Abstract
MAPK activity is important during mitosis for spindle assembly and maintenance of the spindle checkpoint arrest. We previously identified B-Raf as a critical activator of the MAPK cascade during mitosis in Xenopus egg extracts and showed that B-Raf activation is regulated in an M-phase–dependent manner. The mechanism that mediates B-Raf activation at mitosis has not been elucidated. Interestingly, activation of 95-kDa B-Raf at mitosis does not require phosphorylation of Thr-599 and Ser-602 residues (Thr-633 and Ser-636 in Xenopus B-Raf), previously shown to be essential for B-Raf activation by Ras. Instead, we provide evidence for Cdk1/cyclin B in mediating mitotic activation of B-Raf. In particular, Cdk1/cyclin B complexes associate with B-Raf at mitosis in Xenopus egg extracts and contribute to its phosphorylation. Mutagenesis and in vitro kinase assays demonstrated that Cdk1/cyclin B directly phosphorylates B-Raf at Serine-144, which is part of a conserved Cdk1 preferential consensus site (S144PQK). Importantly, phosphorylation of Ser-144 is absolutely required for mitotic activation of B-Raf and subsequent activation of the MAPK cascade. However, substitution of a phospho-mimicking amino acid at Ser-144 failed to produce a constitutive active B-Raf indicating that, in addition of Ser-144 phosphorylation, other regulatory events may be needed to activate B-Raf at mitosis. Taken together, our data reveal a novel cell cycle mechanism for activating the B-Raf/MEK/MAPK cascade.
INTRODUCTION
The Raf family of serine/threonine kinases act as key intermediates for transmitting extracellular signals to the mitogen-activated protein kinase (MAPK) cascade. Three isoforms of Rafs are present in vertebrates: A-Raf, B-Raf, and C-Raf (or Raf-1), which contain large conserved regions (CR) that are functionally divided into the N-terminal regulatory domain (CR1 and CR2 regions) and the C-terminal catalytic domain (CR3 region; Daum et al., 1994; Morrison and Cutler, 1997). All three Raf proteins are detected in most cell types at various expression levels (Storm et al., 1990; Luckett et al., 2000; Wojnowski et al., 2000). Cell culture and gene knockout studies in mice suggest that the three Raf proteins have overlapping and unique regulatory functions (Hagemann and Rapp, 1999). Targeted disruption of B-Raf or C-Raf genes in mice is embryonic lethal, whereas A-Raf−/− mice die shortly after birth (Pritchard et al., 1996; Wojnowski et al., 1997, 1998, 2000). Because all Raf proteins are expressed in most embryonic and adult tissues, differences in their subcellular localization and protein interactions may account for their unique functions.
Of all three Rafs, B-Raf is the major activator of MEK (MAPK/ERK kinase; Catling et al., 1994; Jaiswal et al., 1994; Huser et al., 2001), even in cells where its expression is low (Reuter et al., 1995). This is consistent with B-Raf having a higher affinity than the other Rafs, for MEKs 1 and 2 (Papin et al., 1998) and being more efficient at phosphorylating MEKs under in vitro conditions (Marais et al., 1997; Papin et al., 1998). B-Raf contains structural features that may contribute to its being a potent MEK activator. In contrast to A- and C-Rafs, B-Raf possesses a “phosphomimetic” aspartate (D449) at the position equivalent to tyrosine-341 in C-Raf and is constitutively phosphorylated at residue S446, which is equivalent to S338 of C-Raf (Wellbrock et al., 2004). These differences contribute to B-Raf's high basal kinase activity and allow it to be “primed” for activation. In addition, B-Raf contains a long extended N-terminal region not present in the other Rafs, which may facilitate protein interactions with downstream components of the MAPK cascade.
The details of B-Raf activation have been best studied in the context of mitogen stimulation involving Ras interaction, membrane translocation, protein interaction, and site-specific phosphorylations (Mercer and Pritchard, 2003). Phosphorylation is a critical step for B-Raf activation. Ras-mediated activation of B-Raf requires the phosphorylation of conserved residues Thr599 and Ser602 within the activation segment of the kinase domain (Zhang and Guan, 2000). However, B-Raf can be activated independent of Ras mechanisms. For instance, in certain cell types, cyclic AMP and the small GTPase Rap1 have been shown to activate B-Raf (Ohtsuka et al., 1996; Vossler et al., 1997; Garcia et al., 2001). Recently, we demonstrated that B-Raf is activated at mitosis in Xenopus cycling egg extracts, which is essential for mitotic activation of the MAPK pathway (Borysov et al., 2006). In turn, mitotic roles for MAPK have been demonstrated for spindle formation, normal mitotic progression, and activation of the spindle assembly checkpoint (Minshull et al., 1994; Takenaka et al., 1997; Guadagno and Ferrell, 1998; Horne and Guadagno, 2003; Zhao and Chen, 2006). Thus, understanding how B-Raf activation is regulated at mitosis is integral to understanding its roles at mitosis.
In this study, we investigate the mechanism through which B-Raf activation is regulated at mitosis. Using the Xenopus egg extract system to recapitulate mitotic activation of the MAPK cascade, we provide evidence that supports a key role for Cdk1/cyclin B in regulating the activation of B-Raf at mitosis. We propose that Cdk1 targets a conserved N-terminal activating site in B-Raf, which is essential for mediating activation of the B-Raf/MEK/MAPK cascade during mitosis.
MATERIALS AND METHODS
Preparation of Xenopus Egg Extracts
S-phase–arrested and cycling Xenopus egg extracts were prepared from mature Xenopus oocytes that were parthenogenetically activated with the Ca2+ ionophore A23187 (0.2 μg/ml) for 2.5 min at room temperature as previously described (Borysov et al., 2006). To cycle S-phase–arrested egg extracts into a stable M-phase state, glutathione-S-transferase (GST) nondegradable sea urchin Δ90 cyclin B1 chimeric protein was added to a final concentration of 75–100 nM. Cytostatic factor (CSF)-arrested Xenopus egg extracts were prepared as described (Murray, 1991). The MEK specific inhibitor U0126 was used at a final concentration of 50 μM in Xenopus egg extracts as previously described (Horne and Guadagno, 2003). Kapil Bhalla generously provided Flavopiridol that was used in Xenopus egg extracts at 10 μM to inhibit mitotic Cdk1.
Immunoblot Analysis
Primary antibodies used for Western blots include: mouse monoclonal anti-phospho ERK (extracellular signal–regulated kinase; T202/Y204; Cell Signaling, Beverly, MA), mouse monoclonal myc-tag (Cell Signaling); rabbit polyclonal anti-B-Raf (Santa Cruz Biotechnology, Santa Cruz, CA; sc9002), anti-ERK2 and anti-phospho B-Raf (T599/S602; Santa Cruz); and a mouse monoclonal anti-Cdk1 (Calbiochem, La Jolla, CA). Rabbit anti-Xenopus MEK was prepared by Zymed Laboratories (South San Francisco, CA) against an N-terminal 16-amino acid sequence of Xenopus MEK1. Sheep anti-Xenopus cyclin B1 antibodies were a generous gift from James Maller (University of Colorado). Secondary antibodies to species-specific alkaline phosphatase–conjugated IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and anti-rabbit IgG (Sigma, St. Louis, MO) were detected with the CDP-Star chemiluminescence substrate (Roche Diagnostics, Alameda, CA).
