Abstract
Hsc70 with its cochaperone, either auxilin or GAK, not only uncoats clathrin-coated vesicles but also acts as a chaperone during clathrin-mediated endocytosis. However, because synaptojanin is also involved in uncoating, it is not clear whether GAK is an essential gene. To answer this question, GAK conditional knockout mice were generated and then mated to mice expressing Cre recombinase under the control of the nestin, albumin, or keratin-14 promoters, all of which turn on during embryonic development. Deletion of GAK from brain, liver, or skin dramatically altered the histology of these tissues, causing the mice to die shortly after birth. Furthermore, by expressing a tamoxifen-inducible promoter to express Cre recombinase we showed that deletion of GAK caused lethality in adult mice. Mouse embryonic fibroblasts in which the GAK was disrupted showed a lack of clathrin-coated pits and a complete block in clathrin-mediated endocytosis. We conclude that GAK deletion blocks development and causes lethality in adult animals by disrupting clathrin-mediated endocytosis.
INTRODUCTION
Clathrin-mediated endocytosis is one of the major mechanisms used by cells to internalize cargo. Many proteins are involved in the complex series of events that begins with recruitment of cargo into the nascent clathrin-coated pit (CCP). The CCP invaginates and then constricts to produce a clathrin-coated vesicle (CCV) that is finally uncoated. Two proteins that may play a major role in this process are Hsc70 and its cochaperones, either the neuronally expressed auxilin or the ubiquitously expressed GAK, which is an acronym for cyclin-G–associated kinase. Both auxilin and GAK are multidomain proteins belonging to the J-domain class of proteins that present substrates to Hsc70 (Eisenberg and Greene, 2007). In contrast to most other members of this class of proteins, the J-domains of auxilin and GAK, which contain the characteristic HPD motif, are C-terminal rather than N-terminal. GAK, unlike auxilin, has an N-terminal kinase domain that phosphorylates the clathrin adaptor proteins (APs), AP1 and AP2, on their μ chain (Umeda et al., 2000;Korolchuk and Banting, 2002). Adjacent to the kinase domain is the PTEN-like domain, which can bind to lipids (Lee et al., 2006;Massol et al., 2006). Between the PTEN-like and J-domains is the domain that binds clathrin, AP1, AP2, and dynamin (Scheele et al., 2001;Newmyer et al., 2003;Kametaka et al., 2007). A truncation mutant consisting of just the clathrin-binding domain and the J-domain retains the ability of GAK to support ATP-dependent Hsc70 uncoating of CCVs (Greener et al., 2000; Umeda et al., 2000).
Although Hsc70 and auxilin were initially thought to be involved only in clathrin uncoating (Ungewickell et al., 1995;Greener et al., 2000), evidence from various studies has suggested that they may also be involved in several other key processes that occur during clathrin-mediated endocytosis. Hsc70 and auxilin may be required for clathrin exchange occurring during invagination and constriction; this clathrin exchange could catalyze the rearrangement of the structure of the CCPs (Wu et al., 2001;Yim et al., 2005). Hsc70 and auxilin may also chaperone clathrin after it dissociates from CCVs, so that clathrin does not aggregate in the cytosol, and finally it may facilitate the rebinding of clathrin and APs onto membranes during nucleation of new CCPs (Jiang et al., 2000;Lee et al., 2005).
To examine the function of GAK in mammalian cells, several laboratories have depleted GAK using RNA interference (RNAi), but there is little consensus as to the phenotype of the GAK-depleted cell. In the first study (Zhang et al., 2004), cell lines in which GAK was stably depleted showed a 50-fold increase in the levels of expression of both epidermal growth factor receptor (EGFR) and tyrosine kinase, as well as significant changes in downstream EGFR signaling. EGF uptake was inhibited in this cell line, but, surprisingly, there was only minor inhibition of transferrin uptake. In two later studies, GAK was transiently depleted from HeLa cells using RNAi oligonucleotides. In a study from the Drubin laboratory, transient depletion of GAK caused about a 30% inhibition in transferrin uptake and 50% reduction in the rate of maturation of cathepsin D, an aspartic peptidase that is present in the trans-Golgi network (TGN) as a proenzyme and matures during trafficking to lysosomes (Zhang et al., 2005). Similarly, the Bonifacino laboratory (Kametaka et al., 2007) observed a decrease in the rate of cathepsin D maturation upon GAK depletion. However, these studies did not observe an effect of GAK depletion on the distribution of clathrin either at the plasma membrane (Zhang et al., 2005) or the TGN (Kametaka et al., 2007).
In contrast, a more profound phenotype was obtained by our laboratory using vector-based RNAi to knock down GAK in HeLa cells (Lee et al., 2005). GAK-depleted cells showed a significant reduction both in the number of CCPs at the plasma membrane and the amount of clathrin at the TGN, as well as a reduction in the rate of clathrin exchange on the remaining CCPs. In addition, there was a dramatic reduction in puncta containing AP2 and epsin at the plasma membrane and the amount of AP1 and GGA3 at the TGN. Because GAK works catalytically, it is possible that the observed phenotypic differences upon GAK depletion in these studies are due to the level of GAK remaining in the cells.
The function of auxilin has also been examined in Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster, whole organisms that contain only one gene homologous to auxilin. In yeast, deletion of the auxilin gene causes the accumulation of CCVs, impaired cargo delivery to the vacuole, an increase in the amount of clathrin associated with vesicles relative to cytoplasmic clathrin, and slow cell growth (Pishvaee et al., 2000;Gall et al., 2000). When RNAi was used to inhibit auxilin expression in C. elegans, there was a marked reduction in receptor-mediated endocytosis of yolk protein by oocytes (Greener et al., 2001). In addition, the auxilin-depleted worms showed developmental defects, altered distribution of clathrin, and inhibition of clathrin exchange. In Drosophila, the deletion of auxilin caused lethality, whereas a point mutation in the PTEN-like domain of auxilin impaired its function (Hagedorn et al., 2006). This mutant auxilin, which showed genetic interaction with Hsc70 and clathrin, caused a disruption of the notch-signaling pathway due to defective endocytosis of delta ligand. Interestingly, this defect in notch signaling caused by mutant auxilin is rescued by overexpression of clathrin heavy chain (Eun et al., 2008).
To determine whether GAK has an essential function in the mouse, we carried out the targeted disruption of GAK gene. The conventional GAK knockout mouse was embryonic lethal, making it necessary for us to generate conditional GAK knockout mice. These mice were mated to mice expressing Cre recombinase under the control of different tissue-specific promoters to disrupt the GAK gene. The mice died shortly after birth when GAK was depleted from developing brain, liver, or skin. Moreover, by using a tamoxifen-inducible form of Cre recombinase, we found that knocking out GAK in the adult mouse produced lethality. These results show that GAK plays an essential role in the organs of both developing and mature mice. To investigate the nature of this essential role, we knocked out GAK in mouse embryonic fibroblasts (MEFs) derived from conditional GAK knockout mice. Clathrin-mediated endocytosis was completely blocked in the GAK-disrupted MEFs, whereas structurally these cells show a marked reduction in CCPs on the plasma membrane. We conclude that GAK deletion blocks development and causes lethality in adult animals by inhibiting clathrin-mediated endocytosis.
MATERIALS AND METHODS
Construction of the Targeting Vector and Generation of GAK-deficient Embryonic Stem Cells
To screen the mouse GAK genomic DNA clone from the BAC mouse II library, an ∼480-base pair probe was subcloned from a mouse GAK EST clone (IMAGE Clone ID no. 3483345, ATCC, Manassas, VA) by using primers designed from the rat GAK cDNA sequence. Two individual clones, 370/C06 and 278/G08, which have GAK genomic DNA in the pBeloBacII vector, were obtained by BAC Mouse II library screening (Incyte Genomics, Palo Alto, CA). The full genomic DNA sequence of mouse GAK on chromosome 5 was obtained from Ensembl Gene Browser (Ensembl gene: OTTMUSG00000027010).
