Abstract
The antidepressant and cocaine sensitive plasma membrane monoamine transporters are the primary mechanism for clearance of their respective neurotransmitters and serve a pivotal role in limiting monoamine neurotransmission. To identify molecules in pathways that regulate dopamine transporter (DAT) internalization, we used a genetic complementation screen in Xenopus oocytes to identify a mitogen-activated protein (MAP) kinase phosphatase, MKP3/Pyst1/DUSP6, as a molecule that inhibits protein kinase C–induced (PKC) internalization of transporters, resulting in enhanced DAT activity. The involvement of MKP3 in DAT internalization was verified using both overexpression and shRNA knockdown strategies in mammalian cell models including a dopaminergic cell line. Although the isolation of MKP3 implies a role for MAP kinases in DAT internalization, MAP kinase inhibitors have no effect on internalization. Moreover, PKC-dependent down-regulation of DAT does not correlate with the phosphorylation state of several well-studied MAP kinases (ERK1/2, p38, and SAPK/JNK). We also show that MKP3 does not regulate PKC-induced ubiquitylation of DAT but acts at a more downstream step to stabilize DAT at the cell surface by blocking dynamin-dependent internalization and delaying the targeting of DAT for degradation. These results indicate that MKP3 can act to enhance DAT function and identifies MKP3 as a phosphatase involved in regulating dynamin-dependent endocytosis.
INTRODUCTION
The classical monoamine neurotransmitters dopamine, norepinephrine, and serotonin modulate a range of behavioral functions including locomotion, emotion, and learning. Plasma membrane monoamine transporters mediate the reuptake of neurotransmitters after synaptic release and thus determine the spatial and temporal extent of receptor activation, which ultimately drives these behaviors (Torres et al., 2003). Therapeutic drugs, such as antidepressants, and addictive drugs such as cocaine, act by inhibiting monoamine transporters and provide a compelling reason for understanding the regulation of transport during normal neurotransmission and in pathological states.
Many studies have shown that the activity of monoamine transporters can be regulated acutely by intracellular second-messenger systems, and some of these have implicated direct protein–protein interactions in the regulation of carrier function. One of the best studied examples of acute transporter regulation is the activation of protein kinase C (PKC) by phorbol esters that results in a decrease in dopamine transport in cellular systems and rat striatal synaptosomes (Mortensen and Amara, 2003). The down-regulation of activity occurs through the internalization of transporter molecules from the cell surface through a dynamin-dependent process (Daniels and Amara, 1999; Sorkina et al., 2005). Although one recent study has implied that the direct phosphorylation of the dopamine transporter (DAT) can regulate its intrinsic activity (Khoshbouei et al., 2004), others have ruled out the premise that the direct phosphorylation of the dopamine transporters after PKC activation triggers the internalization of the DAT (Granas et al., 2003). In the latter study the deletion of all potential phosphorylation sites in the N-terminus of DAT eliminates PKC-mediated phosphorylation, but did not eliminate PKC-mediated down-regulation. Thus, other proteins, particularly those linked to endocytosis and trafficking of membrane proteins, are more likely to be the direct substrates for PKC-mediated phosphorylation.
Recent studies have identified several specific regions within DAT that could mediate a direct interaction between DAT and regulatory proteins linked to membrane protein internalization. For example, several amino acids in the DAT C-terminus have been found to be important for both the constitutive and the PKC-regulated internalization of the transporter (Holton et al., 2005; Sorkina et al., 2005). Other work has shown that PKC activation causes DAT to become ubiquitylated in a process that requires the ubiquitin ligase Nedd4-2 (Sorkina et al., 2006), indicating that the addition of ubiquitin moieties could serve to trigger the internalization and degradation of DAT (Miranda et al., 2005, 2007). It should be noted that the degradation of DAT after PKC activation has not been examined in dopaminergic neurons. Other proteins linked to the process of DAT internalization include dynamin, clathrin heavy chain, rab GTPases, epsins, and eps15 (Daniels and Amara, 1999; Sorkina et al., 2005, 2006), and the identification of additional proteins involved in PKC-regulated endocytosis continues to be a central focus for understanding plasma membrane monoamine transporter regulation and physiology.
MATERIALS AND METHODS
Expression Cloning
mRNA from the cell line SK-N-SH was used to construct a cDNA library inserted into the pSPORT vector according to manufacturer's protocol (Invitrogen, Carlsbad, CA). This vector contains a T7 promoter for in vitro transcription. To identify active cDNAs, pools of clones were plated and DNA was isolated. This DNA was used to produce in vitro–transcribed cRNA for injection into Xenopus oocytes.
Molecular Biology
The coding sequences of all phosphatases were cloned, and all transporters were subcloned into pOTV using normal primer-based RT-PCR or PCR cloning. The resulting plasmids were linearized and used to prepare cRNA for injection into oocytes. MKP3 was subcloned into pFLAG-CMV-2 to N-terminally tag the protein with FLAG. For production of stable Madin-Darby canine kidney (MDCK) cell lines both MKP1 (a kind gift from Prof. S. M. Keyse, University of Dundee, United Kingdom; Alessi et al., 1993) and MKP3 were subcloned into an IRES vector expressing the phosphatase together with the blasticidin resistance gene. Point mutations were generated using the QuikChange Site-Directed Mutagenesis Kit according to the manufacturer's protocol (Stratagene Cloning Systems, La Jolla, CA).
Primary Neuronal Cultures
Primary cultures from substantia nigra and ventral tegmental areas were prepared from 2- to 4-d-old Sprague Dawley rat pups as described previously (Prasad and Amara, 2001).
RNA Interference in MN9D Cells
MN9D cells were obtained from Drs. Alfred Heller and Lisa Won (University of Chicago) and propagated as described (Choi et al., 1992). The cells were transiently cotransfected with DAT cDNA and one of the following MISSION short hairpin RNA (shRNA) plasmids: TRCN0000055040 (shRNA-B); TRCN0000055041 (shRNA-C), which produces shRNAs that targets the coding region of MKP3; or the SHC002 MISSION Non-Target shRNA Control Vector (shRNA-nontarget; Sigma Aldrich, St. Louis, MO). To produce an MKP3 knockdown cell line, MN9D cells were transfected with the MISSION shRNA plasmid TRCN0000055041 (shRNA-C). A pool of shRNA-expressing cells was selected using 5 μg/ml puromycin. These cells were transfected with cDNAs for transporters using Fugene HD transfection reagent (Roche Applied Science, Indianapolis, IN) according to manufacturer's protocol. Functional uptake assays were performed 72–96 h after transfection.
Uptake Assays
Defollicated Xenopus oocytes were injected with 50 ng of cRNA. Uptake of radiolabeled [3H]dopamine (60 Ci/mmol; Perkin Elmer-Cetus, Wellesley, MA) was assayed in oocytes for 10 min in a frog Ringer's buffer with 10 μM RO41-0960 (COMT inhibitor) at room temperature. Nonspecific accumulation of substrate was determined with water-injected oocytes for each condition. Uptake assays in all mammalian cells were carried out in PBS containing 1 mM MgCl2, 0.1 mM CaCl2, and 10 μM RO41-0960. Uptake assays were performed at room temperature for 10 min using radiolabeled [3H]dopamine (60 Ci/mmol; Perkin Elmer-Cetus) at concentrations between 50 and 100 nM. Nonspecific accumulation of substrate was determined with either naïve or mock-transfected cells.
