye and extended anteriorly a second tail plate at an angle of about 90° from the anterior–posterior body axis (Fig. 5f). This new tail plate had no stylet or seminal vesicles but showed 55 duo-gland adhesive systems at its posterior tip (Fig. 5i), which the animal used for adhering to the substrate. After 6 weeks, the base of the new tail was broader, with the tip elongated and slimmer than before. The base of the new tail continually broadened and had lost its duo-gland adhesive systems almost 16 weeks after the incision. At this time, the tip of the plate had also broadened to make place for a strayed egg (Fig. 5g). Grooves appeared in the head region of the animal. After about 17 weeks, the second tail was reduced to rudiments extending near the posterior side of the anterior–posterior axis. Twenty weeks after the incision, the additional tail plate was completely gone. No further changes were observed until the last recording, which took place 25 weeks after incision (Fig. 5h). Besides a multitude of grooves all over the body and especially in the head region, the animal had shown no noticeable problems.
Posterolongitudinal incision
By incising along the anterior–posterior axis (n=28), the tail plate could be permanently split in 21.43% (n=6) of the cut animals (Fig. 6d). In 32.14% (n=9), no clear split but merely a bump-like indentation or a thumb-like protrusion resulted from posterolongitudinal incision (Fig. 6e). One third of the indentations receded over time, leaving a normal tail plate. Almost half of the incised animals (42.86%, n=12) could not be distinguished from control animals after 1 week.
The number of duo-gland adhesive systems at the caudal tip of the tail increased significantly compared to untreated standard animals in 32.14% (n=9) of regenerates, even in animals where no other significant marks of the incision remained. The maximum number of duo-gland adhesive systems observed was 230, an increase of 77% compared to control animals.
In one specimen, a stylet was found in each of the tail plates 1 week after incision (Fig. 6b,c). Its tail plate had been split almost symmetrically, with the cut reaching up to the testes level. The stylets were a mirror image of each other, and both were connected with the vas deferens and had vesiculae seminales filled with sperm. Self-fertilization did not occur. The cut in this and also another specimen induced the formation of a second female genital opening.
All additional tail plates could be used by the animals to adhere to the substrate using duo-gland adhesive systems, without noticeable modification of behavior.
Longevity of control animals
The maximum life span of untreated individual M. lignano was determined to be 42 weeks (almost 10 months) in laboratory cultures, starting with a single patch of about 40 1-day-old hatchlings. Possible culture limitations like bacteria or parasites may have shortened the life span of the specimens; therefore, 42 weeks should be considered the lower limit of the possible maximum life span of M. lignano.
About 38 weeks after the start of the experiment, distinct changes in the morphology of the long-time survivors became evident. The transparent body changed to an opaque gray, the movement slowed down to almost a halt, the epidermis built bulges especially in the anterior region of the body, and the overall size of the animal decreased. Despite optimal culture conditions, the specimens did not recover from these symptoms. It appears likely that these morphological changes were associated with the advanced age of the specimens.
The minimal size sufficient for complete regeneration
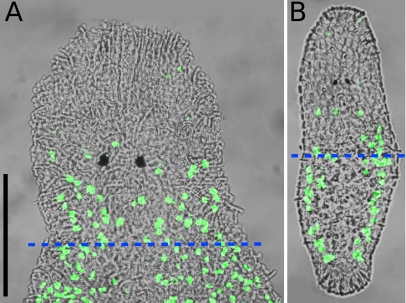
In live squeeze preparations of adult animals, the average pharynx length was 91.17±10.34 μm (n=21). BrdU-labeling of non-regenerating standard animals revealed an average number of 43.57±5.35 (n=7) S-phase cells in the area from the anterior rostrum tip to the posterior end of the pharynx (Fig. 7a). According to Bode et al. (accepted for publication), about 27% of all neoblasts in M. lignano are in S-phase, so the number of remaining neoblasts in posterior regenerates amputated at the pharynx level is about 160. The number of all cells in the posterior regenerate after amputation is estimated to be about 4,000 with the following calculation: Ladurner et al. (2000) found in macerations of 1.2–1.5 mm long adults an average number of 24,708 cells (n=19). The average length of rostrum plus pharynx is 215 μm (Ladurner et al. 2005). If an even distribution of cells along the antero-posterior axis is assumed, 215 μm divided through the average length of 1,350 μm and multiplied with the average cell number of 24,708 gives a number of 3,935 cells for a piece including rostrum and pharynx. Thus, in adult M. lignano, a small population of about 160 neoblasts in a regenerating piece of about 4,000 cells is responsible for the regeneration of the remaining 20,000 cells in 3 weeks.