For the conventional knockout of GAK, a BAC clone 370/C06, containing GAK genomic DNA, was used as a template for PCR to get the targeting arms. The 2.7-kb fragment containing the promoter region and partial exon 1 and the 3.2-kb fragment containing introns and exon 2, 3, and 4 were cloned into pKO Scrambler NTKV-1903 (Stratagene, La Jolla, CA) as scrambler A and B, respectively (Figure 1A).
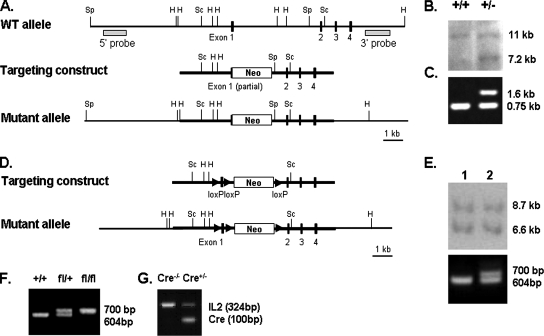
Figure 1.
Establishment of GAK conventional and conditional knockout mice cell lines. (A) Targeting construct and mutant allele used in generating conventional knockout mouse from wild-type gene. Exons are indicated by filled boxes. Restriction enzyme sites are denoted as follows: Sp, SphI; H, HindIII, Sc, SacI. (B) Southern blot analysis of ES cells in which the DNA was digested with SphI. The 5′ probe detects a 7.2- and an 11-kb band for the mutant and wild-type allele, respectively. (C) PCR analysis of mice generated by mating GAK+/− heterozygotes. Mice DNA were analyzed using two sets of primers, one to recognize the wild-type gene (0.75 kb) and the other to recognize the gene with the neomyocin insertion (1.6 kb). (D) Scheme of targeting construct and mutant allele used in generating the conditional knockout mouse. Arrowheads indicate LoxP recognition sites; and neo is the neomycin resistance gene. (E) Southern blot analysis of genomic DNA from ES cells in which the DNA was digested with HindIII. The 3′ probe detects an 8.7- and a 6.6-kb band for the mutant and wild-type allele, respectively. The larger size band shows integration of the neomycin gene. PCR analysis of the positive ES clones gives 700- and 604-base pair fragments for clones with and without integration of the LoxP sites, respectively. (F) PCR genotyping from the mating of heterozygote mice to obtain GAKfl/fl mice. (G) Genotyping of the Cre allele in the Cre+/−, GAKfl/fl mice. IL1 is the interleukin 1 control for the PCR analysis.
For the conditional knockout of GAK, the targeting construct for the GAK conventional knockout was used as a backbone vector and pBS246 loxP cassette plasmid was used to obtain loxP sequences. The following cloning strategy was used. The 1.3-kb fragment containing partial exon1 and neomycin gene was subcloned between two loxP sites in pBS246. The partial exon1 was then exchanged with full exon1 plus one loxP site that was cloned in the pBS246 to produce the loxP–full exon1–loxP–neomycin–loxP sequence. Subsequently, the partial exon1 and neomycin cassette of the conventional targeting construct was replaced by the loxP–full exon1–loxP–neomycin–loxP sequence (Figure 1D).
For both the conditional and conventional GAK knockout, the linearized targeting construct was then introduced into the embryonic stem (ES) cell line from 129S6SvEv mouse strain. The transfectants were first selected by a G418 resistance cassette, followed by Southern Blot analysis (Figure 1, B and E). To detect the ES clone with the desired loxP genomic integration site, PCR was done to detect the 700-base pair band for the flox-exon1-flox gene product (Figure 1E).
Generation and Genotyping of GAK Knockout Mice
To obtain the GAK knockout mice, the positive ES cell clone was microinjected into blastocysts and then reimplanted into pseudopregnant foster females. The resulting chimeras were bred with C57BL/6 mice to obtain F1 offspring, and germ line transmission was determined by Southern blot analysis and/or PCR.
All Cre mouse lines were purchased from Jackson Laboratory (Bar Harbor, ME). For the conditional knockout of GAK in the brain, liver, and skin, we used transgenic mice carrying the gene encoding Cre recombinase under the control of nestin, albumin, and keratin promoters, respectively. To introduce the Cre gene to GAK flox mouse, Cre+/− males were bred with GAKfl/fl females, and then Cre+/−, GAKfl/+ males were bred with GAKfl/fl females to get Cre+/−, GAKfl/fl (Supplementary Figure S1). The Cre mice lines used in this study were as follows: Nes-Cre; B6.Cg-Tg(Nes-Cre)1Kln/J (Jax Mice Stock no. 003771), Alb-Cre; B6.Cg-Tg(Alb-Cre)21Mgn/J (Jax Mice Stock no. 003574), Krt14-Cre, STOCK Tg(KRT14-Cre)1Amc/J (Jax Mice Stock no. 004782), ERT-Cre; and STOCK Tg(Cre/Esr1)5Amc/J (Jax Mice Stock no. 004453).
Genotyping of the offspring was performed by PCR or Southern blot analysis using DNA from tail snips in the adult mice or DNA extracted from embryos. For the conventional knockout mice, the PCR products gave a wild-type band (747 base pairs) and the neomycin gene (1626 base pairs; Figure 1C). For the conditional knockout, we screened the offspring for both flox and Cre alleles by PCR analysis (Figure 1, F and G).
All mice were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Histological Studies and Immunostaining
The mouse embryos, tissue samples and organs were fixed in 10% phosphate-buffered Formalin. For fixation of adult mouse brain, animals were perfused with cold PBS followed by fixative (10% phosphate-buffered Formalin). The brains were then dissected out from the skull and immersed in the same fixative overnight at room temperature. Paraffin sections (5 μm) were prepared and then deparaffinized by xylene for further processing. The sections were stained with H&E for histological analysis (Histoserv, Gaithersburg, MD).
For immunostaining, sections were blocked for 1 h at room temperature with PBS containing 0.1% bovine serum albumin/5% normal goat serum. The sections were then treated overnight at 4°C with the following antibodies: anti-auxilin polyclonal (generated in our laboratory), anti-GAK polyclonal antibody (Lee et al., 2005), anti-TGN38 (gift from G. Banting), anti-clathrin heavy chain (X22, Affinity BioReagents, Golden, CO), anti-GM130 (BD Biosciences, San Jose, CA), anti-keratin 5 (Abcam, Cambridge, MA), anti-keratin1 (Abcam), anti-filaggrin (Covance Laboratories, Madison, WI), anti-loricrin (Covance), and anti-nestin (Abcam). The sections were then treated with fluorescent secondary antibodies including Alexa Fluor 594–, 488–, or 568–conjugated goat anti-rabbit or goat anti-mouse IgG (Molecular Probes, Eugene, OR). Slides were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO) to stain the nucleus. Rabbit or mouse IgG was applied in place of the primary antibody as a negative control. After washing, the coverslips were mounted using a ProLong Antifade kit (Molecular Probes). To detect glycogen in the liver and lipid in the skin samples, periodic acid-Schiff (PAS) Staining System (Sigma-Aldrich), and oil red-O staining (slides prepared by Histoserv, Gaithersburg, MD) were used, respectively. TUNEL assay was carried out on paraffin section using Apo-bromodeoxyuridine (BrdU) in situ DNA fragmentation assay kit (MBL International, San Diego, CA; Medical Biological Laboratories). The images were obtained using a Leica MZ FL III fluorescence stereomicroscope (Deerfield, IL) or Zeiss LSM 510 confocal microscope (Thornwood, NY).
