Abstract
Neuroimaging evidence suggests that the parietal lobe has an important role in memory retrieval, yet neuropsychology is largely silent on this topic. Recently, we reported that unilateral parietal lobe damage impairs various forms of visual working memory when tested by old/new recognition. Here, we investigate whether parietal lobe working memory deficits are linked to problems at retrieval. We tested two patients with bilateral parietal lobe damage in a series of visual working memory tasks that probed recall and old/new recognition. Stimuli were presented sequentially and several stimulus categories were tested. The results of these experiments show that parietal lobe damage disproportionately impairs old/new recognition as compared to cued recall across stimulus categories. The observed performance dissociation suggests that the posterior parietal lobe plays a particularly vital role in working memory retrieval.
Introduction
The claim that the parietal lobe plays an important role in visual working memory (WM) relies on three pieces of evidence. First, neurophysiological studies from non-human primates report similar WM delay-related activity in portions of the parietal and prefrontal lobes (Chafee & Goldman-Rakic, 1998; Gnadt & Andersen, 1988; Quintana & Fuster, 1999) and lesions to these areas lead to similar impairments of spatial WM (reviewed in Curtis & D’Esposito, 2004). Second, a large number of functional MRI studies report broad bilateral activations across much of the posterior parietal cortex (PPC) during visual WM tasks (reviewed in Wager & Smith, 2003). These activations have been interpreted as reflecting attentional demands (reviewed in LaBar, Gitelman, Parrish, & Mesulam, 1999; Naghavi & Nyberg, 2005), WM maintenance (Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000; Song & Jiang, 2006; Todd & Marois, 2004; Xu & Chun, 2006) or of information accrual (Xu, 2007). Finally, right parietal damage is known to cause spatial WM deficits (De Renzi, Faglioni, & Previdi, 1977; Husain et al., 2001; Malhotra et al., 2005; Pisella, Berberovic, & Mattingley, 2004; Ravizza, Behrmann, & Fiez, 2005) and in some cases, object WM deficits (Berryhill & Olson, in press).
Despite this wealth of findings, the mechanistic role of the parietal lobe in visual WM is poorly understood. Here we assess whether the PPC has an important role in WM retrieval by comparing performance across different retrieval tasks while holding memory encoding and maintenance constant. We specifically compared recall to recognition - a comparison has not been examined in the extant literature. Most behavioral studies, and fMRI studies of visual WM that report PPC activations have only examined old/new recognition performance (Awh, Barton, & Vogel, 2007; Jiang, Olson, & Chun, 2000; Olson & Jiang, 2002; Song & Jiang, 2006; Todd & Marois, 2004; Vogel, Woodman, & Luck, 2001; Xu & Chun, 2006). Neuropsychological studies pertinent to this topic have usually relied on old/new recognition tasks in patients with right PPC damage (Berryhill & Olson, in press; Finke, Bublak, & Zihl, 2006; Pisella et al., 2004), and in these cases, deficits have been consistently reported for spatial WM tasks and less frequently for object WM tasks.
Studies investigating visual WM recall performance have overwhelming assessed spatial WM (see Table 1 for a review of the literature). In one commonly used task, the Corsi block task, participants point to a sequence of locations in the same order as that produced by the experimenter (Corsi, 1972; reviewed in Berch, Krikorian, & Huha, 1998). Although the Corsi block task has been heavily used in neuropsychology, most studies fail to adequately describe or localize the lesion, stating only that right hemisphere damage leads to impaired spatial recall (De Renzi et al., 1977; De Renzi & Nichelli, 1975; Hanley, Young, & Pearson, 1991). More recent studies with MRI-verified lesion locations have reported that either left (Baldo & Dronkers, 2006) or right (Malhotra et al., 2005) inferior parietal lobe damage can cause impaired Corsi block performance. Another frequently used test is the pattern span task in which subjects are asked to reproduce the pattern of a partly filled matrix after a delay period. PPC damage is reported to cause impaired recall in this task (Della Sala, Gray, Baddeley, Allamano, & Wilson, 1999).
Table 1.
Neuropsychological studies of spatial working memory as assessed by recall or recognition in patients with putative parietal lobe damage. Note that in many studies precise cerebral localization was not provided. Only published articles testing recognition or recall of spatial WM were included. Abbreviations: Bi = bilateral, Case = case study, Corsi = Corsi block-tapping task, hemi = hemisphere, inf = inferior, L = left, R = Right, PPC = posterior parietal cortex, PFC = prefrontal cortex, 2AFC = two-alternative forced-choice.
| Authors | Task | Lesion Sites | Impaired Performance |
|---|---|---|---|
| De Renzi & Nichelli, 1975 | Recall: Corsi | L hemi, R hemi | R hemi |
| De Renzi et al., 1977 | Recall: Corsi | R hemi, R hemi with visual field defect | R hemi with visual field defect |
| Hanley et al., 1991 | Recall: Corsi | R hemi, Case | R hemi |
| Markowitsch et al., 1999 | Recall: Corsi | L angular gyrus, Case | No difference |
| Della Sala et al., 1999 | Recall: Pattern Span, Corsi | R hemi, L hemi, Bi hemi | No difference |
| Kessels et al., 2000 | Recall: Corsi | R hemi, L hemi, Bi hemi, subcortical | R hemi |
| Postma et al., 2000 | Recall: Perceptual Localization (2AFC) | R hemi, L hemi | R hemi, L hemi |
| Kessels et al., 2002 | Recall: Object-Location Conjunction, Locations, Corsi, Maze learning | L hemi, R hemi, Bi hemi, Anterior (frontal, temporal), Posterior (occipital, parietal) | Conjunction: L hemi, R hemi, Posterior Location: R hemi, Corsi: no difference Maze: R hemi, Bi Hemi |
| Pisella et al., 2004 | Old/new recognition: Locations | R PPC + neglect, non-PPC + neglect | R PPC neglect |
| Malhotra et al., 2005 | Recall/Recognition: Vertical Corsi | R PPC + neglect; R PPC non-neglect | R lateral PPC neglect |
| Van Asselen et al., 2006 | Recall: Corsi | R PFC, L PFC, R PPC, L PPC | No differences |
| Nys et al., 2006 | Recall: Corsi | R hemi + neglect, L hemi + neglect, Bi hemi + neglect, | L hemi + neglect, R hemi + neglect, Bi hemi + neglect |
| Baldo & Dronkers, 2006 | Recall: Corsi | L inf PPC, L inf frontal | L inf PPC |
| Berryhill & Olson, 2008 | Old/new recognition: Locations | R PPC | R PPC |
Taken together, these findings convincingly demonstrate that spatial WM, whether tested by recall or recognition, is impaired after PPC damage. However, it known whether these findings generalize to non-spatial forms of visual WM. More generally, there has been no systematic comparison between recall and recognition in the context of PPC damage.
This is an important variable to assess for the following reasons. Recall and recognition rely on different recollective processes that presumably draw on different computations in different parts of the brain. For instance, there is an extensive literature documenting differential performance on recall as compared to recognition tasks in the context of long-term memory. Normal adults typically exhibit recall performance that is inferior to recognition performance (Hollingworth, 1913). This performance differential is exaggerated by damage to the prefrontal cortex or medial temporal lobes (reviewed in Aggleton & Brown, 1999; Aggleton & Shaw, 1996; Brown & Aggleton, 2001; Mayes, Montaldi, & Migo, 2007; Skinner & Fernandes, 2007; Turner, Cipolotti, Yousry, & Shallice, 2007; but see Haist, Shimamura, & Squire, 1992; Kopelman et al., 2007; Kopelman & Stanhope, 1998; Manns, Hopkins, Reed, Kitchener, & Squire, 2003; Manns & Squire, 1999; Stark & Squire, 2003). These findings have fostered an empirical and theoretical debate about the role of different brain regions in different forms of recollection.