B-Raf and Cdk1 Activity Assays
MEK kinase activity of B-Raf immunoprecipitates was measured in an in vitro–linked kinase assay as described (Guan et al., 2000). Briefly, purified B-Raf immunocomplexes were incubated with 1.0 μg of recombinant unactive GST-MEK1 (Upstate Biotechnology, Lake Placid, NY) in 25 μl of reaction buffer (25 mM HEPES, pH 7.5, 10 mM MgCl2, 25 mM β-glycerophosphate, 5 mM EGTA, 1 mM DTT, 5 mM NaF, 1 mM Na3VO4, and 0.1 mM ATP) for 20 min at room temperature. The reaction mix was briefly centrifugated to pellet immunocomplexes. Next, 20 μl of the supernatant was mixed with 10 μl of the reaction buffer containing 9.0 μg of recombinant unactive GST-ERK, and the reaction was continued for another 15 min. Finally, 3-μl aliquot of the reaction was mixed with 30 μl of reaction buffer containing 50 μg of myelin basic protein (MBP) and 5 μCi of [γ-32P]ATP and incubated for another 10 min at room temperature. The kinase reaction was stopped with SDS sample buffer, separated on 15% SDS-PAGE, and transferred to a PVDF membrane. The levels of MBP phosphorylation was visualized by autoradiography and quantified by using ImageQuant software. Cdk1/cyclin B activity was measured in an in vitro histone H1 kinase assay as described (Borysov et al., 2006).
Immunodepletion and Immunoprecipitation
B-Raf immunodepletion and immunoprecipitation procedures were performed as described (Borysov et al., 2006). For coimmunoprecipitation studies, 20 μl of Xenopus egg extracts were mixed with 4 μg of anti-Cdk1 (Calbiochem) or 6 μg of anti-cyclin B1 antibodies. Extracts were diluted 1:1 in EB buffer (80 mM β-glycerol phosphate, pH 7.3, 15 mM MgCl2, 20 mM EGTA, 25 mM NaF, 1 mM Na3VO4) supplemented with 0.1% Triton X-100 and 10 μg/ml each pepstatin, leupeptin, and chymostatin. The immune complexes were recovered on protein A agarose beads (Sigma) and washed three times with EB buffer containing 0.1% Triton X-100.
Generation and Precipitation of Wild-Type and Mutant Xenopus B-Raf Proteins
Myc-tagged Xenopus B-Raf phosphorylation mutants were generated by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Site-directed mutations were confirmed by DNA sequencing of both strands performed by the Molecular Biology Core Facility at Moffitt Cancer Center. Wild-type (WT) and (S144A, S144D, S144E, S329A) mutant myc-B-Raf proteins were translated from in vitro–transcribed mRNAs in B-Raf–depleted CSF-arrested Xenopus egg extracts supplemented with rabbit reticulocyte lysate using protocols as previously described (Borysov et al., 2006). Levels of recombinant myc-B-Raf proteins, relative to B-Raf protein found in 1 μl of egg extract, were assessed by Western blot analysis. Generally, the recombinant myc-B-Raf protein mixtures were diluted 1:10 or 1:15 into S- or M-phase egg extracts. To isolate myc-tagged B-Raf proteins from egg extracts, 10–20-μl aliquots of egg extracts containing myc-B-Raf protein were diluted 1:20 in buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 25 mM β-glycerophosphate, 10 mM MgCl2, 10% glycerol, 5 mM EGTA, 1 mM DTT, 1 mM Na2VO4, 5 mM NaF, 0.1% Triton X-100, and 10 μg/ml each pepstatin, leupeptin, and chymostatin) and incubated on ice with myc-tag monoclonal antibodies (Cell Signaling) for 2 h. Protein A Sepharose beads were added, and incubation was continued for another 12–16 h with gentle inversion at 4°C. Immunocomplexes were washed twice with the same buffer and twice with the reaction buffer (25 mM HEPES, pH 7.5, 25 mM β-glycerophosphate, 10 mM MgCl2, 5 mM EGTA, 1 mM DTT, 1 mM Na2VO4, and 5 mM NaF) before accessing by Western analysis or applying to in vitro kinase assays (see below).
In Vitro B-Raf Phosphorylation Assays
Purified human (His)6-B-Raf (Upstate Biotechnology, Lake Placid, NY) was incubated in the absence or presence of 100 U of active recombinant Cdk1/cyclin B (New England Biolabs, Beverly, MA) in 30 μl of kinase buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.1 mM ATP, and 0.01% Brij 35) with radioactive [γ-32P]ATP (5 μCi/reaction) for 30 min at 30°C. Reactions were stopped by the addition of SDS sample buffer and heating at 95°C for 5 min and separated by SDS-PAGE, and B-Raf phosphorylation was visualized by autoradiography. For experiments in Figure 6B, recombinant Xenopus kinase-dead myc-tagged B-Raf proteins were immunoprecipitated (IPed) from S-phase extracts and subjected to in vitro kinase reactions with 100 U of active Cdk1/cyclin B complexes as described above.
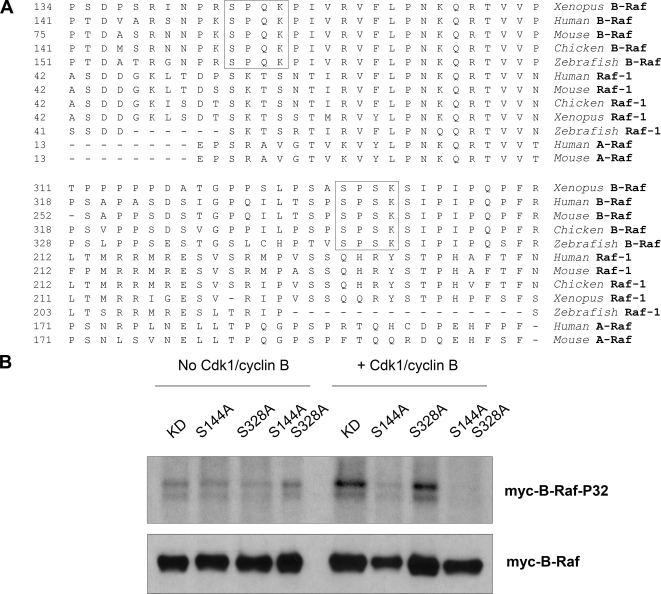
Figure 6.
