Abstract
NADPH oxidase (Nox) family enzymes are one of the main sources of cellular reactive oxygen species (ROS), which have been shown to function as second messenger molecules. To date, seven members of this family have been reported, including Nox1-5 and Duox1 and -2. With the exception of Nox2, the regulation of the Nox enzymes is still poorly understood. Nox1 is highly expressed in the colon, and it requires two cytosolic regulators, NoxO1 and NoxA1, as well as the binding of Rac1 GTPase, for its activity. In this study, we investigate the role of the tyrosine kinase c-Src in the regulation of ROS formation by Nox1. We show that c-Src induces Nox1-mediated ROS generation in the HT29 human colon carcinoma cell line through a Rac-dependent mechanism. Treatment of HT29 cells with the Src inhibitor PP2, expression of a kinase-inactive form of c-Src, and c-Src depletion by small interfering RNA (siRNA) reduce both ROS generation and the levels of active Rac1. This is associated with decreased Src-mediated phosphorylation and activation of the Rac1-guanine nucleotide exchange factor Vav2. Consistent with this, Vav2 siRNA that specifically reduces endogenous Vav2 protein is able to dramatically decrease Nox1-dependent ROS generation and abolish c-Src-induced Nox1 activity. Together, these results establish c-Src as an important regulator of Nox1 activity, and they may provide insight into the mechanisms of tumor formation in colon cancers.
INTRODUCTION
Reactive oxygen species (ROS) are no longer considered only as major contributors to pathological tissue damage but also as intracellular second messengers in a variety of cellular receptor signal transduction pathways (Bedard and Krause, 2007). ROS, including superoxide anion and hydrogen peroxide, have been shown to play roles in proliferation, apoptosis, differentiation, and migration (Suh et al., 1999; Abid et al., 2000; Arnold et al., 2001; Sadok et al., 2008). Several intracellular sources can contribute to the enzymatic production of ROS, including cyclooxygenases, cytochrome P450, lipoxygenases, mitochondrial respiration, xanthine oxidase, and NADPH oxidases (Bedard and Krause, 2007).
Members of the Nox family are transmembrane proteins that catalyze the NADPH-dependent one-electron reduction of oxygen to form superoxide (Bokoch and Knaus, 2003; Lambeth, 2004; Sumimoto et al., 2005). To date, seven members of this family have been described: Nox1-5 and dual oxidase (Duox) 1 and -2. Nox2, expressed by phagocytic leukocytes, is currently the only Nox enzyme whose regulation is reasonably well understood. Microorganisms and inflammatory mediators trigger leukocyte signaling cascades that induce the assembly of four cytosolic regulatory proteins (p40, p47, p67, and Rac GTPase), with the Nox2 core enzyme to stimulate superoxide formation (Bokoch, 2005). This membrane-associated enzyme is a cytochrome b heterodimer consisting of gp91 (=Nox2) and p22 subunits.
The regulation of other Nox enzymes seems to differ significantly from Nox2, and their regulatory mechanisms are still poorly defined (Bokoch and Knaus, 2003). The search for homologues of the Nox2 regulatory proteins p47 and p67 led to the identification of p41 (or NoxO1)—the p47 homologue, and p51 (or NoxA1)—the homologue of p67, both abundant in gastrointestinal tissues (Banfi et al., 2003; Geiszt et al., 2003; Takeya et al., 2003). NoxA1, NoxO1, and Rac1 GTPase have all been shown to be required for full Nox1 activity (Cheng et al., 2006; Miyano et al., 2006; Ueyama et al., 2006). Recently, a mechanism for suppressing Nox1 activity through the cAMP-dependent protein kinase (PKA)-mediated phosphorylation of NoxA1 has been described (Kim et al., 2007).
Despite much investigation, the function of Nox1 and Nox1-derived ROS in the colon epithelium is still poorly understood. Nox1-derived ROS have been suggested to regulate local innate immunity (Rokutan et al., 2006), inflammation (Bedard and Krause, 2007), cell growth (Suh et al., 1999; Arnold et al., 2001; Tominaga et al., 2007; de Carvalho et al., 2008), carcinogenesis (Rokutan et al., 2006), and vascular tone (Sorescu et al., 2002; Gavazzi et al., 2006). Nox1-derived H2O2 has been reported to function as an intracellular mediator regulating cell growth and transformation, e.g., reduced expression of endogenous Nox1 by small interfering RNA (siRNA) decreases vascular smooth muscle proliferation (Arnold et al., 2001). Nox1 and its regulators NoxO1 and NoxA1 are highly expressed in human gastric and intestinal adenocarcinomas but not in normal gastric mucosa, suggesting that the Nox proteins may be potential markers of neoplastic transformation (Tominaga et al., 2007). Moreover, a very recent study has demonstrated a key role for Nox1-derived ROS in human colon cell migration and tumor invasion (Sadok et al., 2008).
Another protein long known to be highly expressed and active in many human tumor types, including those affecting the colon, is the cytoplasmic tyrosine kinase c-Src (Irby and Yeatman, 2000). c-Src has been suggested to play a key role in tumor development and in the acquisition of the metastatic phenotype (Summy and Gallick, 2003). When Tyr527 in the c-Src autoregulatory domain becomes phosphorylated, it binds to its own Src homology 2 domain in such a way that kinase activity is inhibited. The mutation of Tyr527 to Phe prevents such inhibition, representing a constitutively active form of Src (SrcYF) (Thomas and Brugge, 1997). It has been widely demonstrated that c-Src is sensitive to redox stress through inactivation of protein tyrosine phosphatases (PTPases) that control its phosphorylation status (Nakashima et al., 2002).
The molecular mechanisms underlying the transforming potential of Src have been extensively explored (Cartwright et al., 1987; Irby and Yeatman, 2000). Expression of an oncogenic, activated form of Src results in tyrosine phosphorylation of numerous intracellular substrates and the activation of signaling pathways, such as the Ras-extracellular signal-regulated kinase and nuclear factor-κB pathways, which ultimately control transcriptional regulation and cell proliferation (Abram and Courtneidge, 2000). The regulation of the activity the small GTPase Rac1 by c-Src represents one of many mechanisms through which c-Src controls cell function. Activated Src modulates Rac1 activity through the tyrosine phosphorylation of guanine nucleotide exchange factors (GEFs), including Vav2 and Tiam1 (Schuebel et al., 1998; Servitja et al., 2003), and by inhibition of Rac1 membrane-cytosol recycling via the cytosolic GTPase regulator RhoGDI (DerMardirossian et al., 2006). GEFs activate Rac GTPases by promoting the conversion of their inactive GDP-bound form to their active GTP-bound state (Cherfils and Chardin, 1999). Mouse fibroblasts transformed with activated Src show higher levels of active Rac1 (Servitja et al., 2003). However, the significance in vivo of the c-Src-mediated regulation of active Rac1 levels, and how such regulation might affect various c-Src and Rac1-dependent cellular responses, is still poorly defined.
Here, we show that c-Src plays an important role in regulating Nox1-dependent ROS generation in human HT29 colonic adenocarcinoma cells. We demonstrate that endogenous c-Src induces Nox1-dependent, Rac1-mediated ROS generation in HT29 cells by increasing the levels of active Rac1 through the activation of the Rac1-GEF Vav2 by tyrosine phosphorylation.