In contrast, there is little data and even less theory describing the computational functions of the parietal lobe in memory. In regards to the working memory literature, at this point in time, the data suggest that the PPC has some important role in spatial working memory, regardless of whether recall or recognition is probed. There is also evidence that the right PPC plays an important role in object WM. This is demonstrated by the poor performance shown by unilateral right PPC patients on old/new recognition tasks. However we do not know whether the lesion-associated visual WM deficits are due to problems at encoding, maintenance, or retrieval.
The aim of this study was to provide a systematic evaluation of the effects of parietal lobe damage on visual WM retrieval processes. Retrieval was manipulated by comparing performance on similar recall and recognition tasks while holding encoding and maintenance demands constant. The performance of two patients with bilateral PPC damage was compared to that of age- and education- matched controls across several visual WM tasks, using several stimulus categories. The results show that bilateral parietal lobe damage spares visual WM recall while impairing visual WM recognition.
General Methods
Participants
Patients with bilateral parietal lobe damage are extremely rare. The survival rate after suffering the type of cerebrovascular event that produces bilateral parietal lobe damage is low. We were able to identify to patients with bilateral parietal lobe damage in our database. These patients have been discussed previously (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007); we summarize their profiles here.
Patient EE555 is a 39-year-old former teacher with 16 years of education. Between April and June 2004, she suffered three infarcts in the watershed between the posterior and middle cerebral arteries. EE555’s MRI revealed symmetrical lesions in lateral aspects of the inferior parietal lobe, extending from superior aspects of the occipital lobe through the angular gyrus (Brodmann areas (BA) 39) in and around inferior and middle portions of the intraparietal sulcus (IPS). Damage did not encroach into the midline (e.g. precuneus). EE555’s lesions are depicted in Figure 1.
Figure 1.
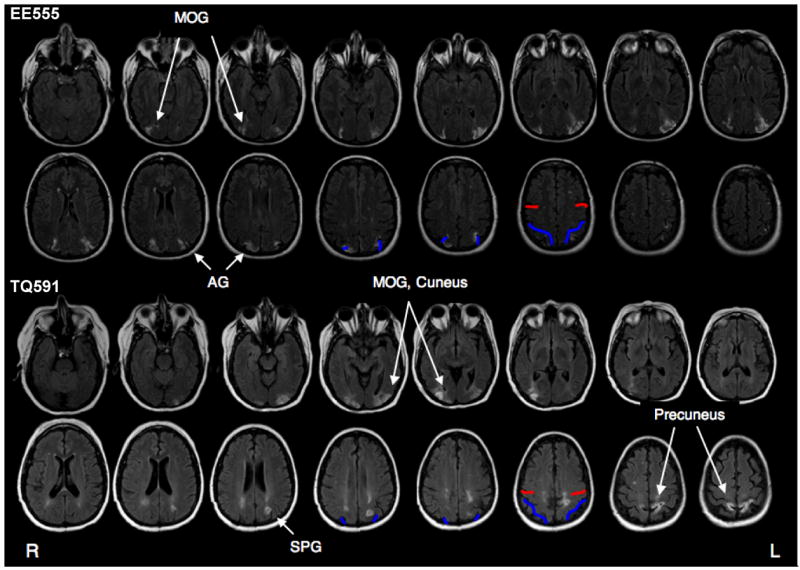
Patient Lesion Traces. Lesions are shown on T2 fluid-attenuated inversion recovery (FLAIR) images in which the lesions appear as white higher intensity patches in parietal regions. Anatomical landmarks are marked in red and blue. Red lines mark the central sulcus, blue lines mark the intraparietal sulcus. Radiological convention is followed (left is on the right). Abbreviations: AG = angular gyrus, MOG = middle occipital gyrus, SPG = superior parietal gyrus.
EE555’s physical and perceptual symptoms are currently stable. Patient EE555’s primary deficit is simultanagnosia as defined by her inability to apprehend the contents of a visual scene, her abnormal performance on line cancellation tasks, in which she crosses off items only at the center, and her tendency towards local bias, as illustrated by her report of the local but not global elements in Navon letters. Language comprehension and speech fluency are unimpaired as assessed by her conversational skills, and performance on the auditory tests of the Western Aphasia Battery (Kertesz, 1982), which were uniformly at ceiling. Reading and writing are impaired due to her simultanagnosia and spatial disorientation. Her visual acuity is normal. EE555 was tested 1.5 – 2.5 years post insult.
Patient TQ591 is a 49-year-old former preschool teacher with 15 years of education. She suffered bilateral parieto-occipital damage due to CNS cerebral vasculitis in March 2006. TQ591’s MRI revealed signs of previous subacute posterior cerebral artery infarctions. The primary lesions are in bilateral parietal regions; see Figure 1. The left parietal lesion extends into IPS (BA 39) and precuneus (BA 7). There are two right lesion sites: the inferior lesion is in superior aspects of the occipital lobe (BA 18 and 19), and the superior lesion is in the superior parietal lobe (BA 7). In both hemispheres, the lesions extend slightly into temporo-occipital (BA 19) regions and parietal white matter.
TQ591’s cortical damage is now considered stable. TQ591’s primary deficit is simultanagnosia, similar to that described for patient EE555. Language comprehension and speech fluency are unimpaired as assessed by her conversational skills, ability to follow instructions and comply with requests, and performance on the Western Aphasia Exam (Kertesz, 1982). Reading and writing are somewhat impaired due to her simultanagnosia and spatial disorientation (she loses her place on a page). Her eyesight is corrected-to-normal. TQ591 was tested 6 – 12 months post insult.
Standardized Test Performance
The subtests of the Wechsler memory scale (WMS-III, Wechsler, 1997b) that do not require comparing complex visual elements were conducted and index scores calculated for the following measures: auditory immediate, auditory delayed, auditory recognition delayed and working memory (see Table 2 for standardized test performance). Patient EE555 performed at least one standard deviation below the mean across all measures. TQ591’s performance was more than one standard deviation below the mean on the auditory immediate and working memory indices but normal on the auditory delayed and auditory recognition delayed tests. These findings provide an initial indication that posterior parietal lobe damage may slightly impair memory performance.
Table 2.
Neuropsychological Test Scores. WMS scores for patients EE555 and TQ591. Scores are from auditory subtests. WM=working memory; recog=recognition; norm=normal; abn=abnormal. Note that scores are index scores in which 100 is the population average, except for forward and backward digit span which are the raw digit lengths. CVLT-II scores represent normalized performance with 0 representing the population mean and a standard deviation (sd) of 1. Z-scores greater than 2 sd below control performance are considered abnormal. Adnormal scores are indicated by bold font.
| Test | Subtest | EE555 | TQ591 |
|---|---|---|---|
| WMS-III | Immediate | 86 | 80 |
| Delayed | 77 | 97 | |
| WM | 83 | 79 | |
| Recog delayed | 55 | 110 | |
| Forward Span | 6 | 8 | |
| Backward | 4 | 4 | |
| Span | |||
| CVLT-II | Immediate free recall, trial 5 | −2.5 | −.5 |
| Short delay free recall | −1.5 | −1.5 | |
| Long delay free recall | −3.0 | 0 | |
| Long delay | −5.0 | −.5 | |
| yes-no recog | |||
| hits | |||
| Total | .5 | −.5 | |
| Intrusions | |||
| Forced-choice | 100% | 100% |
The California Verbal Learning Test-II (Delis, Kramer, Kaplan, & Ober, 2000) is a measure of an adult’s ability to learn and remember verbal material, such as word lists. The CVLT-II data provides additional insight into verbal short-term and long-term memory. Patient EE555’s performance on the CVLT-II indicates that she has impaired verbal memory at both short and long delays. She is most impaired on measures of delayed recognition. In contrast, Patient TQ591’s performance on the CVLT-II indicates that she has spared immediate free recall for verbal information, but moderately impaired verbal free recall memory at short delays. At long delays, her free recall and recognition memory is normal.