Fig. 7.
Animals labeled with BrdU in a 30-min pulse experiment. Overlay of fluorescence image and interference contrast image. a Adult standard animal. On average, about 44 labeled nuclei (green) are in the area from the rostrum tip to the posterior end of the pharynx (dashed blue line). b One-day-old hatchling. On average, about 14 labeled nuclei (green) are in the area from the rostrum tip to the half of the body length (dashed blue line). Scale bar is 100 μm. Sub-panels a and b are of the same scale
The average number of S-phase cells in hatchlings up to 1 day old was found to be 69.07±13.73 (n=15) in BrdU-pulse experiments (Fig. 7b). In the area from the tip of the rostrum to half of the body length, 13.73±5.44 (n=15) BrdU-labeled cells were counted, whereas in the area from the tip of the rostrum to two thirds of the body length the number of S-phase cells was 36.53±9.99 (n=15).
In maceration experiments, the average cell number of 1-day-old hatchlings was found to be 2,959.08±676.43 (n=4). The 1-day-old hatchlings were observed to regenerate after cutting away the posterior half, leaving about 1,500 cells in the regenerating piece. Assuming the percentage of S-phase neoblasts among all neoblasts in hatchlings to be the same as in adults (27%, Bode et al., accepted for publication), the remaining number of neoblasts in these posterior regenerates was about 50.
Discussion
Summary of regeneration in M. lignano
Regeneration in M. lignano, an obligatory sexually reproducing “microturbellarian”, always involved wound closure, the building of a blastema, and the subsequent restoration of the amputated tissues and organs. Only the rostrum could be regenerated anteriorly, provided the brain tissue remained undamaged by amputation. Regeneration proved to be successful posteriorly starting from the posterior-most pharynx level. Repeated regeneration and regeneration in juveniles were possible. Amputees without gonads or gut tissue were able to regenerate functional gonads and the gut. If animals were cut transversally between brain and most of the pharynx, regeneration of both parts failed. Longitudinal incisions could provoke the duplication of body axes and organs, including organs that could not be regenerated by transversal amputations, such as brain and eyes.
Repeated regeneration and rejuvenation
Repeated amputation and subsequent regeneration of M. lignano neither lead to the depletion of the stem cell pool nor to the reduction of the regeneration capacity. We can consider an approximate total number of neoblasts in adults of 1,600 (Bode et al., accepted for publication) and an average amputation level at the middle of the body, each time halving the number of neoblasts. After ten amputations (1600 -> 800 -> 400 -> 200 -> 100 -> 50 -> 25 -> 12 -> 6 -> 3 -> 1), just a single neoblast would have remained in the entire animal if the neoblast pool was not replenished.
Is it possible that repeated regeneration has the potential to rejuvenate the animal by inciting the production of new and younger stem cells? For triclads and for catenulids, repeated regeneration has shown to prolong the life of the regenerating animals (Haranghy and Balázs 1964 and literature therein). Martínez (1996) found evidence for rejuvenation in repeatedly amputated oligochaetes, and Minois (2000) discussed the positive effects of mild stress (e.g., amputation) on longevity. In M. lignano, the animals subjected to successive amputations have already surpassed the maximal life span of untreated animals by 2 months and are not showing decisive signs of aging. However, the loss of eyes, hypertrophic testes, and epidermal grooves and outgrowths of repeated regenerates could be explained as age-related phenomena. As only the tissues posterior of the pharynx were amputated, the head region of the animals was not renewed by regeneration. Therefore, the loss of eyes could be related to the advanced age of the specimens, fueled by exposure to ultraviolet light in the climate chamber. Regeneration of eyes in M. lignano could only be observed in a single specimen (Fig. 5e–h), so the failure of eye regeneration in the repeated regenerates can be attributed to a general regenerative deficiency rather than to the depletion of a hypothetical neoblast subpopulation responsible for eye regeneration.
Another possibility to test whether the refilling of the stem cell pool has a positive effect on the lifetime are starvation experiments (Haranghy and Balázs 1964). The body size and the number of mitotic cells decrease in starved M. lignano (Nimeth et al. 2004) and also in starved D. mediterranea (now Schmidtea mediterranea) (Baguñà 1976), while the cell size remains constant (Baguñà and Romero 1981; Oviedo et al. 2003)—findings that hint at a reduced number of neoblasts in starved animals. As the animals grow back to normal adult size after feeding, the neoblasts will be replenished as well, leading to a possible rejuvenation of the animals (Lillie 1900; Child 1914; Newmark and Sánchez Alvarado 2002).