MATERIALS AND METHODS
Reagents
Cell culture medium, fetal bovine serum, supplements and Hanks' balanced salt solution (HBSS; catalog no. 24020-117) were from Invitrogen (Carlsbad, CA). Plasmids for transfection were purified using the Qiafilter system (QIAGEN, Valencia, CA). The SrcY527F-transformed NIH-3T3 (NIHSrc) cell line has been described previously (Lock et al., 1998). HT29 colonic adeno-carcinoma cells (catalog no. HTB-38) and other carcinoma cell lines were purchased from American Type Culture Collection (Manassas, VA). The following reagents were purchased as indicated: PP2 Src inhibitor (529576), its nonfunctional analogue PP3 (529574), and the Rac1-GEF inhibitor NSC23766 (553502) were from Calbiochem (EMD Biosciences, Darmstadt, Germany); and diphenyliodonium (DPI; D2926), horseradish peroxidase (HRP; 77330) and luminol (09253) were from Sigma-Aldrich (St. Louis, MO). The following antibodies were purchased as indicated: rabbit polyclonal phospho-Src (p-Src) antibody (Tyr418) was from Invitrogen (Carlsbad, CA; 44660G), rabbit polyclonal c-Src antibody was from Santa Cruz Biotechnology (Santa Cruz, CA; Sc-18), monoclonal enhanced green fluorescent protein was from Invitrogen (EGFP 3E6 antibody), and monoclonal Rac1 antibody 23A8 (05-389) and anti-phosphotyrosine (p-Tyr) antibody 4G10 (05-321) were from Millipore (Billerica, MA). Mouse monoclonal actin antibody (691002) was from MD Bioscience; rabbit polyclonal Tiam1 antibody (ST1070) was from Calbiochem (EMD Biosciences); and rabbit polyclonal phospho-Vav (p-Vav) antibody (Tyr174) (ab47282), rabbit monoclonal Vav protein antibody (ab40875), and rabbit monoclonal Vav2 antibody were from Abcam (Cambridge, MA); 9E10 anti-myc antibody was prepared in-house. Rabbit polyclonal Nox1 antibody was a gift from David Lambeth (Emory University).
Plasmid DNA, Reverse Transcription-Polymerase Chain Reaction (RT-PCR), and siRNAs
All Nox1 and NoxA1 mutant constructs were generated by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) as described previously (Kao et al., 2008). pcDNA3.1-Nox2 was also described in Kao et al. (2008), whereas pcDNA3.1-Nox3 and pcDNA3.1-mNox1 were gifts from Botond Banfi (University of Iowa, Iowa City, IA). Expression plasmids for Nox4 and Nox5 were kindly provided by Ulla Knaus (The Scripps Research Institute). Human Myc-tagged NoxO1 and NoxA1 were originally obtained from Tom Leto (National Institutes of Health). Human Myc-tagged constitutively active Vav2 (Vav2-CA), lacking the N-terminal region (amino acids 1-183), and mVav plasmid were gifts from Dr. A. Altman (La Jolla Institute for Allergy and Immunology). EGFP-tagged constitutively active Rac1 (RacQ61L) and dominant-negative Rac1 (RacN17) were described previously (Kim et al., 2007). Constitutively active Src (SrcY527F) and dominant-negative Src (SrcKM) were generously provided by David Schlaepfer (The Scripps Research Institute). Constitutively active GEF-H1 (GEF-H1-CA) was described in Birkenfeld et al. (2007).
For RT-PCR, total RNA was purified from HT29 cells with the use of the RNeasy kit (QIAGEN), and 2 μg was reverse transcribed with SuperScript II enzyme (Invitrogen) according to the manufacturer's instructions. RT-PCR was performed using the following Nox- and actin-specific PCR primer sets: hNox1 forward 5′-aca atg gga aac tgg gtg g-3′ and hNox1 reverse 5′-caa gat aga agc aaa ggg ggt gac-3′; hNox2 forward 5′-act ggg ctg tgt gaa taa gag gg-3′ and hNox2 reverse 5′-ggg cca gac tca gag ttg g-3′; hNox3 forward 5′-act gcc ctg aca gat gat t-3′ and hNox3 reverse 5′-cct gct cac tca tcc gtg tt-3′; hNox4 forward 5′-cac aga agg ttc caa gca gga-3′ and hNox4 reverse 5′-agt cag gtc tgt tct ctt gcc-3′; hNox5 forward 5′-ggt gct gat gct cag agg ctg-3′ and hNox5 reverse 5′-gga cac ctt cga tcc agg agt g-3′; and actin forward 5′-ggc gac gag gcc cag a-3′ and actin reverse 5′-cga ttt ccc gct cgg-3′. Cycling conditions were as follows: 5-min denaturation at 95°C, followed by 30 s at 95°C, 30 s at 60°C, 30 s at 72°C for 35 cycles and by 5 min at 72°C. PCR products were checked for the correct size on 1% agarose gels.
Stealth control and Nox1-specific siRNA were designed by Block-IT Stealth RNAi designer (Invitrogen) and purchased from Invitrogen by using the following Nox1-target sequences: control oligonucleotide (oligo) and Oligo#1 (5′-cgagcgtctgctctctgcttgaat-3′), Oligo#2 (5′-gccgcacactgagaaagcaattgga-3′) and Oligo#3 (5′-cagaaggttgtgattaccaaggttg-3′). Stealth control and Vav2-specific siRNAs were predesigned and purchased from Invitrogen (catalog no. VAV2HSS111250-2). ON-TARGETplus SMARTpool Src siRNA was purchased from Dharmacon RNA Technologies (J-003175-13/16).
Cell Culture, Transfection, and Treatments
Human embryonic kidney (HEK)293, human HCT-116, DLD1, SW480, SW620, HT29 colonic adenocarcinoma, and human CCD481 colon epithelial cells were maintained in DMEM (Invitrogen) containing 10% heat-inactivated fetal bovine serum (Invitrogen), 2 mM glutamine, and antibiotics (100 U/ml penicillin and 100 g/ml streptomycin) at 37°C in 5% CO2. For transfection, HEK293 cells were plated in six-well plates (Falcon; BD Biosciences Discovery Labware, Bedford, MA) at appropriate density and grown overnight and then transfected by using Lipofectamine 2000 following the manufacturer's instructions. HT29 cells were instead plated in 10-cm-diameter plates (Falcon; BD Biosciences Discovery Labware) at appropriate density, grown overnight, and transfected by using Lipofectamine 2000 (for plasmid DNA transfection) or RNAiMax (Invitrogen) for siRNA transfection following the manufacturer's instructions (in experiment in Figure 5C in which siRNA and DNA plasmids are introduced in the same time, RNAiMax was used to transfect cells). Twenty-four and 72 h after transfection, the cells were processed accordingly.
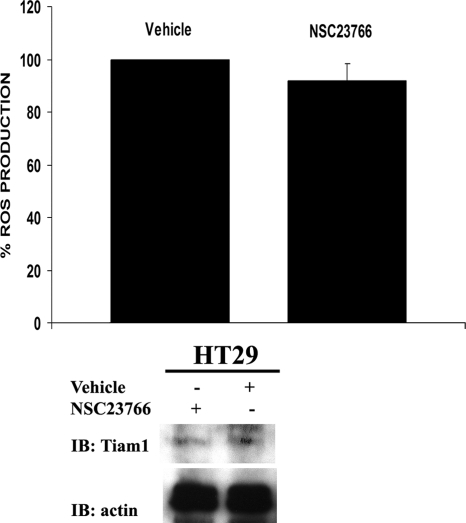
Figure 5.
The Src-regulated Rac-GEF Tiam 1 is not involved in HT29 cell Src-induced ROS generation. HT29 cells were treated with 100 μM NSC23766, an inhibitor of the Rac-Tiam1 interaction, or with vehicle and ROS generation was measured by CL-assay (top). One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD. Bottom, under these conditions the treatment with NSC23766 did not change Tiam1 expression levels in HT29 cells.
For inhibitor studies, cells were treated as follows: 10 μM dimethyl sulfoxide (DMSO)-dissolved DPI or with DMSO alone for 30 min, 10 μM DMSO-dissolved Src inhibitor PP2 or its nonfunctional analog PP3 or DMSO alone for 16 h, or 100 μM water-dissolved Rac-GEF binding inhibitor NSC23766 or vehicle alone for 2 h.