Derivation and Treatment of MEFs
Mouse embryos (11 d old) in 2 ml PBS were passed through a syringe with 18-gauge needle four to six times and then transferred into a 15-ml centrifuge tube. PBS was added to the tube to make a volume of 5 ml. After centrifugation, the supernatant was removed, and the pellet was resuspended in 2 ml cell culture medium (DMEM supplemented with 10% fetal bovine serum, 1× None Essential Amino Acids (Biofluids, Rockville, MD), 1× Diamond Vitamin Solution (Biofluids), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin). Each embryo was spread to each well of a 12-well plate precoated with 0.2% gelatin. Cells were maintained in an incubator, and fresh medium was routinely added until we obtained a stable cell line. The stable MEF line was cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were infected twice with adenovirus expressing Cre recombinase (Microbix, Toronto, Ontario) for 7 d before they were seeded into glass chambers for experiments. Cells then were transfected with green fluorescent protein (GFP)-constructs using Fugene HD (Roche Applied Science, Indianapolis, IN). The following plasmids were used: GFP-clathrin light chain (Wu et al., 2003), GFP-AP2 (Wu et al., 2003), and GFP-Eps15 and GFP-Eps15R.
Western Blotting
To check the expression level of auxilin and GAK at the embryonic stage, the heads of embryonic mice were homogenized in PBS immediately after being killed. When GAK was knocked out of the liver, the liver was taken from the newborn mouse and washed several times with PBS to remove the blood. SDS-PAGE was performed on the brain or liver cytosol using 4–20% gels (Invitrogen, Carlsbad, CA), and then the proteins were transferred onto nitrocellulose and immunoblotted with anti-GAK and anti-auxilin polyclonal antibodies generated in our laboratory. The Western blots performed on the MEFs were done using either anti-GAK polyclonal (Lee et al., 2005) or anti-GAK monoclonal (MBL) antibody. The proteins were transferred to nitrocellulose membrane (Amersham, Piscataway, NJ) by using Trans-Blot semidry system (Bio-Rad, Richmond, CA). The same protein was loaded on each lane using the Bradford assay (Sigma) and where indicated, anti-beta actin monoclonal (Abcam) was loaded as an internal control. The protein bands were detected by using the ECL blotting substrate (Pierce, Rockford, IL) to HRP-conjugated secondary antibodies or infrared secondary antibodies (Li-Cor Bioscience, Lincoln, NE). These Western blots were imaged using the ChemiImager densitometer (Alpha Innotech, San Leandro, CA) and the Odyssey infrared detection system (Li-Cor Bioscience), respectively.
Induction with Tamoxifen
To express Cre recombinase in the adult mice, a tamoxifen-inducible Cre-expressing mouse was bred into a GAKfl/fl background. Tamoxifen was injected intraperitoneally with 1 mg tamoxifen (Sigma) dissolved in corn oil (Sigma) at a concentration of 10 mg/ml for 5 consecutive days into 6-wk-old adult Cre−/−, GAKfl/fl and Cre+/−, GAKfl/fl mice (Hayashi and McMahon, 2002).
Transmission Electron Microscopy
For ultrastructural analysis, organs were fixed in 1% paraformaldehyde/2.5% glutaraldehyde (Sigma) in 130 mM phosphate buffer (pH 7.35) overnight. After embedding in Epon, the thin sections were stained with 2% uranyl acetate and Reynold's lead citrate solution. Micrographs were taken by using a JEOL EX II electron microscope at 80 kV (Peabody, MA). Quantitative analyses were performed to determine the extent of fragmentation of the Golgi. The Golgi fragmentation was defined as disconnected, small and round Golgi fragments dispersed in the cell (Liazoghli et al., 2005). Golgi fragmentation was blindly scored by two individuals who independently counted the fragmented Golgi in electron micrographs, which were numbered so that the genotypes remained unknown to the individuals during the scoring.
RESULTS
Generation of GAK Knockout Mice
To generate conventional GAK knockout mice, homologous recombination was used to disrupt the GAK gene. As shown in Figure 1A, exon 1 was disrupted by insertion of the neomycin gene. Heterozygous ES cells were screened using genomic Southern blot analysis (Figure 1B). Two positive clones were obtained from screening 200 ES cells. These clones were then injected into C57BL/6 blastocysts to establish chimeric mice.
Heterozygous GAK mutant mice, which were screened by PCR analysis, were obtained from crossing chimeric mice with C57BL/6 mice (Figure 1C). These mice were healthy and fertile and lived a normal life span. To produce homozygous GAK knockout mice (GAK−/−), the heterozygous mice were mated, and the genotypes of the offspring were analyzed by PCR analysis. When we genotyped 3-wk-old mice in four litters consisting of 49 mice, there were only GAK+/+ and GAK+/− mice (Table 1). We also found no knockout mice (GAK−/−) at E5.5 (embryonic day 5.5) when 21 embryonic mice were genotyped from two litters. These results show that disruption of the GAK gene is embryonic lethal. Moreover, because the conventional GAK knockout embryos died early in gestation, these embryos either did not implant or died early in development. Therefore, we had to make a conditional GAK knockout mouse.
Table 1.
Genotyping result from conventional GAK knockout mouse
| Genotype |
|||||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | Litters | Total no. of pups | |
| Age | |||||
| P21 | 13 | 36 | — | 4 | 49 |
| E5.5 | 7 | 14 | — | 2 | 21 |
The Cre-lox recombination system was used to produce a disruption in the GAK gene as shown in Figure 1D. To generate the knockout mouse, we designed a vector with three loxP sites, two of them flanking exon1 and the third downstream of the neomycin gene. After injection of this construct into blastocysts, we obtained five ES cells that had integrated the neomycin gene, but PCR analysis showed that only one of these clones had integrated all three loxP sites (Figure 1E). This clone was injected into C57BL/6 blastocysts to obtain chimeric mice. Mice heterozygous for the GAK allele were then obtained by crossing the chimeric mice with C57BL/6 mice. GAKfl/fl mice, which were obtained from the breeding of the heterozygote mice (Figure 1F), showed no phenotypic differences from the wild-type mice. The GAK gene was disrupted by mating the GAKfl/fl to mice expressing Cre recombinase (Cre+/−). To control the expression of Cre recombinase in different tissues, we used the nestin promoter to express Cre recombinase in the brain, the albumin promoter to express Cre recombinase in the liver, the keratin-14 promoter to express Cre recombinase in the epidermis, and a tamoxifen-inducible form of Cre recombinase to knockout GAK in adult mice.
GAK Depletion in the Brain
GAK was turned off in the developing brain by using the nestin promoter to express Cre recombinase in neuroepithelial stem cells. Nestin, which is an intermediate filament protein, is expressed in cells as early as E7 and pronounced expression is seen from E10 to E12, shown in mice expressing GFP under the nestin promoter. Peak expression is around E14.5 (Mignone et al., 2004). Genotyping of embryos at E18 showed that about one-quarter of the mice (seven of 33 pups from five litters) were Cre+/−, GAKfl/fl, the genotype of the brain-specific GAK knockout mice. This ratio is consistent with Mendelian genetics (see mating scheme in Supplementary Figure S1). Newborn GAK knockout mice showed no obvious phenotypic or behavioral differences from its littermates. However, by the third day, some of the mice started to act sluggish and these mice died within a day. The genotype of the nonviable mice was Cre+/−, GAKfl/fl; i.e., brain-specific GAK knockout mice. Consistent with this observation, we found no GAK knockout mice when we genotyped 39 mice from eight litters at 3 wk of age (Table 2). This shows that expression of GAK during brain development is essential for mouse viability.
Table 2.