Some sexually reproducing triclads can live for several years (Haranghy and Balázs 1964), while species propagating by fission essentially have an infinite life span (Brøndsted 1969). For the sexually reproducing proseriate flatworm Otomesostoma auditivum, Pechlaner (1957) reports a life span of 9–10 months. The same maximum life expectancy of about 10 months was found for untreated M. lignano. With its comparably short life span and a well-studied stem cell system, M. lignano seems to be a suitable model organism to study ageing and longevity at the cellular level.
The minimal cell number needed for regeneration
The minimal size of a triclad capable of regeneration was determined by Montgomery and Coward (1974) to be about 10,000 cells using Dugesia dorotocephala. Baguñà (1976) displays for S. mediterranea that about 10% of all cells are neoblasts. Assuming a similar ratio in D. dorotocephala, then there are about 1,000 neoblasts in a piece of 10,000 cells. Montgomery and Coward (1974) regarded the formation of two eyespots as a sign for successful regeneration but did not observe the further development of the regenerates. The only pieces regenerating two eyespots were also those kept in nutrient-supplemented medium; none of the pieces ranging in size from 10,000 up to 62,500 cells regenerated two eyespots in sterile saline medium (6 mM NaCl, 0.048 mM CaCl2, and 0.012 mM NaHCO3 at pH 7.0). The nutrient-supplemented medium was made of sterile saline medium containing 1% minimal essential medium (MEM) of non-essential amino acids, 1% MEM essential amino acids, and 0.5% glucose.
Betchaku (1970) explains the inability of very small pieces of D. dorotocephala to regenerate with the inability to stratify the different tissue layers. If the remaining epidermis cannot cover the wound surfaces, the gastrodermis may push its way out, permanently preventing wound closure.
In adult M. lignano, only about 4,000 cells, including about 160 neoblasts, are required for successful regeneration. To our knowledge, the pharynx-level regenerates of M. lignano have set a new record for the smallest number of cells required for regeneration in an adult bilaterian species, though possibly regenerating gastrotrichs may have an even lower cell number (Manylov 1995, no cell numbers are given). In 1-day-old M. lignano juveniles, pieces of about 1,500 cells, including about 50 neoblasts, have been found to regenerate. These numbers are, short of blastomere ablations in early cleavages, the lowest total cell and stem cell numbers required for complete regeneration in bilaterian juveniles.
Regarding the minimal cell number required for regeneration, the obvious advantage of M. lignano compared to triclads is the smaller size of functional organs and the spatial closeness of vital organs like brain and pharynx. In triclads, organs are generally larger, and head and pharynx are separated by some distance, so that in small regenerating pieces, all vital structures have to be regenerated, whereas in pharynx-level regenerates of M. lignano only a small gut has to be formed to ensure survival. In transversal sections, the wound surface is also relatively small compared to the remaining surface area of the epidermis, so the wound surface can be readily covered with epidermis. Very small pieces of triclads usually have open wound surfaces on four sides. However, it can be speculated whether the number of 4,000 cells can still be undercut in adult M. lignano by additionally amputating parts of the neoblast-free rostrum in pharynx-level regenerates and by using a nutrient-supplemented culture medium.