In both patients, forward and backward digit span was normal. It should be noted that digit span measure is known to be well-preserved in diverse lesion populations, including amnesics (Black, 1986).
Control Participants
Twelve normal control subjects participated in each experiment (Experiment 1a: Mean (M) age = 42.5, M years of education (edu) = 14.6, 4 males; Experiment 1b: M age = 45.0, M edu = 14.8, 6 males; Experiment 2a: M age = 47.3, M edu = 13.9, 6 males; Experiment 2b: M age = 39.7, M edu = 15.4, 7 males; Experiment 3: M age = 47.3, M edu = 14.1, 7 males).
Stimuli
Three stimulus categories were used in Experiment 1a–2b: colors, abstract shapes and common objects. The color category consisted of 20 circular color patches selected from the full color spectrum. The shape category consisted of 36 black, bilaterally symmetrical abstract shapes generated by a computer algorithm that has been previously employed in studies of visual WM (Jiang et al., 2000). The common objects or ‘tool’ category consisted of 36 grayscale photographs and was limited to the subordinate category of tools in order to be consistent with the other stimulus categories. Stimuli measured approximately 6 cm by 6 cm and were presented on a white background. In Experiment 3, colorized line drawings of common objects were used (Rossion & Pourtois, 2004).
To accommodate the simultanagnosia in both patients and the fact parietal damage slows visual processing (Peers et al., 2005), all stimuli in all experiments were presented sequentially at the center of the monitor at the rate of 1000 ms/item.
Perceptual Control Tasks
To insure that EE555 and TQ591could accurately perceive the stimuli that were used in the WM tasks, two perceptual control tasks were administered. (1) Perception of tools. Thirty-six grayscale photographs of household tools were presented on a white background using ePrime software (Psychology Software Tools). The task was to verbally label each tool under free viewing conditions. The experimenter recorded the responses. EE555 was able to identify 89% of the tools. TQ591 identified 72% of the tools. Errors for both subjects were for low-frequency items: clamps, a leather punch, a wood plane, an eggbeater, and a whisk. (2) Perception of colors and abstract shapes. Multicolored geometric shapes were printed on cards. There were eight different shapes and eight different colors creating a set of 24 cards. The task was to match a particular card based on color, shape, or color-shape. Both subjects were accurate when asked to match colors (100%), shapes (100%) or color/shape conjunctions (100%).
Analysis
In the recall experiments, Experiments 1a and 2a, the dependent measure is raw accuracy. In Experiment 1a, chance performance was .25. In Experiment 2a, chance performance was nearly 0. In the recognition experiments, Experiments 1b, 2b and 3, the dependent measure is corrected recognition (CR). CR is equal to the hit rate (responding “yes” on a match trial) minus the false alarm rate (responding “yes” on a non-match trial). Chance performance corresponds to a CR value of 0. Trials were excluded if no response was registered within two standard deviations of the mean reaction time (Excluded trials: controls 2.9%, patients 3.3%).
Data was analyzed with non-parametric permutation analyses approximating independent sample t-test (Experiment 2a, 3) and a repeated measures analysis of variance (Experiments 1ab, 2b). Permutation tests are an alternative to parametric tests for cases of small numbers of participants and can be accurately used for sample sizes larger than one. For the repeated-measures ANOVA tests, a permutation test was used in which we first computed the F statistic under the standard mixed two-factor ANOVA model. Then the observed values were randomly permuted across the patient and control subjects. The F statistics were recomputed for the permuted data set and a one-tailed count over 1000 replicates was used to compute the significance values (Legendre, Oden, Sokal, Vaudor, & Kim, 1990; Manly, 1997). For the t-test version, no t-value is calculated. In the first stage of analysis, the null hypothesis (that there is no difference between the patient and control groups) is tested using a t-test (Experiment 3). During the second stage, two groups were randomly defined and subjected to the same comparison. This continues until 1000 random samples are taken. The reported p-values refer to the proportion of scores below the observed experimental value (Good, 1994). Statistical analyses were conducted using Matlab (The MathWorks, Natick, MA). SPSS (SPSS, Chicago, IL) was used to perform paired t-tests and one-sample t-tests for the comparison of recall and recognition task performance.
Experiment 1a: Order Recall
In our first study, subjects were required to remember item order. This task was chosen because there is neuroimaging evidence that the parietal lobe is involved in order WM. Marshuetz and colleagues found order WM related activity in bilateral superior parietal regions (Brodmann areas 7 and 40), which they hypothesize is involved in tracking the temporal spacing between items in order WM tasks (Marshuetz, 2005; Marshuetz, Smith, Jonides, DeGutis, & Chenevert, 2000; see also Majerus et al., 2007; Majerus et al., 2006). One recent neuropsychological study found that bilateral parietal lobe damage impairs order WM (Malcolm & Barton, 2007). To assess whether parietal lobe damage affects order recall, subjects encoded four sequentially presented items and after a brief delay, were required to recall the temporal position of a particular item.
Methods
Task
Prior to the onset of the actual experiment, all subjects performed several practice trials to become familiar with the trial design.
Each trial began with a central fixation cross (1000 ms), followed by the sequential presentation, in the center of the screen, of four randomly selected stimuli (1000 ms/item) from one stimulus category (color, shape, tool) at the center of the monitor. Items appeared contiguously with no inter-stimulus interval. After a 1000 ms masked delay interval, a probe stimulus was presented at central fixation until a response was made, (see Figure 2). The task was to report the temporal position the probe item had occupied during the memory sequence (position 1, 2, 3, or 4). Responses were unspeeded in this and all subsequent experiments. Each position was equally likely to be probed, making chance performance equal to 25% correct. After the response was entered, the next trial was initiated by pressing the space bar. There were 20 trials per stimulus category.
Figure 2.
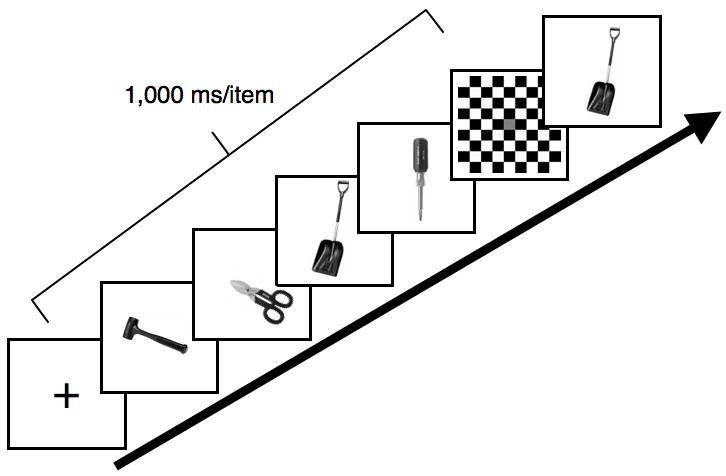
Trial design of Experiment 1a. On each trial, four colors, abstract shapes, or tools were presented sequentially (1000 ms each) and after a brief delay of 1000 ms, a probe image was presented and remained on the computer screen until subjects responded as to which temporal position the probe item had previously occupied. In this example, the correct response would be 3.