Cdk1/cyclin B directly phosphorylates Xenopus B-Raf in vitro at a conserved Serine-144 residue. (A) Two putative Cdk phosphorylation sites in Xenopus B-Raf (marked by squares) are conserved in vertebrates for B-Raf but not Raf-1 or A-Raf. The N-terminal portion of amino acid sequences of Xenopus B-Raf (AAZ06667), human B-Raf (P15056), mouse B-Raf (CAB81555), chicken B-Raf (Q04982), zebrafish B-Raf (BAD16728), human Raf-1 (P04049), mouse Raf-1 (NP084056), chicken Raf-1 (CAA30069), Xenopus Raf-1 (P09560), zebrafish Raf-1 (BAD34647), human A-Raf (TVHUAF), and mouse A-Raf (P04627) were aligned by using MegAlign software. (B) Cdk1/cyclin B directly phosphorylates Xenopus B-Raf in vitro at a conserved Serine-144 residue. Immunoprecipitated wild-type (WT) and mutant myc-B-Raf proteins, isolated from S-phase Xenopus egg extracts, were subjected to an in vitro phosphorylation in the absence or presence of purified active Cdk1/cyclin B (New England Biolabs). Phosphorylation of myc-B-Raf proteins was visualized by autoradiography. Myc-tag Western blotting was performed as a loading control.
Phosphatase Treatment of B-Raf Immunoprecipitates
To dephosphorylated B-Raf IPed complexes, 50 U of recombinant lambda protein phosphatase (Upstate Biotechnology) was added to a reaction mixture containing 50 μl of phosphatase buffer as previously described (Borysov et al., 2006). B-Raf immunoprecipitates (±phosphatase treatment) were washed three times with 40 volumes of buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2) containing phosphatase inhibitors (25 mM NaF and 10 mM Na3VO4). B-Raf immunoprecipitates were analyzed either by Western blots or an in vitro–linked kinase assay to measure B-Raf–associated kinase activity.
RESULTS
Mitotic Activation of B-Raf Involves Phosphorylation
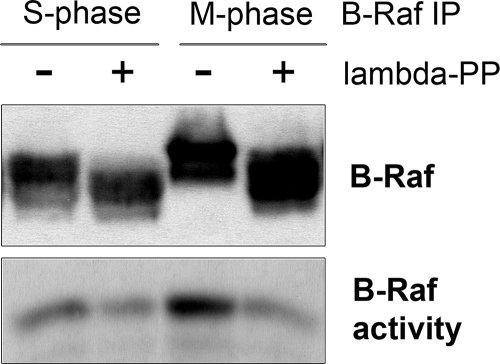
In previous studies, we showed that B-Raf undergoes hyperphosphorylation during its activation at mitosis in Xenopus egg extracts (Borysov et al., 2006). Part of this phosphorylation stems from a negative feedback loop involving ERK to regulate the inactivation of B-Raf. However, the mechanism responsible for mediating the activation of B-Raf during mitosis was not addressed. On the basis of the importance of phosphorylation in regulating the activity of Raf kinases (Chong et al., 2003), we postulated that it might also play a role in regulating mitotic activation of B-Raf. Therefore, we isolated active B-Raf complexes from Xenopus M-phase egg extracts and incubated them with lambda protein phosphatase before measuring B-Raf activity by an in vitro–linked kinase assay. The results show that dephosphorylation of mitotic B-Raf abolished its kinase activity (Figure 1). In addition, the basal activity of B-Raf isolated from S-phase extracts was also sensitive to phosphatase treatment. This may reflect the dependence of B-Raf basal activity on a constitutive phosphorylation within the N-region (Marais et al., 1997; Tran et al., 2005). Thus, our data indicate that phosphorylation is critical for regulating M-phase–specific activation of B-Raf.
Figure 1.
Mitotic B-Raf activity requires phosphorylation. B-Raf was IPed from S- and M-phase–arrested Xenopus egg extracts and incubated in an in vitro dephosphorylation reaction with recombinant lambda protein phosphatase. Then, phosphatase-treated samples were subjected to immunoblot analysis for B-Raf (top panel) or an in vitro–linked kinase assay to measure B-Raf–associated kinase activity (bottom panel).
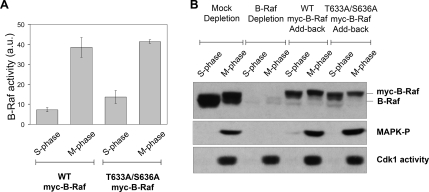
Ras-mediated activation of human B-Raf requires phosphorylation of two critical residues, Thr-599 and Ser-602, located in the kinase domain (Zhang and Guan, 2000). Both of these residues are conserved in Xenopus B-Raf (Thr-633 and Ser-636, respectively) and therefore we asked whether they played a role during mitotic activation of B-Raf. Briefly, recombinant WT or (T633A/S636A) mutant myc epitope–tagged B-Raf proteins were added to a B-Raf–depleted S-phase Xenopus egg extract that was then cycled into a stable mitotic state with nondegradable cyclin B. The myc-tagged B-Raf WT or (T633A/S636A) mutant proteins were isolated from S- and M-phase extracts to assess for B-Raf kinase activity by an in vitro–linked kinase assay. From this analysis it appears that the absence of both phosphorylation sites had little effect on mitotic activation of B-Raf compared with WT myc-B-Raf (Figure 2A). Next, we tested whether the nonphosphorylatable myc-B-Raf mutant could rescue MAPK activation at mitosis in B-Raf–depleted Xenopus egg extracts. The B-Raf–depleted extracts were supplemented with buffer, WT, or nonphosphorylatable (T633A/S636A) B-Raf and cycled into mitosis with nondegradable cyclin B. The depletion of B-Raf blocked mitotic activation of MAPK, which was rescued by the addition of WT B-Raf protein as shown previously (Borysov et al., 2006). Similar to WT, the nonphosphorylatable B-Raf (T633A/S636A) mutant was able to restored MAPK activation in M-phase Xenopus egg extracts (Figure 2B). Taken together, our data indicate that the conserved phosphorylation sites Thr-633 and Ser-636 are not required for mitotic activation of the B-Raf/MEK/MAPK cascade.
Figure 2.
Mitotic activation of Xenopus 95-kDa B-Raf does not require phosphorylation at conserved Thr-633 and Ser-636 residues. (A) Xenopus nonphosphorylatable B-Raf (T633A/S636A) mutant undergoes activation in M-phase egg extracts. Recombinant myc-tag Xenopus B-Raf proteins (WT or T633A/S636A mutant) were precipitated from B-Raf–depleted S- or M-phase– arrested extracts with a myc-epitope antibody and assessed for B-Raf kinase activity by an in vitro–linked kinase assay. B-Raf activity is expressed in arbitrary units (a.u.) as the mean ± SD from three independent experiments. Equal loading of myc-B-Raf immunoprecipitates was confirmed by B-Raf Western blotting (not shown). M-phase was confirmed by measuring Cdk1-H1–associated kinase activity. (B) The nonphosphorylatable B-Raf (T633A/S636A) mutant is able to rescue MAPK activation at mitosis in B-Raf–depleted Xenopus egg extracts. Mock, B-Raf–depleted extracts, and B-Raf–depleted extracts supplemented with recombinant WT or T633A/S636A mutant myc-B-Raf (top panel) were driven into mitosis with recombinant nondegradable cyclin B. M-phase egg extracts were confirmed by measuring Cdk1-associated H1 kinase activity (bottom panel). Activation of the MAPK cascade was determined by Western blotting for phospho-MAPK levels (middle panel).