Immunofluorescence Imaging
After either vehicle or 1 μM PMA treatment, MDCK cells were fixed in freshly prepared 4% paraformaldehyde in PBS. Paraformaldehyde-fixed cells were washed in PBS and incubated overnight at 4°C with the primary anti-FLAG antibody (Sigma-Aldrich). After incubation with primary antibody, the cells were washed with PBS and incubated for 1 h in blocking buffer containing secondary antibody conjugated to rhodamine red-X (Jackson Immuno Research Laboratories, West Grove, PA). After incubation with secondary antibody, the cells were washed in PBS and mounted on glass slides with ProLong (Invitrogen) antifade reagent. Images were produced using confocal microscopy (MRC 1024 system; Bio-Rad, Hercules, CA). Image analysis and quantitation of intracellular fluorescence was carried out using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Cell Surface Biotinylation Assay
Cell surface expression of DAT was assayed with modifications of the method described previously (Daniels and Amara, 1999). After drug treatment cells were washed and incubated with 2 mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford, IL). The cells were quenched with 100 mM glycine buffer, washed with PBS, and lysed in lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 and protease inhibitor cocktail; Roche Applied Science). The cell lysate was incubated on ice and centrifuged at 14,000 × g before incubation with NetrAvidin Resin (Pierce) overnight at 4°C. The beads were separated from the supernatant by centrifugation at 5000 × g for 15 min, washed three times with lysis buffer, twice with a high-salt wash buffer, and once with a no-salt wash buffer. Proteins were separated on SDS-PAGE gels and immunoblotted. Expression of DAT was probed with a rabbit polyclonal antiserum against DAT. Antibodies against the transferrin receptor were obtained from Zymed/Invitrogen, and the antibody against the Na+/K+ ATPase was from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City). EAAT3 was detected using a rabbit polyclonal antibody against the C-terminus. Surface DAT degradation was monitored using the same biotinylation protocol as above with the modification that biotinylation was carried out before drug treatments. After drug treatments cells were lysed and biotinylated proteins were isolated and analyzed as above.
Ubiquitylation of DAT
To examine the ubiquitylation of DAT, cells were lysed in ice-cold lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 10 mM N-ethyl-maleimide, and protease inhibitor cocktail). After drug treatments, cell lysates were immunoprecipitated with a DAT-specific polyclonal rabbit antibody, and samples were separated on SDS-PAGE gels and immunoblotted. Ubiquitylated DAT was detected using a mAb to ubiquitin (P4D1) from Santa Cruz Biotechnology (Santa Cruz, CA). Total DAT was detected with a green fluorescent protein (GFP) antibody from Clontech (Mountain View, CA).
Immunoblotting to Detect Phosphorylation State of ERK
For immunoblotting experiments oocytes were lysed in ice cold lysis buffer (100 mM NaCl, 50 mM β-glycerophosphate, pH 7.4, 10 mM EDTA, 2 mM NaF, 1 mM sodium orthovanadate, and protease inhibitor cocktail; Roche Applied Science) and centrifuged at 700 × g for 2 min, and the resulting supernatant was mixed with 2× sample buffer. MDCK protein samples were produced by washing of cells directly in their well using PBS and lysed in 2× sample buffer. All samples were separated by SDS-PAGE and immunoblotted. Antibodies from Cell Signaling Technology (Danvers, MA) were used to detect the level of and the activation state of ERK1/2. The mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor PD184352 was a kind gift from Prof. Philip Cohen (University of Dundee, Scotland).
Statistical Analysis
GraphPad prism software (San Diego, CA) was used to determine statistical significance using Student's t test or one-way ANOVA followed by Bonferroni's multiple comparison test.
Post Hoc Microarray Analyses
To confirm the expression of MKP3 in dopaminergic neurons, we performed post hoc analyses of data from two publicly available sets of microarray data. One dataset produced by Greene et al. (2005) was obtained from the NIH Neuroscience microarray consortium (http://arrayconsortium.tgen.org/np2/home.do). The raw data from this dataset was analyzed using the Affymetrix (Santa Clara, CA) GCOS software statistical expression algorithm. We also obtained expression data for MKP3 (Gene ID: 93285_at) from the supplemental dataset produced by Miller et al. (2004).
RESULTS
Expression Cloning Identifies MKP3 as Modulator of Dopamine Transporter Activity
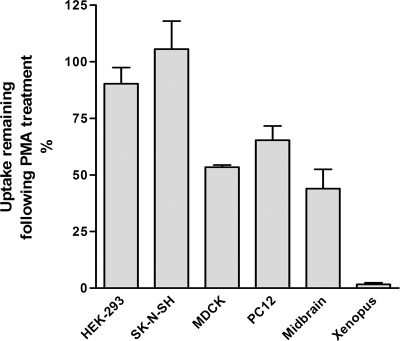
To investigate whether the cellular environment in which the transporters are expressed is important for the reduction in activity of transporters mediated by PKC activation, we assayed the ability of phorbol 12-myristate 13-acetate (PMA), a PKC activator, to regulate the dopamine uptake activity of both endogenous and transfected dopamine and norepinephrine transporters (DAT and NET) in several different cell types. The magnitude of decrease in substrate transport observed after PMA treatment varied widely between different cell types (Figure 1). In Xenopus oocytes there was a dramatic down-regulation after a 30-min incubation with 1 μM PMA, with nearly complete elimination of dopamine uptake activity. In primary cultures of midbrain dopaminergic neurons, treatment with PMA reduced activity to 50%. A similar reduction was observed in MDCK cells stably expressing the DAT. In contrast, HEK-293 cells transiently expressing the DAT displayed little or no down-regulation of uptake activity after PMA treatment. SK-N-SH and PC-12 cells, which express the NET endogenously, displayed dramatically different sensitivities to PMA treatment, with no significant decrease in dopamine uptake activity observed in SK-N-SH cells, but a 40% reduction in uptake activity in PC-12 cells. Other studies previously noted a modest down-regulation and internalization of transporters in SK-N-SH and HEK-293 cells after PKC activation by PMA treatment (Apparsundaram et al., 1998; Granas et al., 2003). In the experiments presented here cells were incubated with PMA for 30 min at room temperature and not at 37°C as in previous studies. When cells were incubated at 37°C, we also found small, but significant down-regulation (data not shown).
Figure 1.
Dopamine and norepinephrine transporters are differentially down-regulated by PKC activation in different cell systems. Cells were pretreated with 1 μM PMA for 30 min. The uptake of dopamine, which is an efficient substrate for both DAT and NET, was carried out at room temperature for 10 min (except for primary cultures where the uptake was for 5 min). Results are shown as average ± SD from five individual experiments.
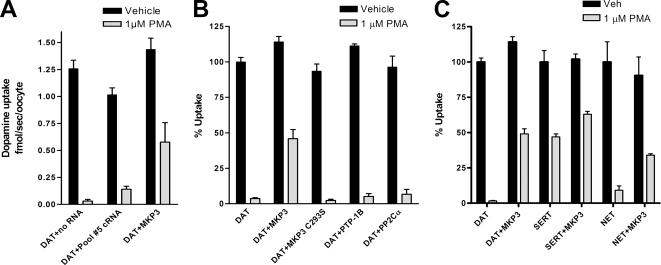
The variability in PMA-induced regulation of transport activity suggested that proteins that interfere with PKC-mediated internalization might exist in cell lines displaying less down-regulation, such as the SK-N-SH line. Thus, we used a complementation assay in Xenopus oocytes to isolate the cDNAs encoding these modulatory proteins. In this assay we coexpressed a cRNA expression library of 800,000 clones from the SK-N-SH cell line together with DAT in Xenopus oocytes. The cDNA library was initially divided into pools of 40,000 individual library clones. In vitro–transcribed cRNA from pools were coinjected together with hDAT cRNA into Xenopus oocytes, and the magnitude of PMA-induced down-regulation of dopamine transport was assessed (Figure 2A). Pools showing a reduction in the PMA-induced down-regulation of transport activity were continuously subdivided and pooled until one single clone was identified and found to encode the MAP kinase phosphatase MKP3/Pyst1/DUSP6 (MKP3).
Figure 2.