Note that although it is common to use concurrent verbal memory loads to minimize the possibility that visual items are remembered by verbal labels (Luck & Vogel, 1997; Olson & Jiang, 2002) this type of task is difficult to implement in older and patient populations and introduces additional variables that may confound the effects of interest. Each stimulus category varied in the degree to which verbal encoding could reasonably be used, from a high level in the tool category, an intermediate level in the color category, and none in the shape category.
Results and Discussion
Because this experiment employed a recall task in which false alarms were not possible, the dependent measure was raw accuracy. The accuracy values for each group (control, patient) and temporal position (1, 2, 3, 4) were subjected to permutation analyses (see Figure 3), collapsing across stimulus category. The groups did not differ (F1, 12 = 62.17, p = .10, M patients = .64, M controls = .69). The main effect of temporal position was significant (F3, 36 = 5.67, p = .002) although no pairwise comparisons reached significance, performance was best when the probe item appeared at the 4th temporal position (p = .07). The interaction of group and temporal position reached significance (F3, 36 = 3.06, p = .05) as the patient group showed a stronger primacy effect than did the control subjects. To determine if there were accuracy differences for different stimulus types, the data were collapsed across temporal position and a permutation analysis examined group (control, patient) and stimulus category (color, shape, tool). Again, the groups did not differ (F1, 12 = 9.65, p = .86). There was a main effect of stimulus category (F2, 24 = 8.92, p = .001), such that performance on the tool category was better than performance with shape stimuli (p = .04). The interaction of group and stimulus category failed to reach significance however (F2, 24 = 1.86, p = .23), see Figure 3.
Figure 3.
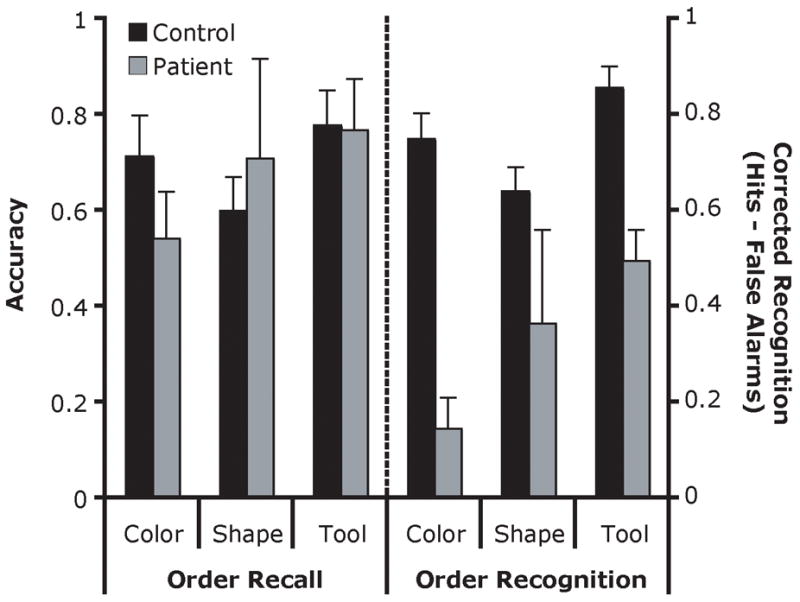
Order recall and recognition performance (Experiments 1a and 1b) as a function of stimulus category. The order recall performance is measured by accuracy (left axis), and the order recognition is measured by corrected recognition (right axis). Error bars indicate the standard error of the mean (SEM).
Finally, the errors were examined in order to evaluate differences between the patient and control groups. Both groups erroneously chose temporal neighbors of the correct answer (i.e. responded ‘3rd’ when the answer was actually ‘2nd’) more often than temporally distant non-neighbor (M neighbor errors: patients: .61; controls: .64). The patients showed particularly strong recency and primacy effects as demonstrated by the lower proportion of errors when the correct answer was in the first or last position (M patients: .29; M controls: .45) than when it was embedded at the second and third positions.
These results suggest that the patients’ performance was unimpaired in a visual order WM task when recall was probed.
Experiment 1b: Order Recognition
In Experiment 1a, patients demonstrated normal order recall performance. To assess whether order recognition performance is similarly spared by parietal lobe damage, we conducted a recognition version of the order task.
Methods
Task
The stimuli and task design were identical to that of Experiment 1a with the following exceptions: after the delay period, the four previously viewed items were repeated in either the same or a different order. The task was to make an unspeeded response as to whether the two order sequences were the same or different by pressing one key for ‘same’, another key for ‘different’. The second order could differ from the first order by 0 (same order), 2, 3, or 4 changes in position. After a response was made, a key press initiated the next trial. Ten trials per stimulus category were conducted.
Results and Discussion
The corrected recognition scores for each group were subjected to permutation analyses (see Figure 3). Unlike the findings of Experiment 1a, the patients were impaired relative to controls (F1, 12 = 744.62, p = .03; M patients = .34, M controls = .75). The main effect of stimulus category also reached significance (F2, 24 = 4.46, p = .03), this was due to overall better performance on the tool stimuli than shapes (p = .04). The interaction of group × stimulus category did not reach significance (F2, 24 = 1.03, p = .40). When performance was assessed as a function of the number of changes in the presented order (either 0, 2, 3, or 4), it was found that patients were worse at detecting changes (F1, 12 = 996.84, p < .001) and that their performance did not steadily improve as the number of changes increased (M 0 changes = .75, M 2 changes = .54, M 3 changes = .42, M 4 changes = .65). In contrast, more changes improved control subjects’ performance (M 0 = .85, M 2 = .77, M 3 = .96, M 4 = .92). The interaction of group and number of changes did not reach significance (F3, 36 = 2.14, p = .15).
These results show that the patients’ performance was impaired in an order recognition task. This finding provides preliminary evidence that PPC lesions may differentially affect recall as compared to recognition.
Experiment 2a: Object Recall
One possible criticism of the order recognition task is that poor performance was due to the higher memory load imposed by maintaining one order while observing a second order. In fact, some researchers might consider Experiment 1b a long-term memory task, given the large number of items presented. In Experiments 2a, 2b and 3, we probed the recall/recognition dissociation further using paradigms designed to extend our findings and allay methodological concerns. Experiment 2a examined WM for objects using a recall task that imposed no temporal ordering demands. Following a brief delay, subjects were required to verbally report the identities of four items.
Methods
Task
Four tool images were presented sequentially (1000 ms/image) and followed by a masked delay (1000 ms). After the mask, a cue appeared and prompted the subject to name aloud the four previously viewed images. The experimenter recorded responses. Correct answers consisted of accurately named items from the encoding phase, regardless of order. Intrusions were also tallied. Only tool stimuli were used because the items in this category are easily named. After the responses had been made and recorded, a key press initiated the next trial. A total of 10 trials were performed.