Cdk1/Cyclin B Triggers Activation of B-Raf in Xenopus Egg Extracts
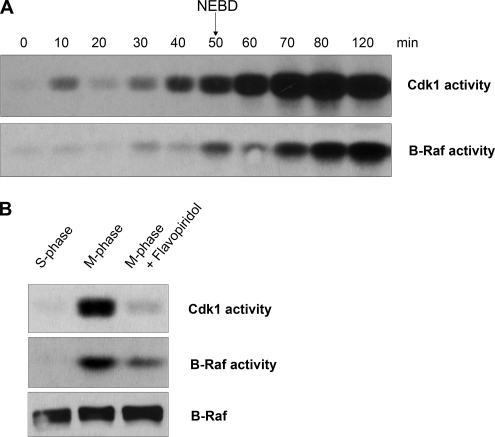
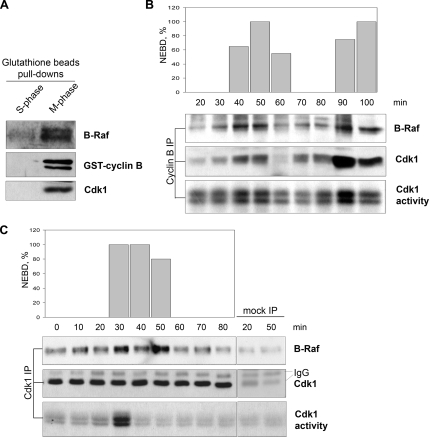
We examined whether Cdk1/cyclin B might promote mitotic activation of B-Raf. To do this, recombinant nondegradable cyclin B was added to S-phase Xenopus egg extracts to activate Cdk1 and drive the extract into a stable M-phase state. Aliquots were collected at 10-min intervals to assess entry into M-phase and to assess Cdk1 and B-Raf kinase activation. The egg extract entered M-phase at 50 min, as evident by nuclear envelop breakdown (NEBD) from nuclei formed from sperm DNA and by the abrupt activation of Cdk1/cyclin B occurring at 40–50 min (Figure 3A). A modest amount of B-Raf activity was detected in egg extracts during S-phase and at the onset of M-phase (50 and 60 min), which likely reflects either its high basal activity due to both constitutive phosphorylation and the presence of a phospho-mimicking aspartic acid residue in the positive regulatory N-terminal region (Mason et al., 1999) or partial activation. In contrast, B-Raf activity was markedly increased at M-phase by 70–80 min after the addition of nondegradable cyclin B (Figure 3A). Therefore, we conclude that Cdk1 activation (40–50 min) precedes the mitotic activation of B-Raf (70 min). Consistent with this, previous results in Xenopus cycling extracts show sequential activation of Cdk1, B-Raf, and MAPK during mitosis (Borysov et al., 2006). Is Cdk1/cyclin B activity necessary for maintenance of B-Raf activity during mitosis? To address this question, M-phase–arrested extracts were treated with Flavopiridol, a Cdk-specific inhibitor (Shapiro, 2004). Abolishing Cdk1 activity in M-phase–arrested extracts led to a substantial reduction of B-Raf activity (Figure 3B), suggesting that the enzymatic activity of Cdk1/cyclin B is required to regulate B-Raf activity during mitosis. Together, these results indicate that Cdk1/cyclin B triggers (directly or indirectly) mitotic activation of B-Raf.
Figure 3.
B-Raf activity in M-phase egg extracts is coupled to Cdk1 activity. (A) Time course of Cdk1/cyclin B and B-Raf activation after GST-Δ90 cyclin B addition to S-phase–arrested Xenopus egg extracts. B-Raf was IPed from aliquots collected at the indicated time points and subjected to an in vitro–linked kinase assay. Equal loading of B-Raf immunoprecipitates per kinase assay was confirmed by B-Raf Western blotting (not shown). Cdk1/cyclin B activity was measured in in vitro histone H1 kinase assay. M-phase was monitored by nuclear envelop breakdown of nuclei formed from added sperm DNA. (B) Cdk1/cyclin B activity is necessary for B-Raf activation during mitosis. Cdk1 activity in M-phase–arrested extracts was inhibited with 10 μM Flavopiridol. B-Raf was immunopurified from S-phase, M-phase, and M-phase extracts treated with Flavopiridol to assess its activity by an in vitro–linked kinase assay. Equal loading of B-Raf immunoprecipitates per kinase assay was confirmed by B-Raf Western blotting. Cdk1/cyclin B activity was measured in in vitro histone H1 kinase assay.
B-Raf Associates with Cdk1/Cyclin B Complexes during Mitosis
To determine whether Cdk1/cyclin B associates with B-Raf, we recovered GST-cyclin B/Cdk1 complexes from Xenopus M-phase egg extracts with glutathione Sepharose beads. Indeed, GST-cyclin B coprecipitated Cdk1 and B-Raf (Figure 4A). As an extension of these studies, we analyzed endogenous Cdk1/cylin B and B-Raf interactions in Xenopus cycling egg extracts that oscillate between S- and M-phases. Immunoprecipitation (IP) of either cyclin B or Cdk1 was performed from aliquots of extract collected during the cell cycle and analyzed by Western blotting for coprecipitation of B-Raf protein or subjected to an in vitro H1 kinase assay to measure Cdk1 activity. Cell cycle progression of the extracts was also monitored for nuclei formation and NEBD for assessing S-phase and mitosis, respectively. The results showed that B-Raf coprecipitates with either cyclin B (top panel) or Cdk1 (bottom panel) IP complexes (Figure 4, B and C). The peak of B-Raf association was consistently observed during M-phase and was substantially reduced at S-phase. Importantly, only a negligible amount (if any) of B-Raf was detected in mock IPs using IgG as a control (Figure 4C), suggesting that the coprecipitation of B-Raf is not nonspecific. Taken together, we conclude that a portion of B-Raf is associated with Cdk1/cyclin B complexes in Xenopus M-phase egg extracts.
Figure 4.
B-Raf associates with active Cdk1/cyclin B complexes during mitosis. (A) Western analysis of B-Raf, cyclin B, and Cdk1 from a GST-cyclin B pulldown. Glutathione beads in S-phase extracts were used as a negative control for background of nonspecific binding. Cyclin B (B) or Cdk1 (C) complexes were IPed from aliquots of Xenopus cycling extracts and subjected to B-Raf and Cdk1 Western blotting. Mitosis was assessed by Cdk1-associated H1 kinase activity and nuclear envelope breakdown (NEBD). Mock IPs used rabbit IgG.