Genotyping result from conditional GAK knockout mouse
| Genotype |
||||||
|---|---|---|---|---|---|---|
| Cre−/− GAKfl/+ | Cre−/− GAKfl/fl | Cre+/− GAKfl/+ | Cre+/− GAKfl/fl | Litters | Total no. of pups | |
| Cre line | ||||||
| Nestin | 9 | 16 | 14 | — | 8 | 39 |
| Albumin | 18 | 10 | 16 | — | 8 | 44 |
| Keratin 14 | 9 | 7 | 10 | — | 5 | 26 |
Mice were genotyped at 3 wk old (P21).
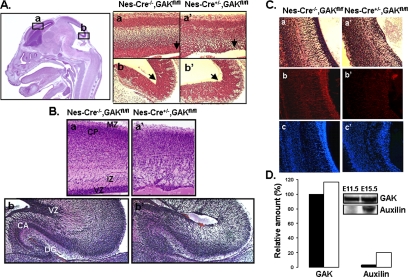
Brains of both embryonic and newborn GAK knockout mice were examined for histological defects. Figure 2A shows the H&E staining of brains from Cre−/−, GAKfl/fl and Cre+/−, GAKfl/fl littermates at E15.5. The Cre−/−, GAKfl/fl mice still express GAK and had no apparent histological defects. On the other hand, brains from the Cre+/−, GAKfl/fl mice, which have a disrupted GAK gene in their brain tissue, showed marked morphological changes in the ventricular zones of both the forebrain and the hindbrain, consistent with the expression pattern of nestin. The cells in this zone were not as dense as in the control mice. With further development of the embryo, the neuroepithelial cells in the ventricular zone divide and migrate to the peripheral layers where they differentiate into neurons. Consistent with this development sequence, the newborn (P1) brain-specific GAK knockout mice showed a dramatic loss of cells in the cerebral cortex, which gave them a spongy appearance along with a dramatic reduction in the thickness of the ventricular zone (Figure 2B). The hippocampus also showed a marked reduction in cell density in both the dentate gyrus and cornu ammonis. The neuronal cortex did not show significant apoptosis when newborn brains were stained with BrdU even though propidium iodide staining of the same area showed a reduction in cell number (Supplementary Figure S2A). These results suggest that the neuroprogenitor cells are not proliferating in the ventricular zone. Further support of this decrease in progenitor cells come from nestin staining in the GAK knockout mice compared with control mice (Figure 2C, b and b′). Nestin staining was dramatically reduced in the ventricular zone of GAK knockout mice, whereas staining with DAPI of the same histological sections showed much fewer cells in this layer. Therefore, these cranial sections showed that depletion of GAK causes a dramatic decrease in the population of progenitor cells in the CNS.
Figure 2.
Morphological effects of knocking-out GAK from neuroepithelial cells. (A) H&E staining of a sagittal section of the head obtained from E15.5 d mice. The boxed regions are higher magnification of the control (a and b) and conditional knockout (a′ and b′) mice. Arrow indicates ventricular zones in the forebrain (a) and hindbrain (b). (B) H&E staining of brain sections obtained from mice at P1. Images are of the coronal section of the cerebral cortex (a and a′) and sagittal section of the hippocampus (b and b′) from control (a and b) and knockout (a[prime and b′) mice. The regions labeled are as follow: VZ, ventricular zone; IZ, intermediate zone; CP, cortical plate; MZ, marginal zone; DG, dentate gyrus, and CA, cornu ammonis. (C) The same section of the cerebral hemisphere stained with H&E (a and a′), anti-nestin antibodies (b and b′), and DAPI (c and c′). Compared with the control (a–c), the knockout mice (a′–c′) show abnormal proliferation and migration of the neuroprogenitor cells. (D) Western blot analysis of auxilin and GAK in mice brains during development. Cell lysates were prepared from the heads of E11.5 and E15.5 mice. Levels were normalized to that of GAK at E11.5.
It was unexpected that knocking out GAK in the brain would produce such a profound effect on brain development because auxilin, along with GAK, is present in neurons. However, in analyzing the protein levels of these two cochaperones by Western blots, we found that at E11.5, neurons are primarily expressing GAK (Figure 2D), the same time in brain development that the nestin promoter is turning on Cre recombinase and disrupting the GAK gene. Therefore, even though by day E15.5 the levels of auxilin have markedly increased, disruption of the GAK gene may have already produced profound histological changes in the brain. The requirement for GAK may therefore occur simply because auxilin is produced at relatively low levels during the early stages of the developing mouse brain. However, another possible explanation is that GAK, in particular the kinase domain of GAK, plays a specific role at the TGN that cannot be replaced by auxilin.
GAK Depletion in the Liver
To determine the effect of depleting GAK from liver, we bred the GAKfl/fl mice to mice expressing Cre recombinase under the control of the albumin promoter. As the liver develops, the hepatoblasts differentiate into hepatocytes and biliary epithelial cells. The albumin promoter, which is active in both hepatoblasts and hepatocytes, shows a 20-fold increase in transcriptional activity from E9.5 to E12.5. The level of albumin then continues to increase as the liver develops and simultaneously the biliary tree and the hepatic bile duct form (Cascio and Zaret, 1991).
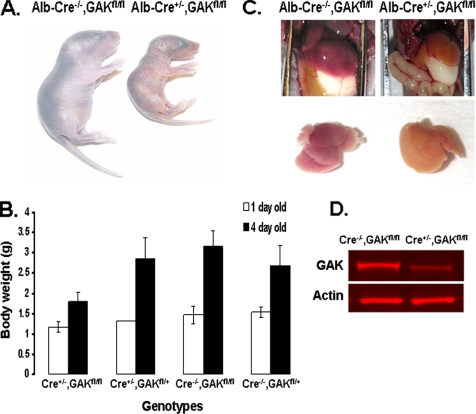
Genotyping of newborn mice showed that liver-specific GAK knockout mice were present at birth, but by 3 wk of age, no mice with this genotype were viable (Table 2). Initially, the liver-specific GAK knockout mice were indistinguishable from their littermates; i.e., the knockout mice all developed milk spots and showed no behavioral differences. However, a day or two after birth, the liver-specific GAK knockout mice were readily apparent in that they became jaundiced and were much smaller than the rest of the litter (Figure 3A). As shown in Figure 3B, the knockout mice showed a relatively small increase in weight compared with the rest of the litter during the first 4 d after birth, and by the fifth day after birth, the liver-specific GAK knockout mice all died. The livers of these mice had a yellowish coloration, while their size was similar to that of the control mice (Figure 3C). Even though the conditional GAK knockout newborn mice showed no obvious phenotype, the protein level of GAK in their liver was markedly reduced (Figure 3D). Quantitation of the Western blot shows that there was one-third as much GAK in the knockout liver compared with the control. Although there was a reduction in GAK expression, livers from knockout newborn mice did not show significant apoptosis (Supplementary Figure S2B). Furthermore, cell counts of liver preparations showed no significant difference between the knockout and control mice (Supplementary Figure S2C). These results show that even though GAK levels were partially reduced at birth and the livers were not markedly apoptotic, the reduction in GAK in the liver was sufficient to cause mortality shortly after birth.
Figure 3.
Phenotype of 4-d-old albumin-Cre conditional knockout mouse. (A) Appearance of the control and GAK knockout mice. (B) Weight of the different genotypes of mice at P1 and P4 obtained from the mating of albumin-Cre+/−, GAKfl/+ to Cre−/−, GAKfl/fl mice. At day 1, the weights were obtained from the average of three mice for the Cre+/−, GAKfl/fl genotype, three mice for the Cre+/−, GAKfl/+ genotype, six mice for the Cre−/−, GAKfl/fl genotype, and six mice for the Cre−/−, GAKfl/+ genotype. At day 4, the weights were obtained from the average of 15 mice for the Cre+/−, GAKfl/fl genotype, 12 mice for the Cre+/−, GAKfl/+ genotype, 9 mice for the Cre−/−, GAKfl/fl genotype, and 10 mice for the Cre−/−, GAKfl/+ genotype (C) Peritoneal cavity showing the livers and dissected livers from control and knockout mice. (D) Western blot of GAK probed with anti-GAK and anti-actin antibodies of liver preparations from Cre−/−, GAKfl/fl and Cre+/−, GAKfl/fl newborn mice.