Results and Discussion
The raw accuracy scores for each group (control, patient) were subjected to permutation analyses (see Figure 4). The results showed that patients and controls had similar levels of accuracy (p = .23, M patients = .69, M controls = .78). An examination of the erroneous responses indicated that the patients reported fewer intrusive responses than the controls (M patients = 1.0, M controls = 4.8). This difference was marginally significant (p = .06)1. These results confirm the findings from Experiment 1a, suggesting that the patients’ performance is unimpaired in recall tasks of visual WM.
Figure 4.
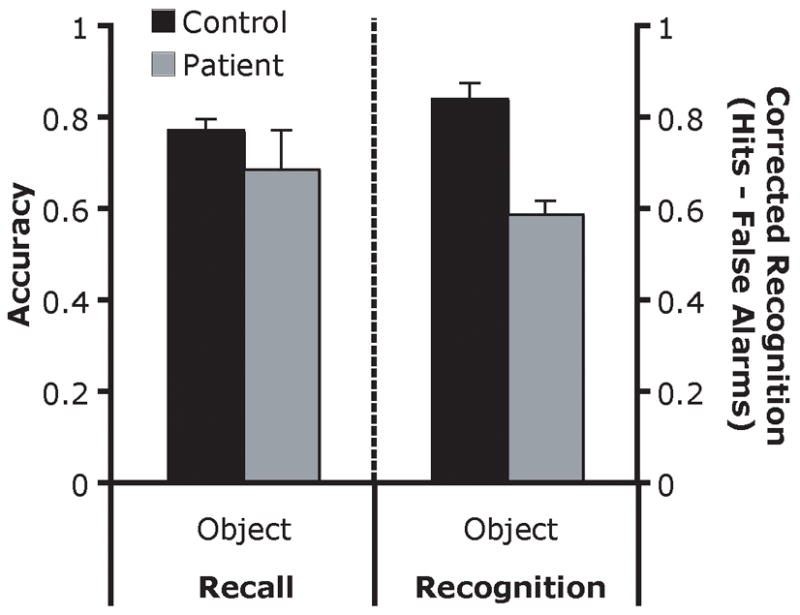
Object recall and recognition performance (Experiments 2a and 2b). The pattern of behavior observed for tools was also observed with color and shape stimulus categories in the object recognition experiment (data not shown). Error bars indicate SEM.
Experiment 2b: Object Recognition
In Experiment 2b object memory was assessed in an old/new recognition paradigm. The task and stimuli are identical to that used in Experiment 2 of Berryhill and Olson (in press).
Methods
Task
Trials were similar to those used in Experiment 2a except for the probe task. On one half of all trials, the probe image was a new item that had not been in the memory set; in the other half of trials the probe image was an old item. Thus, chance performance was 50%. The task was to report whether the probe image was old or new. Four-item trials were organized into three 20-trial blocks. To make this experiment directly comparable to Experiment 1b, three 20-trial blocks of color and abstract shape recognition trials were also tested. Subjects performed several practice trials to become familiar with the trial design.
Results and Discussion
The corrected recognition scores for each group (control, patient) and stimulus category (color, shape, tool) were subjected to permutation analyses (see Figure 4). The patient group was significantly impaired relative to the control group (F1, 12 = 637.51, p = .01, M patients = .40, M controls = .65). There was also a main effect of stimulus category (F2, 24 = 32.43, p < .001) such that performance on the tool stimuli was better than color (p = .003) and shape (p = .001) performance. It should be noted that for the color stimuli, but not for the tool or shape stimuli, patient EE555’s performance drove the group effect (see Table 3). There was no interaction of group × stimulus category (F2, 24 < 1, n.s.). This was confirmed by evaluation of the difference scores between the control and patient groups, which differed by a constant value across stimulus categories. In order to make this experiment directly comparable to Experiment 2a, only the performance in the tool category are shown in Figure 4. A permutation analysis of only the tool data revealed a significant effect of group (p = .02, M patients = .59, M controls = .84). The pattern of performance was identical across stimulus types.
Table 3.
Hits followed by false alarms for each recognition task.
| Exp. 1b. Order Recognition | Exp. 2b. Object Recognition | Exp. 3. Non-Repeating Object Recognition | |||||
|---|---|---|---|---|---|---|---|
| Color | Shape | Tools | Color | Shape | Tools | Common Objects | |
| EE555 | .86, .33 | .67, .50 | 1.0, .43 | .96, .74 | .97, .74 | 1.0, .38 | .79, .22 |
| TQ591 | .83, .75 | 1.0, .44 | .56, 0 | .54, 07 | .75, .50 | .71, .15 | .73, .04 |
| Controls | .80, .05 | .80, .16 | .92, .07 | .80, .22 | .74, .20 | .90, .05 | .92, .03 |
These results show that the patients’ performance was impaired at visual WM tasks testing recognition across three very different stimulus categories - colors, abstract shapes, and tools. Overall performance was highest in the tool condition and lowest in the novel shape condition. However, deficits were observed to a similar degree for all three stimulus types, suggesting that the WM deficit exists when a verbal strategy could be employed for nameable items, such as tools, and even when such a strategy would be challenging, such as with the novel shape stimuli.
Experiments 1a–2b: Combined Analyses
To directly compare patient’s performance on the recall and recognition tasks, their performance was converted into z-scores and t-tests were performed (see Figure 5). This showed that only the recognition scores differed from that of the controls (t3 = −9.4, p = .003). When the patient’s scores on the recall (Order Recall, Object Recall) and recognition (Order Recognition, Object Recognition) tasks were directly compared, significantly worse performance was found in the recognition tasks compared to the recall tasks (t3 = 3.1, p = .05).
Figure 5.
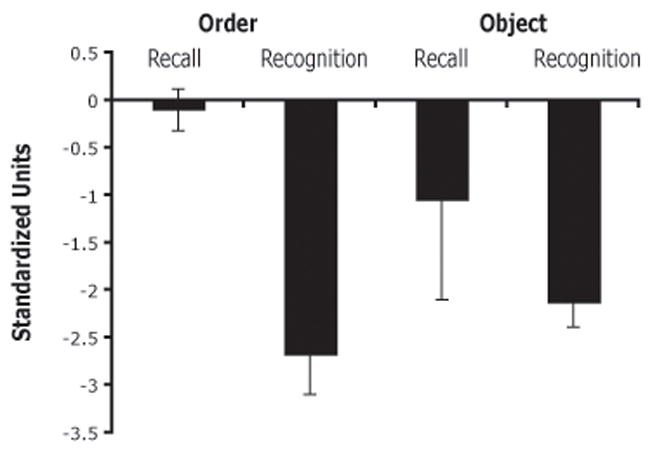
Recall and recognition performance. Patients’ scores are in standardized units (z-scores) with regards to control subject performance. By convention, impaired performance is when z > 1.96.
Experiment 3: Non-Repeating Object Recognition WM
In Experiments 1a–2b only a limited number of stimuli were used, giving rise to repeated exposures across trials. One explanation for the poor patient performance on the recognition tasks is that parietal lobe damage causes source memory impairments such that patients confuse the probe item on trial N with target items previously viewed on other trials. If true, patients should perform normally on recognition tasks that use unique stimuli in each trial. This was tested in Experiment 3.
Methods
Task
The trial design was identical to that used in Experiment 2b. The only difference was the stimuli which were colorized line drawings of animals, objects, and buildings (Rossion & Pourtois, 2004), shown without repetition. There was a single block of 56 trials. Two controls were excluded due to a failure to understand and comply with instructions.