Mitotic Phosphorylation of B-Raf Is Mediated by Cdk1
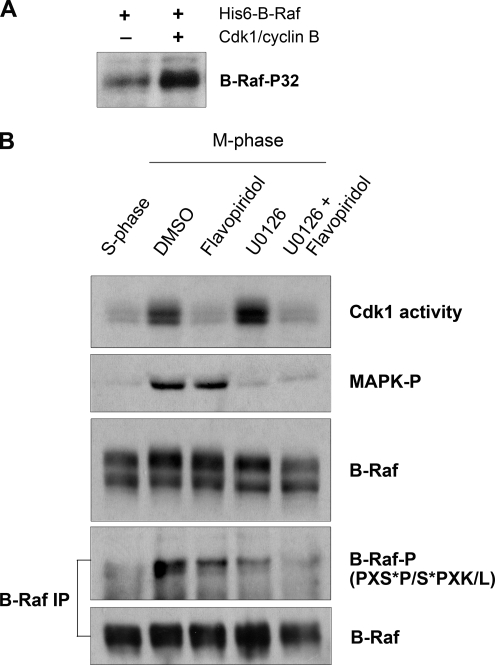
On the basis of the finding that Cdk1 associates with B-Raf, we asked whether Cdk1 might regulate B-Raf directly through phosphorylation. To examine this possibility, we incubated purified active Cdk1/cyclin B and full-length recombinant B-Raf in kinase buffer supplemented with [γ-32P]ATP. Reaction products were separated by SDS-PAGE, electrotransferred onto a PDVF membrane, and detected by autoradiography. A modest amount of B-Raf 32P-labeling was detected without the addition of Cdk1, which is probably due to autophosphorylation (Figure 5A). Importantly, 32P-labeling of B-Raf substantially increased in the presence of active Cdk1, suggesting that Cdk1 can directly mediate phosphorylation of B-Raf under in vitro conditions.
Figure 5.
Cdk1/cyclin B directly phosphorylates B-Raf in vitro and in M-phase–arrested Xenopus egg extracts. (A) Cdk1/cyclin B directly phosphorylates recombinant human (His)6-B-Raf in vitro. 32P-labeled B-Raf was visualized by autoradiography. (B) B-Raf is phosphorylated by Cdk1 and MAPK in M-phase–arrested Xenopus egg extracts. M-phase extracts treated with 10 μM Flavopiridol and/or 50 μM U0126 were assessed for B-Raf phosphorylation. B-Raf phosphorylation was analyzed by changes in electrophoretic mobility of B-Raf (middle panel) and reactivity of B-Raf immunoprecipitates with a phospho-Cdk/MAPK substrate antibody (PXS*P/S*PXK/L; B-Raf-P). Cdk1/cyclin B activity was assessed by in vitro kinase assay. Active MAPK was determined by phospho-MAPK immunoblotting.
Next, we analyzed the status of B-Raf phosphorylation in M-phase–arrested extracts where Cdk1/cyclin B activity was selectively inhibited by Flavopiridol treatment. Under these conditions, the electrophoretic mobility of mitotic B-Raf was slightly reduced (Figure 5B, middle panel, third lane), indicating that its phosphorylation was partly dependent on Cdk1 activity. As a second approach, we took advantage of a commercially available phospho-Cdk1/MAPK substrate antibody, which recognizes both Cdk1- and MAPK-phosphorylated substrates at the consensus sequences S*PXK/L and PXS*P, respectively. Because this phospho-antibody recognizes both Cdk1 and MAPK phosphorylation, we set up experimental conditions to ascertain between Cdk1- and MAPK-mediated phosphorylations. B-Raf was IPed from S- or M-phase–treated extracts and subjected to Western analysis. Phosphorylation of B-Raf at PXS*P/S*PXK/L sites was dramatically increased from S- to M-phase (Figure 5B, compare lanes 1 and 2 of the bottom panels). Importantly, inhibition of Cdk1 or MAPK led to a reduction in B-Raf reactivity to the phospho-Cdk1/MAPK substrate antibody (Figure 5B, compare lanes 2, 3, and 4). Furthermore, simultaneous inhibition of both Cdk1/cyclin B and MAPK blocked mitotic phosphorylation of B-Raf at the Cdk/MAPK phosphorylation sites. Taken together, our results suggest that Cdk1/cyclin B directly phosphorylates B-Raf during mitosis in Xenopus egg extracts.
Cdk1 Directly Phosphorylates Xenopus B-Raf In Vitro at a Conserved Serine-144 Residue
Cdk1 is a serine/threonine proline-directed protein kinase that preferentially phosphorylates substrates containing the consensus motif S/T-P-X-K/L (Nigg, 1991). The N-terminal region of Xenopus B-Raf contains two preferential Cdk1 consensus SPXK sites that are perfectly conserved among vertebrates (Figure 6A). Further comparison among other Raf family members shows that the two Cdk1 consensus sites are specific for B-Raf because they are not found in either Raf-1 (C-Raf) or A-Raf. Site-directed mutagenesis and in vitro Cdk1 kinase reactions were performed to test directly whether either of the serine residues (Ser-144 and Ser-328) within the two Cdk1 consensus sites are possible targets for Cdk1/cyclin B phosphorylation. Kinase-dead (KD) versions of WT and nonphosphorylatable B-Raf mutants (S144A, S328A, and S144A/S328A) were used for in vitro Cdk1 kinase reactions. The presence of active Cdk1 led to robust labeling of B-Raf KD over background levels (minus Cdk1) as shown in Figure 6B. Phosphorylation of B-Raf mutant S144A, but not S328A, was abolished in the presence of Cdk1. Thus, our data demonstrate that under in vitro conditions Ser-144 of Xenopus B-Raf is the primary target of Cdk1 phosphorylation.
Assessing a Mitotic Role for Phosphorylation of B-Raf at Ser-144
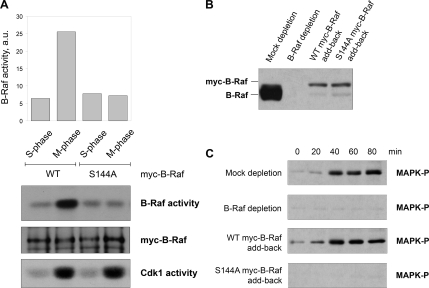
The results described in the previous section imply that phosphorylation at Serine-144 may regulate mitotic activation of B-Raf. If this is true, than the B-Raf S144A mutant should be resistant to mitotic activation. To test this, recombinant myc-B-Raf proteins (WT or S144A mutant) were introduced into S-phase extracts depleted of endogenous B-Raf and cycled into M-phase by the addition of nondegradable GST-cyclin B. The myc-tagged B-Raf proteins were IPed from S- or M-phase egg extracts and subjected to an in vitro–linked kinase assay to measure B-Raf–associated kinase activity. As expected, WT myc-B-Raf underwent activation at M-phase, as shown by a fivefold increase in B-Raf–associated kinase activity (Figure 7A). In contrast, the nonphosphorylatable myc-B-Raf (S144A) mutant failed to activate at mitosis (Figure 7A).
Figure 7.