Western Blot
Transfected or control cells were washed extensively in ice-cold phosphate-buffered saline (PBS) and lysed in appropriate amount of radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1%, Nonidet P-40, 0.25% sodium deoxycholate, and 1 mM EDTA) supplemented with 1 mM leupeptin, 1 mM aprotinin, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Then, 4× Laemmli sample buffer was added to the whole cell lysates without clarification, and samples were resolved by 10% SDS-PAGE. Gels were transferred to nitrocellulose membranes using the electrophoretic transfer cell (Bio-Rad, Hercules, CA) at 100 V for 1 h. After blocking with nonfat dry milk (5%), proteins were probed overnight using their respective antibodies at appropriate dilution. Anti-Nox1, anti-Myc, anti-total Src, anti-p-Src (Tyr418), anti-p-Vav (Tyr174), anti-p-Tyr, anti-Vav proteins, anti-Tiam, and anti-Rac1 were used 1/1000, anti-green fluorescent protein (GFP) was used 1/5000, anti-actin and anti-Vav2 were used 1/10,000 and 1/2000, respectively. The excess antibody was removed by sequential washing of the membranes in Tween-PBS, and then a 1:5000 dilution of the appropriate horseradish peroxidase-conjugated secondary antibody (Pierce Chemical, Rockford IL) was added to filters for 1 h at room temperature. The excess was removed by sequential washing of the membranes in T-PBS, and the signals were detected by chemiluminescence using the ECL system (Pierce Chemical). Blots were stripped and reprobed as necessary.
Measurement of ROS
ROS were measured using a luminol-based chemiluminescence assay (CL-assay), essentially as described previously (Kim et al., 2007), although in the absence of an activating stimulus. Briefly, HEK293 or HT29 cells were cultured for 16 h (for plasmid DNA transfection), 48 h (for siRNA/DNA plasmid transfection), or 72 h (for siRNA transfection), and then they were harvested by incubation with trypsin/EDTA for 1 min at 37°C. After being washed with phosphate-buffered saline (without MgCl2 and CaCl2), the cells were removed from the well, pelleted at 1000 ×g for 5 min and resuspended in HBSS containing calcium and magnesium. Then, 5 × 105 HEK293 or 2 × 105 HT-29 cells per assay were dispensed in white 96-well plate (Berthold Technologies, Bad Wildbad, Germany) and mixed with 250 μM luminol and 1 U of HRP (final concentration) in 200 μl total final volume in each well. Chemiluminescence was recorded using 96-well plate luminometer (Berthold) 5 min after the addition of HRP/luminol mixture for 30 min at room temperature without any stimulation. The data output consisting of the emission intensities for each well was imported into a spreadsheet program (such as Excel; Microsft, Redmond, WA) for further processing.
Rac Activation Pull-Down (PAK1 p21 Binding Domain; PBD) Assay
The glutathione transferase (GST) PBD fusion protein was expressed in Escherichia coli strain BL21 and isolated bound to glutathione-Sepharose beads (Stofega et al., 2006). Then 1 × 106 HT29 cells were plated in 150-mm-diameter plates (Falcon; BD Biosciences Discovery Labware), grown overnight, and treated with 10 μM Src inhibitor PP2, PP3, or DMSO. After 16 h, cells were washed twice in ice-cold PBS and lysed in 0.6 ml of PBD lysis buffer (25 mM Tris, pH 7.5, 200 mM NaCl, 30 mM MgCl2, 1 mM dithriothreitol [DTT], and 1% Nonidet P-40) supplemented with 1 mM leupeptin, 1 mM aprotinin, 1 mM sodium orthovanadate, and 1 mM PMSF. On centrifugation for 10 min at 14,000 rpm, guanosine 5′-O-(3-thio)triphosphate (GTPγS) and guanosine diphosphate (GDP) loading controls were prepared by incubating 100 μg of cell lysates for 15 min at 30°C in the presence of 60 mM EDTA and 0.1 mM GTPγS or 1 mM GDP (Roche Diagnostics, Indianapolis, IN). The loading was stopped by the addition of MgCl2 to 30 mM. Then, 1.5 mg of crude cell lysates or guanine nucleotide-loaded controls were added to 500 μl of PBD binding buffer (25 mM Tris, pH 7.5, 1 mM DTT, 30 mM MgCl2, 40 mM NaCl, and 0.5% Nonidet P-40) with 10 μg of PAK1 PBD-GST glutathione beads. The binding reaction was incubated for 1 h at 4°C, and then the beads were washed three times with washing buffer (25 mM Tris-HCl, pH 7.5, 1 mM DTT, 30 mM MgCl2, and 40 mM NaCl). The pellets were finally resuspended in 4× Laemmli sample buffer and analyzed on 12% SDS-PAGE as described above.
Statistical Analysis
In this study, representative experiments from three independent experiments are shown. Results for each experiment are given as mean of triplicates ± SD. Statistically significant differences between sample groups are determined using t tests (Excel, Microsoft). A p value of <0.01 was considered significant.
RESULTS
In Human Colon Cancer HT29 Cells, ROS Generation Is Dependent on the Nox1 Pathway
Nox1 and its known regulators NoxO1 and NoxA1 are most highly expressed in colon epithelium in vivo (Rokutan et al., 2006; Bedard and Krause, 2007). Many human colon cell lines, such as CaCO2, HT29, DLD1, and so on, are reported to express high levels of these proteins constitutively or upon stimulation with interferon-γ or lipopolysaccharide (Kuwano et al., 2006). In particular, human colon cancer HT29 cells have been shown to express a high level of Nox1 and its regulators NoxA1 and NoxO1 at the mRNA and protein level (Perner et al., 2003) (Figure 1A). Moreover, we have determined by RT-PCR analysis that the HT29 cells used in our study do not express other members of the NADPH oxidase family (Supplemental Figure S1).
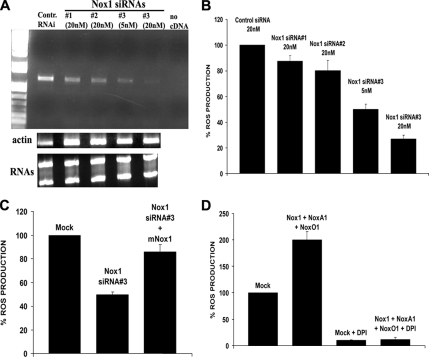
Figure 1.
HT29 cell ROS generation is dependent on the Nox1 pathway. (A) The Nox1 siRNA#3 was the most effective in specifically knocking down the level of Nox1 mRNA in a dose-dependent manner. HT29 cells were transfected with the indicated amounts of three different Nox1-specific siRNAs or with control siRNA, and total RNA was extracted after 72 h as in Materials and Methods. RT-PCR was performed using Nox1-specific primers (top) or actin-specific primers (middle) and PCR products were run on a 1% agarose gel. Bottom, total RNA from each extraction was run on 1% agarose, showing no difference in the amount of the total RNA between different experimental conditions. (B) The transfection of Nox1-specific siRNA #3 results in the strongest dose-dependent reduction of cellular ROS production. HT29 cells were transfected (as indicated) with different amounts of three different Nox1-specific siRNAs or with control siRNA, and after 72 h ROS generation was measured by CL-assay as described in Materials and Methods. One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD. (C) The inhibition of ROS generation caused by Nox1 siRNA#3 in HT29 cells is rescued by the overexpression of mNox1. HT29 cells were transfected with control siRNA or Nox1 siRNA#3 (20 nM) as described in Materials and Methods. After 48 h, Nox1 siRNA-transfected cells were transfected again with expression plasmid for mNox1 or with empty vector by Lipofectamine 2000. Twenty-four hours later ROS generation was measured. One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD. (D) Treatment of HT29 cells with 10 μM DPI, a flavoenzyme inhibitor of NADPH oxidases, blocks both the increase in ROS production due to the transfection of Nox1, NoxA1, and NoxO1, and it reduces the amount of cellular ROS in mock-transfected HT29 cells. HT29 cells were transfected as indicated with empty vector or with the expression vector for Nox1, NoxO1, and NoxA1. After 24 h, cells were treated with 10 μM DPI or DMSO, and ROS production was monitored by CL assay. One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD.
