Abstract
Phosphatidylinositol 3-phosphate (PI(3)P) plays an important role in insulin-stimulated glucose uptake. Insulin promotes the production of PI(3)P at the plasma membrane by a process dependent on TC10 activation. Here, we report that insulin-stimulated PI(3)P production requires the activation of Rab5, a small GTPase that plays a critical role in phosphoinositide synthesis and turnover. This activation occurs at the plasma membrane and is downstream of TC10. TC10 stimulates Rab5 activity via the recruitment of GAPEX-5, a VPS9 domain–containing guanyl nucleotide exchange factor that forms a complex with TC10. Although overexpression of plasma membrane-localized GAPEX-5 or constitutively active Rab5 promotes PI(3)P formation, knockdown of GAPEX-5 or overexpression of a dominant negative Rab5 mutant blocks the effects of insulin or TC10 on this process. Concomitant with its effect on PI(3)P levels, the knockdown of GAPEX-5 blocks insulin-stimulated Glut4 translocation and glucose uptake. Together, these studies suggest that the TC10/GAPEX-5/Rab5 axis mediates insulin-stimulated production of PI(3)P, which regulates trafficking of Glut4 vesicles.
INTRODUCTION
Polyphosphoinositides serve as versatile second messengers that regulate various cellular processes, including cell signaling, vesicle trafficking, and cytoskeletal dynamics. Polyphosphoinositides are generated from phosphatidylinositol (PI) by the action of specific PI kinases that catalyze the phosphorylation of one of the three hydroxyls at positions 3, 4, and 5 on the inositol ring. In some cases, these lipids may also be generated by specific phosphatases that dephosphorylate the higher ordered phospholipids (Di Paolo and De Camilli, 2006). In response to insulin stimulation, the class I PI3-kinase phosphorylates phosphatidylinositol 4,5-bisphosphate (PI4,5P2), generating PI3,4,5P3, which promotes the recruitment and activation of the Ser/Thr kinase PDK1. PDK1, along with the Raptor/Rictor complex, in turn phosphorylates and activates the downstream protein kinases Akt1–3, and PKCζ/λ (Kanzaki et al., 2004; Mora et al., 2004; Manning and Cantley, 2007). In addition to the well-characterized role of PI3,4,5P3 in insulin action, phosphatidylinositol 3-phosphate [PI(3)P] has also been recognized as an important regulator of Glut4 vesicle trafficking (Chaussade et al., 2003; Maffucci et al., 2003; Sweeney et al., 2004; Kanda et al., 2005; Kong et al., 2006; Falasca et al., 2007).
The Rho-family GTPase TC10 is highly expressed in insulin-responsive tissues such as muscle and fat and is rapidly activated by insulin (Murphy et al., 1999; Chang et al., 2001, 2006). In its active (GTP-bound) form, TC10 recruits multiple effectors that influence the cellular localization of the Glut4 glucose transporter. Besides regulating the tethering of Glut4 vesicles at the plasma membrane by assembling the exocyst complex (Inoue et al., 2003, 2006), TC10 may also regulate intracellular retention of Glut4 vesicles by recruiting the multidomain adapter protein CIP4 (Cdc42-interacting protein 4) to the plasma membrane (Chang et al., 2002; Lodhi et al., 2007). Furthermore, the activated form of TC10 induces production of PI(3)P at the plasma membrane (Maffucci et al., 2003; Falasca et al., 2007), although the mechanism(s) underlying this event remains unclear.
TC10 has also been implicated in Rab5 signaling (Bucci et al., 1992; de Toledo et al., 2003). This small G protein is present in different membrane compartments, including the plasma membrane, clathrin-coated vesicles and early endosomes. As a multifunctional GTPase, Rab5 recruits a network of effectors that regulate internalization of proteins from the cell surface (Bucci et al., 1992), homotypic fusion of early endosomes (Gorvel et al., 1991), formation of clathrin-coated vesicles (McLauchlan et al., 1998), and motility of early endosomes on microtubules (Nielsen et al., 1999). Rab5 also plays a critical role in phosphoinositide synthesis and turnover by recruiting two distinct PI3-kinases, VPS34 and PI3K-p110β (Christoforidis et al., 1999), as well as PI phosphatases, in particular the type II PI5-phosphatases (OCRL and INPP5B) and the type 1α PI(3,4,)P2 4-phosphatase (Shin et al., 2005; Hyvola et al., 2006). Thus, Rab5 coordinates a cascade of enzymes involved in polyphosphoinositide synthesis and turnover. Because the spatial and temporal distribution of phosphoinositides is critical for insulin-stimulated glucose transport, Rab5 has been recognized as an important regulator of insulin action (Huang et al., 2001; Su et al., 2006).
We recently identified a novel gene encoding the protein GAPEX-5, a guanine nucleotide exchange factor for the Rab5 subfamily of GTPases. GAPEX-5, in complex with the TC10 effector CIP4, is recruited to the plasma membrane in response to insulin in 3T3-L1 adipocytes via the activation of TC10 (Lodhi et al., 2007). We describe here the insulin-dependent, compartmentalized stimulation of Rab5 by TC10 via the translocation of GAPEX-5, leading to PI(3)P production.
MATERIALS AND METHODS
Reagents
Anti-hemagglutinin (HA) and anti-Myc monoclonal (9E10) and polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-GAPEX-5 polyclonal antibody was previously described (Lodhi et al., 2007). Enhanced chemiluminescence (ECL) reagents were purchased from NEN (Boston, MA), GST-Pak-1 CRIB agarose beads purchased from Cytoskeleton (Denver, CO), and GST-EEA1/NT cDNA was a generous gift of Dr. Philip Stahl (Washington University, St. Louis, MO). GST-2xFYVE plasmid was a generous gift of Dr. Harald Stenmark (Institute of Cancer Research, Oslo, Norway). cDNAs for Rab5-CAAX constructs were obtained from Dr. Alexander Sorkin (University of Colorado) and subcloned into a monomeric RFP-tagged plasmid (Chen et al., 2007).
Cell Culture and Transient Transfections
Cos-1 cells were grown in DMEM containing 10% fetal bovine serum. Cos-1 cells were transfected with FuGene6 reagent following the manufacturer's instructions (Roche Diagnostics, Alameda, CA). Mouse 3T3-L1 preadipocytes were maintained in DMEM containing 10% calf serum, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate (Invitrogen, Carlsbad, CA). To induce differentiation of 3T3-L1 cells to adipocytes, 3T3-L1 fibroblasts 3 d after confluency were treated with 1 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM isobutyl-1-methylzanthine as previously described (Baumann et al., 2000). The adipocytes were transfected with cDNAs and Stealth small interfering RNA (siRNA duplexes; Invitrogen) by electroporation as previously described (Inoue et al., 2003, 2006).
Immunoprecipitation and Immunoblotting
Cell lysates were prepared as described (Lodhi et al., 2007). The lysates were then incubated with the indicated antibodies for 1 h at 4°C. The immune complexes were precipitated with protein A/G agarose (Santa Cruz Biotechnology) for 1 h at 4°C, washed extensively with lysis buffer, resolved in 4–20% gradient SDS/PAGE, and analyzed by immunoblotting. All immunoblots were developed by ECL.
GST-EEA1 Pulldown Assays
Cells were lysed using a buffer containing 20 mM HEPES, pH 7.5, 100 mM NaCl, 1 mM DTT, 5 mM MgCl2, 5% glycerol, and 1% Triton-X-100, supplemented with protease inhibitor cocktail (Complete Mini, EDTA-free; Roche). Lysates were diluted with an equal volume of the lysis buffer lacking Triton X-100 and incubated with 15 μg of GST-EEAI/NT at 4°C with rocking. After 1 h, the beads were washed four times using the lysis buffer (containing Triton X-100). The pulldowns and lysates were subjected to electrophoresis and analyzed by immunoblotting using an anti-Rab5 antibody.