Results and Discussion
The corrected recognition scores were subjected to two-tailed permutation analysis comparing the two groups (see Figure 6). This analysis found that the patients were significantly impaired (p = .03, M patients = .63, M controls = .89).
Figure 6.
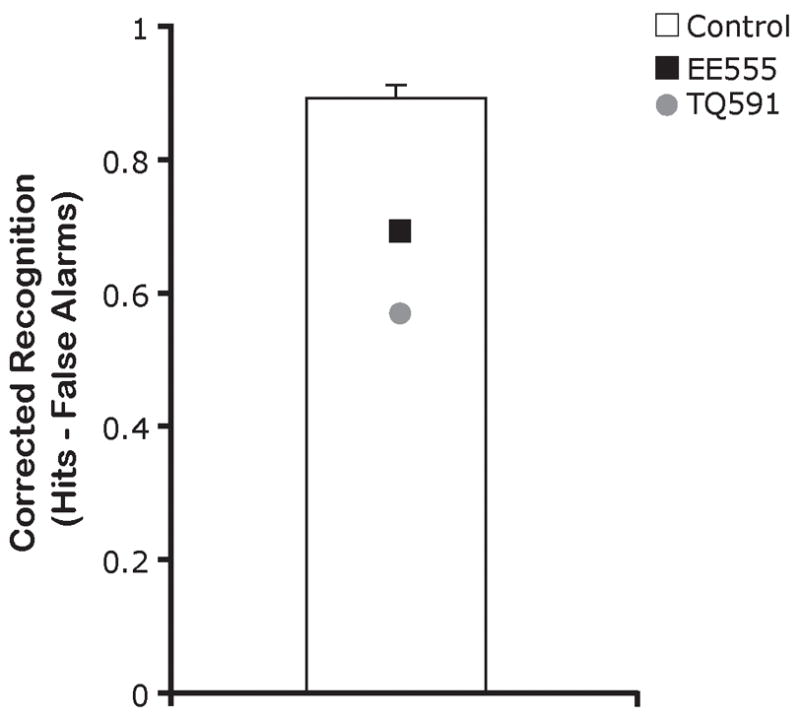
Results of Experiment 3, non-repeating stimuli recognition. Corrected recognition performance as a function of group membership. Error bars indicate SEM.
These results confirm that the patients were generally impaired in recognition WM tasks. This cannot be attributed to increased intrusions from one trial to the next because patients exhibited recognition impairments even when there were no stimulus repetitions.
General Discussion
The present findings show that the human posterior parietal lobe has a significant role in human visual WM, replicating findings from a different group of patients with right parietal lobe damage (Berryhill & Olson, in press). The PPC’s role in WM can be most closely linked with retrieval processes because performance varied as a function of probe task. Performance was intact when visual WM was tested by cued recall (Experiments 1a and 2a) but impaired when tested by old/new recognition tasks (Experiments 1b, 2b, 3). These findings generalized across several tasks and types of visual stimuli, such as colors, novel shapes, and common objects. It is difficult to attribute the impaired recognition performance to source memory errors (e.g. intrusions from previous trials) because performance was impaired even when the stimuli were unique every trial (Experiment 3).
These findings reveal the essential role of the parietal lobe in visual WM retrieval. The intact performance on the recall experiments – employing the same stimuli and encoding tasks as in the recognition experiments – signify that information was accurately encoded. These same patients were also tested on a detailed autobiographical memory test and deficits were observed for information that was encoded long before they incurred parietal lesions, once again, indicating impaired memory retrieval (Berryhill et al., 2007).
Our interpretation of the present data suggests impaired memory retrieval. This view stands in contrast to the interpretations of previous fMRI findings in which PPC activations were associated with maintenance of visual information in WM (Leung, Oh, Ferri, & Yi, 2007; Moore, Cohen, & Ranganath, 2006; Todd & Marois, 2004; Woodward et al., 2006; Xu & Chun, 2006). One explanation for this is that activity during the WM maintenance period reflects an attentional tagging process important for memory retrieval. Of course, the fact that the patients accurately retrieved information in the recall tasks indicates that they are capable of memory retrieval. In other words, they do not have gross, all-encompassing memory-retrieval deficits, which is predicted by the fact that neither patient is amnestic. Rather, they have a selective deficit made evident by certain retrieval conditions, namely, old/new recognition.
Generality of Findings
One open question is whether the observed retrieval deficit is specific to the stimulus modality (visual and non-spatial) and time delay (short) tested here, or whether it is a general deficit. Our review of the literature suggests that the findings reported in this manuscript will not generalize to spatial WM tasks: unilateral PPC damage impairs spatial WM, whether tested by recall or recognition (see Table 1). It has long been noted that the parietal lobe has a special role in apprehending and acting upon spatial representations (Critchley, 1953). As such, spatial WM may be more reliant on the parietal lobe for all aspects of mnemonic processing - encoding, maintenance, and retrieval - as compared to other forms of visual memory.
It is difficult to assess whether the observed retrieval deficits will generalize to verbal WM. Our only data that speaks to this point is that both patients have unimpaired forward and backward digit spans. However, there are numerous reports of impoverished verbal WM in the context of left inferior-lateral PPC damage (Majerus et al., 2006; Smith & Jonides, 1998; Vallar & Papagno, 2002). These studies have not attempted to link the deficits to failures at particular points in mnemonic processing. Future studies should assess whether parietal-based verbal WM impairments are best explained as retrieval failures.
Last, on occasion it has been reported that certain types of PPC lesions (left inferior) can damage WM while leaving LTM intact (Basso, Spinnler, Vallar, & Zanobio, 1982; Bisiacchi, Cipolotti, & Denes, 1989; Shallice & Warrington, 1970; Warrington, Logue, & Pratt, 1971). This piece of evidence is frequently used to defend the multistore model of memory. The implication of these findings is that PPC damage should not cause long-term memory impairments of any sort. Contrary to this, we have evidence that PPC retrieval deficits exist in LTM. First, Experiment 1b cannot be easily classified as a WM or LTM task because subjects were exposed to four stimuli, then to another four stimuli, exceeding the limits of the short-term store and also potentially overwriting the first memory trace. In this task, parietal patients performed as poorly as they did on Experiments 2b and 3, which were clearly WM tasks. Second, as noted earlier, both patients’ exhibit impaired retrieval of autobiographical memories under certain retrieval contexts (Berryhill et al., 2007). Third, both patients are deficient in the ability to imagine or construct future events, a memory process that is thought to rely on the retrieval of episodic memories (Berryhill, Picasso, & Olson, submitted). These findings bolster the claims of fMRI studies linking PPC activity to LTM retrieval processes (reviewed in Cabeza, 2008; Wagner, Shannon, Kahn, & Buckner, 2005; Vilberg & Rugg, 2008) while calling into question the dissociation between short-term and long-term memory reported in prior studies of patients with unilateral damage to the parietal lobe (Shallice & Warrington, 1970).
Although these findings are suggestive, future studies should assess the effects of PPC damage on LTM retrieval in more detail. It is tempting to generalize the deficits reported here to all types of recognition memory; just as medial temporal lobe damage indiscriminately impairs explicit memory (Squire, 1992; Squire, Stark, & Clark, 2004). However it is more likely that parietal lobe memory deficits will be somewhat specific (Haramati, Soroker, Dudai, & Levy, 2007), given the role of this region in perception and attention.