Expression cloning identifies MAP kinase phosphatase 3 (MKP3) as modulator of PMA-induced down-regulation of neurotransmitter transporters. (A) Xenopus oocytes coinjected with DAT and pools of cRNA from an SK-N-SH library were treated with vehicle or 1 μM PMA for 30 min, and uptake was carried out for 10 min. Results are shown as average ± SD from five individual oocytes. The initial pool no. 5 containing 40,000 individual clones shows significant inhibition of the PMA-induced down-regulation of coexpressed DAT activity. Pools that contained activity were further subdivided and rescreened for activity until a single clone MKP3 was identified. (B and C) Xenopus oocytes coinjected with combinations of transporters and phosphatases were treated with vehicle or 1 μM PMA for 30 min, and uptake was carried out for 10 min. Results are shown as average ± SD from five individual oocytes. (B) A catalytically inactive mutant of MKP3 (MKP3 C293S), a tyrosine phosphatase (PTP-1B) and a serine/threonine phosphatase (PP2Cα) does not prevent PKC-mediated regulation of the dopamine transporter (DAT). (C) The dopamine transporter (DAT), the serotonin transporter (SERT), and the norepinephrine transporter (NET) are all regulated by both PKC and MKP3 in Xenopus oocytes.
MAP kinase phosphatases are dual specificity phosphatases belonging to the superfamily of tyrosine phosphatases, which all inactivate MAP kinases by dephosphorylating a threonine and a tyrosine residue (Dickinson and Keyse, 2006; Kondoh and Nishida, 2006). When expressed along with DAT in oocytes, MKP3 prevented the PMA-induced down-regulation. PMA still induced a down-regulation with an IC50 of 8 nM, similar to the IC50 of 4 nM found in oocytes injected with the hDAT cRNA alone, but in the presence of MKP3 the magnitude of down-regulation was dramatically reduced from nearly 100% to 52 ± 6%.
Although no specific inhibitors of MKP3 exist, we used the vanadate analogue bpv(phen), a general in vitro inhibitor of cysteine-based tyrosine and dual specificity phosphatases (Wiland et al., 1996), to test the involvement of this superfamily of tyrosine phosphatases in the PKC-mediated regulation of neurotransmitter transporters. SK-N-SH cells that express both NET and MKP3 endogenously were pretreated with 100 μM bpv(phen) before activation of PKC with PMA. Although vehicle-treated SK-N-SH cells displayed no significant change in uptake activity in response to PMA, cells pretreated with bpv(phen) displayed a 54% reduction in activity after PMA (data not shown), confirming the involvement of tyrosine phosphatases in PKC-mediated regulation of NET activity.
Only Catalytically Active MAP Kinase Phosphatase Can Prevent the PMA-induced Down-Regulation of the Dopamine Transporter
Because bpv(phen) can inhibit other phosphatases in addition to MKP3, we next examined whether other tyrosine phosphatases or even serine/threonine phosphatases could modulate the PKC induced down-regulation of DAT activity in Xenopus oocytes. We chose to investigate PP2Cα a prototypic serine/threonine phosphatase expressed in the brain (Price and Mumby, 1999) and PTP-1B a prototypic tyrosine phosphatase involved in the dephosphorylation of several receptor tyrosine kinases (Tonks, 2003; Figure 2B). Coexpressing either PP2Cα or PTP-1B with DAT in Xenopus oocytes had no effect on the PMA-induced down-regulation of DAT, suggesting the effect observed above in SK-N-SH cells is the effect of bpv(phen) inhibiting the endogenous MKP3 in these cells.
To further explore the mechanism of action of MKP3 in regulating DAT activity, we used a mutant of MKP3 in which the catalytic activity was removed by mutating a single cysteine in the catalytic site to a serine (C293S). This mutant phosphatase binds and traps MAP kinases, but because the dephosphorylating activity of the mutant is highly reduced, it does not inactivate its MAP kinase substrates (Brunet et al., 1999). Thus, although the C293S mutant does not prevent the MAP kinase from activating cytosolic targets, it disrupts the nuclear translocation of the activated MAP kinase by sequestering it in the cytosol, preventing activation of nuclear targets of the MAP kinases. In contrast to the effects observed with wild-type MKP3, the MKP3-C293S mutant did not prevent PKC-mediated down-regulation of DAT (Figure 2B), indicating that a catalytically active phosphatase is required for blocking the inhibition of DAT activity. Because the MKP3 mutant sequesters its MAP kinase substrates within the cytoplasm, this experiment also addresses whether translocation of MAP kinase to the nucleus and activation of downstream nuclear targets is required for PKC-mediated regulation of DAT. The mutant was unable to prevent PKC-mediated down-regulation and thus, these results also suggest that nuclear translocation of MAP kinases is not required for the effect of PKC on transporter activity.
PKC Regulation of All Plasma Membrane Monoamine Transporters Is Modulated by MKP3
To determine whether the effect of MKP3 expression was specific for dopamine transporters or whether MKP3 could also modulate PMA-induced down-regulation of other monoamine transporters, we coexpressed three monoamine transporters in Xenopus oocytes alone or with MKP3 (Figure 2C). The effect of PMA on transport activity varied greatly between the different transporters. DAT and NET were the most sensitive, with almost complete inhibition of transport activity, 98 ± 1 and 91 ± 7%, respectively. The serotonin transporter (SERT) was moderately affected with only 47 ± 5% of the uptake activity remaining after PMA treatment. Although differences in expression of the three transporters could contribute to the variation in sensitivity to PMA, coexpression of MKP3 with either DAT, NET, or SERT consistently reduced the PMA-mediated decrease in activity. With the SERT and the NET we found modest effects of MKP3 expression, with 17 and 25% increases in serotonin and norepinephrine uptake, respectively. However, DAT showed the most dramatic effect with a recovery of about 48% of its maximal uptake activity.
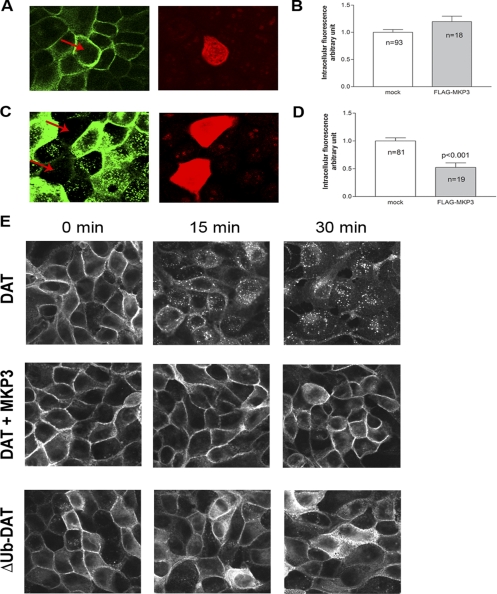
Overexpression of MKP3 Prevents DAT Internalization in MDCK Cells
We next transiently expressed FLAG-tagged MKP3 into MDCK cells stably expressing GFP-tagged DAT to examine how MKP3 influences the trafficking of DAT in the system we have used previously to study PKC regulation of DAT (Daniels and Amara, 1999). MDCK cells can be grown to form a polarized epithelium with tight junctions in a very distinct honeycomb pattern, making them extremely useful as a model for membrane protein trafficking and sorting. Representative confocal images of GFP-DAT in MDCK cells are shown in Figure 3, A and C. We have previously shown in MDCK cells that after PKC activation dopamine transporters are rapidly endocytosed through a dynamin-dependent and clathrin-mediated process resulting in a very distinctive punctate intracellular pattern (Daniels and Amara, 1999). The internalized DAT molecules present within these puncta initially colocalize with internalized transferrin receptors, but subsequently transit through an endosomal pathway into lysosomes where they are ultimately degraded (Daniels and Amara, 1999). The dramatic increase in intracellular puncta reflecting internalized GFP-DAT occurs within 30 min of treatment with 1 μM PMA (Figure 3C) and precisely parallels the decrease in transporter surface expression. Vehicle-treated cells showed virtually no intracellular puncta (Figure 3A). The intracellular accumulation of DAT in response to PMA treatment was not apparent in cells transiently transfected with FLAG-tagged MKP3, shown in red on Figure 3C. Quantitative analysis of the amount of intracellular fluorescence using ImageJ (NIH; Figure 3D) shows that after PMA treatment there was a significantly lower level of intracellular fluorescence in MKP3-expressing cells with almost double the amount of intracellular fluorescence in cells that did not express MKP3. In control cells that were not treated with PMA, we did not find a significant difference in intracellular fluorescence between MKP3- expressing cells and naïve cells (Figure 3, A and B).