Immunofluorescence Microscopy
To detect Myc-tagged CIP4 or GAPEX-5, the cells were stained with anti-Myc polyclonal antibody (Santa Cruz Biotechnology) at 2 μg/ml. To detect HA-GAPEX-5 or HA-Rab5, the cells were stained with anti-HA mAb (Santa Cruz Biotechnology) at 2 μg/ml. After incubation with primary antibodies, cells were incubated with Alexa488 or Alexa594 goat anti-mouse or anti-rabbit IgG at 2 μg/ml (Molecular Probes, Eugene, OR). Coverslips were mounted in Vectashield mounting media (Vector Laboratories, Burlingame, CA). Images were captured by using an Olympus FV300 laser scanning confocal microscope (Melville, NY).
2-Deoxyglucose Uptake Assay
Determination of 2-deoxyglucose uptake was performed as previously described (Baumann et al., 2000). Briefly, electroporated 3T3-L1 adipocytes were serum-starved for 3 h before a 30-min incubation with 100 nM insulin. Glucose uptake was monitored by first adding 2-[14C]deoxyglucose for 5 min. The reaction was quenched with an excess of cold 2-deoxyglucose. Cells were washed with PBS and lysed in water. The radioactivity was counted using a Beckman scintillation counter (Fullerton, CA).
HPLC
Inositol labeling, extraction, and quantification were performed using a modification of a previously described procedure (Whiteford et al., 1996). Briefly, 3T3-L1 adipocytes in six-well dishes were labeled for 24 h with 30 μCi/well [3H]myoinositol (GE Healthcare, Waukesha, WI) in inositol-free media with 10% dialyzed fetal bovine serum. Cells were then stimulated with 100 nM insulin for 10 min and then scraped into 0.3 ml of ice-cold 4.5% perchloric acid and lysed for 15 min at room temperature. Two wells of lysate were combined and centrifuged for 15 min at 13,000 rpm and then washed with 100 mM EDTA. Pellets were then resuspended in 50 μl of water and deacylated in a 4:4:1 solution of methanol:methylamine:butanol at 56°C for 45 min and then dried under vacuum. Deacylated glycerophosphoinositols were then resuspended in 0.3 ml water, extracted twice with a 20:4:1 solution of butanol:ethyl ether:ethyl formate, and dried again. Soluble glycerophosphoinositols were then resuspended in water and separated on a Partisphere 5-SAX column with a 0–0.8 M ammonium phosphate, pH 3.8 gradient. Glycerophosphoinositols were presented as a percent of total glycerophosphoinositol.
Plasma Membrane Sheet Overlay
GST-2xFYVE Protein was purified on glutathione agarose (GE Healthcare) according to the manufacturer's instructions, except that 10 μM ZnCl2 was included in all buffers. Protein was then labeled with digoxygenin-NHS ester (DIG; Roche) according to standard protocols (Bridges et al., 2006) and dialyzed extensively to remove excess label. 3T3-L1 plasma membrane sheets were prepared as described (Watson et al., 2001; Chiang et al., 2002; Inoue et al., 2006). In brief, cells were seeded onto glass coverslips for 3–5 d. Cells were then treated with insulin as described above, washed twice with ice cold PBS, and then treated with 0.2% Triton X-100 in 25 mM MES (pH 6.0) and 150 mM NaCl on ice for 5 min. Cells were then washed twice with PBS, fixed in 4% paraformaldehyde for 15 min, quenched in 100 mM glycine for 5 min, and then blocked in 1% bovine serum albumin (BSA) for 1 h. Coverslips were then incubated with purified, DIG-labeled GST-2xFYVE protein (10 μM in 1% BSA) overnight at 4°C. Sheets were then washed, and anti-DIG rhodamine-conjugated secondary antibodies were used to visualize GST-2xFYVE signals. For quantification, sheet fluorescence was measured using Fluoview software and normalized to either basal control cells (for knockdowns) or same field, overexpression negative cells (for overexpression experiments). Typically 10–25 sheets were measured per experiment. All images were taken at the same photomultiplier tube and gain settings.
RESULTS
Insulin Activates Rab5 at the Plasma Membrane
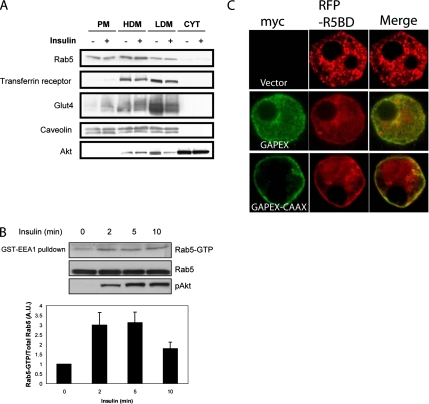
Although most Rab5 is found in early endosomes, a pool of the protein is also localized to the plasma membrane and clathrin-coated vesicles (Chavrier et al., 1990; Bucci et al., 1992). To evaluate the relative distribution of Rab5 in 3T3-L1 adipocytes, we probed different subcellular fractions for Rab5 protein by Western blotting (Figure 1A). Although a majority of Rab5 was found in high density microsomes, the remainder was equally distributed between the low-density microsome and plasma membrane fractions. We examined the effect of insulin on the activation state of Rab5 in these subcellular fractions (Figure 1B). 3T3-L1 adipocytes were stimulated with insulin for various times. Cells were then lysed, and the activation state of Rab5 was assayed by pulldown with GST-EEA1, which interacts only with the active, GTP-bound form of the protein (Simonsen et al., 1998). Insulin stimulation of adipocytes resulted in the rapid (within 2 min) activation of Rab5 at the plasma membrane which persisted for up to 10 min, although there was no effect of the hormone on the total levels of Rab5 protein found in this fraction (Figure 1A). This time course of activation was similar to that seen for phosphorylation of Akt by insulin. Quantification of the amount of active Rab5 relative to total Rab5 indicates that insulin produced a threefold increase in Rab5 activity (Figure 1B).
Figure 1.
Insulin and GAPEX-5 activate Rab5 at the plasma membrane. (A) Subcellular distribution of Rab5 in 3T3-L1 adipocytes. Adipocytes were stimulated with or without 100 nM insulin for 5 min and then fractionated as described in Materials and Methods. Subcellular distribution of Rab5 was determined by immunoblotting using an anti-Rab5 antibody. For comparison, the subcellular distribution of transferrin receptor, Glut4, caveolin, and total Akt was determined using their respective antibodies. PM, plasma membrane; HDM, high-density microsome; LDM, low-density microsome; CYT, cytosol. (B) Insulin activates Rab5 at the plasma membrane in 3T3-L1 adipocytes. 3T3-L1 adipocytes were stimulated with insulin for the indicated times, and the plasma membrane fraction was isolated as described in Materials and Methods. GST-EEA1 was used to pull down active Rab5. Pulldowns and lysates were resolved by SDS-PAGE and immunoblotted using anti-Rab5 or anti-pAkt antibodies as indicated. Blots were quantified and the activity ratio of membrane Rab5 is shown below. (C) Plasma membrane targeted Gapex-5 promotes activation of Rab5. 3T3-L1 adipocytes were transfected with RFP-tagged R5BD alone or together with myc-tagged wild-type GAPEX-5 or GAPEX-5/CAAX. After 24 h, the cells were fixed, immunostained and analyzed by confocal microscopy.