To assess whether ROS generation is dependent on the Nox1 pathway, HT29 cells were transfected with three different Nox1-specific siRNAs or with control siRNA. As shown in Figure 1A, Nox1 siRNA#3 was the most effective in strongly and specifically knocking down HT29 Nox1 mRNA levels in a dose-dependent manner. Consistent with this, the transfection of Nox1 specific siRNA#3 also results in a strong reduction of cellular ROS production in a dose-dependent manner (Figure 1B). Interestingly, this phenotype is rescued by the overexpression of mouse Nox1 (mNox1) in HT29 cells, as shown in Figure 1C. These data indicate that Nox1 is responsible for the majority of ROS produced in these cells.
To further confirm this, HT29 cells were cotransfected with Nox1 and its regulators NoxO1 and NoxA1 or with empty vector, and ROS production was monitored by the luminol-based CL-assay. As shown in Figure 1D, treatment of HT29 cells with an inhibitor of NADPH oxidase, 10 μM DPI in DMSO for 30 min (Robertson et al., 1990), blocks the increase in ROS production due to the transfection of Nox1, NoxA1, and NoxO1, and it reduces the amount of cellular ROS in mock-transfected cells compared with DMSO-treated cells.
The Non-receptor Tyrosine Kinase Src Induces ROS Generation in HT29 Cells
A variety of rapidly growing human tumor cells release large amounts of ROS that seem to be required for proliferation through poorly defined mechanisms (Szatrowski and Nathan, 1991; Laurent et al., 2005). Nonreceptor tyrosine kinase Src was found to be overexpressed and highly active in a number of human tumors, including those affecting the colon. To investigate whether the expression and the activity of the nonreceptor tyrosine kinase Src was related to increased ROS production, we compared ROS generation in NIH3T3 cells stably expressing c-Src to NIH3T3 wild-type cells. As shown in Figure 2A, NIH3T3 cells that stably express c-Src produce significantly more ROS than wild-type cells, suggesting that c-Src may be involved in ROS generation in this cell line.
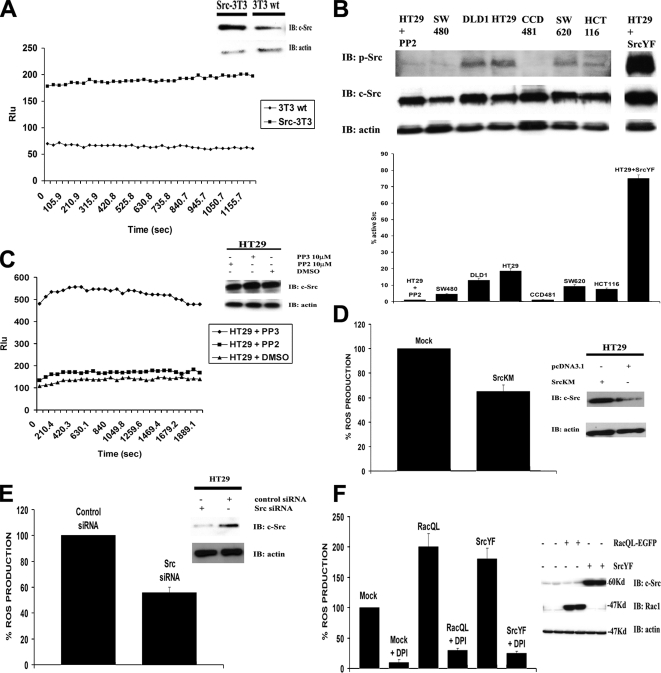
Figure 2.
c-Src regulates cellular ROS generation. (A) NIH3T3 cells stably expressing c-Src (Src-3T3) produce significantly more ROS than wild-type NIH3T3 (3T3) cells. ROS production was measured by CL-assay in Src-3T3 and 3T3 cells without additional stimulation (left). The different levels of Src expression in these two cell lines was confirmed by WB, as described in Materials and Methods (right). One representative experiment from three separate experiments is shown. (B) Variation of endogenous c-Src activity in different human colon cell lines. Top, protein extracts (20 μg) from several human colon cell lines were analyzed by SDS-PAGE using anti-p-Src antibody (Tyr418). PP2-treated and SrcYF-transfected HT29 cells were used as negative and positive control, respectively. Blots were reprobed with anti-total Src and anti-actin antibody (bottom). Bottom, the results from three independent experiments performed as described above were quantified. First, the values from p-Src and total Src were normalized to actin. Successively, the ratio between actin-normalized p-Src and total-Src was calculated, and the values were normalized to the value of PP2-treated HT29 cells and are shown as mean ± SD. (C) In HT29 cells, the inhibition of Src activity by PP2 treatment significantly decreases ROS generation compared with HT29 cells treated with the nonfunctional inhibitor PP3. HT29 cells were treated with 10 μM Src inhibitor PP2 or its nonfunctional analogue PP3, and ROS production was monitored by CL-assay (left). Right, PP2 and PP3 treatment in HT29 cells did not affect c-Src protein expression level. One representative experiment from three independent experiments is shown. (D) The overexpression of Src dominant-negative (SrcKM) in HT29 cells significantly inhibits ROS generation compared with mock-transfected cells. HT29 cells were transfected as indicated with empty vector or with expression plasmid for SrcKM. After 24 h, cells were harvested and ROS production was measured by CL-assay (left). The Western blot in the right panel shows the level of SrcKM overexpression (c-Src immunoblot) and equal amount of loading (actin immunoblot). One representative experiment from three independent experiments is shown. (E) Src-specific siRNA, which strongly decreases Src protein level also inhibits ROS generation in HT29 cells. HT29 cells were transfected as described in Materials and Methods with Src-specific siRNA or with control siRNA (20 nM), and after 72 h the ROS formation was quantified by CL-assay (left). Right, Src-specific siRNA significantly decreases endogenous Src protein levels without affecting actin levels. (F) The treatment of HT29 cells with DPI blocks the increase in ROS production due to the transfection of both SrcYF and RacQL, and it reduces the amount of cellular ROS in mock-transfected compared with DMSO-treated cells. HT29 cells were transfected as indicated with empty vector or with constitutive active Rac1 (RacQL) or with constitutive active Src (SrcYF). After 24 h, cells were treated with 10 μM DPI, and ROS production was measured (left). The Western blot (right) shows that the block caused by DPI to the SrcYF- and RacQL-mediated increase in ROS production in HT29 cells is not due to any effect on expression of the transfected proteins. One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD.
Several human colon cancers also show increased Src activation (Bolen et al., 1987a). To ascertain whether in different colon cell lines the activity of Src was up-regulated, we analyzed by anti-phospho-Src (p-Src) antibody (Tyr418) immunoblot the DLD1, SW480, SW620, HCT116, HT29 human colonic adenocarcinoma cell lines, and normal human CCD481 colon epithelial cells. Figure 2B (top) shows varying levels of Src phospho-activation in the analyzed colon cell lines. Interestingly, human HT29 cells, in which the Nox1 pathway is responsible for the production of the majority of the ROS (Figure 1), show a high level of active Src compared with other human adenocarcinoma and epithelial colon cell lines (Figure 2B, bottom). Furthermore, at least for some of the analyzed cell lines, such as HT29, DLD1, and CCD481, we observed a correlation between the levels of active Src and the amount of ROS produced, as shown in Supplemental Figure S2.
To determine whether c-Src was indeed able to affect ROS generation in HT29 cells, these cells were treated for 16 h with 10 μM Src inhibitor PP2, with its nonfunctional analog PP3, or with DMSO alone. The results in Figure 2C (left) demonstrate that the inhibition of Src activity in HT29 cells by PP2 treatment significantly decreased ROS generation compared with treatment with PP3 or DMSO alone, without affecting c-Src expression level (Figure 2C, right).