Conserved residue Ser-144 is critical for mitotic activation of B-Raf and the MAPK cascade. (A) Mutation of Ser-144 residue blocks mitotic activation of B-Raf. Wild-type (WT) and Ser-144-Ala (S144A) myc-B-Raf proteins were incubated in S- and M-phase–arrested extracts depleted of endogenous B-Raf. Recombinant myc-tagged B-Raf proteins were IPed with a myc-epitope antibody and then subjected to either Western blotting with a myc-epitope antibody or an in vitro–linked kinase assay to measure B-Raf activity. Cdk1/cyclin B activity was measured by an in vitro histone H1 kinase assay. (B) Levels of B-Raf protein in mock-depleted, B-Raf–depleted, and B-Raf–depleted extracts supplemented with recombinant WT or S144A mutated myc-B-Raf. (C) myc-B-Raf (S144A) mutant, unlike WT myc-B-Raf, does not rescue MAPK activation in M-phase extracts depleted of endogenous B-Raf. Egg extracts were cycled into mitosis with the addition of nondegradable cyclin B protein. At indicated times, equal aliquots of extract were collected and assessed for MAPK activation by immunoblotting with a phospho-MAPK antibody.
Because we showed previously that depletion of B-Raf blocks mitotic activation of the MAPK cascade (Borysov et al., 2006), we asked whether the B-Raf S144A mutant was capable of rescuing this effect when added back to B-Raf–depleted extracts. For this experiment, recombinant WT or S144A mutant B-Raf proteins were introduced into B-Raf–depleted extracts (Figure 7B) that were then driven into M-phase by the addition of recombinant nondegradable GST-cyclin B. Aliquots of extracts were collected every 20 min for analysis of MAPK activation by immunoblotting with phospho-MAPK antibodies. As expected, depletion of endogenous B-Raf abrogated activation of MAPK in M-phase–arrested extracts, which could be rescued by adding back recombinant WT myc-B-Raf (Figure 7C). In contrast, the addition of an equivalent amount of nonphosphorylatable S144A myc-B-Raf mutant failed to rescue MAPK activation at mitosis in B-Raf–depleted extracts. Taken together, our data suggest that phosphorylation of Ser-144 is critical for mitotic activation of B-Raf and enables B-Raf to activate the MAPK cascade in Xenopus M-phase egg extracts.
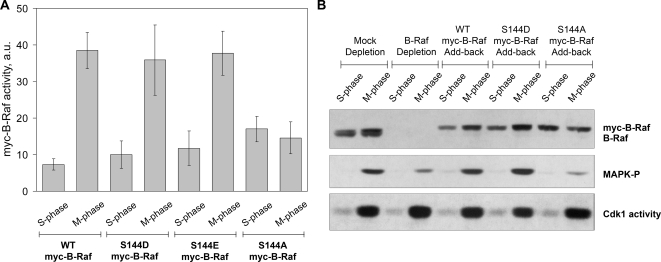
Finally, we asked whether phosphorylation of the Ser-144 residue was sufficient for B-Raf activation. To test this, phospho-mimetic mutants (S144D and S144E) were generated by site-directed mutagenesis and assessed for kinase activity in Xenopus egg extracts. If phosphorylation of Ser-144 is sufficient, then the phospho-mimetic B-Raf mutant should be constitutively active in both S-phase and mitotic egg extracts. Our results showed, instead, that the kinase activities of the recombinant myc-tagged B-Raf S144D or S144E mutants were regulated at M-phase similarly to the WT myc-B-Raf (Figure 8A). Consistent with this, add-back of the B-Raf S144D mutant to Xenopus egg extracts depleted of endogenous B-Raf led to MAPK activation during mitosis but not S-phase (Figure 8B). Taken together, we conclude that phosphorylation of B-Raf at Ser-144 residue is required but not sufficient for mitotic activation of B-Raf.
Figure 8.
Phospho-mimetic mutations at Ser-144 residue do not constitutively active B-Raf. (A) B-Raf kinase activity in S- and M-phase Xenopus egg extracts. Recombinant myc-B-Raf proteins (WT, S144D, S144E, and S144A myc-B-Raf) incubated in B-Raf–depleted S- or M-phase egg extracts were assessed for B-Raf activity in an in vitro–linked kinase assay. Kinase activities of myc-B-Raf proteins were graphed as the average activity ± SD from three independent experiments. B-Raf activity expressed in arbitrary units (a.u.). (B) Phosphomimicking S144D B-Raf mutant promotes MAPK activation in M-, but not S-phase, extracts. Recombinant myc-B-Raf proteins were translated in B-Raf–depleted CSF-arrested extracts as described in Materials and Methods. S- and M-phase extracts depleted of B-Raf were incubated with recombinant myc-B-Raf (WT, S144D, and S144A) proteins for 1 h at room temperature. MAPK activation (MAPK-P) and myc-B-Raf protein levels were assessed by Western analysis using phospho-MAPK and B-Raf antibodies. Cdk1 activity was measured by standard in vitro Cdk1-H1–associated kinase assay. Rabbit IgG was used for mock depletion control. An equivalent volume of CSF extract (B-Raf depletion) was added back to B-Raf–depleted S- or M-phase extracts as a negative control. Note: a modest amount of phospho-MAPK was detected in lanes 4 and 10. This is due likely to very low levels of B-Raf remaining in the extract after immunodepletion or to very low levels of c-Mos present in the CSF extract used to translate the myc-B-Raf proteins.
DISCUSSION
Previously we reported that B-Raf is critical for mitotic activation of the MAPK pathway and showed that its activity was regulated both positively and negatively at mitosis (Borysov et al., 2006). In this study, the mitotic mechanism for the activation of B-Raf was investigated in Xenopus egg extracts. We show here that a conserved Cdk1 consensus site (S144PQK) in Xenopus B-Raf is required for mediating its activation during mitosis. Consistent with this, Cdk1/cyclin B was found to associate with B-Raf in Xenopus cycling egg extracts during entry into mitosis. Furthermore, Cdk1 directly phosphorylated B-Raf under in vitro conditions, and mutation of Ser-144 to an alanine residue completely abolished the phosphorylation of B-Raf by Cdk1. Together, we propose that Cdk1 participates in the mitotic activation of the B-Raf/MEK/MAPK pathway through its direct phosphorylation of a novel activating site.
Conserved Thr-599 and Ser-602 Sites Are Dispensable for Mitotic B-Raf Activation
Phosphorylation plays an important role in regulating the activity and function of Raf kinases. B-Raf sites regulated by phosphorylation have been well characterized during mitogen stimulation of the Ras-Raf-MEK-ERK pathway in mammalian tissue culture cells. In this context, Ras-dependent phosphorylation of conserved residues Thr-599 and Ser-602 in human B-Raf is essential for its activation (Zhang and Guan, 2000, 2001). The results from our studies suggest that these two phosphorylation sites are dispensable for mitotic activation of B-Raf in Xenopus egg extracts (Figure 2A). In fact, the recombinant myc-B-Raf (T633A/S636A) mutant protein worked as well as the WT version of B-Raf in restoring mitotic activation of the MAPK cascade in Xenopus egg extracts depleted of endogenous B-Raf protein (Figure 2B). Thus, Thr-633 and Ser-636 residues of Xenopus B-Raf are dispensable for mitotic activation of the B-Raf/MEK/MAPK pathway, indicating that a Ras-independent signaling mechanism controls mitotic activation of B-Raf.