GAPEX-5, also known as RME-6 and RAP6, is a VPS9-domain–containing Rab5 guanyl nucleotide exchange factor (GEF; Sato et al., 2005; Hunker et al., 2006; Su et al., 2006; Lodhi et al., 2007). Because GAPEX-5 translocates to the plasma membrane in response to insulin in adipocytes (Lodhi et al., 2007), we investigated whether this exchange factor might be involved in the regulation of Rab5 by the hormone. We expressed in 3T3-L1 adipocytes a red fluorescent protein (RFP)-tagged Rab5-binding domain of Rabaptin-5 (RFP-R5BD) as a probe for activation. This domain of Rabaptin-5 has been previously used as an in vivo sensor of Rab5 activation state (Galperin and Sorkin, 2003), because it selectively interacts with GTP-bound Rab5 and not with other Rab proteins, including Rab5 subfamily GTPases Rab22 and Rab31 (Vitale et al., 1998; Lodhi et al., 2007). 3T3-L1 adipocytes were transfected with RFP-R5BD alone or together with Myc-GAPEX-5 or a GAPEX-5 mutant containing a C-terminal CAAX sequence that was shown to recruit the protein to the plasma membrane (Lodhi et al., 2007). In the absence of GAPEX-5 overexpression, RFP-R5BD was predominantly localized to intracellular compartments. Transfection of cells with wild type resulted in a small increase in the plasma membrane staining of RFP-R5BD, whereas expression of membrane-targeted GAPEX-5 profoundly increased the signal, compared with empty vector (Figure 1C). The R5BD does not interact with GAPEX-5 (data not shown), suggesting that the translocation of this reporter is a result of Rab5 activation at the plasma membrane.
GAPEX-5 Forms a Stable Complex with TC10
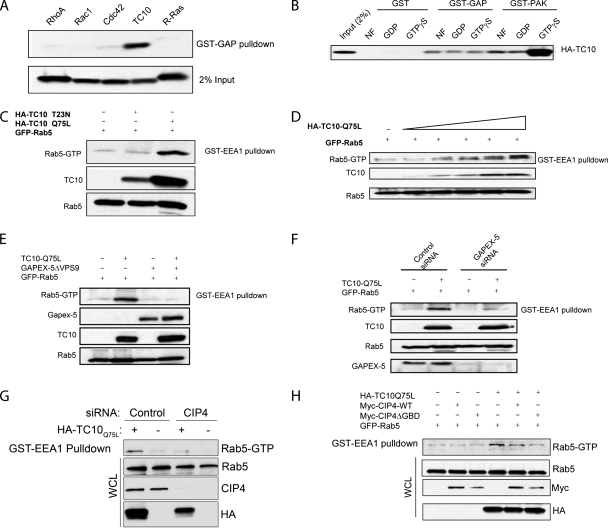
The presence of both Ras GAP and Rab GEF domains in GAPEX-5 suggests that the protein may link signaling from different families of G proteins. Multiple sequence alignment revealed that the RasGAP domain of GAPEX-5 is homologous to known RasGAPs such as p120GAP and NF1, as well as to IQGAP (Figure S1B), a RasGAP-domain–containing protein that lacks GAP activity, but interacts with Rho-family GTPases (Hart et al., 1996). To characterize the GTPase binding specificity of the GAP domain of GAPEX-5, we used a GST-tagged GAP domain of GAPEX-5 to pull down various HA-tagged G proteins expressed in Cos-1 cells (Figure 2A). Interestingly, the GAP domain of GAPEX-5 selectively interacted with TC10, but not with RhoA, Rac1, Cdc42, or R-Ras. The GAP domain also failed to interact with H-Ras (Figure S1C), as previously reported (Sato et al., 2005).
Figure 2.
TC10 interacts with GAPEX-5 and regulates Rab5 activation. Interaction of small G proteins with the GAP domain of GAPEX-5. (A) Cos-1 cells transfected with various HA-tagged small G proteins. Lysates were prepared and incubated GST-GAP domain of GAPEX-5. The pulldowns and lysates were resolved by SDS-PAGE and immunoblotted using an anti-HA antibody. (B) Cos-1 cells overexpressing HA-tagged TC10 were lysed using an NP40 lysis buffer (25 mM Tris-HCl, pH 7.5, 137 mM NaCl, 10% glycerol, 1% NP40, 5 mM MgCl2) and left nucleotide free (NF) or incubated with 2 mM GDP or 1 mM GTPγS in the presence of 10 mM EDTA at 30°C for 15 min. The loading reaction was stopped by adding 60 mM MgCl2. Pulldowns were performed using GST, GST-GAP domain of GAPEX-5, or GST-Pak-1 CRIB as indicated. The pulldowns and lysates were resolved by SDS-PAGE and immunoblotted using an anti-HA antibody. (C) Constitutively active TC10 promotes activation of Rab5. Cos-1 cells were transfected with GFP-Rab5a alone or together with HA-TC10 (Q75L) or HA-TC10 (T23N). Lysates were subjected to pulldown using GST-EEA1/NT. Rab5 and TC10 were detected by immunoblotting using anti-GFP or anti-HA antibody, respectively. (D) Effect of increasing amount of HA-TC10 (Q75L) on Rab5 activation was determined as described in C. (E) Cos-1 cells were transfected with GFP-Rab5a alone or together with HA-TC10 (Q75L) and/or myc-GAPEX-5ΔVPS9 as indicated. The active, GTP-bound Rab5 was pulled down using GST-EEA1/NT. Rab5, TC10, and Gapex-5 were detected using antibody against GFP, HA, or myc, respectively. (F) Cells were transfected with an siRNA oligonucleotide for GAPEX-5 or a matching scrambled siRNA. Forty-eight hours later, the cells were transfected with GFP-Rab5a alone or together with HA-TC10 (Q75L) as indicated. After another 24 h, the cells were harvested and used in pulldown with GST-EEA1/NT. Rab5 and TC10 were detected using an anti-GFP or anti-HA antibody, respectively. GAPEX-5 was detected using a polyclonal GAPEX-5 antibody. (G) Knockdown of CIP4 reduces TC10-mediated Rab5 activation. Cos-1 cells were transfected with scrambled or CIP4 siRNA. After 48 h, the cells were transfected with GFP-Rab5 alone or together with HA-TC10 Q75L as indicated. After another 24 h, lysates were subjected to pulldown using GST-EEA1. (H) Dominant interfering mutant of CIP4 blocks TC10-mediated Rab5 activation. Cos-1 cells were transfected as indicated. After 24 h, the lysates were subjected to pulldown using GST-EEA1.
To determine whether the interaction of GAPEX-5 with TC10 is nucleotide-dependent, we incubated lysates of Cos-1 cells overexpressing HA-TC10 with GDP or GTPγS and used a GST-tagged GAP domain of GAPEX-5 to pull down the small GTPase. The interaction of TC10 with GAPEX-5 was not influenced by nucleotide-binding state, although TC10 did interact with its effector Pak-1 in a nucleotide-dependent manner in this experiment, indicating that the protein was capable of loading GTP in vitro (Figure 2B).
TC10 Activates Rab5 via GAPEX-5
The ability of GAPEX-5 to form a stable complex with TC10 is reminiscent of the interaction of Rin1 with H-Ras, which potentiates the Rab5 GEF activity of Rin1 (Tall et al., 2001). Thus, we decided to determine whether TC10 can regulate Rab5 activation through its association with GAPEX-5. First, we investigated whether overexpression of TC10 mutants can affect the activation state of Rab5. Cos-1 cells were transfected with green fluorescent protein (GFP)-Rab5 alone or together with dominant negative or constitutively active mutants of HA-TC10, and the activation state of Rab5 was determined by pulldown assay using GST-EEA1. Interestingly, overexpression of constitutively active TC10 (Q75L), but not its dominant negative mutant (T23N), increased the interaction of EEA1 with Rab5, suggesting that TC10 activates Rab5 (Figure 2C). This effect of TC10 was dose-dependent (Figure 2D).
To determine whether GAPEX-5 is required for TC10-mediated Rab5 activation, we disrupted GAPEX-5 function by expression of a dominant-interfering mutant or siRNA-mediated knockdown. Because the GAP domain of GAPEX-5 is sufficient for its interaction with TC10, we investigated whether a GAPEX-5 mutant lacking the VPS9 domain, which still interacts with TC10, could act as a dominant negative mutant and block TC10-mediated Rab5 activation. Cos-1 cells were transfected with GFP-Rab5 alone or together with constitutively active TC10 and/or GAPEX-5ΔVPS9. Although overexpression of active TC10(Q75L) increased Rab5 activity, coexpression of the GAPEX-5 mutant blocked the ability of TC10 to promote Rab5 activation (Figure 2E).