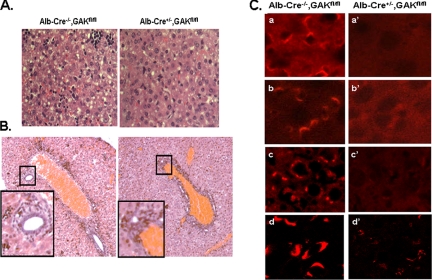
Histochemical analysis showed that the livers from the knockout mice had a morphology that was very different from that of control mice. In particular, H&E staining (Figure 4A) of the liver cells from the knockout mice appeared more homogeneous than those of the control mice, suggesting that hepatoblasts in the livers of the knockout mice did not differentiate as much as control hepatoblasts during embryonic development. In addition, detailed examination of the biliary tree showed that the bile ducts were not as well organized in the livers of the knockout mice (Figure 4B), which probably caused the jaundiced appearance of the GAK knockout mice.
Figure 4.
Histology of the livers of 4-d-old control and albumin-Cre conditional knockout mice. (A) H&E staining of liver section from control and GAK knockout newborn mice. (B) H&E staining of biliary duct network in liver from control and GAK knockout mice. Inset is the enlargement of the biliary duct. (C) Confocal images of sections from liver of control and GAK knockout mice immunostained with the following antibodies: GAK (a and a′), clathrin (b and b′), TGN38 (c and c′), and GM130 (d and d′).
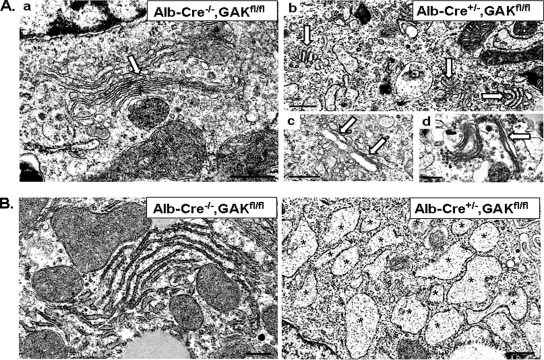
Immunostaining of the liver was also performed on the histological sections. As expected, the livers from the knockout mice showed a marked decrease in GAK staining compared with control livers (Figure 4C). In addition, comparison of the immunostaining of control and knockout livers showed that clathrin and TGN38, two proteins associated with the TGN, were markedly reduced, as well as GM130, a cis-Golgi matrix protein. To better visualize the morphological changes, we examined electron micrographs of liver samples from control and liver-specific GAK knockout mice. The Golgi cisternae appeared much more fragmented in the knockout hepatocytes than in the control hepatocytes (Figure 5Ab). Specifically, when the Golgi cisternae were analyzed, 74% (74/101) of the Golgi in the knockout hepatocytes showed this fragmented phenotype, whereas only 10% (5/52) of the Golgi in the control hepatocytes showed this phenotype. Although fragmented Golgi was the major phenotype observed in the hepatocytes from the knockout mice, some cisternae in the hepatocytes were distended (Figure 5Ac) and other cisternae seemed elongated (Figure 5Ad).
Figure 5.
Electron micrographs of liver sections from control and albumin-Cre GAK conditional knockout 4-d-old mice show morphological changes due to GAK depletion. (A) EM images of liver sections showing Golgi (labeled with white arrows) in control (a) and GAK knockout (b–d) cells. Golgi appears fragmented in b, swollen in c, and elongated in d. (B) Rough ER sections of liver in control and knockout liver cells. Rough ER (marked with asterisks) appears swollen in the knockout liver cells. Scale bar, 500 nm.
One unexpected observation from the electron micrographs was that there was dramatic swelling of the rough ER in the knockout livers (Figure 5B, see asterisks). This unusual phenotype has been reported to occur when cells are treated with brefeldin A (BFA) for long periods of time (Alvarez and Sztul, 1999) or with both BFA and the protein kinase A inhibitor, H89 (Puri and Linstedt, 2003). Therefore, the swollen rough ER might be due to a disruption of the Golgi caused by GAK depletion. Liver sections from 4-d-old mice were also examined for glycogen storage and for neutral lipids. The livers of the GAK knockout mice failed to accumulate glycogen, which indicates either a breakdown in the glycogen synthesis pathway or increased degradation of glycogen (Supplementary Figure S3A). There was no increase in oil Red O staining of the knockout livers, indicating overall no significant accumulation of neutral lipids in these livers (Supplementary Figure S3B). This suggests that the yellow color of the liver was due to a buildup of bilirubin, probably due to malformed bile ducts.
GAK Depletion from Epidermis
The Cre−/−, GAKfl/fl mice were also bred to mice that express Cre recombinase under the control of the keratin 14 promoter. Keratin 14 is present in the progenitor cells that differentiate to form the epidermis. As cells from this layer differentiate, they migrate to the cell surface where they become enucleated and then slough off. If a protein essential for differentiation or viability of the epithelial cells is lacking, it would produce a mouse with a defective epidermis, resulting in loss of the permeability barrier that is essential for maintenance of body fluids.
In fact, at 3 wk of age, there were no viable skin-specific GAK knockout mice expressing Cre recombinase under the control of the keratin 14 promoter (Table 2). When mice were examined immediately after birth, all of the mice appeared healthy, but shortly thereafter, the skin-specific GAK knockout mice took on a shiny wrinkled appearance as if they were dehydrating (Figure 6Aa). This defective epidermal barrier was confirmed in Figure 6A by using a dye permeability assay (Hardman et al., 1998). All of the skin-specific GAK knockout mice died within hours after birth with a phenotype consistent with a defective epidermal barrier (Segre et al., 1999).
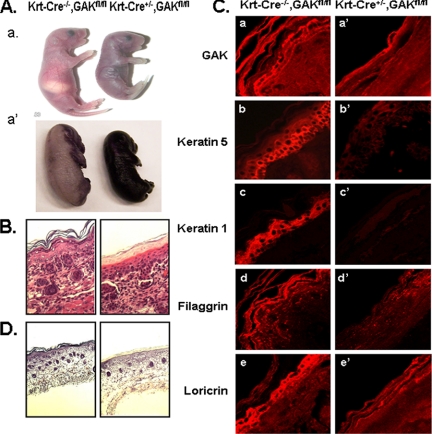
Figure 6.
Phenotype of newborn control and keratin 14-Cre GAK knockout mice. (A) Comparison of control and keratin-Cre conditional GAK knockout mice at P1 (a). Defect in epidermis barrier of GAK knockout mice (E17.5) shown by dye permeability assay (a′). (B) H&E staining of epidermis from control and keratin-Cre GAK knockout newborn mice. (C) Confocal images of immunostained control and GAK knockout epidermis from newborn mice. Epidermis was stained using anti-GAK (a and a′), anti-keratin 5 (b and b′), anti-keratin 1 (c and c′), anti-fillagrin (d and d′), and anti-loricrin (e, e′) antibodies. (D) Oil red O staining of neutral lipids in the epidermis of control and GAK knockout mice.