Biochemical Link between Cdk1/cyclin B and B-Raf
The results presented in this study indicate a biochemical link between Cdk1 and B-Raf activation during mitosis. First, we showed that addition of recombinant cyclin B to Xenopus S-phase egg extracts triggered sequential activation of Cdk1 and B-Raf (Figure 3A). This is consistent with previous studies that showed sequential activation of Cdk1, B-Raf, and p42 MAPK in Xenopus cycling egg extracts (Guadagno and Ferrell, 1998; Borysov et al., 2006). Likewise, blocking Cdk1 activity in M-phase Xenopus egg extracts resulted in a marked reduction of B-Raf activity (Figure 3B), further supporting that Cdk1 acts upstream of B-Raf. The association B-Raf and Cdk1/cyclin B complexes during M-phase in Xenopus egg extracts (Figure 4) indicates a direct regulatory link between the two protein kinases. Indeed, we showed that Cdk1 can mediate phosphorylation of B-Raf, both in M-phase Xenopus egg extracts (Figure 5B) and under in vitro conditions (Figure 5A). Furthermore, mutagenesis analysis demonstrated that Cdk1 phosphorylates B-Raf at a conserved Cdk1 preferential site (S144PQK; Figures 6). Therefore, B-Raf is a direct target of Cdk1/cyclin B. Conversely, cyclin B is phosphorylated at its cytoplasmic retention sequence by MAPK to allow for nuclear entry of Cdk1/cyclin B complexes at the onset of mitosis (Walsh et al., 2003), indicating that both signaling pathways can regulate each other during the cell cycle.
During meiosis of frog oocyte development, Cdk1 phosphorylates the germ cell–specific MEK kinase, c-Mos, at Ser-3 residue, and this was shown to be critical for the stabilization and activation of c-Mos (Castro et al., 2001; Yue and Ferrell, 2006). Subsequent to fertilization, c-Mos is targeted for degradation and remains essentially undetectable throughout the mitotic cell cycles (Watanabe et al., 1989, 1991; Borysov et al., 2006). We propose that during the mitotic cell cycle Cdk1 then plays a role to regulate B-Raf for transient activation of the MAPK cascade during mitosis. Whether B-Raf is regulated by Cdk1 at mitosis in somatic cells remains to be tested, but immunofluorescence studies in tissue culture cells indicate that Cdk1/cyclin B1 (Riabowol et al., 1989; Rattner et al., 1990; Jackman et al., 2003) and B-Raf (Borysova and Guadagno, unpublished results) localize to similar spindle structures during mitosis including the centrosomes and kinetochores. Importantly, physiological functions for B-Raf in regulating spindle formation and the spindle checkpoint arrest have been revealed in recent studies that target its expression by siRNA (Borysova and Guadagno, unpublished data) or ectopically express the constitutively active B-RafV600E mutant (Cui and Guadagno, 2007). In agreement with these findings, similar mitotic functions have been attributed to MAPK (Takenaka et al., 1997; Wang et al., 1997; Horne and Guadagno, 2003). Thus, we propose that mitotic regulation of B-Raf has important implications for coordinating mitotic events through the MAPK pathway.
B-Raf Activation at Mitosis Requires Phosphorylation at Ser-144
How does Cdk1 regulate B-Raf? On the basis of our data, we propose that Cdk1 directly phosphorylates a novel activating site on B-Raf. Two potential Cdk1 consensus phosphorylation sites (S144PQK and S328PSK) reside in B-Raf, but not Raf-1 or A-Raf (see Figure 6A). In this study, we show Cdk1 phosphorylates B-Raf at Ser-144, but not Ser-328, under in vitro conditions (Figure 6B). Mutation of Ser-144 demonstrates that it positively regulates mitotic activation of B-Raf (Figure 7A) and subsequent activation of the MAPK cascade (Figure 7C) in Xenopus egg extracts. In contrast, the Ser-328 residue does not appear to be required for B-Raf activation during mitosis (see Supplementary Figure S1). The conservation of the S144PQK motif in B-Raf, but not other Raf members, among vertebrates may help explain why B-Raf is required for controlling mitotic activation of the MAPK pathway in Xenopus egg extracts. Indeed, Raf-1, which lacks this Cdk1 preferential consensus site, is not essential for mitotic activation of MAPK cascade in Xenopus egg extracts (Yue and Ferrell, 2004; Borysov et al., 2006). Hence, our studies reveal a novel regulatory site in the N-terminal region of B-Raf that is essential for promoting its activation in the context of mitosis.
The molecular basis for how phosphorylation at this Cdk1 phosphorylation site contributes to mitotic activation of B-Raf remains to be addressed in future studies. Current models of Raf activation suggest that regulatory phosphorylations promote conformational changes that open the C-terminal kinase domain from the inhibitory N-terminal regulatory domain (Kolch, 2000). Therefore, we speculate that the presence of a negative charge at the Serine-144 residue may promote, at least in part, the aforementioned intramolecular changes necessary for B-Raf activation. Alternatively, phosphorylation at Ser-144 creates a binding site for a B-Raf-interacting protein that, in turn, promotes mitotic activation of B-Raf. In addition, the analysis of the phospho-mimetic B-Raf mutants (S144E or S144D) described in this study (Figure 8) indicates that phosphorylation of Ser-144 alone may not be sufficient to mediate B-Raf activation. Therefore, it is possible that other regulatory step(s) are required for mitotic activation of B-Raf. This would be consistent with our observations showing a delay between Cdk1 and B-Raf activation (Figure 2A).
In summary, we identify a new B-Raf regulatory site that facilitates mitotic activation of the B-Raf/MEK/MAPK pathway. A role for Cdk1/cyclin B as a mitotic activator of B-Raf opens a new perspective for understanding how B-Raf signaling is regulated during mitosis, which is quite distinct from its activation during cell cycle entry via Ras (Roovers and Assoian, 2000). The ability of B-Raf to respond to mitotic signals from Cdk1/cyclin B would allow for temporal and spatial regulation of MAPK signaling at spindle structures during M-phase progression.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kapil Bhalla (MCG Cancer Center, Georgia) for providing Flavopiridol and James Maller (University of Colorado, Denver) for anti-Xenopus cyclin B antibodies; we also thank members of the Guadagno laboratory for helpful advice and comments. We acknowledge the support of the Molecular Biology Core Facility at H. Lee Moffitt Cancer Center. This work was supported in part by National Institutes of Health Grant GM62542 and Bridge funding from Moffitt to T.M.G.
Abbreviations used:
- CR
conserved regions
- CSF
cytostatic factor
- GST
glutathione S-transferase
- IP
immunoprecipitation
- IPed
immunoprecipitated
- WT
wild type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0679) on April 23, 2008.