To determine whether endogenous GAPEX-5 is required for TC10-mediated Rab5 activation, we knocked down GAPEX-5 in cells transfected with HA-TC10(Q75L) and GFP-Rab5, and determined the Rab5 activation state by pulldown with GST-EEA1. Interestingly, the depletion of GAPEX-5 abolished the ability of TC10 to promote Rab5 activation (Figure 2F). These data, combined with the effect of the GAPEX-5ΔVPS9 mutant, suggest that GAPEX-5 is required for TC10-mediated Rab5 activation.
Although only the GTP-loaded form of TC10 promotes Rab5 activation, the interaction of TC10 with GAPEX-5 is not nucleotide-dependent. This suggests that an adaptor protein might recruit GAPEX-5 to active TC10. Previous studies suggested that CIP4 may serve this role. CIP4 constitutively interacts with GAPEX-5 and is recruited to the plasma membrane upon activation of TC10 (Chang et al., 2002; Lodhi et al., 2007). To explore whether CIP4 is required for TC10-mediated Rab5 activation, we disrupted CIP4 function by siRNA-mediated knockdown or expression of a dominant-interfering mutant (Figure 2, G and H). Interestingly, knockdown of CIP4 reduced TC10-mediated Rab5 activation (Figure 2G). Previous studies indicate that mutation of a critical isoleucine residue in the second coiled-coil domain of CIP4 (CIP4ΔGBD) abolishes its interaction with TC10 (Chang et al., 2002). This mutant of CIP4 retains the ability to interact with GAPEX-5 (data not shown). Coexpression of CIP4ΔGBD blocked TC10-mediated Rab5 activation (Figure 2H). Surprisingly, overexpression of wild-type CIP4 also reduced the ability of TC10 to activate Rab5. It is possible that the overexpressed CIP4 protein competes with the endogenous GAPEX-5 for binding to TC10. Nevertheless, these studies indicate that CIP4 may play an important role in mediating Rab5 activation by TC10.
Insulin Stimulates PI(3)P Production in 3T3-L1 Adipocytes
PI(3)P is constitutively present in endosomes, where it performs essential housekeeping functions in membrane transport (Gillooly et al., 2001). Recent studies suggest that localized generation of PI(3)P at the plasma membrane may also occur in response to acute insulin stimulation, in a process dependent on TC10 (Maffucci et al., 2003; Falasca et al., 2007).
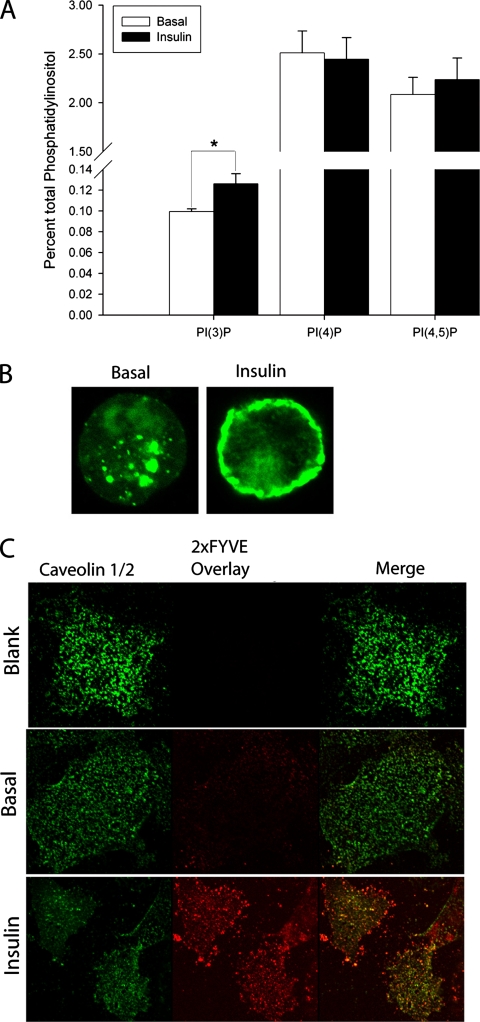
To measure phosphoinositide levels in control and insulin-stimulated adipocytes, we labeled 3T3-L1 adipocytes with [3H]inositol, treated the cells with or without insulin, and quantified the relative levels of each phosphoinositide species. There was a small but significant increase in the levels of PI(3)P after 10 min of insulin stimulation in adipocytes. In contrast, the levels of PI(4)P and PI(4,5)P2 were not significantly changed upon insulin stimulation (Figure 3A).
Figure 3.
Insulin stimulates phosphatidylinositol 3-phosphate formation in adipocytes. (A) 3T3-L1 adipocytes were treated with 100 nM insulin for 10 min and relative levels of PI(3)P, PI(4)P, and PI(4,5)P2 were determined as described by HPLC. Data are mean ± SE of five separate experiments. (B) 3T3-L1 adipocytes were transfected with GFP-2xFYVE plasmid and stimulated with 100 nM insulin for 10 min. Cells were fixed and analyzed by confocal microscopy. (C) Plasma membrane sheets from 3T3-L1 adipocytes probed with purified GST-2xFYVE protein. Sheets left untreated (blank and control) or treated with 100 nM insulin for 10 min. Sheets were fixed and then stained using anti-caveolin and DIG-labeled GST-2xFYVE (except blank, which was treated with DIG-labeled GST) and visualized with fluorescently tagged secondary antibodies and confocal microscopy.
To examine the location of this de novo generated PI(3)P, we used a GFP-2xFYVE domain fusion protein that specifically interacts with PI(3)P in cells (Gillooly et al., 2000). Although most of the fusion protein was detected inside cells under basal conditions, treatment with insulin produced a redistribution of the probe from intracellular to peripheral locations in adipocytes (Figure 3B), suggesting that the majority of the insulin-dependent increase in PI(3)P occurred at the plasma membrane. To confirm this result, we also assayed the levels of this phospholipid by generating plasma membrane sheets from 3T3-L1 adipocytes and probing for PI(3)P using exogenous, labeled GST-2xFYVE fusion protein (Figure 3C). As previously described (Watson et al., 2001; Chiang et al., 2002; Inoue et al., 2006), these plasma membrane sheets were identified by staining with antibodies to caveolin 1, the concentration of which was unaffected by insulin treatment. Insulin caused a marked increase in GST-2xFYVE binding to plasma membrane sheets. Interestingly, there was good overlap between the staining with anti-caveolin antibodies and the GST-2xFYVE domain, suggesting that the lipid is likely to be generated primarily in lipid raft domains of the plasma membrane. These data, combined with the results of the GFP-2xFYVE translocation and HPLC studies, suggest that insulin stimulation results in the production of PI(3)P at the plasma membrane in adipocytes.
GAPEX-5 Regulates PI(3)P Production at the Plasma Membrane in 3T3-L1 Adipocytes
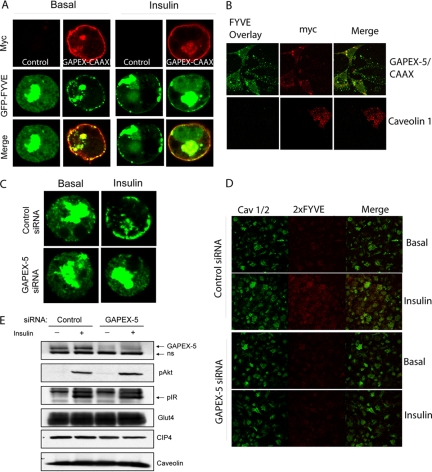
Because GAPEX-5 is an activator of Rab5, a GTPase that has been reported to control phosphoinositide turnover and production (Nielsen et al., 1999; Shin et al., 2005), we investigated whether GAPEX-5 can mediate PI(3)P formation in response to insulin in adipocytes. 3T3-L1 adipocytes were transfected with GFP-2xFYVE alone or together with myc-GAPEX-5/CAAX and then stimulated with or without insulin for 5 min. As described previously (Lodhi et al., 2007), Myc-GAPEX-5 was detected primarily at the plasma membrane (Figure 4A). GFP-FYVE was predominantly localized to intracellular compartments in the absence of insulin. After insulin stimulation, there was a profound increase in GFP-2xFYVE staining at the plasma membrane, confirming that insulin treatment results in the formation of PI(3)P at the cell surface. Transfection of cells with the membrane-targeted GAPEX-5 produced GFP-2xFYVE staining at the cell surface in the presence or absence of insulin (Figure 4A). We also analyzed the role of GAPEX-5 in generation of PI(3)P by overlay assay. 3T3-L1 adipocytes were transfected with myc-GAPEX-5/CAAX or myc-Caveolin 1 (as a negative control) and stained for membrane PI(3)P by GST-2xFYVE fusion protein overlay. Constitutively membrane-bound GAPEX-5 expression resulted in an increase in GST-2xFYVE binding to the plasma membrane sheet, indicative of the formation of PI(3)P at the plasma membrane, whereas expression of myc-Caveolin was without effect (Figure 4B). Quantitatively, myc-GAPEX-5/CAAX–expressing cells had 4.3 (±1.3)-fold more fluorescence as did myc-negative cells in the same field.