To further confirm that c-Src is directly involved in ROS generation in HT29 cells, these cells were transfected with the dominant-negative form of Src (SrcKM) or with c-Src–specific siRNA. As shown in Figure 2, D and E, respectively, transfection with dominant-negative Src or with Src-specific siRNA are both able to reduce ROS generation in HT29 cells compared with mock- or control siRNA-transfected cells. The level of inhibition obtained was consistent with the relative HT29 cell transfection efficiencies observed.
Additional evidence that Src induces ROS formation in HT29 cells is shown by the experiment in which HT29 cells were transfected with constitutively active Src (SrcY527F or SrcYF), constitutively active Rac1 (RacQ61L or RacQL), or empty vector and ROS generation was determined. As shown in Figure 2F, the presence of SrcYF induces an increase in ROS generation comparable with that obtained with RacQL, indicating that c-Src alone is able to induce Nox1-dependent ROS generation in HT29 cells. Consistent with this, treatment of HT29 cells with DPI, an inhibitor of NADPH oxidase, blocks the increase in ROS production induced by the expression of both SrcYF and RacQL, and reduces the amount of cellular ROS in mock-transfected compared with DMSO-treated cells.
Src Induces Nox1-dependent ROS Generation through a Rac1-dependent Mechanism
It has previously been described that Src-induced cell responses can be mediated through Rac GTPase-dependent mechanisms (Gianni et al., 2003). Consistent with this, cell transformation induced by v-Src is inhibited by blockade of the Rac1 pathway (Servitja et al., 2003). Rac1 is required along with NoxO1 and NoxA1 for full Nox1 activity. Recently, a putative Rac binding site on Nox1 has been identified by mutational analysis (Kao et al., 2008), revealing that the integrity of specific residues, particularly Lys421, Tyr425, and Lys426 (Nox2 numbering), is required for Rac1-dependent NADPH oxidase activity. These residues are conserved in the Rac-regulated Nox1, Nox2, and Nox3 enzymes, but not in other Nox proteins.
To address whether c-Src-induced ROS production is mediated through a Rac-dependent mechanism, HEK293 cells were cotransfected with expression vectors for 1) the Nox1 regulators NoxO1 and NoxA1; 2) constitutively active SrcYF or RacQL; and 3) Nox1 mutants unable to bind Rac1 (K421E, Y425A and K426E), the NADPH binding site Nox1 (Y442A) mutant, or a randomly chosen Nox1 (T448A) mutant not known to be involved in regulatory cofactor binding. As indicated in Figure 3A, the presence of NoxO1, NoxA1, Nox1 wild-type, and SrcYF stimulates the formation of superoxide anion. In contrast, the expression at similar levels of Nox1 mutants unable to bind Rac1 (K421E, Y425A, and K426E), or the Nox1 NADPH binding site mutant (Y442A), were completely unable to support SrcYF-induced ROS generation. On the contrary, expression of the nearby Nox1 (T448A) mutant fully supported SrcYF-stimulated ROS production.
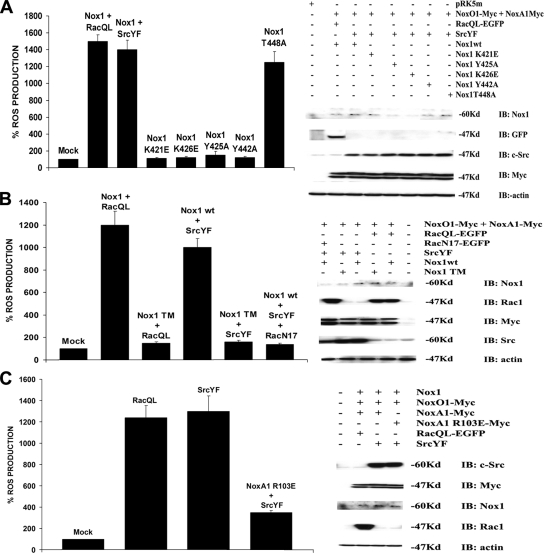
Figure 3.
c-Src induces Nox1-dependent ROS generation through a Rac1-dependent mechanism. (A) The integrity of Nox1 residues required for Rac1 binding is necessary for Src-mediated ROS generation in HEK293 cells. HEK293 cells were transfected with empty vector or with NoxO1 and NoxA1 and, where indicated, with SrcYF, RacQL, Nox1 wild-type, the Nox1 mutants unable to bind Rac1 (K421E, Y425A, K426E), the Nox1 mutant in the NADPH binding site (Y442A), or with Nox1 bearing a mutation outside the Rac and NADPH binding domains (T448A). ROS production was monitored after 24 h (left). Right, comparable expression levels of all the transfected proteins. (B) Blockade of the Rac1 pathway abolishes the Nox1-dependent ROS generation in HEK293 cells. HEK293 cells were transfected with empty vector or with NoxO1 and NoxA1, and, where indicated, with RacQL, SrcYF, the dominant-negative Rac (RacN17), and Nox1 wt or Nox1 TM bearing all three the mutations responsible for Rac1 binding. ROS generation was measured after 24 h (left). The Western blot (right) indicates similar expression levels of all the above-mentioned proteins. (C) The presence of the NoxA1 mutant R103E, which is unable to bind Rac, completely blocks the induction in the Nox1-dependent superoxide formation caused by SrcYF. HEK293 cells were transfected with empty vector or with NoxO1 and Nox1, and, where indicated, with RacQL, SrcYF, and with NoxA1 wild type or with NoxA1 R103E. ROS formation was determined after 24 h (left). Right, all transfected proteins are expressed at similar levels. Results are representative of one experiment from three separate experiments, and data are given as mean of triplicates ± SD.
To verify that c-Src induces Nox1-dependent superoxide production in a Rac1 dependent manner, we monitored ROS generation in HEK293 cells cotransfected with Nox1 wild-type, its regulatory proteins NoxO1 and NoxA1, SrcYF and the dominant-negative form of Rac (RacN17). As shown in Figure 3B (left), the presence of RacN17 dramatically abolishes the SrcYF-mediated ROS generation. Again, SrcYF was also unable to stimulate the non-Rac binding (K421A/Y425A/K426EA triple mutant; Nox TM).
Additional evidence that c-Src induces ROS generation through a Rac-dependent mechanism came from experiments in which HEK293 cells were cotransfected with Nox1, NoxO1, SrcYF and with either NoxA1 wild-type or NoxA1 R103E, a mutant form of NoxA1 unable to bind Rac because of the mutation R103E in its tetratricopeptide repeat domain (Takeya et al., 2003). As indicated in Figure 3C (left), the presence of NoxA1 R103E abrogates the induction of Nox1-dependent superoxide formation caused by SrcYF. Importantly, none of the above-mentioned effects were due to changes in the expression levels of the regulatory components or Rac1Q61L itself (Figure 3, right), nor was activity restored by increasing the expression of NoxO1, NoxA1, or Rac1Q61L (data not shown).
Endogenous c-Src Activity Regulates the Levels of Active Rac in HT29 Cells
NIH3T3 fibroblasts stably transformed with v-Src show higher levels of Rac1-GTP (Servitja et al., 2003). We hypothesized that in the HT29 human colon cancer cell line, c-Src induces Nox1-dependent ROS production by increasing the levels of Rac1-GTP through the activation of specific Rac1-GEF.