Consistent with the observed defect in the epidermis barrier, the morphology of the skin from the skin-specific GAK knockout mice showed profound histological changes. H&E staining showed that the GAK knockout epidermis lacked the extensive differentiation observed in skin from control mice (Figure 6B). Specifically, it lacked the multiple cell layers that normally make up the epidermis, and in addition, the epidermal corneum was not well developed, which probably explains the desiccation of the knockout mice. Immunostaining of different marker proteins confirmed the lack of differentiation of the epidermal layers in the GAK knockout mouse (Figure 6C). First, by immunostaining for GAK, we confirmed that GAK expression was markedly reduced throughout the epidermis in the knockout mice. Second, keratin 5 and keratin 1, cytoskeletal proteins of the basal and suprabasal epidermis, respectively, were markedly reduced, as were the suprabasal proteins, fillagrin and loricrin. In addition, there was a reduction in neutral lipids in the epidermis of the skin-specific knockout mice (Figure 6D). All of these morphological changes suggest that when keratinocytes lack GAK, the epidermis does not undergo normal differentiation.
GAK Knockout in Adult Mice
The conditional GAK knockout mice studies showed that GAK was essential for the proper development of the brain, liver, and skin but did not show whether GAK had an essential role in the adult mouse. To answer this question, the GAKfl/fl mice were bred to mice expressing tamoxifen-inducible Cre recombinase. Tamoxifen binds to the estrogen receptor ligand-binding domain conjugated to the Cre recombinase, and this causes the translocation of Cre recombinase into the nucleus. As expected, these mice showed no phenotypic defects in the absence of tamoxifen.
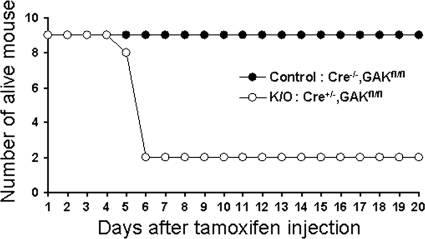
To disrupt the GAK gene in adult mice, mice at 6 wk of age were injected intraperitoneally with tamoxifen (1 mg/mouse) for 5 consecutive days (Hayashi and McMahon, 2002). As shown in Figure 7, the mice with the Cre−/−, GAKfl/fl genotype were all viable, whereas seven of nine mice with the Cre+/−, GAKfl/fl genotype died by the sixth day after the start of injections. These results show that GAK is not only essential for developing mice, but is also essential protein for the viability of adult mice.
Figure 7.
Knocking out GAK in the adult mouse causes lethality. GAK was disrupted in the 6-wk-old tamoxifen-inducible GAK knockout mice by injecting tamoxifen. Mice viability is plotted as a function of time measured 5 d after injecting nine control and nine knockout mice.
Characterization of GAK-disrupted MEFs
Having observed that knocking out GAK caused lethality in both developing and adult mice, we next examined the cause of this lethality by making MEFs from the GAKfl/fl mice. The MEFs were then treated with adenovirus expressing Cre recombinase to disrupt the GAK gene. Thus far, as detailed in the Introduction, knocking down GAK using RNAi has given different phenotypes, depending on the method used to deplete GAK. We were therefore interested in characterizing MEFs derived from conditional knockout mice because they do not have the problems that occur in cells using RNAi, namely incomplete depletion of GAK and a possible knocking down of extraneous proteins.
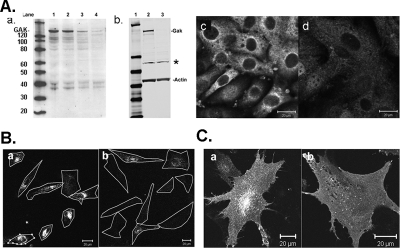
MEFs that were generated from mice with the GAKfl/fl genotype were treated with adenovirus expressing Cre recombinase to disrupt the GAK gene in these cells. A GAK knockdown phenotype can then be observed after the remaining GAK in the cell is degraded. GAK protein levels in the adenovirus-treated cells were measured by Western blots. As shown in Figure 8A, the GAK level in the adenovirus-treated cells decreased over time to <5% of that present in control MEFs (Figure 8A). To ensure that no truncated fragments of GAK were being produced in the adenovirus-treated MEFs, the immunoblot was probed using a polyclonal antibody against the C-terminal J-domain of GAK instead of the anti-GAK mAb (Figure 8Ab). This blot shows that not only was there no full-length GAK in the adenovirus-treated cells, but there were also no truncated GAK gene products, although there was one nonspecific band (see asterisk) both in the control and in the adenovirus-treated cells (Figure 8Ab, lanes 2 and 3). Immunostaining the MEFs with anti-GAK polyclonal antibody confirmed the absence of GAK; the residual staining might be due to the protein associated with the nonspecific band observed in the Western blot (Figure 8Ac and Ad).
Figure 8.
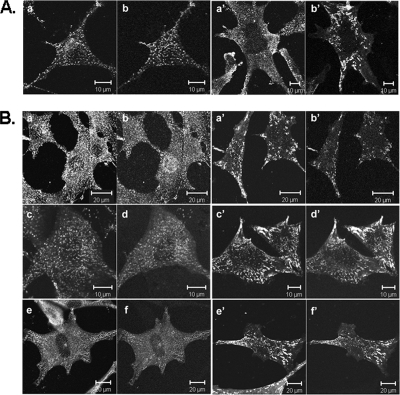
Disruption of the GAK gene in MEFs inhibits transferrin uptake. (A) Western blots of GAK in cytosol of MEFs showing expression levels before and after treatment with adenovirus expressing Cre recombinase. The Western blots were immunostained with anti-GAK antibodies, using either an mAb (a) or a polyclonal antibody (b). The same amount of protein was loaded in each lane. Gel b is also stained with anti-actin antibody. The immunoblots were developed either by using the ECL method (a) or Odyssey infrared imaging method. In a, the lanes are as follows: lane 1, molecular marker; lane 2, GAK control; lane 3, day 3 after adenovirus treatment; lane 4, day 7 after adenovirus treatment; and lane 5, day 11 after adenovirus treatment. In b, the lanes are as follows: lane 1, molecular weight marker; lane 2, GAK control; and lane 3, 11 d after adenovirus treatment. This Western blot was also immunostained for actin. The asterisk in panel b indicates a nonspecific band recognized by the GAK polyclonal antibody. (c) Immunostaining of GAK in control (b) and adenovirus-treated MEFS (d). (B) Transferrin internalization is inhibited in the GAK knockout MEFs. Control or GAK knockout MEFS were incubated with rhodamine-conjugated transferrin from 15 min at 37°C. The cell outlines are shown. (C) Confocal images of GFP-transferrin receptor in control (a) and knockout (b) MEFs. Scale bar, 20 μm.
The effect of GAK depletion on receptor-mediated endocytosis was determined by examining transferrin uptake. As expected, based on the RNAi studies, transferrin uptake was almost completely blocked in the GAK-disrupted MEFs (Figure 8B). Consistent with this block in endocytosis, the transferrin receptor had a diffuse appearance on the plasma membrane with no apparent internalized pool of receptor in the GAK-depleted cells (Figure 8C). In contrast, in control cells, transferrin receptor showed a punctate appearance on the plasma membrane, and in addition, there was a large internalized pool of receptor.
Imaging of the plasma membrane showed that disrupting the GAK gene profoundly altered the structure of the CCPs. Figure 9A shows that the plasma membrane was no longer covered with clathrin puncta, but instead the clathrin appeared mostly cytosolic. Furthermore, the AP2 no longer colocalized with the clathrin in small puncta, but instead was clustered on the plasma membrane. These AP2 clusters contained the clathrin accessory proteins, epsin, Eps15, and Eps15R (Figure 9B), whereas in the control MEFs these proteins did not have this clumped appearance. By transfecting the MEFs with GFP-clathrin light chain, fluorescence recovery after photobleaching experiments (FRAP) showed that, in contrast to the clathrin puncta of the plasma membrane of control MEFs, there was no clathrin exchange on the few remaining clathrin puncta present on the plasma membrane of the adenovirus-treated cells (Supplementary Figure S4).