Figure 4.
GAPEX-5 regulates PI(3)P production at the plasma membrane in adipocytes. (A) 3T3-L1 adipocytes were transfected with GFP-2xFYVE domain alone or together with myc-GAPEX-5/CAAX. After 24 h, the cells were serum-starved and then stimulated with or without 100 nM insulin for 5 min. The cells were fixed and then stained using a mAb against myc, followed by Alexa594 goat anti-mouse IgG secondary antibody. The fluorescence of GFP-2xFYVE was directly visualized. The cells were analyzed by confocal microscopy. (B) Overexpression of mycGAPEX-5/CAAX causes membrane accumulation of PI(3)P. 3T3-L1 adipocytes were electroporated with myc-Caveolin or myc-GAPEX-5/CAAX for 24h. Membrane sheets were generated and visualized using anti-myc antibodies and GST-2xFYVE overlay. Panels show both myc positive and negative cells. (C) Knockdown of GAPEX-5 blocks insulin-stimulated GFP-2xFYVE translocation. 3T3-L1 adipocytes were transfected with GAPEX-5 siRNA or scrambled siRNA together with GFP-2xFYVE domain. After 72 h, the cells were serum-starved and then stimulated with or without 100 nM insulin for 5 min. The cells were fixed and the fluorescence of GFP-2xFYVE was directly visualized. The cells were analyzed by confocal microscopy. (D) Gapex-5 knockdown blocks plasma membrane PI(3)P formation as measured by GST-2xFYVE overlay. Adipocytes were transfected with control or GAPEX-5-specific siRNA. After 72 h, the cells were treated with 100 nM insulin for 5 min and relative levels of PI(3)P were visualized by GST-2xFYVE overlay and anti-caveolin staining. (E) GAPEX-5 knockdown by siRNA. 3T3-L1 adipocytes were electroporated with control or GAPEX-5 siRNA for 72 h and blotted with anti-GAPEX-5, CIP4, and caveolin antibodies. The phosphorylation of IR was detected using the phospho-Tyr (4G10) antibody. The phosphorylation of Akt was detected using phospho-Akt473 antibody. ns, a nonspecific band that cross-reacts with the GAPEX-5 antibody.
To determine if endogenous GAPEX-5 can influence insulin-stimulated PI(3)P formation, adipocytes were transfected with GAPEX-5-specific or scrambled siRNA, together with GFP-2xFYVE. After 72 h, the cells were serum-starved and then stimulated with or without insulin for 5 min. The knockdown of GAPEX-5 blocked insulin-stimulated increase in the GFP-2xFYVE fluorescence at the plasma membrane (Figure 4C). Knockdown also blocked the insulin-stimulated increase in membrane sheet PI(3)P as measured by GST-2xFYVE overlay (Figure 4D). Quantification of fluorescence from the sheets indicated that in control cells there was a 2.3 (±0.3)-fold increase upon insulin treatment; this effect was completely blocked in GAPEX-5 knockdown cells (GAPEX-5 knockdown cells treated with insulin are 1.0 ± 0.2-fold the fluorescence of control basal cells). As shown in Figure 4E, the knockdown of GAPEX-5 had no effect on the phosphorylation of the insulin receptor (IR) or Akt by insulin, nor did it affect expression levels of Glut4, CIP4, or caveolin.
Before its insulin-dependent translocation to the plasma membrane, GAPEX-5 maintains the Rab5 family GTPase Rab31 in an active state, resulting in the intracellular retention of Glut4 (Lodhi et al., 2007). To determine its effect on insulin-stimulated PI(3)P production, we knocked down Rab31 in adipocytes and analyzed the translocation of GFP-2xFYVE. The knockdown of Rab31 had no effect on GFP-2xFYVE translocation (data not shown).
Plasma Membrane–targeted Rab5 Promotes PI(3)P Formation at the Cell Surface
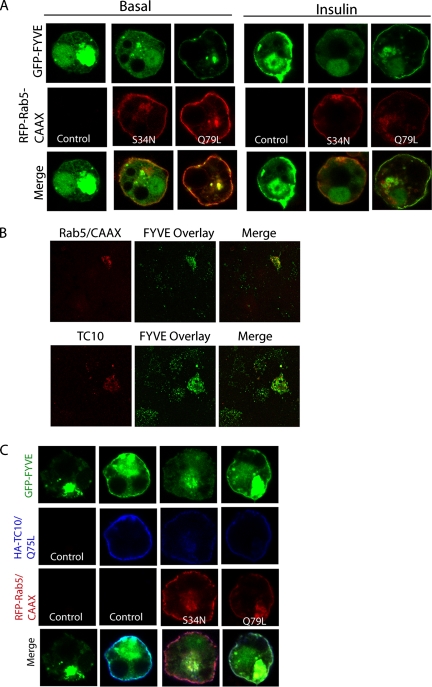
We next investigated the role of plasma membrane-localized Rab5 on insulin-stimulated PI(3)P formation at the cell surface (Figure 5). To target Rab5 to the plasma membrane, we used an RFP-tagged chimera of mutant forms of Rab5 fused to the last 20 residues of K-Ras (Rab5-CAAX). Adipocytes were transfected with GFP-2xFYVE alone or together with an RFP-tagged Rab5-CAAX mutant, and the cells were stimulated with or without insulin. In cells transfected with GFP-2xFYVE alone, insulin stimulation resulted in an increase in the plasma membrane localization of the FYVE domain. Interestingly, overexpression of the constitutively active Rab5 mutant (Q79L) produced an increase in membrane GFP-2xFYVE localization in the presence or absence of insulin. Overexpression of the dominant negative Rab5-CAAX (S34N) also resulted in a small increase in GFP-2xFYVE translocation (Figure 5A). It is possible that the dominant negative Rab5 mutant blocks internalization of endosomes containing PI(3)P in some of the cells. Nevertheless, the fact that the constitutively active Rab5 mutant dramatically promotes translocation of GFP-2xFYVE suggests that Rab5 activation results in localized formation of PI(3)P at the plasma membrane.
Figure 5.
TC10-mediated PI(3)P formation in adipocytes requires Rab5 activation. (A) Effect of plasma membrane–targeted Rab5 on GFP-2xFYVE translocation. 3T3-L1 adipocytes were transfected with GFP-2xFYVE domain alone or together with RFP-Rab5-CAAX constitutively active (Q79L) or dominant negative (S34N) mutant. After 24 h, the cells were serum-starved and then stimulated with or without 100 nM insulin for 5 min. The fluorescence of GFP and RFP was detected by confocal microscopy. (B) Effect of constitutively active TC10 or Rab5/CAAX on plasma membrane PI(3)P levels. 3T3-L1 adipocytes were transfected with HA-TC10 Q75L or RFP-Rab5/CAAX Q79L for 24 h. Membrane sheets were generated and analyzed by GST-2xFYVE overlay and anti-HA antibodies (for TC10; RFP-Rab5 was visualized directly). Panels show both GTPase-positive and -negative cells. (C) Effect of Rab5 mutants on TC10-mediated PI(3)P formation. 3T3-L1 adipocytes were transfected with GFP-2xFYVE domain alone, GFP-2xFYVE and HA-TC10 Q75L or GFP-2xFYVE and HA-TC10 Q75L together with RFP-Rab5-CAAX constitutively active (Q79L) or dominant negative (S34N) mutant. After 24 h, the cells were serum-starved and then stimulated with or without 100 nM insulin for 5 min. After fixing and staining using an anti-HA antibody, the cells were analyzed by confocal microscopy. The fluorescence of GFP and RFP were directly visualized.