Alternative Explanations
One alternative explanation for the present findings is that the patients exhibited impaired performance when they had to rely on visual processing, but intact performance when they could employ a verbal strategy such as the recall tasks of Experiment 1a and 2a. This explanation does not fully explain the observed findings because verbal strategies could easily be used whenever tools or common objects served as stimuli, yet performance was impaired in a number of instances in which such stimuli were used (Experiments 1b and 2b, tool conditions, Experiment 3).
A second explanation is that the presence of a probe image on the recognition trials erased the fragile memory trace of the encoded stimulus set. This hypothesis predicts that patient performance will always suffer when the encoded stimuli are followed by additional interference-producing stimuli such as a probe image. The “fragile memory trace” hypothesis is countered by the findings of the first recall task, Experiment 1a. In this task, four items were presented and after a brief delay, another item was shown and the task was to report the sequential position that item had previously occupied. Patients performed normally on this task, even though there was a probe image following the encoded stimuli.
Anatomical Considerations
Although it is parsimonious to conclude that the PPC is the critical neural region for the observed WM recognition deficits, it remains possible that the results reported here were due to underlying damage to fiber tracts between parietal and frontal or medial temporal regions. In consideration of this alternative, it is important to note that damage to frontal and hippocampal regions is associated with greater recall than recognition deficits in LTM tasks; reviewed in (Aggleton & Brown, 1999; Aggleton & Shaw, 1996; Mayes et al., 2007; Skinner & Fernandes, 2007), or less frequently, with equivalent recall and recognition deficits (Haist et al., 1992; Kopelman et al., 2007; Kopelman & Stanhope, 1998; Manns et al., 2003; Manns & Squire, 1999; Stark & Squire, 2003), the opposite of the present findings. We found one neuropsychological report of the opposite finding, impaired recognition and preserved recall, in a single patient with extensive frontal damage (Delbecq-Derouesne, Beauvois, & Shallice, 1990). The bilateral parietal patients do not behave like frontal patients – for example, in Experiment 2a they supply fewer intrusive responses than the control subjects. As such, we believe that the observed deficits in recognition visual WM are due to PPC damage.
Conclusions and Future Considerations
There are several questions that should be investigated more carefully in future work on parietal lobe memory retrieval mechanisms. First, are these deficits similar to deficits that occur after other types of brain damage? We were unable to identify an appropriate lesion control population so we cannot address this important question. Second, what aspect of memory retrieval is disrupted after parietal lobe damage? In a previous study of these patients we suggested that internal attention was disrupted (Berryhill et al., 2007) but there are other plausible explanations. Third, which subregion of the PPC is most critical for recognition memory deficits? Our patients both have PPC lesions but beyond that, their lesions differ. Patient EE555’s lesions are more inferior whereas patient TQ591’s lesions are more superior. Patient EE555 exhibits memory impairments on a wide range of memory tests: the auditory subscales of the WMS, CVLT, autobiographical memory tests, and the present data. Patient TQ591 does not exhibit consistent deficits on the auditory subscales of the WMS or CVLT, but does exhibit memory impairments on the other tasks. There are a number of possible explanations for the differences between the two patients. Inferior portions of the PPC may be important for amodal memory while the superior PPC may be important only for visual memory. Evidence for this view comes from disparate performance by patient TQ591 on the visual memory tests compared to the verbal memory tests – the WMS and CVLT. Alternatively, inferior PPC may be important for memory retrieval regardless of time delay while superior PPC may be more important for short-delay memory retrieval. This explanation is suggested by differences in the degree of impairments exhibited by the patient TQ591 on short- and long- delay memory tasks.
In sum, our study shows that visual WM is impaired under certain retrieval contexts, namely, old/new recognition. This impairment was consistent across a number of conditions and task manipulations. The finding that the parietal lobe plays an important role in memory retrieval is supported by many neuroimaging studies that have only recently found support from neuropsychological cases.
Acknowledgments
We thank Junhyong Kim and Eiling Yee for help with permutation analyses and Youssef Ezzyat, Page Widick and Olu Faseyitan for help with MRIcro. We thank Lisa Phuong for testing control subjects. We thank Marianna Stark and Branch Coslett for their advice and assistance, and EE555 and TQ591 for their time. This research was supported by RO1 MH071615-01 to I. Olson.
Footnotes
Both patients exhibited normal levels of intrusions on the CVLT.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22(3):425–444. discussion 444–489. [PubMed] [Google Scholar]
- Aggleton JP, Shaw C. Amnesia and recognition memory: a re-analysis of psychometric data. Neuropsychologia. 1996;34(1):51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci. 2007;18:622–628. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20(5):529–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Basso A, Spinnler H, Vallar G, Zanobio ME. Left hemisphere damage and selective impairment of auditory verbal short-term memory. A case study. Neuropsychologia. 1982;20(3):263–274. doi: 10.1016/0028-3932(82)90101-4. [DOI] [PubMed] [Google Scholar]
- Berch DB, Krikorian R, Huha EM. The Corsi block-tapping task: methodological and theoretical considerations. Brain Cogn. 1998;38(3):317–338. doi: 10.1006/brcg.1998.1039. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. The Right Parietal Lobe Is Critical For Visual Short-Term Memory. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2008.01.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and Episodic Memory: Bilateral Damage Causes Impaired Free Recall of Autobiographical Memory. Journal of Neuroscience. 2007;(27):14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Picasso L, Olson IR. Bilateral Parietal Lobe Damage Impairs the Ability to Imagine the Future submitted. [Google Scholar]
- Bisiacchi PS, Cipolotti L, Denes G. Impairment in processing meaningless verbal material in several modalities: the relationship between short-term memory and phonological skills. Q J Exp Psychol A. 1989;41(2):293–319. doi: 10.1080/14640748908402367. [DOI] [PubMed] [Google Scholar]
- Black FW. Digit repetition in brain-damaged adults: clinical and theoretical implications. J Clin Psychol. 1986;42(5):770–782. doi: 10.1002/1097-4679(198609)42:5<770::aid-jclp2270420516>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of lateral posterior parietal regions in episodic memory retrieval: The dual attention hypothesis. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79(6):2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Critchley M. The Parietal Lobes 1953 [Google Scholar]
- Curtis CE, D’Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogn Affect Behav Neurosci. 2004;4(4):528–539. doi: 10.3758/cabn.4.4.528. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P, Previdi P. Spatial memory and hemispheric locus of lesion. Cortex. 1977;13(4):424–433. doi: 10.1016/s0010-9452(77)80022-1. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Nichelli P. Verbal and non-verbal short-term memory impairment following hemispheric damage. Cortex. 1975;11(4):341–354. doi: 10.1016/s0010-9452(75)80026-8. [DOI] [PubMed] [Google Scholar]
- Delbecq-Derouesne J, Beauvois MF, Shallice T. Preserved recall versus impaired recognition. A case study. Brain. 1990;113(Pt 4):1045–1074. doi: 10.1093/brain/113.4.1045. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II: California Verbal Learning Test Second Edition Adult Version. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Della Sala S, Gray C, Baddeley A, Allamano N, Wilson L. Pattern span: a tool for unwelding visuo-spatial memory. Neuropsychologia. 1999;37(10):1189–1199. doi: 10.1016/s0028-3932(98)00159-6. [DOI] [PubMed] [Google Scholar]