Acknowledgements
Discussions with Roland Peter, Christian Gärber, and Roland Aufschnaiter were valuable for writing this paper. Lukas Schärer and Bernd Pelster are to be thanked for generously sharing their equipment. In addition, B. E. wants to express his gratitude towards Sachiko Ishida, with whom he cut his first Macrostomum. This work was supported by FWF projects 15204 and 16618 granted to R. R.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00427-006-0079-2
References
- Agata K, Tanaka T, Kobayashi C, Kato K, Saitoh Y (2003) Intercalary regeneration in planarians. Dev Dyn 226:308–316 [DOI] [PubMed]
- Baguñà J (1976) Mitosis in the intact and regenerating planarian Dugesia mediterranea n. sp. I. Mitotic studies during growth, feeding and starvation. J Exp Zool 195:53–64 [DOI]
- Baguñà J, Romero R (1981) Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia 84:181–194 [DOI]
- Betchaku T (1970) The cellular mechanism of the formation of a regeneration blastema of fresh-water planaria, Dugesia dorotocephala, I. The behavior of cells in a tiny body fragment isolated in vitro. J Exp Zool 174:253–280 [DOI] [PubMed]
- Brøndsted HV (1969) Planarian regeneration. Pergamon, Oxford
- Child CM (1914) Starvation, rejuvenescence and acclimation in Planaria dorotocephala. Arch Entwicklungsmech Org 38:418–446 [DOI]
- Egger B, Ishida S (2005) Chromosome fission or duplication in Macrostomum lignano (Macrostomorpha, Plathelminthes)—remarks on chromosome numbers in “archoophoran turbellarians”. J Zool Syst Evol Res 43:127–132 [DOI]
- Egger B, Salvenmoser W, Nimeth K, Adamski Z, Ladurner P, Rieger R (2005) Role and dynamics of stem cells during regeneration in the flatworm Macrostomum lignano. Folia Histochem Cytobiol 43(Suppl 1):40
- Haranghy L, Balázs A (1964) Ageing and rejuvenation in planarians. Exp Gerontol 1:77–91 [DOI]
- Hyman LH (1951) The invertebrates. II. Platyhelminthes and rhynchocoela. The acoelomate bilateria. McGraw-Hill, New York
- Ladurner P, Rieger R, Baguñà J (2000) Spatial distribution and differentiation potential of stem cells in hatchlings and adults in the marine platyhelminth Macrostomum sp.: a bromodeoxyuridine analysis. Dev Biol 226:231–241 [DOI] [PubMed]
- Ladurner P, Schärer L, Salvenmoser W, Rieger RM (2005) Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha): a new model organism among the lower Bilateria and the use of digital video microscopy in taxonomy of meiobenthic Platyhelminthes. J Zool Syst Evol Res 43:114–126 [DOI]
- Lillie FR (1900) Some notes on regeneration and regulation in planarians. Am Nat 34:173–177 [DOI]
- Manylov OG (1995) Regeneration in gastrotricha—I. Light microscopical observations on the regeneration in Turbanella sp. Acta Zool (Stockh) 76:1–6
- Martínez DE (1996) Rejuvenation of the disposable soma: repeated injury extends lifespan in an asexual annelid. Exp Gerontol 31:699–704 [DOI] [PubMed]
- Minois N (2000) Longevity and aging: beneficial effects of exposure to mild stress. Biogerontology 1:15–29 [DOI] [PubMed]
- Montgomery JR, Coward SJ (1974) On the minimal size of a planarian capable of regeneration. Trans Am Microsc Soc 93:386–391 [DOI] [PubMed]
- Morris J, Nallur R, Ladurner P, Egger B, Rieger R, Hartenstein V (2004) The embryonic development of the flatworm Macrostomum sp. Dev Genes Evol 214:220–239 [DOI] [PubMed]
- Newmark PA, Sánchez Alvarado A (2000) Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev Biol 220:142–153 [DOI] [PubMed]
- Newmark PA, Sánchez Alvarado A (2002) Not your father’s planarian: a classic model enters the era of functional genomics. Nat Rev Genet 3:210–219 [DOI] [PubMed]
- Nimeth KT, Mahlknecht M, Mezzanato A, Peter R, Rieger R, Ladurner P (2004) Stem cell dynamics during growth, feeding and starvation in the basal flatworm Macrostomum sp. (Platyhelminthes). Dev Dyn 230:91–99 [DOI] [PubMed]
- Orii H, Mochii M, Watanabe K (2003) A simple “soaking method” for RNA interference in the planarian Dugesia japonica. Dev Genes Evol 213:138–141 [DOI] [PubMed]
- Oviedo NJ, Newmark PA, Sánchez Alvarado A (2003) Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev Dyn 226:326–333 [DOI] [PubMed]
- Pechlaner R (1957) Die Regenerationsfähigkeit von Otomesostoma auditivum (Forel et Duplessis) (Turbellaria). Arch Entwicklungsmech Org 150:104–114 [DOI] [PubMed]
- Pfannkuche O, Thiel H (1988) Sample processing. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution, Washington, pp 134–145
- Reddien PW, Sánchez Alvarado A (2004) Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 20:725–757 [DOI] [PubMed]
- Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sánchez Alvarado A (2005) Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell 8:635–649 [DOI] [PMC free article] [PubMed]
- Saló E, Baguñà J (2002) Regeneration in planarians and other worms: new findings, new tools, and new perspectives. J Exp Zool 292:528–539 [DOI] [PubMed]
- Sánchez Alvarado A (2000) Regeneration in the metazoans: why does it happen? Bioessays 22:578–590 [DOI] [PubMed]
- Westheide W, Purschke G (1988) Organism processing. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution, Washington, pp 146–160