REFERENCES
- Borysov S. I., Cheng A. W., Guadagno T. M. B-Raf is critical for MAPK activation during mitosis and is regulated in an M phase-dependent manner in Xenopus egg extracts. J. Biol. Chem. 2006;281:22586–22596. doi: 10.1074/jbc.M601432200. [DOI] [PubMed] [Google Scholar]
- Castro A., Peter M., Magnaghi-Jaulin L., Vigneron S., Galas S., Lorca T., Labbe J. C. Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell. 2001;12:2660–2671. doi: 10.1091/mbc.12.9.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling A. D., Reuter C. W., Cox M. E., Parsons S. J., Weber M. J. Partial purification of a mitogen-activated protein kinase kinase activator from bovine brain. Identification as B-Raf or a B-Raf-associated activity. J. Biol. Chem. 1994;269:30014–30021. [PubMed] [Google Scholar]
- Chong H., Vikis H. G., Guan K. L. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003;15:463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- Cui Y., Guadagno T. M. B-Raf(V600E) signaling deregulates the mitotic spindle checkpoint through stabilizing Mps1 levels in melanoma cells. Oncogene. 2007;27:3122–3133. doi: 10.1038/sj.onc.1210972. [DOI] [PubMed] [Google Scholar]
- Daum G., Eisenmann-Tappe I., Fries H. W., Troppmair J., Rapp U. R. The ins and outs of Raf kinases. Trends Biochem. Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Garcia J., de Gunzburg J., Eychene A., Gisselbrecht S., Porteu F. Thrombopoietin-mediated sustained activation of extracellular signal-regulated kinase in UT7-Mpl cells requires both Ras-Raf-1- and Rap1-B-Raf-dependent pathways. Mol. Cell Biol. 2001;21:2659–2670. doi: 10.1128/MCB.21.8.2659-2670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T. M., Ferrell J. E., Jr. Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Figueroa C., Brtva T. R., Zhu T., Taylor J., Barber T. D., Vojtek A. B. Negative regulation of the serine/threonine kinase B-Raf by Akt. J. Biol. Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- Hagemann C., Rapp U. R. Isotype-specific functions of Raf kinases. Exp. Cell Res. 1999;253:34–46. doi: 10.1006/excr.1999.4689. [DOI] [PubMed] [Google Scholar]
- Horne M. M., Guadagno T. M. A requirement for MAP kinase in the assembly and maintenance of the mitotic spindle. J. Cell Biol. 2003;161:1021–1028. doi: 10.1083/jcb.200304144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huser M., Luckett J., Chiloeches A., Mercer K., Iwobi M., Giblett S., Sun X. M., Brown J., Marais R., Pritchard C. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 2001;20:1940–1951. doi: 10.1093/emboj/20.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M., Lindon C., Nigg E. A., Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Jaiswal R. K., Moodie S. A., Wolfman A., Landreth G. E. The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol. Cell. Biol. 1994;14:6944–6953. doi: 10.1128/mcb.14.10.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- Luckett J. C., Huser M. B., Giagtzoglou N., Brown J. E., Pritchard C. A. Expression of the A-raf proto-oncogene in the normal adult and embryonic mouse. Cell Growth Differ. 2000;11:163–171. [PubMed] [Google Scholar]
- Marais R., Light Y., Paterson H. F., Mason C. S., Marshall C. J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J. Biol. Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Mason C. S., Springer C. J., Cooper R. G., Superti-Furga G., Marshall C. J., Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer K. E., Pritchard C. A. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim. Biophys. Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N. K., Murray A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Cutler R. E. The complexity of Raf-1 regulation. Curr. Opin. Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Nigg E. A. The substrates of the cdc2 kinase. Semin. Cell Biol. 1991;2:261–270. [PubMed] [Google Scholar]
- Ohtsuka T., Shimizu K., Yamamori B., Kuroda S., Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J. Biol. Chem. 1996;271:1258–1261. doi: 10.1074/jbc.271.3.1258. [DOI] [PubMed] [Google Scholar]
- Papin C., Denouel-Galy A., Laugier D., Calothy G., Eychene A. Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J. Biol. Chem. 1998;273:24939–24947. doi: 10.1074/jbc.273.38.24939. [DOI] [PubMed] [Google Scholar]
- Pritchard C. A., Bolin L., Slattery R., Murray R., McMahon M. Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr. Biol. 1996;6:614–617. doi: 10.1016/s0960-9822(02)00548-1. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Lew J., Wang J. H. p34cdc2 kinase is localized to distinct domains within the mitotic apparatus. Cell Motil. Cytoskelet. 1990;17:227–235. doi: 10.1002/cm.970170309. [DOI] [PubMed] [Google Scholar]
- Reuter C. W., Catling A. D., Jelinek T., Weber M. J. Biochemical analysis of MEK activation in NIH3T3 fibroblasts. Identification of B-Raf and other activators. J. Biol. Chem. 1995;270:7644–7655. doi: 10.1074/jbc.270.13.7644. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989;57:393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Roovers K., Assoian R. K. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shapiro G. I. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin. Cancer Res. 2004;10:4270s–4275s. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- Storm S. M., Cleveland J. L., Rapp U. R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene. 1990;5:345–351. [PubMed] [Google Scholar]
- Takenaka K., Gotoh Y., Nishida E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J. Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N. H., Wu X., Frost J. A. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J. Biol. Chem. 2005;280:16244–16253. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- Vossler M. R., Yao H., York R. D., Pan M. G., Rim C. S., Stork P. J. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Walsh S., Margolis S. S., Kornbluth S. Phosphorylation of the cyclin b1 cytoplasmic retention sequence by mitogen-activated protein kinase and Plx. Mol. Cancer Res. 2003;1:280–289. [PubMed] [Google Scholar]
- Wang X. M., Zhai Y., Ferrell J. E., Jr. A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J. Cell Biol. 1997;137:433–443. doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Hunt T., Ikawa Y., Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991;352:247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Stancato L. F., Larner A. C., Rapp U. R., Zimmer A. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev. 2000;91:97–104. doi: 10.1016/s0925-4773(99)00276-2. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Stancato L. F., Zimmer A. M., Hahn H., Beck T. W., Larner A. C., Rapp U. R., Zimmer A. Craf-1 protein kinase is essential for mouse development. Mech. Dev. 1998;76:141–149. doi: 10.1016/s0925-4773(98)00111-7. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Zimmer A. M., Beck T. W., Hahn H., Bernal R., Rapp U. R., Zimmer A. Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- Yue J., Ferrell J. E., Jr. Mos mediates the mitotic activation of p42 MAPK in Xenopus egg extracts. Curr. Biol. 2004;14:1581–1586. doi: 10.1016/j.cub.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Yue J., Ferrell J. E., Jr. Mechanistic studies of the mitotic activation of mos. Mol. Cell Biol. 2006;26:5300–5309. doi: 10.1128/MCB.00273-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. H., Guan K. L. Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J. 2000;19:5429–5439. doi: 10.1093/emboj/19.20.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. H., Guan K. L. Regulation of the Raf kinase by phosphorylation. Exp. Lung Res. 2001;27:269–295. doi: 10.1080/019021401300054046. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen R. H. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr. Biol. 2006;16:1764–1769. doi: 10.1016/j.cub.2006.07.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.