Figure 9.
Alteration in the localization of clathrin, clathrin adaptors, and accessory proteins in GAK knockout MEFs. (A) Confocal images of clathrin and AP2 in control (a and b) and GAK knockout (a′ and b′) MEFs. Cells, transfected with GFP-AP2, were immunostained for clathrin. Cells were simultaneously imaged for clathrin (a and a′) and GFP-AP2 (b and b′). (B) Localization of AP2 and accessory proteins in control (a–f) and GAK knockout (a′-f″) MEFs. Cells were transfected with either GFP-Eps15 (c, d, c′, and d′) or GFP-Eps-15R (e, f, e′, and f″) followed by fixation and immunostaining for AP2. Cells were stained for AP2 (a, a′, c, c′, e, and e′) and epsin (b and b′). Cells were simultaneously imaged for AP2 and epsin (a, b, a′, and b′), AP2 and GFP-Eps15 (c, d, c′, and d′), and AP2 and GFP-Eps15R (e, f, e′, and f′).
DISCUSSION
Although GAK and Hsc70 certainly play a role in uncoating CCVs both in vivo and in vitro, it has not yet been established whether they are actually required for uncoating CCVs in vivo. By hydrolyzing phosphatidylinositol (4,5)-bisphosphate, synaptojanin also plays a role in uncoating CCVs, and it is possible that synaptojanin alone can carry out uncoating even in the absence of GAK and Hsc70 activity. There is also evidence that GAK and Hsc70 have chaperone activity that may be required for both clathrin and APs to bind to membranes to form pits. However, again it is not clear whether these chaperone functions of Hsc70 and GAK are essential in vivo. The role of GAK and Hsc70 in clathrin-mediated endocytosis has been studied using RNAi, but contradictory results have been obtained. Our laboratory (Lee et al., 2005) found that GAK and Hsc70 were required for clathrin-mediated endocytosis in general and for the binding of clathrin and APs to membranes in particular, whereas two other laboratories found that GAK depletion did not affect clathrin and AP binding (Zhang et al., 2005;Kametaka et al., 2007). To better understand the role of GAK in the cell, we decided that constructing a GAK knockout mouse was probably the best way to determine whether GAK in conjunction with Hsc70 has an essential function in mice.
The De Camilli laboratory has made mouse knockouts of several neuronal specific proteins involved in synaptic vesicle trafficking including synaptojanin 1, amphiphysin 1, phosphatidylinositol phosphate kinase type Iγ, and dynamin-1 (Cremona et al., 1999;Di Paolo et al., 2002, 2004; Ferguson et al., 2007). None of these knockouts was embryonic lethal nor did any of them cause developmental defects. Rather they generally caused defects in synaptic transmission. Mouse knockouts of the β-subunit of AP2 or the μ1A subunit of AP1 produced embryonic lethality suggesting that these subunits are absolutely required for clathrin-mediated endocytosis (Zizioli et al., 1999;Mitsunari et al., 2005). However, conditional knockout mice were not made for these subunits so their requirement during embryonic development or on adult mice is not yet proven.
In our study, because disruption of GAK in germ line cells caused embryonic lethality, we constructed a conditional GAK knockout mouse. When GAK expression was disrupted by expressing Cre recombinase, our results showed that GAK was essential for the development of the brain, liver, and skin; in all cases, the knockout newborns were viable but died within 5 d after birth. In fact, disruption of the GAK gene in the skin caused death within hours after birth, apparently due to desiccation of the mice. This is very similar to the phenotype observed when the Klf4 transcriptional factor, which is highly expressed in differentiating layers of epidermis, is disrupted in the mouse (Segre et al., 1999).
When GAK was disrupted in the brain and the liver, the mice were viable for several days. The brain-specific GAK knockout mice appeared healthy with no obvious defects for the first 2 d after birth, but they then became sluggish and died. Histological studies of their brains clearly showed developmental defects with a marked loss of cells in the ventricular zone of the brain. However, it is not clear what causes the lethality in these mice, especially because nestin is expressed in endocrine and vascular endothelial cells in addition to neurons (Takahashi et al., 2008). The liver-specific GAK knockout mice were distinguishable from their littermates within a day or two after birth in that they did not gain weight. The knockout mice then became jaundiced and died by the fourth day after birth. In addition to developmental defects in the liver, these knockout mice also had defective glycogen storage. Again, the specific cause of death is not known, but it is important to note that even though the albumin promoter is turned on very early in the development of the liver, unlike nestin it is not a very strong promoter (Postic and Magnuson, 2000), inducing only modest expression of Cre recombinase until after birth. Therefore GAK expression may not be completely blocked in the developing liver, but the expression levels are reduced enough to cause major problems. GAK was also essential for viability of the adult mouse as shown by injecting tamoxifen in order to obtain nuclear Cre recombinase expression. Although the cause of the lethality induced by GAK disruption in the adult is not known, these results show that GAK is not only required for development but also has essential functions in the adult mouse.
Having established that GAK has an essential function in both developing and adult mice, the next question is what is this essential function. The function of GAK on the cellular level was determined by characterizing MEFs in which the GAK gene was conditionally disrupted by Cre recombinase. The MEFs had very few clathrin puncta on their plasma membrane and showed a block in transferrin uptake; rather than being internalized, the transferrin receptor was diffusive on the plasma membrane. We also found that AP2, epsin, Eps15, and Eps15R all had an aberrant distribution, clustering together on the plasma membrane without clathrin. However, this effect that does not occur in HeLa cells when clathrin rather than GAK is depleted by RNAi (Motley et al., 2003), so it was not just due to the absence of clathrin. Therefore our results suggest that in the absence of GAK a number of proteins associated with the CCPs bind abnormally to the plasma membrane, thereby preventing CCP formation and blocking endocytosis.
Our results with the MEFs are consistent both with our GAK RNAi studies in HeLa cells and with our studies on the effect of depleting or mutating auxilin in yeast, C. elegans, and Drosophila; all of these studies showed that auxilin and GAK play a major role in clathrin-mediated endocytosis (Eisenberg and Greene, 2007). Furthermore, the MEF data strongly suggest that the developmental defects caused by the conditional GAK knockout are due to a defect in clathrin-mediated endocytosis. Such a defect could inhibit the uptake of an essential transcription factor necessary for the development of a specific tissue. This would be consistent with the phenotype observed when auxilin was mutated in Drosophila; this mutation caused a disruption in the notch-signaling pathway due to a defect in the endocytosis of the delta ligand (Hagedorn et al., 2006).
Our characterization of the GAK knockout MEFs, as well as the GAK knockout mouse, now provides strong evidence that GAK is not only involved in uncoating clathrin but also acts to chaperone clathrin and adaptors so that they can rebind to the plasma membrane to form new CCPs. Furthermore, the MEF data support the view that GAK and Hsc70 are required for the clathrin exchange that occurs during CCP invagination and constriction (Wu et al., 2001) because the few remaining pits present on the plasma membrane after GAK deletion showed no clathrin exchange. The GAK knockout mouse and MEFs derived from this mouse provide a new model system to study the various functions of these cochaperones and the role of the different domains comprising these proteins. Using this system, we can now start to examine the question as to whether GAK and auxilin show functional redundancy in regards to the multiple function of these cochaperones.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Chengyu Liu for assistance in making the knockout mice, Myoung-Soon Hong for the electron microscopy, Drs. George Banting (University of Bristol, Bristol, United Kingdom) and Matthew Seaman (Cambridge University, Cambridge, United Kingdom) for the anti-TGN38 antibody, Dr. Benmerah (Université Paris Descartes) for the GFP-Eps15 and Eps15R constructs, and Drs. Mary Anne Conti, Xufei Ma, Robert Shamburek, Maria Morasso, Matthew Daniels, and Eric Snapp for helpful discussions.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1115) on April 23, 2008.