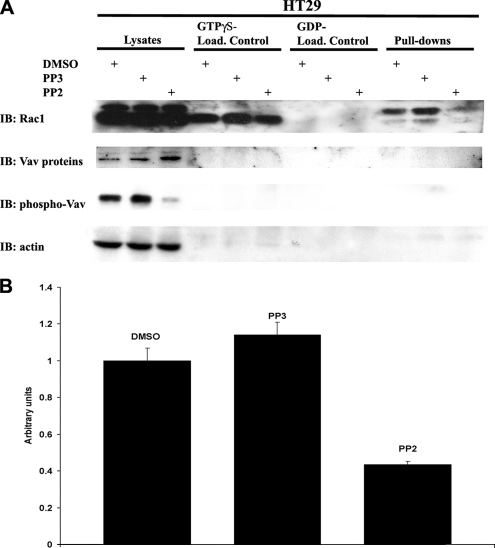
To first establish whether endogenous Src activity in HT29 cells regulates the levels of active Rac1, these cells were treated with Src inhibitor PP2 or its nonfunctional analogue PP3 or DMSO vehicle control alone for 16 h. For the analysis of active Rac1 levels, we used the well-established assay involving the GST fusion of the PBD prebound to glutathione Sepharose beads to affinity precipitate the activated GTP-bound forms of Rac1 (Stofega et al., 2006). Using this assay, we observed remarkably lower levels of Rac1-GTP in HT29 cells treated with PP2 compared with PP3- or DMSO-treated cells, as indicated in Figure 4(A and B). Total Rac1 expression was comparable between three cell populations and loading cell lysates with a nonhydrolysable GTP analogue GTPγS or with GDP showed an equal GDP or GTP binding capacity of total Rac1 between different treatments (Figure 4A, top). Correlating with the decrease in active Rac1 observed, PP2 treatment also efficiently decreased the tyrosine phosphorylation of the Src substrate, Vav, in HT29 cells (Figure 4A, bottom; phospho-Vav). Vav family members belong to the Rac-GEF superfamily and their activation by Src-mediated tyrosine phosphorylation is well established. Therefore, we conclude that the inhibition of endogenous Src activity significantly decreases the levels of active Rac1 in HT29 cells and that this may occur through effects on activity of the exchange factor Vav2.
Figure 4.
c-Src activity regulates the levels of Rac1-GTP in HT29 cells. (A) The inhibition of Src activity significantly lowers the levels of active Rac1 in HT29 cells. HT29 cells were treated Src inhibitor PP2, its nonfunctional analogue PP3, or DMSO alone. Total Rac and Rac1-GTP levels were measured by PBD assay as indicated in Materials and Methods. A nonhydrolysable analogue of GTP, GTPγS, was used as positive loading control and GDP was used for the negative control. Inhibition of Src activity was confirmed by Western blotting of the Src tyrosine phosphorylation target pTyr418-Vav, whereas similar levels of total Vav protein in HT29 cells was confirmed by Vav immunoblot. Finally, equal loading of lanes was confirmed by actin immunoblot. (B) PP2 treatment significantly lowers the levels of active Rac1 in HT29 cells compared with treatment with PP3 or DMSO alone. The results from three independent experiments, performed as described above, were quantified, normalized to the value of DMSO-treated cells, and they are shown as mean ± SD.
Vav2 and Not Tiam1 Is the Rac-GEF Involved in Src-induced ROS Generation in HT29 Cells
It has previously been demonstrated that in NIH3T3 cells transfected with active Src or stably expressing v-Src, members of the Rac-GEF superfamily (such as Vav2 and Tiam1) are tyrosine-phosphorylated and consequently activated (Servitja et al., 2003). To assess whether the ubiquitously expressed Tiam1 is involved in Src-mediated ROS generation in HT29 cells, we treated HT29 cells with 100 μM Rac activation inhibitor NSC23766 for 2 h. Under these conditions, NSC23766 acts as a potent and selective inhibitor for the Rac1–Tiam1 interaction (IC50 = 50 μM), thus leading to the inhibition of Tiam1-mediated Rac activation (data not shown; Gao et al., 2004). As shown in Figure 5, NSC23766 treatment of HT29 cells under the above-mentioned conditions does not cause any significant change in ROS generation compared with vehicle-treated cells (Figure 5, top), indicating that Tiam1 is not the Rac-GEF responsible for Src-mediated ROS augmentation in HT29 cells. NSC23766 treatment did not affect Tiam1 expression levels (Figure 5, bottom).
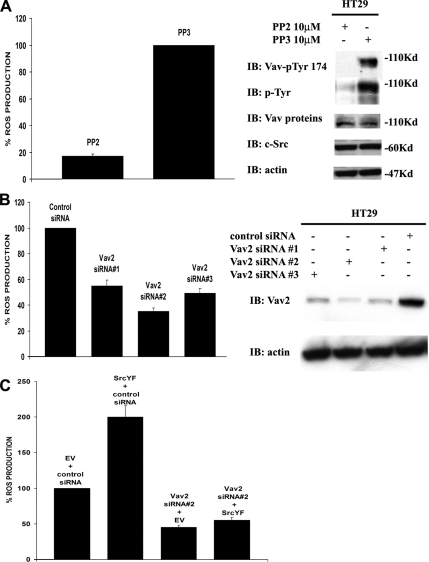
Vav2 is the only member of the Vav GEF family expressed in HT29 cells (determined by immunoblot; data not shown). To ascertain the possibility that in HT29 cells Vav2 is targeted by c-Src, we first confirmed that endogenous Vav2 is phosphorylated by Src in these cells. As shown in Figure 6A, the inhibition of HT29 c-Src activity by PP2 treatment reduces ROS generation compared with PP3-treated cells (Figure 6A, right) and DMSO-treated cells (data not shown); and abolishes Vav2 tyrosine phosphorylation (Figure 6A, left), without affecting the expression levels of endogenous Vav2 and c-Src.
Figure 6.
c-Src activates Vav2 to regulate Nox1-dependent ROS formation. (A) Inhibition of Src activity in HT29 cells by PP2 treatment reduces ROS generation and blocks endogenous Vav2 tyrosine-phosphorylation. HT29 cells were treated with 10 μM Src inhibitor PP2 or with its nonfunctional analogue PP3, and ROS production was monitored (left). One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD. The inhibition of Src activity by PP2 blocks tyrosine-phosphorylation of endogenous Vav2 without affecting the expression level of endogenous c-Src and Vav2 (right). The results shown are typical of at least three separate experiments. (B) Vav2-specific siRNAs that strongly decrease endogenous Vav2 protein levels also potently decrease ROS generation in HT29 cells. HT29 cells were transfected as described in Materials and Methods with three different Vav2-specific siRNAs (20 nM), and after 72 h the ROS formation was quantified by CL-assay (left). The right panel shows that all three Vav2-specific siRNAs significantly decrease endogenous Vav2 protein levels, with oligo#2 being the most effective. One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD. (C) SrcYF-induced ROS generation in HT29 cells is blocked by the transfection of Vav2-specific siRNA#2, which strongly decreases the level of endogenous Vav2 protein. HT29 cells were transfected by RNAiMax with empty vector or SrcYF (as indicated), and with control siRNA or with Vav2 siRNA#2. After 48 h, ROS generation was measured by CL-assay. One representative experiment from three separate experiments is shown, and results are given as mean of triplicates ± SD.
To confirm this finding, we investigated the effects of the knockdown of endogenous Vav2 protein. To this end, HT29 cells were transfected with three different Vav2-specific siRNAs or with control siRNA. As illustrated in Figure 6B (left), all three Vav2 siRNAs were able to significantly and specifically decrease endogenous Vav2 protein levels, with oligo#2 being the most effective. Interestingly, the amount of ROS produced by HT29 cells transfected with each of these three Vav2-specific siRNAs is significantly decreased compared with control siRNA-transfected cells, and it mirrors the pattern of Vav2 protein knockdown, with oligo#2 again displaying the most significant effect (Figure 6B, right). Furthermore, this effect caused by Vav2 oligo#2 in HT29 cells is rescued by the overexpression of mouse Vav (mVav) as shown in Supplemental Figure S3.
Finally, to test whether Vav2 was the Rac-GEF that c-Src activates to induce Nox1-dependent ROS generation in HT29 cells, we examined whether the decrease of endogenous Vav2 was sufficient to block Src-mediated ROS induction in these cells. To this end, HT29 cells were transfected with SrcYF and/or Vav2 siRNA#2 and ROS generation was monitored. As shown in Figure 6C, transfection of SrcYF or Vav2 siRNA#2 alone dramatically induced or decreased respectively ROS generation compared with mock-transfected cells, as expected. Interestingly, cotransfection of SrcYF along with Vav2 siRNA#2 abolished SrcYF-mediated ROS induction in HT29 cells, confirming that Vav2 is the primary Rac-GEF involved in the increased ROS production induced by c-Src in these cells in vivo.