To confirm these data, 3T3-L1 adipocytes were transfected with RFP-Rab5/CAAX or constitutively active HA-TC10 (Q75L), which is constitutively membrane localized (Watson et al., 2001), and membrane PI(3)P levels were analyzed by GST-2xFYVE overlay, as described above. As Figure 5B shows, the active forms of both Rab5/CAAX and TC10 caused a substantial increase in plasma membrane GST-2xFYVE staining (Rab5/CAAX positive sheets were 4.8 ± 0.9-fold the fluorescence of Rab5/CAAX negative sheets; TC10 positive sheets were 3.7 ± 0.1-fold the signal of TC10 negative sheets in the same field). Together these data suggest that overexpression of both active TC10 and Rab5 at the plasma membrane stimulate PI(3)P production. Consistent with the above observations, siRNA-mediated knockdown of TC10 also inhibited GFP-2xFYVE domain translocation stimulated by insulin (Figure S1A).
Dominant Negative Rab5 Inhibits TC10-mediated PI(3)P Formation at the Plasma Membrane
Previous studies have demonstrated that insulin induces PI(3)P formation through TC10 activation (Maffucci et al., 2003; Falasca et al., 2007). However, the mechanism underlying this process remains undefined. Because insulin-stimulated PI(3)P formation was relatively resistant to inhibitors of type IA PI3-kinase, it was hypothesized that TC10 might recruit the class II PI3-KC2α, which is less sensitive to these inhibitors. However, TC10 failed to directly interact with this kinase, as well as with VPS34, a type III PI-3K (data not shown). Because TC10 activates Rab5, we tested the hypothesis that TC10 promotes PI(3)P formation through Rab5 signaling. 3T3-L1 adipocytes were cotransfected with GFP-2xFYVE and HA-TC10 (Q75L) in the presence or absence of an RFP-tagged Rab5-CAAX mutant. Overexpression of TC10 (Q75L) resulted in an increase in the GFP-2xFYVE signal at the plasma membrane. Although coexpression of constitutively active Rab5-CAAX did not influence this effect of TC10, the dominant negative Rab5 mutant blocked the TC10-mediated translocation of the GFP-2xFYVE reporter (Figure 5C). These data suggest that TC10 regulates PI(3)P formation through Rab5 activation, likely mediated through GAPEX-5 and CIP4.
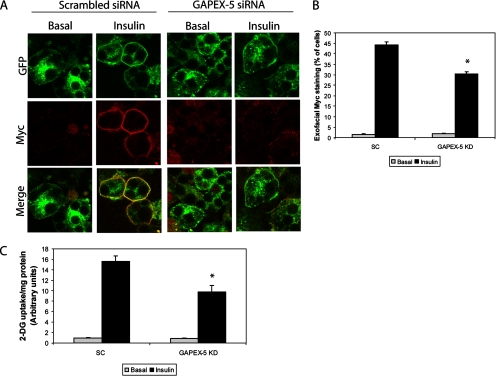
Knockdown of GAPEX-5 Blocks Insulin-stimulated Glut4 Translocation and Glucose Uptake
PI(3)P has been implicated in the translocation of Glut4 to the plasma membrane (Chaussade et al., 2003; Maffucci et al., 2003; Sweeney et al., 2004; Kanda et al., 2005; Kong et al., 2006). Because GAPEX-5 appears to regulate PI(3)P production in response to insulin stimulation, we investigated whether knockdown of GAPEX-5 affects insulin-stimulated Glut4 translocation and glucose uptake. Adipocytes stably expressing myc-Glut4-eGFP (Bogan et al., 2001) were transfected with GAPEX-5-specific or scrambled siRNAs. After 72 h, the cells were serum-starved and then stimulated with or without insulin for 15 min. Interestingly, the knockdown of GAPEX-5 blocked insulin-stimulated translocation of Glut4, without affecting basal trafficking of the transporter (Figure 6A). Quantification of the number of cells with exofacial myc staining revealed that ∼45% of control cells had myc epitope exposure, whereas the knockdown of GAPEX-5 resulted in only 30% of cells with exofacial myc staining (Figure 6B), suggesting that GAPEX-5 depletion blocks insulin-stimulated Glut4 translocation. Consistent with the effect on Glut4 translocation, the knockdown of GAPEX-5 resulted in ∼35% inhibition of insulin-stimulated glucose uptake compared with cells transfected with control siRNA (Figure 6C).
Figure 6.
GAPEX-5 regulates Glut4 translocation. (A) 3T3-L1 adipocytes were transfected with Gapex-5 siRNA or scrambled siRNA. After 72 h, the cells were serum-starved and then stimulated with or without 100 nM insulin for 10 min. The cells were fixed and then stained using a polyclonal anti-Myc antibody, followed by Alexa594 goat anti-rabbit secondary antibody. The cells were analyzed by confocal microscopy. (B) Quantification of the number of cells with exofacial myc-Glut4 staining. (C) Knockdown of Gapex-5 blocks insulin-stimulated glucose uptake. 3T3-L1 adipocytes were transfected with Gapex-5 siRNA or scrambled siRNA. After 72 h, the cells were serum-starved and then stimulated with or without 100 nM insulin for 30 min. The uptake of 2-deoxyglucose was determined as described in Materials and Methods. Data are presented as mean ± SD of triplicate determinations and were reproduced three times.
DISCUSSION
We previously identified GAPEX-5 as a Rab5 subfamily GEF that interacts with CIP4. In untreated adipocytes, GAPEX-5 is predominantly a cytosolic protein that maintains the Rab5 family GTPase, Rab31, in an active state, in the process promoting the intracellular retention of Glut4. Insulin activates TC10, which recruits the CIP4/GAPEX-5 complex to the plasma membrane. This movement of GAPEX-5 results in the reduction of intracellular Rab31 activity, thus permitting Glut4 to translocate to the plasma membrane (Lodhi et al., 2007). The intrinsic ability of GAPEX-5 to translocate in response to a hormonal stimulus suggests that the exchange factor acts on an intracellular target under basal conditions and then on a plasma membrane localized target after its insulin-dependent translocation. Results described here suggest that once translocated to the plasma membrane, GAPEX-5 activates Rab5. In this regard, insulin-stimulated glucose uptake, a process mediated by Rab5 activity (Huang et al., 2001; Su et al., 2006), is blocked by GAPEX-5 knockdown (this study).
In addition to insulin, other hormones have also been shown to promote Rab5 activation. Epidermal growth factor has been reported to increase Rab5 activity both at the plasma membrane and at endosomes (Barbieri et al., 2000; Di Fiore and De Camilli, 2001). Furthermore, stimulation of C2C12 myoblasts with adiponectin promotes the GTP-dependent interaction of Rab5 with APPL1, leading to increased Glut4 translocation (Mao et al., 2006). It will be of interest to examine whether GAPEX-5, as a ubiquitously expressed protein, is generally involved in Rab5 activation in response to hormones.