- Finke K, Bublak P, Zihl J. Visual spatial and visual pattern working memory: neuropsychological evidence for a differential role of left and right dorsal visual brain. Neuropsychologia. 2006;44(4):649–661. doi: 10.1016/j.neuropsychologia.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70(1):216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Good P. Permutation tests: a practical guide to resampling methods for testing hypotheses. New York: Springer-Verlag; 1994. [Google Scholar]
- Haist F, Shimamura AP, Squire LR. On the relationship between recall and recognition memory. J Exp Psychol Learn Mem Cogn. 1992;18(4):691–702. doi: 10.1037//0278-7393.18.4.691. [DOI] [PubMed] [Google Scholar]
- Hanley JR, Young AW, Pearson NA. Impairment of the visuo-spatial sketch pad. Q J Exp Psychol A. 1991;43(1):101–125. doi: 10.1080/14640749108401001. [DOI] [PubMed] [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The Posterior Parietal Cortex in Recognition Memory: A Neuropsychological Study. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hollingworth HL. Characteristic differences between recall and recognition. American Journal of Psychology. 1913;24:532–544. [Google Scholar]
- Husain M, Mannan S, Hodgson T, Wojciulik E, Driver J, Kennard C. Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain. 2001;124:941–952. doi: 10.1093/brain/124.5.941. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Olson IR, Chun MM. Organization of visual short-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:683–702. doi: 10.1037//0278-7393.26.3.683. [DOI] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery. Psychological Corp; 1982. [Google Scholar]
- Kessels RP, Jaap Kappelle L, de Haan EH, Postma A. Lateralization of spatial-memory processes: evidence on spatial span, maze learning, and memory for object locations. Neuropsychologia. 2002;40(8):1465–1473. doi: 10.1016/s0028-3932(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Bright P, Buckman J, Fradera A, Yoshimasu H, Jacobson C, et al. Recall and recognition memory in amnesia: patients with hippocampal, medial temporal, temporal lobe or frontal pathology. Neuropsychologia. 2007;45(6):1232–1246. doi: 10.1016/j.neuropsychologia.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N. Recall and recognition memory in patients with focal frontal, temporal lobe and diencephalic lesions. Neuropsychologia. 1998;36(8):785–795. doi: 10.1016/s0028-3932(97)00167-x. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10(6):695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Legendre P, Oden NL, Sokal RR, Vaudor A, Kim J. Approximate analysis of variance of spatially autocorrelated regional data. Journal of Classification. 1990;7:53–75. [Google Scholar]
- Leung HC, Oh H, Ferri J, Yi Y. Load response functions in the human spatial working memory circuit during location memory updating. Neuroimage. 2007;35(1):368–377. doi: 10.1016/j.neuroimage.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Majerus S, Bastin C, Poncelet M, Van der Linden M, Salmon E, Collette F, et al. Short-term memory and the left intraparietal sulcus: focus of attention? Further evidence from a face short-term memory paradigm. Neuroimage. 2007;35(1):353–367. doi: 10.1016/j.neuroimage.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Majerus S, Poncelet M, Van der Linden M, Albouy G, Salmon E, Sterpenich V, et al. The left intraparietal sulcus and verbal short-term memory: focus of attention or serial order? Neuroimage. 2006;32(2):880–891. doi: 10.1016/j.neuroimage.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Malcolm GL, Barton JJ. Sequence Agnosia” in Balint’s syndrome: defects in visuotemporal processing after bilateral parietal damage. J Cogn Neurosci. 2007;19(1):102–108. doi: 10.1162/jocn.2007.19.1.102. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Jager HR, Parton a, Greenwood R, Playford ED, Brown MM, et al. Spatial working memory capacity in unilateral neglect. Brain. 2005;128:424–435. doi: 10.1093/brain/awh372. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, bootstrap, and monte carlo methods in biology. 2. London: Chapman & Hall; 1997. [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37(1):171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Impaired recognition memory on the Doors and People Test after damage limited to the hippocampal region. Hippocampus. 1999;9(5):495–499. doi: 10.1002/(SICI)1098-1063(1999)9:5<495::AID-HIPO2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Kalbe E, Kessler J, von Stockhausen HM, Ghaemi M, Heiss WD. Short-term memory deficit after focal parietal damage. J Clin Exp Neuropsychol. 1999;21(6):784–797. doi: 10.1076/jcen.21.6.784.853. [DOI] [PubMed] [Google Scholar]
- Marshuetz C. Order information in working memory: an integrative review of evidence from brain and behavior. Psychol Bull. 2005;131(3):323–339. doi: 10.1037/0033-2909.131.3.323. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. J Cogn Neurosci. 2000;12(Suppl 2):130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Moore CD, Cohen MX, Ranganath C. Neural mechanisms of expert skills in visual working memory. J Neurosci. 2006;26(43):11187–11196. doi: 10.1523/JNEUROSCI.1873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14(2):390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nys GM, van Zandvoort MJ, van der Worp HB, Kappelle LJ, de Haan EH. Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain. 2006;129(Pt 8):2148–2157. doi: 10.1093/brain/awl199. [DOI] [PubMed] [Google Scholar]
- Olson IR, Jiang Y. Is visual short-term memory object based? Perception & Psychophysics. 2002;64:1055–1067. doi: 10.3758/bf03194756. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJ, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, et al. Attentional functions of parietal and frontal cortex. Cereb Cortex. 2005;15(10):1469–1484. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Pisella L, Berberovic N, Mattingley JB. Impaired working memory for location but not for colour or shape in visual neglect: a comparison of parietal and non-parietal lesions. Cortex. 2004;40:379–390. doi: 10.1016/s0010-9452(08)70132-1. [DOI] [PubMed] [Google Scholar]
- Quintana J, Fuster JM. From perception to action: temporal integrative functions of prefrontal and parietal neurons. Cereb Cortex. 1999;9(3):213–221. doi: 10.1093/cercor/9.3.213. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Behrmann M, Fiez JA. Right parietal contributions to verbal working memory: spatial or executive? Neuropsychologia. 2005;43(14):2057–2067. doi: 10.1016/j.neuropsychologia.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham D. The Prefrontal Cortex: Response Selection or Maintenance Within Working Memory. Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Shallice T, Warrington EK. Independent functioning of verbal memory stores: a neuropsychological study. Q J Exp Psychol. 1970;22(2):261–273. doi: 10.1080/00335557043000203. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45(10):2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95(20):12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Jiang Y. Visual working memory for simple and complex features: an fMRI study. Neuroimage. 2006;30(3):963–972. doi: 10.1016/j.neuroimage.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13(2):281–292. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Turner MS, Cipolotti L, Yousry T, Shallice T. Qualitatively different memory impairments across frontal lobe subgroups. Neuropsychologia. 2007;45(7):1540–1552. doi: 10.1016/j.neuropsychologia.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Vallar G, Papagno C. Neuropsychological impairments of verbal short-term memory. In: Baddeley A, Kopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. Chichester: John Wiley & Sons; 2002. pp. 249–270. [Google Scholar]
- van Asselen M, Kessels RP, Neggers SF, Kappelle LJ, Frijns CJ, Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44(7):1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from event-related fMRI. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception & Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Logue V, Pratt RT. The anatomical localisation of selective impairment of auditory verbal short-term memory. Neuropsychologia. 1971;9(4):377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third edition: Administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997b. [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139(1):317–325. doi: 10.1016/j.neuroscience.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Xu Y. The role of the superior intraparietal sulcus in supporting visual short-term memory for multifeature objects. J Neurosci. 2007;27(43):11676–11686. doi: 10.1523/JNEUROSCI.3545-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440(7080):91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]