Vav2 Induces Nox1-mediated ROS Generation through a Rac-dependent Mechanism
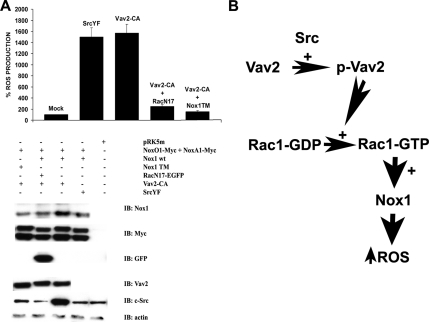
To further demonstrate that Vav2 is the downstream effector through which c-Src exerts its effect on the Nox1-mediated Rac-dependent ROS generation, we sought to abolish Vav2-mediated ROS formation by blocking the Rac1 pathway using the dominant-negative Rac1N17 mutant. To this aim, we monitored ROS generation in HEK293 cells cotransfected with Nox1 wild type, its regulatory proteins NoxO1 and NoxA1, SrcYF, and RacN17. As shown in Figure 7A (top), the presence of RacN17 blocks SrcYF-induced ROS generation, as expected, but it also dramatically abolishes the increased ROS generation caused by the presence of the constitutively active Vav2 (Vav2-CA). Consistent with this, the Vav2-CA-induced ROS production is also significantly impaired when the Nox1 triple mutant (Nox1 TM) is substituted for Nox1 wild type (Nox1 wt). Figure 7A (bottom) demonstrates that this effect is not due to changes in the expression levels of any of the transfected proteins. Of interest, the stimulatory effect of active Vav2 on Nox1-dependent ROS formation in HEK 293 cells is not observed with the constitutively active Rho GEF, GEF-H1 (GEF-H1-CA), as shown in Supplemental Figure S4 (top).
Figure 7.
Vav2 induces Nox1-dependent ROS generation through a Rac-dependent mechanism. (A) The increase in ROS formation caused by the presence of constitutively active Vav2 (Vav2-CA) is blocked in HEK293 cells by the cotransfection of dominant-negative Rac1 (RacN17) and/or is prevented by Nox1 TM unable to bind Rac. HEK293 cells were transfected with empty vector or with NoxO1 and NoxA1, and, where indicated, with SrcYF, Vav2-CA, RacN17, and Nox1 wt or Nox1 TM. The ROS production was checked after 24 h (top). All transfected proteins were expressed at similar levels (bottom). One representative experiment from three separate experiments is shown and results are given as mean of triplicates ± SD. (B) A schematic representation of the mechanism described in this paper through which c-Src induces the Nox1-dependent ROS generation in HT29 cells. c-Src activates the member of the Rac-GEF superfamily Vav2 by tyrosine-phosphorylation, thereby increasing the cellular levels of Rac1-GTP and leading to the generation of superoxide anion by Nox1.
Collectively, these results strongly support the model proposed in Figure 7B. According to this model, c-Src induces Nox1-dependent ROS generation by phosphorylating the Rac-GEF Vav2, thereby increasing cellular levels of Rac1-GTP, which consequently leads to an increase in Nox1-mediated superoxide anion (ROS) production. Whether c-Src has additional regulatory effects on Nox1 activity remains to be determined.
DISCUSSION
The idea that ROS play a key role in neoplastic growth and tumor invasion is supported by much experimental data (Szatrowski and Nathan, 1991; Laurent et al., 2005). A variety of rapidly growing human tumor cells have been shown to release large amounts of ROS that seem to be required for proliferation. Cell transformation induced by ras and other oncogenes requires ROS formation (Irani et al., 1997). Sustained exposure of tumor cells to H2O2 increases cell invasion through the activation of matrix metalloproteinases, the main group of proteolytic enzymes involved in tumor metastasis, and cell motility, through poorly defined mechanisms (Yoon et al., 2002). In many of these studies, the source of ROS was identified as a member of the NADPH oxidase family.
Nox1 and its known regulators NoxO1 and NoxA1 are most highly expressed in colon epithelium in vivo, and there is considerable evidence indicating that Nox1-derived ROS play a key role in the control of cell proliferation in the colon (Suh et al., 1999; Perner et al., 2003). Consistent with this, Nox1 overexpression and increased ROS generation have been observed in a large number of human colon cancers and colon cell lines (Suh et al., 1999). Fibroblasts overexpressing Nox1 show increased ROS levels and exhibit a transformed phenotype, including increased proliferation and aggressive tumor formation in athymic mice (Arnold et al., 2001). The authors demonstrated that Nox1-derived H2O2 functions as an intracellular mediator regulating cell growth and transformation. Conversely, reduced expression of endogenous Nox1 by siRNA methods decreases proliferation of vascular smooth muscle (Menshikov et al., 2006).
Although highly homologous to the phagocyte NADPH oxidase Nox2, the regulation of Nox1 activation is less well understood. Full Nox1 activity has been shown to require the regulatory cofactors NoxO1, NoxA1, and Rac1 GTPase (Cheng et al., 2006; Miyano et al., 2006; Ueyama et al., 2006). Consistent with this, HEK293 cells transfected with Nox1, NoxO1, and NoxA1 produce significant levels of ROS that can be enhanced by expression of an activated form of Rac1 GTPase. Nox1 has also been shown to be activated acutely through stimulation of the angiotensin type I receptor (Lassegue et al., 2001). To a large extent, however, the physiological signals regulating Nox1 activity remain undefined.
In this study, we provide evidence that the cytoplasmic tyrosine kinase c-Src induces Nox1-dependent ROS generation in human HT29 colonic adenocarcinoma cells. Several studies have suggested that c-Src plays an important role in the genesis and progression of colon cancers. In fact, increased c-Src activation has been identified in colonic malignant polyps and in adjacent normal mucosa in colon cancers (Bolen et al., 1987b). We first established that the Nox1 pathway is responsible for the production of the majority of ROS in human HT29 cells (Figure 1). We then demonstrated that c-Src induces ROS production in different cell lines, such as in NIH3T3 stably expressing active Src (Figure 2A) and HT29 cells (Figure 2, C–F), in which the level of Src activation is one of the highest among the human colon cancer cells screened in this study (Figure 2B). It has previously been described that Src-induced cell responses can be mediated through Rac1-dependent mechanism(s) (Gianni et al., 2003; Servitja et al., 2003). Consistent with this, as shown in Figure 3, A–C, Src-induced ROS generation can be prevented 1) by Nox1 mutants unresponsive to Rac1, 2) by NoxA1 mutant unable to bind Rac1, and 3) by dominant-negative RacN17.
Although it has been reported previously that NIH3T3 fibroblasts stably expressing v-Src show higher levels of Rac1-GTP (Servitja et al., 2003), the relevance in vivo of Src-mediated control of Rac1-GTP levels has not been addressed yet. Indeed, we describe a mechanism for c-Src-induced, Nox1-dependent ROS formation in HT29 cells (illustrated in Figure 7B): c-Src induces ROS generation in HT29 cells by increasing the levels of active Rac1 through the activation by phosphorylation of the specific Rac1-GEF Vav2 (also see Figure 4). Furthermore, in Figure 6 we show that Vav2 is the Vav family member responsible for the Src-mediated ROS generation in HT29 cells because 1) Vav2 tyrosine phosphorylation is abolished in PP2-treated HT29 cells; and 2) Vav2-specific siRNA blocks Src-mediated induction of ROS generation. As described in Figure 7, Vav2-induced ROS formation can be abolished by blocking the Rac1 pathway by using the dominant-negative Rac1N17 in HEK293 cells. Treatment of HT29 cells with NSC23766, a potent inhibitor of the Rac1-Tiam1 interaction, did not affect ROS generation (Figure 5).