REFERENCES
- Alvarez C., Sztul E. S. Brefeldin A (BFA) disrupts the organization of the microtubule and the actin cytoskeletons. Eur. J. Cell Biol. 1999;78:1–14. doi: 10.1016/S0171-9335(99)80002-8. [DOI] [PubMed] [Google Scholar]
- Cascio S., Zaret K. S. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991;113:217–225. doi: 10.1242/dev.113.1.217. [DOI] [PubMed] [Google Scholar]
- Cremona O., et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Di Paolo G., Moskowitz H. S., Gipson K., Wenk M. R., Voronov S., Obayashi M., Flavell R., Fitzsimonds R. M., Ryan T. A., De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Di Paolo G., et al. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron. 2002;33:789–804. doi: 10.1016/s0896-6273(02)00601-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Eun S. H., Banks S.M.L., Fischer J. A. Auxilin is essential for Delta signaling. Development. 2008;135:1089–1095. doi: 10.1242/dev.009530. [DOI] [PubMed] [Google Scholar]
- Ferguson S. M., et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Gall W. E., Higginbotham M. A., Chen C., Ingram M. F., Cyr D. M., Graham T. R. The auxilin-like phosphoprotein Swa2p is required for clathrin function in yeast. Curr. Biol. 2000;10:1349–1358. doi: 10.1016/s0960-9822(00)00771-5. [DOI] [PubMed] [Google Scholar]
- Greener T., Grant B., Zhang Y., Wu X., Greene L. E., Hirsh D., Eisenberg E. Caenorhabditis elegans auxilin: a J-domain protein essential for clathrin-mediated endocytosis in vivo. Nat. Cell Biol. 2001;3:215–219. doi: 10.1038/35055137. [DOI] [PubMed] [Google Scholar]
- Greener T., Zhao X., Nojima H., Eisenberg E., Greene L. E. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J. Biol. Chem. 2000;275:1365–1370. doi: 10.1074/jbc.275.2.1365. [DOI] [PubMed] [Google Scholar]
- Hagedorn E. J., Bayraktar J. L., Kandachar V. R., Bai T., Englert D. M., Chang H. C. Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J. Cell Biol. 2006;173:443–452. doi: 10.1083/jcb.200602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman M. J., Sisi P., Banbury D. N., Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- Hayashi S., McMahon A. P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Jiang R., Gao B., Prasad K., Greene L. E., Eisenberg E. Hsc70 chaperones clathrin and primes it to interact with vesicle membranes. J. Biol. Chem. 2000;275:8439–8447. doi: 10.1074/jbc.275.12.8439. [DOI] [PubMed] [Google Scholar]
- Kametaka S., Moriyama K., Burgos P. V., Eisenberg E., Greene L. E., Mattera R., Bonifacino J. S. Canonical interaction of cyclin G-associated kinase with adaptor protein 1 regulates lysosomal enzyme sorting. Mol. Biol. Cell. 2007;18:2911–3001. doi: 10.1091/mbc.E06-12-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk V. I., Banting G. CK2 and GAK/auxilin2 are major protein kinases in clathrin-coated vesicles. Traffic. 2002;3:428–439. doi: 10.1034/j.1600-0854.2002.30606.x. [DOI] [PubMed] [Google Scholar]
- Lee D. W., Wu X., Eisenberg E., Greene L. E. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J. Cell Sci. 2006;119:3502–3512. doi: 10.1242/jcs.03092. [DOI] [PubMed] [Google Scholar]
- Lee D. W., Zhao X., Eisenberg E., Greene L. E. Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors. J. Cell Sci. 2005;118:4311–4321. doi: 10.1242/jcs.02548. [DOI] [PubMed] [Google Scholar]
- Liazoghli D., Perreault S., Micheva K. D., Desjardins M., Leclerc N. Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am. J. Pathol. 2005;166:1499–1514. doi: 10.1016/S0002-9440(10)62366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol R. H., Boll W., Griffin A. M., Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc. Natl. Acad. Sci. USA. 2006;103:10265–10270. doi: 10.1073/pnas.0603369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone J. L., Kukekov V., Chiang A. S., Steindler D., Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mitsunari T., Nakatsu F., Shioda N., Love P. E., Grinberg A., Bonifacino J. S., Ohno H. Clathrin adaptor AP-2 is essential for early embryonal development. Mol. Cell. Biol. 2005;25:9318–9323. doi: 10.1128/MCB.25.21.9318-9323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmyer S. L., Christensen A., Sever S. Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev. Cell. 2003;4:929–940. doi: 10.1016/s1534-5807(03)00157-6. [DOI] [PubMed] [Google Scholar]
- Pishvaee B., Costaguta G., Yeung B. G., Ryazantsev S., Greener T., Greene L. E., Eisenberg E., McCaffery J. M., Payne G. S. A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat. Cell Biol. 2000;2:958–963. doi: 10.1038/35046619. [DOI] [PubMed] [Google Scholar]
- Postic C., Magnuson M. A. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Puri S., Linstedt A. D. Capacity of the golgi apparatus for biogenesis from the endoplasmic reticulum. Mol. Biol. Cell. 2003;14:5011–5018. doi: 10.1091/mbc.E03-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele U., Kalthoff C., Ungewickell E. Multiple interactions of auxilin 1 with clathrin and the AP-2 adaptor complex. J. Biol. Chem. 2001;276:36131–36138. doi: 10.1074/jbc.M106511200. [DOI] [PubMed] [Google Scholar]
- Segre J. A., Bauer C., Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Itoh M. T., Ishizuka B. Human chorionic gonadotropin induces nestin expression in endothelial cells of the ovary via vascular endothelial growth factor signaling. Endocrinology. 2008;149:253–260. doi: 10.1210/en.2007-0774. [DOI] [PubMed] [Google Scholar]
- Umeda A., Meyerholz A., Ungewickell E. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol. 2000;79:336–342. doi: 10.1078/S0171-9335(04)70037-0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Ungewickell H., Holstein S. E., Lindner R., Prasad K., Barouch W., Martin B., Greene L. E., Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Wu X., Zhao X., Baylor L., Kaushal S., Eisenberg E., Greene L. E. Clathrin exchange during clathrin-mediated endocytosis. J. Cell Biol. 2001;155:291–300. doi: 10.1083/jcb.200104085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhao X., Puertollano R., Bonifacino J. S., Eisenberg E., Greene L. E. Adaptor and clathrin exchange at the plasma membrane and trans-Golgi network. Mol. Biol. Cell. 2003;14:516–528. doi: 10.1091/mbc.E02-06-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim Y. I., Scarselletta S., Zang F., Wu X., Lee D. W., Kang Y. S., Eisenberg E., Greene L. E. Exchange of clathrin, AP2 and epsin on clathrin-coated pits in permeabilized tissue culture cells. J. Cell Sci. 2005;118:2405–2413. doi: 10.1242/jcs.02356. [DOI] [PubMed] [Google Scholar]
- Zhang C. X., Engqvist-Goldstein A. E., Carreno S., Owen D. J., Smythe E., Drubin D. G. Multiple roles for cyclin G-associated kinase in clathrin-mediated sorting events. Traffic. 2005;6:1103–1113. doi: 10.1111/j.1600-0854.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gjoerup O., Roberts T. M. The serine/threonine kinase cyclin G-associated kinase regulates epidermal growth factor receptor signaling. Proc. Natl. Acad. Sci. USA. 2004;101:10296–10301. doi: 10.1073/pnas.0403175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizioli D., Meyer C., Guhde G., Saftig P., von Figura K., Schu P. Early embryonic death of mice deficient in gamma-adaptin. J. Biol. Chem. 1999;274:5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.