GAPEX-5 contains an N-terminal RasGAP domain that selectively interacts with TC10, a Rho family GTPase. Interestingly, overexpression of constitutively active TC10 promotes Rab5 activation, and disruption of GAPEX-5 function by expression of a dominant-interfering mutant or siRNA-mediated knockdown abolishes this effect of TC10, suggesting that GAPEX-5 is required for TC10-mediated Rab5 activation. Together, these data suggest that GAPEX-5 acts as a molecular bridge that links TC10 to Rab5. This finding is consistent with experiments demonstrating that TC10β (also known as TCL), an isoform of TC10 (Vignal et al., 2000; Chiang et al., 2002), regulates Rab5-dependent endocytic trafficking of transferrin. Suppression of TC10β expression by siRNA treatment resulted in the failure of transferrin to reach EEA1-positive early endosomes. Instead, transferrin accumulated in Rab5-positive uncoated vesicles. Conversely, overexpression of activated TC10β resulted in transferrin accumulation in EEA1-positive endosomes. Similar results were obtained when the C-terminus of constitutively active TC10 was replaced with that of TC10β (de Toledo et al., 2003). Together, these results indicate that TC10 isoforms may regulate Rab5 activation. We propose that GAPEX-5 may be the missing link between TC10 and Rab5 signaling.
The ability of constitutively active TC10 to increase Rab5 activity suggests that TC10 interacts with and stimulates the exchange activity of GAPEX-5. This is reminiscent of the association of H-Ras with Rin1, a Rab5 exchange factor implicated in EGF receptor endocytosis. H-Ras interacts with the RA domain of Rin1 and potentiates its exchange activity (Tall et al., 2001). Although the interaction of H-Ras with Rin1 is GTP-dependent, the association of TC10 with GAPEX-5 is not. However, CIP4 appears to provide a mechanism for the GTP-dependent recruitment of GAPEX-5 to TC10 (Lodhi et al., 2007).
A previous report suggested that insulin stimulates PI(3)P formation at the plasma membrane through the activation of TC10 (Maffucci et al., 2003). Because insulin-stimulated PI(3)P production is less sensitive to PI3-kinase inhibitors than is the production of PIP3, it was hypothesized that upon activation, TC10 recruits and activates the wortmannin-resistant (Domin et al., 1997), class II enzyme PI3-kinase C2α. Indeed, previous studies have suggested that this PI3-kinase isoform is activated by insulin (Brown et al., 1999) in a TC10-dependent manner (Maffucci et al., 2003; Falasca et al., 2007). Recently, it was demonstrated that PI3-kinase C2α translocates to the plasma membrane in response to insulin stimulation of L6 cells and may mediate insulin-stimulated PI(3)P production at the plasma membrane (Maffucci et al., 2003; Falasca et al., 2007). However, TC10 fails to directly interact with this kinase, as well as with a number of other PI3-kinases and phosphatases (data not shown).
Alternatively, we explored here the possibility that TC10 stimulates PI(3)P production through activation of Rab5, which may recruit and activate a cascade of PI-metabolizing enzymes, including Vps34, PI3K-p110β, PI5-phosphatases (OCRL and INPP5B) and a PI4-phosphatase (INPP4A; Christoforidis et al., 1999; Shin et al., 2005; Hyvola et al., 2006). Consistent with this possibility, overexpression of a plasma membrane-localized, constitutively active Rab5 mutant promoted translocation of GFP-2xFYVE, a reporter of PI(3)P production, whereas overexpression of a dominant negative Rab5 mutant reduced TC10-mediated PI(3)P formation. Taken together, these data suggest that TC10 regulates PI(3)P formation through activation of Rab5 and probably not by directly recruiting PI-metabolizing enzymes.
Rab5 bound to RabGDI has been shown to dramatically increase the production of PI(3)P on membranes isolated from HeLa cells. Previous studies detected a Rab5-specific nucleotide exchange activity on clathrin-coated vesicles (CCVs), which required the presence of the Rab5-RabGDI complex for maximal stimulation of nucleotide exchange (Horiuchi et al., 1995). Although several different Rab5 GEFs have been identified, only GAPEX-5/RME-6 has been associated with CCVs (Sato et al., 2005). Thus, GAPEX-5 may be the GEF that catalyzes nucleotide exchange on Rab5 bound to RabGDI and promotes PI(3)P formation at the plasma membrane. Consistent with this possibility, we report here that overexpression of a plasma membrane targeted GAPEX-5 promotes, whereas siRNA-mediated knockdown of GAPEX-5 inhibits, insulin-stimulated production of PI(3)P at the plasma membrane in adipocytes.
Phosphoinositides play a critical role in insulin-stimulated Glut4 vesicle trafficking. In addition to the well-established role of PIP3 in insulin signaling, PI(3)P has recently emerged as an important regulator of Glut4 translocation. Recent studies suggest that PI(3,4,5)P3 regulates fusion of Glut4 with the plasma membrane, whereas PI(3)P regulates translocation of Glut4 vesicles to the cell surface (Ishiki et al., 2005). Intracellular delivery of exogenous PI(3)P induces Glut4 translocation to the plasma membrane in 3T3-L1 adipocytes and L6 cells (Maffucci et al., 2003; Sweeney et al., 2004; Kanda et al., 2005). Overexpression of a 72-kDa 5-phosphatase was shown to generate PI(3)P and induce Glut4 translocation, suggesting that PI(3)P regulates Glut4 trafficking (Kong et al., 2006). Furthermore, overexpression of myotubularin, a 3-phosphatase that specifically hydrolyzes PI(3)P, blocks insulin-stimulated Glut4 translocation in adipocytes (Chaussade et al., 2003). Because GAPEX-5 appears to regulate insulin-stimulated PI(3)P formation in adipocytes, we investigated the effect of GAPEX-5 knockdown on insulin-stimulated Glut4 translocation and glucose uptake. Interestingly, the depletion of GAPEX-5 blocked both insulin-stimulated Glut4 translocation and glucose uptake in adipocytes.
In summary, our data suggest that GAPEX-5 is a multifunctional regulator of small G protein function and links TC10 to Rab5 signaling. Our results suggest that the TC10/GAPEX-5/Rab5 axis modulates insulin-stimulated production of PI(3)P, which regulates trafficking of Glut4 vesicles. How does Rab5 signaling regulate insulin-stimulated PI(3)P formation? We propose a model in which insulin stimulation results in the activation of Rab5 by the TC10/GAPEX-5 complex. In turn, activated Rab5 leads to PI(3)P formation at the plasma membrane. Future efforts will be directed toward addressing the mechanisms of insulin stimulated membrane PI(3)P formation and the means by which PI(3)P might regulate different stages in the trafficking, tethering, docking or fusion of the Glut4 vesicle.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Allison Darland and Emily Kauffman for technical support and members of Saltiel and Weisman laboratories for helpful discussions and critical analysis of data. This work was supported by an American Diabetes Association Mentor Based Fellowship to A.R.S. and National Institutes of Health Grant R01DK061618-06 to A.R.S.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0105) on April 23, 2008.