Figure 3.
Expression of MKP3 prevents PMA-induced internalization in MDCK cells. Flag-tagged MKP3 was transiently expressed in MDCK cells stably expressing green fluorescent protein (GFP)-tagged dopamine transporter (DAT) and visualized using confocal microscopy. Representative images of GFP-tagged DAT (green) and FLAG-tagged MKP3 (red) are shown in A (vehicle treated) and C (1 μM PMA treated). (B and D) Quantitative analysis of intracellular fluorescence; vehicle treated (B) and 1 μM PMA treated (D). Results are shown as average ± SEM. Amounts of intracellular fluorescence from cells expressing MKP3 are shown in gray and from cells not expressing MKP3 in white. Fluorescence was normalized to fluorescence in non-MKP3–expressing cells (mock). (E) Confocal images visualizing GFP-tagged DAT in MDCK cells treated with PMA at various time points. The cells were stably expressing GFP-tagged DAT (DAT), DAT with MKP3 (DAT+MKP3), or a DAT mutant in which the following N-terminal lysines 19, 27, and 35 had been replaced with arginine (ΔUb-DAT).
Internalization and Not Recycling of DAT Is Attenuated by MKP3
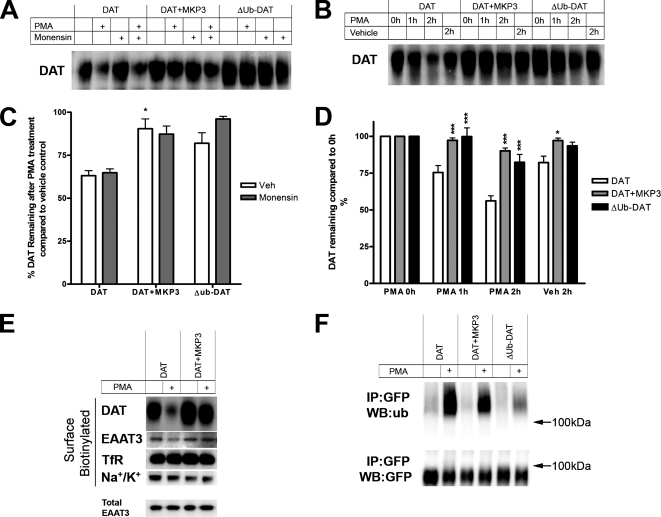
To further explore the actions of MAP kinase phosphatases in the MDCK cell model, we established MDCK cell lines stably expressing GFP-DAT together with either MKP3 or MKP1, another member of the same family of dual specificity phosphatases. Figure 3E shows a time course of PMA-treated MDCK cells either expressing GFP-DAT alone (3E, DAT) or together with MKP3 (3E, DAT+MKP3). Within 15 min of PMA treatment a very characteristic intracellular punctuate pattern of fluorescence appears in the DAT cells, whereas in MKP3-expressing cells most of the GFP-DAT appears to be located on or near the surface even after 30 min of PMA treatment. To establish which steps in DAT trafficking are modulated by MKP3, we used surface biotinylation of DAT in several experimental paradigms to monitor the fate of carriers after internalization. The intent of these experiments was to resolve whether MKP3 inhibits internalization of DAT, enhances recycling of DAT back to the surface, and/or regulates the degradation of DAT as previously reported (Daniels and Amara, 1999; Miranda et al., 2005). To test if MKP3 regulates internalization or recycling, we carried out a conventional biotinylation assay in which surface proteins were biotinylated after drug treatments, purified using streptavidin beads, and visualized using immunoblotting (Figure 4A). The PMA treatment was carried out on cells pretreated either with vehicle or with 25 μM monensin, which blocks the recycling of vesicles back to the surface (Miranda et al., 2004). We found that the down-regulation of surface DAT density after PMA treatment (∼65% remaining) was dramatically reduced in cells expressing MKP3 (∼90% remaining; Figure 4C). Pretreatment of the cells with monensin had no effect on the ability of MKP3 to attenuate the removal of DAT from the surface, suggesting that MKP3 regulates internalization without altering the process of vesicle recycling (Figure 4C).
Figure 4.
Internalization and degradation but not ubiquitylation of the DAT is attenuated by MKP3 expression. (A and C) MDCK cells stably expressing a GFP-tagged dopamine transporter (DAT), a GFP-tagged DAT together with MKP3 (DAT+MKP3), or a GFP-tagged DAT mutant in which the following N-terminal lysines 19, 27, and 35 had been replaced with arginine (ΔUb-DAT) were pretreated with 25 μM monensin or vehicle for 15 min and treated with 1 μM PMA or vehicle for 30 min at 37°C. (A) After the drug treatments, surface proteins were biotinylated, isolated using streptavidin, and immunoblotted with a DAT antibody. (C) Densities of surface DAT in PMA-treated cells were quantified from at least three independent experiments. Results are shown as average ± SEM. Statistically significant differences were calculated using one-way ANOVA followed by Bonferroni's multiple comparison test comparing vehicle-pretreated DAT+MKP3 and ΔUb-DAT cells to vehicle-pretreated DAT cells (*p < 0.05). In all three cell lines there was no significant difference between the vehicle- and the monensin-pretreated cells. (B and D) To investigate the fate of surface DAT, surface proteins of the DAT, DAT+MKP3, and the ΔUb-DAT cell lines were biotinylated before 1 μM PMA or vehicle treatments. (B) After drug treatments biotinylated proteins were isolated and analyzed as above. (D) The densities of remaining surface biotinylated DAT protein species were quantified in at least three independent experiments. Results are shown as average ± SEM. Statistically significant differences were calculated using one-way ANOVA followed by Bonferroni's multiple comparison test comparing DAT+MKP3 or ΔUb-DAT cells with similarly treated DAT cells (*p < 0.05; ***p < 0.001). (E) The effects of PMA and MKP3 on the surface expression of other membrane proteins are shown. Surface-expressed proteins were biotinylated, isolated, and immunoblotted as above using antibodies against the DAT, the transferrin receptor (TfR), the Na+/K+-ATPase (Na+/K+), and a glutamate transporter, EAAT3. (F) To study the ubiquitylation of DAT in the DAT; DAT+MKP3, and the ΔUb-DAT cell lines, cell extracts were immunoprecipitated with a GFP antibody and immunoblotted using either an ubiquitin antibody (ub) or a GFP antibody (GFP).
To determine whether the degradation of DAT is also regulated by MKP3, we inhibited new synthesis with the translation blocker cycloheximide and assessed the degradation of the DAT protein species on immunoblots after PMA treatment. DAT was clearly being degraded in cells expressing MKP3, but we could detect no differences in degradation rates (data not shown). Because the cycloheximide-based assay investigated the fate of both intracellular and membrane-bound DAT, we decided to use a modified assay that examines the degradation rate of only the surface DAT. We biotinylated surface proteins before drug treatments and at various time points after vehicle or PMA treatment, harvested and isolated remaining biotinylated proteins, and visualized DAT by immunoblotting. Using this assay, we detected a significant reduction in the degradation rate of surface DAT after both PMA and vehicle treatments in cells expressing MKP3 (Figure 4, B and D). In PMA-treated cells we observed significant degradation of DAT in the cells expressing DAT alone within 1 h, but in MKP3-expressing cells ∼90% of surface DAT remains even after 2 h of PMA treatment. Taken together these results suggest that MKP3 attenuates the internalization of DAT, thereby delaying the degradation of the protein. This is also in agreement with the time course of DAT trafficking imaged with confocal microscopy in Figure 3E.