Of interest, Src has been shown to be involved in signaling events stimulated by ROS production (Abe et al., 1997). c-Src undergoes activation upon cellular ROS formation through multiple mechanisms (Alvarez et al., 2006). Src activity is regulated through the control of its phosphorylation status by PTPases (Nakashima et al., 2002). The PTPases, which contain sulfhydryl (-SH) groups on cysteine residues in the catalytic domain that undergo oxidative modification to impair catalytic activity, are well-known targets of ROS (Stone and Dixon, 1994). Two major regulatory phosphorylation sites are present in human c-Src: Tyr527 and Tyr418. The phosphorylation of Tyr418 is responsible for c-Src activation, whereas phosphorylation of Tyr527 maintains Src in an inactive state. The inability to dephosphorylate Tyr418 by ROS-inactivated PTPases upon Src-induced ROS generation in colon cancer cells could explain the higher level of Src activation in such cells observed also in this study (Figure 2B). More importantly, c-Src-induced ROS formation by Nox1 could provide an elegant positive feedback mechanism through which c-Src induces its autoactivation by increasing ROS production via Nox1. However, whether such a mechanism contributes to formation and progression of colon cancers remains to be clarified in future experiments.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge J. Nguyen and B. Fowler for excellent technical assistance. Drs. J. D. Lambeth and D. Schlaepfer kindly provided us with Nox1 antibody and Src constructs, respectively. We thank Dr. Ulla Knaus for Nox4 and Nox5 expression plasmids and Dr. Botond Banfi for Nox3 and mouse Nox1 plasmids. We also acknowledge laboratory members Anthony Anselmo and Aimee DeCathelineau for sharing reagents, and other members of Bokoch and Knaus laboratories for helpful suggestions in revising this manuscript. This work was funded by National Institutes of Health grant HL-48008 (to G.M.B.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-02-0138) on May 7, 2008.
REFERENCES
- Abe J., Takahashi M., Ishida M., Lee J. D., Berk B. C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- Abid M. R., Kachra Z., Spokes K. C., Aird W. C. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- Abram C. L., Courtneidge S. A. Src family tyrosine kinases and growth factor signaling. Exp. Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- Alvarez R. H., Kantarjian H. M., Cortes J. E. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–1929. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- Arnold R. S., Shi J., Murad E., Whalen A. M., Sun C. Q., Polavarapu R., Parthasarathy S., Petros J. A., Lambeth J. D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B., Clark R. A., Steger K., Krause K. H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Birkenfeld J., Nalbant P., Bohl B. P., Pertz O., Hahn K. M., Bokoch G. M. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Knaus U. G. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc. Natl. Acad. Sci. USA. 1987a;84:2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., Deseau V., Rosen N. Analysis of pp60c-src in human colon carcinoma and normal human colon mucosal cells. Oncogene Res. 1987b;1:149–168. [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987;49:83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cheng G., Diebold B. A., Hughes Y., Lambeth J. D. Nox1-dependent reactive oxygen generation is regulated by Rac1. J. Biol. Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- Cherfils J., Chardin P. GEFs: structural basis for their activation of small GTP-binding proteins. Trends Biochem. Sci. 1999;24:306–311. doi: 10.1016/s0968-0004(99)01429-2. [DOI] [PubMed] [Google Scholar]
- de Carvalho D. D., Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int. J. Cancer. 2008;122:1757–1764. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C., Rocklin G., Seo J. Y., Bokoch G. M. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol. Biol. Cell. 2006;17:4760–4768. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Dickerson J. B., Guo F., Zheng J., Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi G., Banfi B., Deffert C., Fiette L., Schappi M., Herrmann F., Krause K. H. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Geiszt M., Lekstrom K., Witta J., Leto T. L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- Gianni D., Zambrano N., Bimonte M., Minopoli G., Mercken L., Talamo F., Scaloni A., Russo T. Platelet-derived growth factor induces the beta-gamma-secretase-mediated cleavage of Alzheimer's amyloid precursor protein through a Src-Rac-dependent pathway. J. Biol. Chem. 2003;278:9290–9297. doi: 10.1074/jbc.m211899200. [DOI] [PubMed] [Google Scholar]
- Irani K., Xia Y., Zweier J. L., Sollott S. J., Der C. J., Fearon E. R., Sundaresan M., Finkel T., Goldschmidt-Clermont P. J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Irby R. B., Yeatman T. J. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- Kao Y. Y., Gianni D., Bohl B., Taylor R. M., Bokoch G. M. Identification of a conserved Rac binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J. Biol. Chem. 2008;283:12736–12746. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Diebold B. A., Babior B. M., Knaus U. G., Bokoch G. M. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J. Biol. Chem. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- Kuwano Y., Kawahara T., Yamamoto H., Teshima-Kondo S., Tominaga K., Masuda K., Kishi K., Morita K., Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006;290:C433–C443. doi: 10.1152/ajpcell.00135.2005. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lassegue B., Sorescu D., Szocs K., Yin Q., Akers M., Zhang Y., Grant S. L., Lambeth J. D., Griendling K. K. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Laurent A., et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- Lock P., Abram C. L., Gibson T., Courtneidge S. A. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshikov M., Plekhanova O., Cai H., Chalupsky K., Parfyonova Y., Bashtrikov P., Tkachuk V., Berk B. C. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler. Thromb. Vasc. Biol. 2006;26:801–807. doi: 10.1161/01.ATV.0000207277.27432.15. [DOI] [PubMed] [Google Scholar]
- Miyano K., Ueno N., Takeya R., Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J. Biol. Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- Nakashima I., Kato M., Akhand A. A., Suzuki H., Takeda K., Hossain K., Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid. Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- Perner A., Andresen L., Pedersen G., Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–236. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. K., Cross A. R., Jones O. T., Andrew P. W. The use of diphenylene iodonium, an inhibitor of NADPH oxidase, to investigate the antimicrobial action of human monocyte derived macrophages. J. Immunol. Methods. 1990;133:175–179. doi: 10.1016/0022-1759(90)90357-2. [DOI] [PubMed] [Google Scholar]
- Rokutan K., Kawahara T., Kuwano Y., Tominaga K., Sekiyama A., Teshima-Kondo S. NADPH oxidases in the gastrointestinal tract: a potential role of Nox1 in innate immune response and carcinogenesis. Antioxid. Redox Signal. 2006;8:1573–1582. doi: 10.1089/ars.2006.8.1573. [DOI] [PubMed] [Google Scholar]
- Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim. Biophys. Acta. 2008;1783:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Schuebel K. E., Movilla N., Rosa J. L., Bustelo X. R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servitja J. M., Marinissen M. J., Sodhi A., Bustelo X. R., Gutkind J. S. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J. Biol. Chem. 2003;278:34339–34346. doi: 10.1074/jbc.M302960200. [DOI] [PubMed] [Google Scholar]
- Sorescu D., et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- Stofega M., DerMardirossian C., Bokoch G. M. Affinity-based assay of Rho guanosine triphosphatase activation. Methods Mol. Biol. 2006;332:269–279. doi: 10.1385/1-59745-048-0:269. [DOI] [PubMed] [Google Scholar]
- Stone R. L., Dixon J. E. Protein-tyrosine phosphatases. J. Biol. Chem. 1994;269:31323–31326. [PubMed] [Google Scholar]
- Suh Y. A., Arnold R. S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A. B., Griendling K. K., Lambeth J. D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Miyano K., Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Summy J. M., Gallick G. E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Szatrowski T. P., Nathan C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tominaga K., Kawahara T., Sano T., Toida K., Kuwano Y., Sasaki H., Kawai T., Teshima-Kondo S., Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radical Biol. Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Geiszt M., Leto T. L. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol. Cell. Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. O., Park S. J., Yoon S. Y., Yun C. H., Chung A. S. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J. Biol. Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.