REFERENCES
- Barbieri M. A., Roberts R. L., Gumusboga A., Highfield H., Alvarez-Dominguez C., Wells A., Stahl P. D. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J. Cell Biol. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C. A., Ribon V., Kanzaki M., Thurmond D. C., Mora S., Shigematsu S., Bickel P. E., Pessin J. E., Saltiel A. R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- Bogan J. S., McKee A. E., Lodish H. F. Insulin-responsive compartments containing GLUT4 in 3T3-L1 and CHO cells: regulation by amino acid concentrations. Mol. Cell Biol. 2001;21:4785–4806. doi: 10.1128/MCB.21.14.4785-4806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D., MacDonald J. A., Wadzinski B., Moorhead G. B. Identification and characterization of D-AKAP1 as a major adipocyte PKA and PP1 binding protein. Biochem. Biophys. Res. Commun. 2006;346:351–357. doi: 10.1016/j.bbrc.2006.05.138. [DOI] [PubMed] [Google Scholar]
- Brown R. A., Domin J., Arcaro A., Waterfield M. D., Shepherd P. R. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. J. Biol. Chem. 1999;274:14529–14532. doi: 10.1074/jbc.274.21.14529. [DOI] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chang L., Adams R. D., Saltiel A. R. The TC10-interacting protein CIP4/2 is required for insulin-stimulated Glut4 translocation in 3T3L1 adipocytes. Proc. Natl. Acad. Sci. USA. 2002;99:12835–12840. doi: 10.1073/pnas.202495599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Chiang S. H., Saltiel A. R. TC10α is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology. 2007;148:27–33. doi: 10.1210/en.2006-1167. [DOI] [PubMed] [Google Scholar]
- Chaussade C., et al. Expression of myotubularin by an adenoviral vector demonstrates its function as a phosphatidylinositol 3-phosphate [PtdIns(3)P] phosphatase in muscle cell lines: involvement of PtdIns(3)P in insulin-stimulated glucose transport. Mol. Endocrinol. 2003;17:2448–2460. doi: 10.1210/me.2003-0261. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chen X. W., Leto D., Chiang S. H., Wang Q., Saltiel A. R. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Chiang S. H., Baumann C. A., Kanzaki M., Thurmond D. C., Watson R. T., Neudauer C. L., Macara I. G., Pessin J. E., Saltiel A. R. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001;410:944–948. doi: 10.1038/35073608. [DOI] [PubMed] [Google Scholar]
- Chiang S. H., Hou J. C., Hwang J., Pessin J. E., Saltiel A. R. Cloning and functional characterization of related TC10 isoforms, a subfamily of Rho proteins involved in insulin-stimulated glucose transport. J. Biol. Chem. 2002;277:13067–13073. doi: 10.1074/jbc.M109471200. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., Miaczynska M., Ashman K., Wilm M., Zhao L., Yip S. C., Waterfield M. D., Backer J. M., Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- de Toledo M., Senic-Matuglia F., Salamero J., Uze G., Comunale F., Fort P., Blangy A. The GTP/GDP cycling of rho GTPase TCL is an essential regulator of the early endocytic pathway. Mol. Biol. Cell. 2003;14:4846–4856. doi: 10.1091/mbc.E03-04-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., De Camilli P. Endocytosis and signaling. an inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Domin J., Pages F., Volinia S., Rittenhouse S. E., Zvelebil M. J., Stein R. C., Waterfield M. D. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 1997;326(Pt 1):139–147. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M., Hughes W. E., Dominguez V., Sala G., Fostira F., Fang M. Q., Cazzolli R., Shepherd P. R., James D. E., Maffucci T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J. Biol. Chem. 2007;282:28226–28236. doi: 10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- Galperin E., Sorkin A. Visualization of Rab5 activity in living cells by FRET microscopy and influence of plasma-membrane-targeted Rab5 on clathrin-dependent endocytosis. J. Cell Sci. 2003;116:4799–4810. doi: 10.1242/jcs.00801. [DOI] [PubMed] [Google Scholar]
- Gillooly D. J., Morrow I. C., Lindsay M., Gould R., Bryant N. J., Gaullier J. M., Parton R. G., Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D. J., Simonsen A., Stenmark H. Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J. 2001;355:249–258. doi: 10.1042/0264-6021:3550249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Callow M. G., Souza B., Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Giner A., Hoflack B., Zerial M. A GDP/GTP exchange-stimulatory activity for the Rab5-RabGDI complex on clathrin-coated vesicles from bovine brain. J. Biol. Chem. 1995;270:11257–11262. doi: 10.1074/jbc.270.19.11257. [DOI] [PubMed] [Google Scholar]
- Huang J., Imamura T., Olefsky J. M. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl. Acad. Sci. USA. 2001;98:13084–13089. doi: 10.1073/pnas.241368698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunker C. M., Galvis A., Kruk I., Giambini H., Veisaga M. L., Barbieri M. A. Rab5-activating protein 6, a novel endosomal protein with a role in endocytosis. Biochem. Biophys. Res. Commun. 2006;340:967–975. doi: 10.1016/j.bbrc.2005.12.099. [DOI] [PubMed] [Google Scholar]
- Hyvola N., Diao A., McKenzie E., Skippen A., Cockcroft S., Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J. 2006;25:3750–3761. doi: 10.1038/sj.emboj.7601274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Chang L., Hwang J., Chiang S. H., Saltiel A. R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- Inoue M., Chiang S. H., Chang L., Chen X. W., Saltiel A. R. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol. Biol. Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiki M., Randhawa V. K., Poon V., Jebailey L., Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J. Biol. Chem. 2005;280:28792–28802. doi: 10.1074/jbc.M500501200. [DOI] [PubMed] [Google Scholar]
- Kanda H., Tamori Y., Shinoda H., Yoshikawa M., Sakaue M., Udagawa J., Otani H., Tashiro F., Miyazaki J., Kasuga M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J. Clin. Invest. 2005;115:291–301. doi: 10.1172/JCI22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M., Mora S., Hwang J. B., Saltiel A. R., Pessin J. E. Atypical protein kinase C (PKCzeta/lambda) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J. Cell Biol. 2004;164:279–290. doi: 10.1083/jcb.200306152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A. M., et al. Phosphatidylinositol 3-phosphate [PtdIns3P] is generated at the plasma membrane by an inositol polyphosphate 5-phosphatase: endogenous PtdIns3P can promote GLUT4 translocation to the plasma membrane. Mol. Cell Biol. 2006;26:6065–6081. doi: 10.1128/MCB.00203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi I. J., Chiang S. H., Chang L., Vollenweider D., Watson R. T., Inoue M., Pessin J. E., Saltiel A. R. Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes. Cell Metab. 2007;5:59–72. doi: 10.1016/j.cmet.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffucci T., Brancaccio A., Piccolo E., Stein R. C., Falasca M. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J. 2003;22:4178–4189. doi: 10.1093/emboj/cdg402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B. D., Cantley L. C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- McLauchlan H., Newell J., Morrice N., Osborne A., West M., Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Mora A., Komander D., van Aalten D. M., Alessi D. R. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Murphy G. A., Solski P. A., Jillian S. A., Perez de la Ossa P., D'Eustachio P., Der C. J., Rush M. G. Cellular functions of TC10, a Rho family GTPase: regulation of morphology, signal transduction and cell growth. Oncogene. 1999;18:3831–3845. doi: 10.1038/sj.onc.1202758. [DOI] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J. M., Hyman A. A., Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Sato M., Sato K., Fonarev P., Huang C. J., Liou W., Grant B. D. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat. Cell Biol. 2005;7:559–569. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. W., et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Lippe R., Christoforidis S., Gaullier J. M., Brech A., Callaghan J., Toh B. H., Murphy C., Zerial M., Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Su X., Lodhi I. J., Saltiel A. R., Stahl P. D. Insulin-stimulated Interaction between insulin receptor substrate 1 and p85alpha and activation of protein kinase B/Akt require Rab5. J. Biol. Chem. 2006;281:27982–27990. doi: 10.1074/jbc.M602873200. [DOI] [PubMed] [Google Scholar]
- Sweeney G., et al. Intracellular delivery of phosphatidylinositol (3,4,5)-trisphosphate causes incorporation of glucose transporter 4 into the plasma membrane of muscle and fat cells without increasing glucose uptake. J. Biol. Chem. 2004;279:32233–32242. doi: 10.1074/jbc.M402897200. [DOI] [PubMed] [Google Scholar]
- Tall G. G., Barbieri M. A., Stahl P. D., Horazdovsky B. F. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Vignal E., De Toledo M., Comunale F., Ladopoulou A., Gauthier-Rouviere C., Blangy A., Fort P. Characterization of TCL, a new GTPase of the rho family related to TC10 andCcdc42. J. Biol. Chem. 2000;275:36457–36464. doi: 10.1074/jbc.M003487200. [DOI] [PubMed] [Google Scholar]
- Vitale G., Rybin V., Christoforidis S., Thornqvist P., McCaffrey M., Stenmark H., Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. T., Shigematsu S., Chiang S. H., Mora S., Kanzaki M., Macara I. G., Saltiel A. R., Pessin J. E. Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 2001;154:829–840. doi: 10.1083/jcb.200102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford C. C., Best C., Kazlauskas A., Ulug E. T. D-3 phosphoinositide metabolism in cells treated with platelet-derived growth factor. Biochem. J. 1996;319(Pt 3):851–60. doi: 10.1042/bj3190851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.