We next examined whether the effects of MKP3 were selective for the DAT by assessing the effects of PMA and MKP3 expression on the surface distribution of several endogenously expressed integral membrane proteins. Figure 4E shows that the steady-state surface levels of both the transferrin receptor (TfR) and the sodium potassium ATPase (Na+/K+) remain unchanged after incubation with PMA and, as expected, MKP3 expression had no effect on the surface expression of the two proteins. Intriguingly, the glutamate transporter EAAT3, a member of a distinct neurotransmitter transporter family, showed a decrease in surface expression in response to PMA, and this decrease also could be prevented by overexpression of MKP3. Thus, the effects of MKP3 appear selective for membrane proteins that are modulated by PKC and may reflect a more general regulatory mechanism that is not limited to the DAT.
PKC-induced Ubiquitylation of DAT Is Not Affected by MKP3
Recently it has been established that DAT is ubiquitylated after PMA treatment and that this ubiquitylation is necessary for the internalization of DAT (Miranda et al., 2005, 2007), and thus we hypothesized that MKP3 could be regulating the ubiquitylation of DAT. However, we found no difference in the level of DAT ubiquitylation in cells expressing MKP3 compared with cells expressing DAT alone (Figure 4F). We did find that PMA would increase the ubiquitylation of DAT, but only in a very small subfraction of the DAT population, because the GFP antibody detecting all GFP-tagged DAT detects a band with a different and smaller size constituting nonubiquitylated DAT. This suggests that the ubiquitylation is a dynamic process in which ubiquitinylated DAT exists only transiently and is readily deubiquitinylated by enzymes involved in ubiquitin turnover. To confirm that ubiquitylation is required for DAT internalization in the MDCK cell line, we stably expressed a DAT mutant (ΔUb-DAT) in which all three previously reported N-terminal lysines (positions 19, 27, and 35) that are responsible for the ubiquitin-mediated down-regulation of DAT (Miranda et al., 2007) have been mutated to arginine. As in the previous study, we found that the ubiquitylation of this mutant was reduced and that the PMA-induced down-regulation, internalization and degradation was attenuated, but not completely prevented. Moreover, cells expressing this DAT mutant displayed a very similar behavior to the cells expressing MKP3 and wild type DAT (Figures 3E and 4, A–F). These results are not likely due to differences in the amount of DAT, as comparable expression was observed in DAT, DAT+MKP3, and ΔUb-DAT cell lines (data not shown).
The Effects of MKP3 on DAT Internalization Is Independent of Classical MAP Kinases
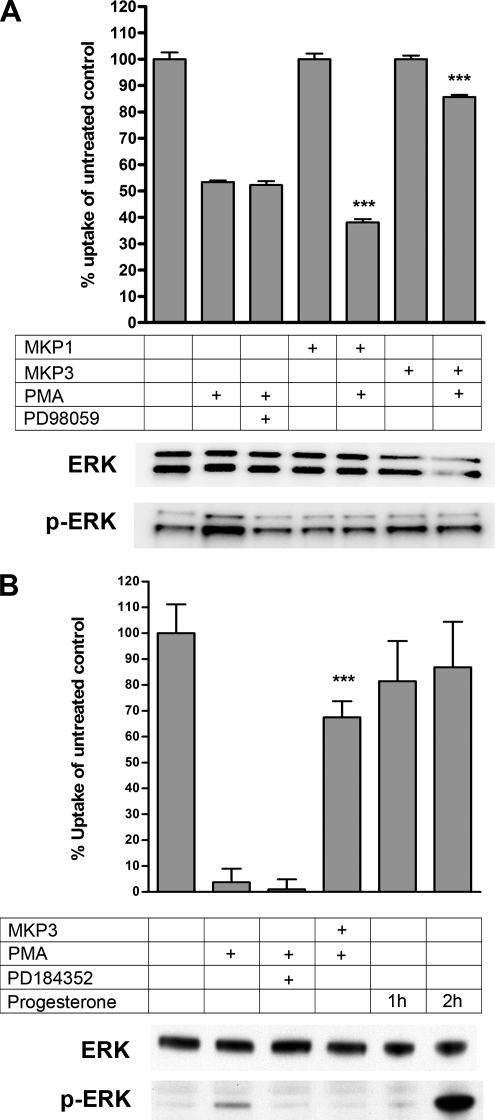
MAP kinases are thought to be the primary target of MKP3, and thus we anticipated that a MAP kinase might be the downstream target of the signaling cascade activated by PMA. Previous work has demonstrated that PKC can activate MAP kinases through activation of raf-1 and its MAP kinase kinase substrates (MEKs), although the precise mechanism and PKC substrates required remain controversial. We therefore investigated the activation state of various MAP kinases after PMA treatment either in the absence or presence of MKP3 in MDCK cells and Xenopus oocytes expressing DAT (Figure 5). The phosphorylation state of ERK1/2 was elevated in untreated MDCK cells when compared with oocytes. PMA treatment of MDCK cells did induce an increase in the phosphorylation and activation of ERK1/2 (Figure 5A), but did not induce activation of the stress-induced JNKs or p38 MAPKs (data not shown). We observed that untransfected cells had levels of activated ERK very similar to cells stably expressing either MKP1 or MKP3. The PMA-induced increase in phosphorylation and activation of ERK1/2 was abolished when MKP1 or MKP3 was stably expressed in PMA-treated MDCK cells, even though MKP1 was unable to prevent PKC-mediated down-regulation of DAT activity (see below). Pharmacological inhibition of the upstream MAP kinase kinase MEK1 using the compound PD98059 also resulted in inhibition of PMA-induced activation of ERK.
Figure 5.
Phosphorylation state of ERK1/2 in MDCK cells and Xenopus oocytes does not correlate with PMA-induced down-regulation of dopamine transporter (DAT). (A) MDCK cells expressing DAT alone or together with MKP1 or MKP3 were incubated for 30 min at 37°C with either vehicle or 1 μM PMA or were preincubated with 20 μM PD98059 for 30 min and then incubated with 1 μM PMA. (B) Xenopus oocytes expressing DAT alone or together with MKP3 were incubated for 30 min at room temperature with vehicle or 1 μM PMA and incubated with 10 μM progesterone for 1 and 2 h or preincubated with 20 μM PD184352 for 30 min and then incubated with 1 μM PMA. In both A and B after drug incubation either radiolabeled dopamine uptake was performed (top graph), or cells were lysed and subjected to SDS-PAGE and immunoblotting (bottom two blots). Uptake was carried out for 10 min. Results are shown as average ± SD from five individual oocytes or three individual MDCK cell experiments. Statistically significant differences were calculated using one-way ANOVA followed by Bonferroni's multiple comparison test comparing similarly treated MDCK cells or oocytes expressing DAT alone with cells or oocytes expressing DAT together with MKP1 or MKP3 (***p < 0.001). ERK-specific and phospho-ERK–specific antibodies (Cell Signaling) were used to detect levels of total ERK1/2 (ERK) and activated phospho-ERK1/2 (p-ERK).
When DAT uptake activity was examined under the same conditions, it became clear that there was no correlation between the activation state of ERK and the down-regulation of DAT. When PMA-induced ERK activation was blocked using either the MEK inhibitor, PD98059, or a stably expressed MKP1, there was no change in the ability of PMA to decrease DAT activity. Surprisingly, instead of preventing DAT down-regulation as observed with MKP3, cells expressing MKP1 displayed an even larger decrease in DAT activity in response to PMA. This result also demonstrates the specificity of MKP3's action in modulating DAT activity in MDCK cells, because MKP1, a related MAP kinase phosphatase does not prevent the down-regulation of DAT activity. This finding is also consistent with the idea that the substrate of MKP3 remains within the cytosol, as MKP1 is a nuclear protein (Brondello et al., 1995). Because our results suggested that blocking ERK activation did not have the same effect as expressing MKP3, we tested the involvement of other members of the MAP kinase family, even those that do not appear to be activated by PKC. In these experiments, inhibition of MAP kinases such as JNKs with SP600125 (50 μM) or p38 MAPK with SB203580 (10 μM) or MEK with U0126 (20 μM) could not prevent the PMA-induced down-regulation of DAT activity (data not shown).
As was found in MDCK cells, there was no correlation with the activation state of MAP kinases and the down-regulation of DAT in Xenopus oocytes (Figure 5B). The MEK1 inhibitor PD184352 had no effect on the down-regulation of transporter activity, but did remove the very limited activation of ERK1/2 after PMA treatment. None of the other MAP kinases were activated by PMA, nor could the p38 MAP kinase inhibitor SB203580 (10 μM) or the SAP/JNK MAP kinase inhibitor SP600125 (50 μM) inhibit the PMA-induced down-regulation (data not shown). Table 1 lists all the compounds tested for their effects on the PMA-mediated down-regulation of DAT in oocytes. The only compounds within this list that can prevent the down-regulation are the two PKC inhibitors bisindolylmaleimide I (GF109203X; 10 μM) and staurosporine (10 μM). One interpretation of these results is that the expression of MKP3 leads to the inhibition of PKC itself. To test this, we assayed the activity of PKC after PMA treatment in oocytes expressing MKP3 or not. We found no difference in basal and PMA-induced PKC activity between the two in a crude assay using an antibody that detects phosphorylated PKC substrates to estimate PKC activity (data not shown).
Table 1.
Compounds tested in Xenopus oocytes for effect on the PKC-mediated down-regulation of the dopamine transporter
| Compounda | Activityb |
|---|---|
| U0126 (20 μM) | MEK inhibitor |
| PD98059 (50 μM) | MEK inhibitor |
| PD184352 (20 μM) | MEK inhibitor |
| 5-Iodotubercidin (10 μM) | ERK2 inhibitor |
| SB203580 (10 μM) | p38 kinase inhibitor |
| SP600125 (50 μM) | SAPK/JNK inhibitor |
| LY294002 (50 μM) | PI3 kinase inhibitor |
| PP2 (20 μM) | Src kinase family inhibitor |
| Dantrolene (25 μM) | Ryanodine receptor antagonist |
| AG490 (100 μM) | RTK, JAK, and STAT inhibitor |
| Roscovitine (50 μM) | Cyclin-dependent kinase inhibitor |
| GM6001 (1 μM) | Matrix metalloproteinase inhibitor |
| Forskolin (50 μM) | Adenylyl cyclase activator |
| Bisindolylmaleimide I (GF109203X; 10 μM) | PKC inhibitor |
| Staurosporine (10 μM) | PKC inhibitor |
a The oocytes were pretreated with the respective compound at the concentration noted for 30 min before PMA treatment.
b The presumed main target for the compound listed.
We also incubated oocytes with progesterone to test the effect of activating ERK by a different pathway that does not involve PKC. The activation of ERK mediated by progesterone takes longer than that observed with PMA, but the effect is much stronger as seen on Figure 5B. Progesterone mediates its effect by stimulating the synthesis of Mos, a MEK1 kinase, which explains why progesterone-mediated ERK activation is delayed when compared with PMA-mediated ERK activation. Despite the robust activation of ERK by progesterone treatment, there was no effect on DAT activity. These results indicate that the PMA-induced down-regulation of neurotransmitter transporters is not mediated through the ERK1/2 pathway or through the JNK or p38 MAPK pathways.
MKP3 Effects on DAT Down-Regulation in a Dopaminergic Cell Line
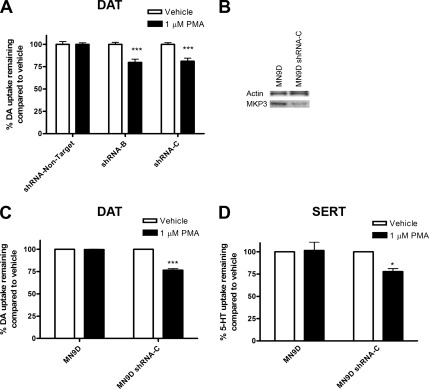
To examine whether the effects of MKP3 on monoamine transport activity can be observed in dopaminergic cells, we used MN9D cells, a dopaminergic neuronal hybrid cell line (Choi et al., 1992). These cells contain dopamine and tyrosine hydroxylase and express DAT at very low levels (Chen et al., 2005). As shown in Figure 6, A, C, and D, MN9D cells transiently transfected with the DAT or the SERT exhibited no down-regulation of transport activity after acute PMA treatment. Interestingly, MKP3 is expressed endogenously at readily detectable levels in these cells (Figure 6B), a finding consistent with the idea that expression of MKP3 disrupts PKC-mediated regulation. To test this hypothesis, we transiently cotransfected MN9D cells with DAT and either a control shRNA directed against no known mouse gene targets (shRNA-Non-Target) or two different shRNAs directed against the MKP3 coding region (shRNA-B and shRNA-C). When MN9D cells are transfected with either MKP3-targeted shRNA, dopamine transport activity is decreased (∼20%) by PMA application (Figure 6A). We also established a pool of MN9D cells (MN9D shRNA-C) stably expressing the shRNA-C. This cell pool expressed significantly lower levels of MKP3 protein as demonstrated by immunoblotting (Figure 6B). In these stably transfected cell pools, PMA induced a decrease in transport activity of the DAT (Figure 6C) and the SERT (Figure 6D) confirming that when MKP3 is reduced the transporters are no longer refractory to down-regulation by PKC in MN9D cells. In cell surface biotinylation experiments we also obtained data consistent with the results of the uptake data present in Figure 6. However, unlike the robust effects we present in Figure 4 for MDCK cells, the changes in the MN9D cells were modest and not always significant within the variability of the biochemical assay (data not shown).
Figure 6.
Dopamine transporter regulation in the dopaminergic MN9D cell line. (A) MN9D cells were transiently cotransfected with DAT cDNA and either a control shRNA directed against no known mouse gene targets (shRNA-Non-Target) or two different shRNAs directed against the MKP3 coding region (shRNA-B and shRNA-C) and assayed for uptake of dopamine (DA). (B) Naïve (MN9D) and pooled stable MKP3 knock-down MN9D cells used in C and D (MN9D shRNA-C) were immunoblotted for MKP3 and actin expression. (C and D) The same cells were transiently transfected with transporter cDNAs and assayed for uptake of dopamine (DA) or serotonin (5-HT). In all uptake assays (A, C, and D) cells were treated with PMA or vehicle 3–4 d after transfection for 30 min, and uptake assays were carried out for 10 min at room temperature. Results are shown as average ± SEM from at least three individual experiments. Statistically significant differences were calculated using Student's t test (*p < 0.05; ***p < 0.001) comparing vehicle- and PMA-treated cells.
DISCUSSION
The cellular context in which a protein is expressed can have a profound impact on its function and regulation. For example, we and others have found significant differences in the sensitivity of the human dopamine transporter (DAT) to PKC activation by phorbol esters (Zahniser and Doolen, 2001; Mortensen and Amara, 2003). By coexpressing DAT with a cRNA library from the human SK-N-SH neuroblastoma cell line, we obtained evidence for a factor expressed in SK-N-SH cells that could prevent or reverse the dramatic internalization of DAT observed in response to PKC activation in Xenopus oocytes. Using the oocyte system, we were able to establish a complementation assay to screen pools of clones from the SK-N-SH cRNA library, which led to the isolation of a MAP kinase phosphatase MKP3 (DUSP6 or PYST1) with an activity that attenuates the PKC-mediated down-regulation of DAT function.
MAP kinase phosphatases (MKPs) are dual specificity phosphatases that dephosphorylate MAP kinases at both threonine and tyrosine residues and thereby inactivate them (Dickinson and Keyse, 2006; Kondoh and Nishida, 2006). Within the family of MKPs, the individual phosphatases show some selectivity between the three conventional families of MAP kinases. For example, MKP3 has a preference for the ERK kinases (Muda et al., 1996), and MKP1 has a preference for the stress activated kinases p38 and JNK/SAPK (Chu et al., 1996). Most of this work is from studies in transfected mammalian cells, and only recently studies have begun to examine the physiological role of MKP3 in vivo. These studies have focused on the involvement of the phosphatase in signaling pathways during development. One study described the effects of targeted disruption of the MKP3 gene in mice (Li et al., 2007). These mice displayed increased ERK activation, and their phenotype led to dominant postnatal lethality and included serious developmental defects such as skeletal dwarfism, coronal craniosynostosis, and hearing loss. Other genetic and mutational approaches to examine the developmental role of MKP3 have been carried out in zebrafish (Tsang et al., 2004), chick embryos (Eblaghie et al., 2003; Smith et al., 2005), and Drosophila (Rintelen et al., 2003).
To understand how MKP3 regulates DAT trafficking in mammalian cells, we used canine kidney MDCK cells as a model system. We had demonstrated previously in these cells that PKC activation stimulates clathrin-mediated endocytosis and trafficking of DAT to lysosomes where it is ultimately degraded (Daniels and Amara, 1999). Expression of MKP3 did indeed result in less intracellular DAT (Figure 4) and using biochemical assays, we showed that the step MKP3 inhibits is the PKC-activated dynamin-dependent endocytosis of DAT (Figures 5 and 7). In mammalian cells clathrin-mediated endocytosis is a well-studied process that involves a variety of proteins including epsins, dynamin, adaptor proteins, and clathrin, as well as several accessory proteins that work as scaffolding proteins (Conner and Schmid, 2003). Not surprisingly, several of these are also required for internalization of DAT (Sorkina et al., 2006). Various steps in clathrin-mediated endocytosis have been shown to be regulated by phosphorylation and/or dephosphorylation of proteins present in the endocytic complex (Cousin and Robinson, 2001). A number of studies have identified phosphoproteins required for dynamin-dependent endocytosis (AP-2, dynamin), as well as associated kinases (PKC, Casein kinase II, cyclin-G–associated kinase, cdk5, and adaptor-associated kinase 1), and phosphatases (Calcineurin and Synaptojanin; reviewed in Conner and Schmid 2003).
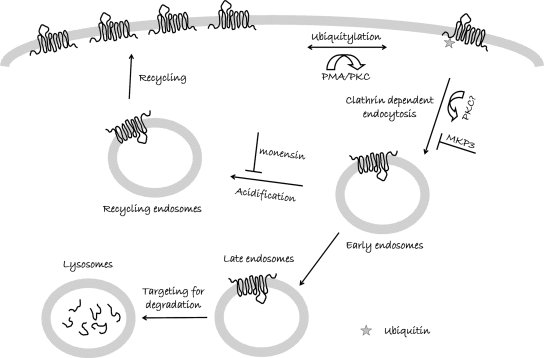
Figure 7.
Model of regulated dopamine transporter trafficking. After activation of PKC the dopamine transporter (DAT) is targeted for internalization and degradation in an ubiquitin dependent manner. We propose that MKP3 regulates the subsequent dynamin-dependent endocytosis step and thereby delays the targeting of DAT to degradation. This is based on the evidence 1) that the blockade of recycling of transporter back to the surface by monensin pretreatment has no effect on PKC- and MKP3-mediated regulation of DAT surface levels; and 2) that MKP3 prevents the PKC-mediated decrease in surface DAT levels, but does not affect the ubiquitylation of DAT.
Because direct phosphorylation of DAT does not appear to trigger its internalization (Granas et al., 2003), other modifications such as ubiquitinylation, have been considered as potential signals for the internalization of DAT. DAT becomes ubiquitinylated after activation of PKC (Miranda et al., 2005) in a process that appears to require the E3 ubiquitin ligase Nedd4-2 (Sorkina et al., 2006). However, intriguingly, we do not find any effect of MKP3 on PMA-induced ubiquitylation of DAT and thus conclude that the process regulated by MKP3 must be downstream from ubiquitylation events that take place while the transporter sits on the plasma membrane (Figure 7). Whether only the initial ubiquitylation of DAT is regulated by PKC activation or whether other steps in internalization are regulated by PKC cannot be established from our data. One possibility is that the machinery for internalization is primed and awaits the ubiquitylated DAT, which is sufficient for activation of internalization process, but that other proteins involved in the internalization of ubiquitylated cargo are regulated by MKP3. It could also be imagined that PKC-dependent phosphorylation of several key proteins occurs simultaneously and multiple processes are activated to produce the internalization of DAT. Thus, one required PKC-dependent step would be turned on by the ubiquitylation of DAT and another step would be turned off by dephosphorylation by MKP3.
To date two different MAP kinase families, p38 and ERK MAP kinases, have been implicated in the regulation of plasma membrane transporters. The p38 MAP kinase was found to inhibit insulin mediated up-regulation of NET in SK-N-SH cells (Apparsundaram et al., 2001), and it was in one study also found to increase membrane insertion of the serotonin transporter (SERT) in a PKC-independent manner in both synaptosomes and HEK-293 cells (Samuvel et al., 2005). In several studies another group found that the activation of the p38 MAP kinase would result in increased intrinsic activity of SERT (Zhu et al., 2004, 2005). The inhibition of the ERK MAP kinases was found in a study to result in a small inhibition of DAT believed to be a result of internalization (Moron et al., 2003), and recently it was similarly found that effects of dopamine receptor activation on DAT regulation were mediated through the MEK/ERK pathway (Bolan et al., 2007). The results of these studies were obtained using inhibitors of MAP kinases that have no effect on the PKC-dependent internalization of DAT in our studies and are likely to involve different mechanisms.
Our results suggest that the three best characterized MAP kinase families are not directly involved in PKC-mediated regulation of the DAT, because the activation state of these kinases does not correlate with the effect of MKP3 on DAT down-regulation in either oocytes or MDCK cells (Figure 6). We did find, as has been found in many cell systems, that ERK is activated by PMA treatment, but using pharmacological inhibitors of the upstream MAP kinase kinase, MEK1 to reduce ERK activation, did not effect the PMA-induced down-regulation of DAT. Moreover, in oocytes, when we used progesterone to activate ERK through a non-PKC–mediated pathway no change in transport activity was observed.
Thus, it seems likely that PKC activates a protein target of MKP related to MAP kinases such as ERK3, ERK7, MOK (DYF-5), MAK, ICK, and DYRK1A (Chen et al., 2001), a hypothesis that is further supported by the fact that only MKP3 and not the closely related MAP kinase phosphatase, MKP1, can prevent the internalization of DAT. Little is known about the signaling roles of these orphan MAP kinases, and even less is known about their involvement in endocytosis and trafficking. One study in Caenorhabditis elegans has examined the orphan MAP kinase DYF-5 and implicated it directly in the docking and undocking of kinesin motors (Burghoorn et al., 2007). Obviously, the identification of the unknown substrate of MKP3 would further our understanding of the mechanism mediating DAT trafficking. It has been found that replacement of the cysteine in the active site of MKPs with serine abolishes catalytic activity, but dramatically stabilizes the otherwise transient interaction between substrate and phosphatase, creating a “substrate-trap” to enable the isolation of substrates of tyrosine-directed phosphatases (Blanchetot et al., 2005).
An issue critical to the physiological relevance of MKP3 to DAT regulation is whether it is expressed in the same cell type. Post hoc analyses (see Materials and Methods) of data on gene expression in laser-captured tyrosine hydroxylase–positive neurons from substantia nigra (SN) and ventral tegmental area (Greene et al., 2005) demonstrate that the DAT and MKP3 are expressed in the same cells. In addition, others have shown that MKP3 expression decreases significantly when dopamine neurons in SN are selectively lesioned by the neurotoxin, 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine (MPTP; Miller et al., 2004).
Our studies in MN9D cells, which exhibit a dopaminergic phenotype (Choi et al., 1992; Chen et al., 2005), also demonstrate that MKP3 is expressed in dopamine neurons; however, in most studies to date, MKP3 displays relatively low basal expression in the brain. Most work on the regulation of intracellular signal transduction has focused on the activity of kinases. However, it has been proposed that the acute activation of phosphatases could be a more effective way of controlling the activity of signaling cascades (Bhalla et al., 2002). This might explain why MKP3 and other MAP kinase phosphatases are relatively nonabundant in the brain, but can be turned on by activity (Boschert et al., 1997). MKP3 is also strongly regulated posttranslationally and displays an enhanced sensitivity to proteasomal degradation that is phosphorylation-dependent (Marchetti et al., 2005). Midbrain dopamine neuron cultures display robust down-regulation (Figure 1) consistent with the idea that MKP3 is turned off in these cells, whereas cells lines such as MN9D or SK-N-SH, which express MKP3, are more refractory to the effects of PKC activation. Because MKP1 and MKP3 mRNAs are up-regulated by acute and chronic treatment of rats with the DAT substrate methamphetamine (Takaki et al., 2001), we hypothesize that MKP3 plays a regulatory homeostatic role in maintaining and stabilizing neurotransmitter transporters on the surface to limit the actions of neurotransmitter during periods of increased neuronal activity.
ACKNOWLEDGMENTS
We thank Prof. John Denu, Prof. Elias Aizenman, Prof. Geoff Murdoch, Prof. Yongjian Liu, Dr. Andreia C.K. Fontana, and all the members of the Amara laboratory for helpful discussions. We also thank Yuqin Yang and Megan Little for their excellent technical assistance. We also thank Prof. Philip Cohen for the generous gift of PD184352, Prof. Stephen Keyse for the generous gift of MKP1, and Drs. Alfred Heller and Lisa Won for the generous gift of MN9D cells. The study was supported by the Alfred Benzon Foundation (O.V.M.) and National Institute on Drug Abuse Grant DA07595 (S.G.A.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-09-0980) on April 23, 2008.
REFERENCES
- Alessi D. R., Smythe C., Keyse S. M. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993;8:2015–2020. [PubMed] [Google Scholar]
- Apparsundaram S., Schroeter S., Giovanetti E., Blakely R. D. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J. Pharmacol. Exp. Ther. 1998;287:744–751. [PubMed] [Google Scholar]
- Apparsundaram S., Sung U., Price R. D., Blakely R. D. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J. Pharmacol. Exp. Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- Bhalla U. S., Ram P. T., Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–1023. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- Blanchetot C., Chagnon M., Dube N., Halle M., Tremblay M. L. Substrate-trapping techniques in the identification of cellular PTP targets. Methods. 2005;35:44–53. doi: 10.1016/j.ymeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bolan E. A., et al. D2 receptors regulate dopamine transporter function via an ERK 1/2-dependent and PI3 kinase-independent mechanism. Mol. Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Boschert U., Muda M., Camps M., Dickinson R., Arkinstall S. Induction of the dual specificity phosphatase PAC1 in rat brain following seizure activity. Neuroreport. 1997;8:3077–3080. doi: 10.1097/00001756-199709290-00014. [DOI] [PubMed] [Google Scholar]
- Brondello J. M., McKenzie F. R., Sun H., Tonks N. K., Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. [PubMed] [Google Scholar]
- Brunet A., Roux D., Lenormand P., Dowd S., Keyse S., Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghoorn J., Dekkers M. P., Rademakers S., de Jong T., Willemsen R., Jansen G. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2007;104:7157–7162. doi: 10.1073/pnas.0606974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. X., Huang S. Y., Zhang L., Liu Y. J. Synaptophysin enhances the neuroprotection of VMAT2 in MPP+-induced toxicity in MN9D cells. Neurobiol. Dis. 2005;19:419–426. doi: 10.1016/j.nbd.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Chen Z., Gibson T. B., Robinson F., Silvestro L., Pearson G., Xu B., Wright A., Vanderbilt C., Cobb M. H. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Choi H. K., Won L., Roback J. D., Wainer B. H., Heller A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc. Natl. Acad. Sci. USA. 1992;89:8943–8947. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Solski P. A., Khosravi-Far R., Der C. J., Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol. Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cousin M. A., Robinson P. J. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Daniels G. M., Amara S. G. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol. Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Dickinson R. J., Keyse S. M. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J. Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- Eblaghie M. C., Lunn J. S., Dickinson R. J., Munsterberg A. E., Sanz-Ezquerro J. J., Farrell E. R., Mathers J., Keyse S. M., Storey K., Tickle C. Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos. Curr. Biol. 2003;13:1009–1018. doi: 10.1016/s0960-9822(03)00381-6. [DOI] [PubMed] [Google Scholar]
- Granas C., Ferrer J., Loland C. J., Javitch J. A., Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol. Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- Greene J. G., Dingledine R., Greenamyre J. T. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol. Dis. 2005;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Holton K. L., Loder M. K., Melikian H. E. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat. Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H., Sen N., Guptaroy B., Johnson L., Lund D., Gnegy M. E., Galli A., Javitch J. A. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS. Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh K., Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Li C., Scott D. A., Hatch E., Tian X., Mansour S. L. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development. 2007;134:167–176. doi: 10.1242/dev.02701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti S., Gimond C., Chambard J. C., Touboul T., Roux D., Pouyssegur J., Pages G. Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol. Cell. Biol. 2005;25:854–864. doi: 10.1128/MCB.25.2.854-864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. M., et al. Dysregulation of gene expression in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse substantia nigra. J. Neurosci. 2004;24:7445–7454. doi: 10.1523/JNEUROSCI.4204-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M., Dionne K. R., Sorkina T., Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol. Biol. Cell. 2007;18:313–323. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M., Sorkina T., Grammatopoulos T. N., Zawada W. M., Sorkin A. Multiple molecular determinants in the carboxyl terminus regulate dopamine transporter export from endoplasmic reticulum. J Biol. Chem. 2004;279:30760–30770. doi: 10.1074/jbc.M312774200. [DOI] [PubMed] [Google Scholar]
- Miranda M., Wu C. C., Sorkina T., Korstjens D. R., Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J. Biol. Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Moron J. A., et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen O. V., Amara S. G. Dynamic regulation of the dopamine transporter. Eur. J. Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Muda M., Boschert U., Dickinson R., Martinou J. C., Martinou I., Camps M., Schlegel W., Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol. Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- Prasad B. M., Amara S. G. The dopamine transporter in mesencephalic cultures is refractory to physiological changes in membrane voltage. J. Neurosci. 2001;21:7561–7567. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. E., Mumby M. C. Brain protein serine/threonine phosphatases. Curr. Opin. Neurobiol. 1999;9:336–342. doi: 10.1016/s0959-4388(99)80049-x. [DOI] [PubMed] [Google Scholar]
- Rintelen F., Hafen E., Nairz K. The Drosophila dual-specificity ERK phosphatase DMKP3 cooperates with the ERK tyrosine phosphatase PTP-ER. Development. 2003;130:3479–3490. doi: 10.1242/dev.00568. [DOI] [PubMed] [Google Scholar]
- Samuvel D. J., Jayanthi L. D., Bhat N. R., Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. G., Sweetman D., Patterson M., Keyse S. M., Munsterberg A. Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite. Development. 2005;132:1305–1314. doi: 10.1242/dev.01699. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Hoover B. R., Zahniser N. R., Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Miranda M., Dionne K. R., Hoover B. R., Zahniser N. R., Sorkin A. RNA interference screen reveals an essential role of Nedd4–2 in dopamine transporter ubiquitination and endocytosis. J. Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M., Ujike H., Kodama M., Takehisa Y., Nakata K., Kuroda S. Two kinds of mitogen-activated protein kinase phosphatases, MKP-1 and MKP-3, are differentially activated by acute and chronic methamphetamine treatment in the rat brain. J. Neurochem. 2001;79:679–688. doi: 10.1046/j.1471-4159.2001.00615.x. [DOI] [PubMed] [Google Scholar]
- Tonks N. K. PTP1B: from the sidelines to the front lines! FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- Torres G. E., Gainetdinov R. R., Caron M. G. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Tsang M., Maegawa S., Kiang A., Habas R., Weinberg E., Dawid I. B. A role for MKP3 in axial patterning of the zebrafish embryo. