Abstract
The preparation of a collection of 131 small molecules, reminiscent of families of long chain N-acyl tyrosines, enamides and enol esters that have been isolated from heterologous expression of environmental DNA (eDNA) in Eschericia coli, is reported. The synthetic libraries of N-acyl tyrosines and their 3-keto counterparts were prepared via solid-phase routes, whereas the enamides and enol esters were synthesized in solution-phase.
Microbes, especially soil-dwelling bacteria, have made enormous contributions to our stock of biologically active small molecules.1 Discovering these molecules usually requires isolating the producing organism, culturing it in the laboratory, and assaying the culture extracts for biological activity. The realization that only a tiny and unrepresentative minority of soil and other microorganisms can be cultured by currently described techniques2 led many laboratories to develop alternative strategies for accessing the small molecules produced by the uncultured majority.3,4 Such processes typically involve obtaining DNA–not the producing organism–directly from the environment (thus termed environmental DNA or eDNA) and incorporating it into alternative hosts to discover gene-host combinations with the capacity to produce biologically active compounds. In our laboratory we have employed this approach to express eDNA-encoded pathways in E. coli, and used an antibiotic assay to identify colonies producing small molecule antibiotics. The most frequently identified metabolites have been long chain N-acyl amino acids,3b,5 especially N-acyl tyrosines (NATs), and the widespread occurrence of these compounds, which had not been previously reported as microbial natural products, raised questions about their biosynthesis and biological function(s).
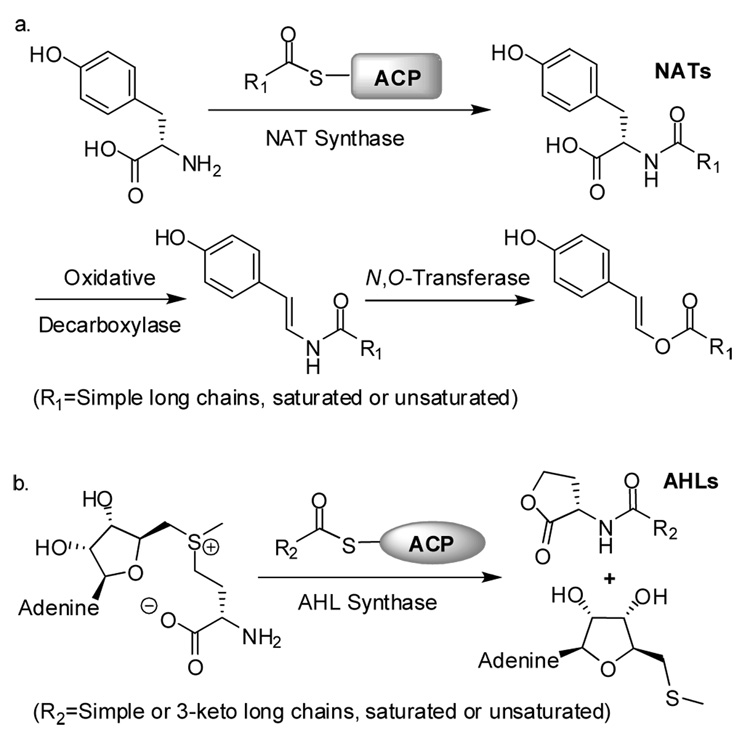
One pathway uncovered using this approach produced NATs that were first converted to N-acyl (E)-enamides through an oxidative decarboxylation, and finally to N-acyl (E)-enol esters through an unusual N,O-exchange5a (Figure 1a). Formation of the amide is catalyzed by an N-acyl synthase that couples free tyrosine to a long chain acid, delivered by an acyl carrier protein (ACP).6 The structure, mechanism, and sequence alignment of this N-acyl synthase5b,6 suggest a relationship to the synthases that produce the acyl homoserine lactones (AHLs, Figure 1b), quorum sensing mediators in many Gram-negative bacteria.7 In both the AHLs and NATs, a common head group–either homoserine lactone or tyrosine–is attached to a variety of long chain acids, and biological activity requires the correct acid fragment. In the E. coli heterologous expression system we use, ACP-bound long chain acids from E. coli pathways can replace those of the original producer, and consequently the identities of the biologically-relevant products of NAT pathways are not known with certainty.5a In order to explore the relationship between acid structure and biological activity in the amide, enamide and enol ester pathway, we developed an efficient chemical synthesis of plausible library members, which is described herein.
Figure 1.
(a) Proposed biosynthetic pathway for the production of long chain N-acyl tyrosines and their conversion to (E)-enamides and (E)-enol esters. (b) Formation of acyl homoserine lactones from biosynthetic precursor S-adenosyl methionine (SAM). [Single column]
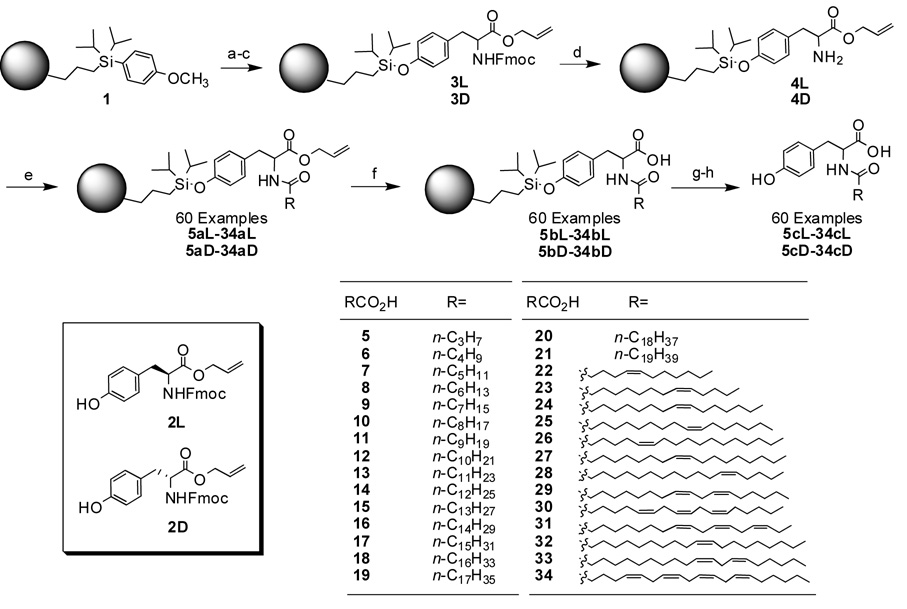
Long chain NATs were prepared via a solid-phase route (Scheme 1). Polystyrene macrobeads of 500–600 µm diameter, functionalized with a trialkylaryl silicon linker (1), were chosen as the solid support.8 First, the resin was activated with excess trifluoromethanesulfonic acid in CH2Cl2, followed by quenching with 2,6-lutidine. The activated beads were then treated for 24 h with a saturated solution of a bis-protected tyrosine substrate, the Fmoc carbamate/allyl ester of either L- or D-tyrosine (2L and 2D, respectively),9 to afford the corresponding resin-bound species (3L and 3D).10 Complete removal of the Fmoc group with 20% (v/v) piperidine in DMF led to the resin-bound allyl esters 4L and 4D. After screening various amide coupling conditions, we elected to carry out this reaction in THF using diethyl phosphocyanidate (DEPC) as the activating agent in the presence of TEA, for 24 h at room temperature. Excess of acid, DEPC and TEA was required. Under these conditions, 30 simple acids with various degrees of unsaturation (5–34) were combined with the resin-bound nucleophiles in parallel to furnish 60 coupling products (5aL–34aL and 5aD–34aD). All couplings were quantitative and free of impurities, as indicated by LC-MS. Parallel removal of the allyl protecting group from these intermediates was achieved with 5 mol % Pd(PPh3)4 in THF and excess morpholine in 6–8 h. The mild deprotection conditions were compatible with the presence of unsaturated long chains. This sensitive reaction required absence of light and initial degassing of the reaction container, to avoid catalyst degradation. Cleavage of the NATs from the beads was carried out using a 70/30 (v/v) HF/pyridine mixture in THF, buffered with additional pyridine. The reaction was typically quenched with TMSOMe, to provide >95% pure products (5cL–34cL and 5cD–34cD) after the supernatant was separated from the beads and the volatile byproducts were evaporated.
Scheme 1.
Solid-phase synthesis of long chain N-acyl tyrosines. Key: (a) CH2Cl2/TfOH/rt/40 min. (b) CH2Cl2/2,6-lutidine/rt/5–10 min. (c) CH2Cl2/2L or 2D/rt/24 h. (d) DMF/piperidine/rt/4 h. (e) RCO2H (5–34)/THF/DEPC/TEA/rt/24 h. (f) THF/Pd(PPh3)4/morpholine/rt/8–12 h. (g) THF/HF/pyridine/rt/2.5 h. (h) TMSOMe/rt/15 min. [Double column]
To augment the library with long chains reminescent of AHLs, solid-phase synthesis of NATs bearing a 3-keto functionality was carried out using a modification of the above procedure (Scheme 2). Long chain 5-acyl-2,2-dimethyl-1,3-dioxane-4,6-diones (5-acyl Meldrum’s acids),11 a masked form of the labile 3-oxo carboxylic acids, were used as building blocks in the amide coupling step. An alternative to conventional heating in the presence of an external base, which is the usual protocol of choice for a solution-phase reaction involving nucleophilic attack on a 5-acyl Meldrum’s acid, was necessary in order to avoid bead degradation. Thus a microwave-assisted method was developed. Optimal reaction conditions required the use of N-methyl pyrrolidone (NMP) as the solvent, which has both excellent absorbance characteristics and ideal interaction with this solid phase, excess of the 5-acyl Meldrum’s acid building block and no external base, at 200 °C with controlled microwave irradiation in a commercial reactor for 10 min. Parallel coupling of 19 different saturated and unsaturated building blocks (35–53) to resin-bound substrates 4L and 4D led to full consumption of the substrate in all cases, and formation of 70–80% of the desired products (35aL–53aL and 35aD–53aD). The 38 intermediates were successfully carried through the allyl deprotection and cleavage steps as described above, to yield 38 final 3-oxo-N-acyl tyrosines (35cL–53cL and 35cD–53cD).
Scheme 2.
Solid-phase synthesis of long chain 3-oxo-N-acyl tyrosines. Key: (a) NMP/5-acyl Meldrum’s acid (35–53)/microwave/200 °C/10 min. (b) THF/Pd(PPh3)4/morpholine/rt/8–12 h. (c) THF/HF/pyridine/rt/2.5 h. (d) TMSOMe/rt/15 min. [Double column]
Since our devised synthetic routes leading to the enamide and enol ester families involved steps that were either heterogeneous or incompatible with the solid-phase, both were realized in solution-phase. The (E)-enamides were prepared as shown in Scheme 3, starting with protection of 4-hydroxycinnamic acid (54) as a silyl ether (55, 85% yield) with TBDMSCl in DMF, in the presence of imidazole. Intermediate 55 was stereoselectively converted to the corresponding (E)-vinyl bromide (56, 83% yield) via a decarboxylative bromination with N-bromosuccinimide.12 This LiOAc-catalyzed Hunsdiecker transformation was carried out in CH3CN/H2O with mild heating. A modification of Buchwald’s conditions for copper-catalyzed amidation13 was employed for coupling of the vinyl bromide to a series of saturated long chain carboxamides (57–63),14 generating the long chain (E)-enamides 57aE–63aE at 65–70% yield with retention of the trans geometry. The amidation was carried out in toluene, using CuI as the copper source, N,N′-dimethylethylenediamine as the bidentate ligand, and Rb2CO3 as the base,13 and required strictly anhydrous conditions and heating at 70 °C for 24 h. Deprotection of the intermediates was achieved with 70/30 (v/v) HF/pyridine in THF/pyridine, and afforded 85–90% of the final (E)-enamides (57bE–63bE).
Scheme 3.
Solution-phase synthesis of long chain N-acyl enamides. (a) TBDMSCl/imidazole/DMF/rt/12 h/85%. (b) NBS/LiOAc/CH3CN/H2O/60 °C/1 h/83%. (c) RCONH2 (57–63)/(CH3NHCH2)2/CuI/Rb2CO3/toluene/80 °C/24 h/65–70%. (d) HF/pyridine/THF/rt/6 h/85–90%. [Single column]
The last class of eDNA-associated compounds, the enol esters, were derived from methyl 4-hydroxyphenyl acetate (64, Scheme 4), which was quantitatively protected under conditions similar to the protection of 54 above to afford TBDMS-silyl ether 65. Intermediate 65 was converted to an aldehyde in two steps: Reduction to an alcohol (66) with LiAlH4 in THF at −78 °C (94% yield), followed by alcohol oxidation to the aldehyde (67) with 2-iodoxybenzoic acid (IBX)15 in DMSO (93% yield). By means of KHMDS in THF/toluene, 67 was converted to a mixture of trans and cis potassium enolates, in accord with a previous report from our lab for a similar system.16 Under the specific reaction conditions employed here, moderate selectivity for the trans enolate (precursor to the only isomeric product present in the natural extract from the eDNA clone) was observed. The enolates were trapped with saturated long chain N-hydroxysuccinimide esters (68–80).17 LC-MS analysis of an aliquot from each reaction revealed in all cases a mixture of products with the same mass, that included (E)- and (Z)-enol esters as well as what appeared to be carbon acylation products. Partial purification of the crude products afforded inseparable mixtures of (E)- and (Z)-enol esters (68aE–80aE and 68aZ–80aZ), for which 1H-NMR indicated a ratio of approximately 2:1. The ratio was independent of acid chain length and reflects the thermodynamic preference for the trans enolate. The (E)/(Z) mixtures were resolved after HF removal of the TBDMS protecting group in pyridine, which proceeded at 85–90% yield. Thirteen deprotected (E)-enol esters (68bE–80bE) and thirteen (Z)-enol esters (68bZ–80bZ) were obtained in pure form.
Scheme 4.
Solution-phase synthesis of long chain N-acyl enol esters. Key: (a) TBDMSCl/imidazole/DMF/rt/12 h/100%. (b) LiAlH4/THF/−78 °C/1 h/94%. (c) IBX/DMSO/rt/4 h/93%. (d) KHMDS/toluene/THF/0 °C/5 min. (e) THF/RCO2Suc (68–80)/rt/30 min/30–40%. (f) HF/pyridine/rt/6 h/85–90%. [Single column]
In summary, solid- and solution-phase methods are described for the preparation of synthetic libraries of tyrosine-derived bacterial metabolites (131 compounds have been delivered), resembling small molecules isolated from heterologous expression of eDNA in E. coli. Preliminary biological studies on library members have shown antibiotic activity against B. subtilis and moderate inhibitory potential of P. aeruginosa biofilm formation. Additional assays are planned and will be reported elsewhere in due course.
Supplementary Material
Acknowledgements
We gratefully acknowledge the NIH for funding of this project (CA24487 to J.C.). We thank Dr. Sean Brady (formerly HMS) and Dr. Lauren Junker (HMS) for helpful discussions and preliminary biological studies on the library, Dr. Li Lai (formerly HMS) for assistance with solid phase handling, and Drs. Ralph Mazitschek (Broad Institute of MIT and Harvard) and Jared Shaw (formerly Broad Institute) for useful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data Detailed experimental procedures and spectroscopic data can be found in the on-line version, at xxxxx.
References and notes
- 1.(a) Clardy J, Fischbach MA, Walsh CT. Nat. Biotechnol. 2006;24:1541. doi: 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]; (b) Clardy J, Walsh C. Nature. 2004;432:829. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]; (c) Koehn FE, Carter GT. Nat. Rev. Drug Discov. 2005;4:206. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]; (d) Butler MSJ. Nat. Prod. 2004;67:2141. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]; (e) Leeds JA, Schmitt EK, Krastel P. Expert Opin. Investig. Drugs. 2006;15:211. doi: 10.1517/13543784.15.3.211. [DOI] [PubMed] [Google Scholar]; (f) Pelaez F. Biochem. Pharmacol. 2006;71:981. doi: 10.1016/j.bcp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 2.(a) Torsvik V, Salte K, Sørheim R, Goksøyr J. Appl. Environ. Microbiol. 1990;56:776. doi: 10.1128/aem.56.3.776-781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Torsvik V, Goksøyr J, Daae FL. Appl. Environ. Microbiol. 1990;56:782. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ward DM, Weller R, Bateson MM. Nature. 1990;345:63. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]; (d) Stackebrandt E, Liesack W, Goebel BM. FASEB J. 1993;7:232. doi: 10.1096/fasebj.7.1.8422969. [DOI] [PubMed] [Google Scholar]; (e) Amann RI, Ludwig W, Schleifer KH. Microbiol. Rev. 1995;59:143. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hugenholtz P, Goebel BM, Pace NR. J. Bacteriol. 1998;180:4765. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Wang G-Y-S, Graziani E, Waters B, Pan W, Li X, McDermott J, Meurer G, Saxena G, Andersen RJ, Davies J. Org. Lett. 2000;2:2401. doi: 10.1021/ol005860z. [DOI] [PubMed] [Google Scholar]; (b) Brady SF, Clardy J. J. Am. Chem. Soc. 2000;122:12903. [Google Scholar]; (c) MacNeil IA, Tiong CL, Minor C, August PR, Grossman TH, Loiacono KA, Lynch BA, Phillips T, Narula S, Sundaramoorthi R, Tyler A, Aldredge T, Long H, Gilman M, Holt D, Osburne MSJ. Mol. Microbiol. Biotechnol. 2001;3:301. [PubMed] [Google Scholar]
- 4.A similar approach has been used for enzyme catalyst discovery: DeSantis G, Zhu Z, Greenberg WA, Wong K, Chaplin J, Hanson SR, Farwell B, Nicholson LW, Rand CL, Weiner DP, Robertson DE, Burk MJ. J. Am. Chem. Soc. 2002;124:9024. doi: 10.1021/ja0259842.
- 5.(a) Brady SF, Chao CJ, Clardy J. J. Am. Chem. Soc. 2002;124:9968. doi: 10.1021/ja0268985. [DOI] [PubMed] [Google Scholar]; (b) Brady SF, Chao CJ, Clardy J. Appl. Environ. Microbiol. 2004;70:6865. doi: 10.1128/AEM.70.11.6865-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brady SF, Clardy J. J. Nat. Prod. 2004;67:1283. doi: 10.1021/np0499766. [DOI] [PubMed] [Google Scholar]; (d) Brady SF, Clardy J. Org. Lett. 2005;7:3613. doi: 10.1021/ol0509585. [DOI] [PubMed] [Google Scholar]
- 6. Van Wagoner RM, Clardy J. Structure. 2006;14:1425. doi: 10.1016/j.str.2006.07.005. Commentary by Churchill MEA. Structure. 2006;14:1342. doi: 10.1016/j.str.2006.08.002.
- 7.For recent reviews on AHLs and bacterial signalling, see: Waters CM, Bassler BL. Annu. Rev. Cell Dev. Biol. 2005;21:319. doi: 10.1146/annurev.cellbio.21.012704.131001. Bassler BL, Losick R. Cell. 2006;125:237. doi: 10.1016/j.cell.2006.04.001.
- 8.Preparation of this resin is described in: Tallarico JA, Depew KM, Pelish HE, Westwood NJ, Lindsley CW, Shair MD, Schreiber SL, Foley MA. J. Comb. Chem. 2001;3:312. doi: 10.1021/cc000107i.
- 9.Substrates 2L and 2D were prepared from commercial Fmoc-tyrosine following the method of: Jensen KJ, Meldal M, Bock K. J. Chem. Soc. Perkin Trans. 1. 1993;17:2119.
- 10.The loading in this step was typically 1.3–1.4 mmol of substrate per gram of resin, approximately equal to the empirical maximum for this type and batch of resin. It was determined by UV analysis as explained in the supplementary data.
- 11.The long chain 5-acyl Meldrum’s acids were prepared from carboxylic acids and Meldrum’s acid, as detailed in the supplementary data, using a modification of a published method: Raillard SP, Chen W, Sullivan E, Bajjalieh W, Bhandari A, Baer TA. J. Comb. Chem. 2002;4:470. doi: 10.1021/cc0200033.
- 12.(a) Kuang C, Yang Q, Senboku H, Tokuda M. Synthesis. 2005;8:1319. [Google Scholar]; (b) Kuang C, Senboku H, Tokuda M. Synlett. 2000;10:1439. [Google Scholar]
- 13.Jiang L, Job GE, Klapars A, Buchwald SL. Org. Lett. 2003;5:3667. doi: 10.1021/ol035355c. [DOI] [PubMed] [Google Scholar]
- 14.The carboxamide building blocks were prepared from fatty acids and NH3 with DEPC in 1,4-dioxane/DMF, as described in the supplementary data.
- 15.For an efficient preparation of IBX from 2-iodobenzoic acid, see: Frigerio M, Santagostino M, Sputore S. J. Org. Chem. 1999;64:4537.
- 16.Brady SF, Clardy J. Org. Lett. 2003;5:121. doi: 10.1021/ol0267681. [DOI] [PubMed] [Google Scholar]
- 17.The N-hydroxysuccinimide ester building blocks were prepared from fatty acids and N-hydroxysuccinimide with DCC in THF, as described in the supplementary data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.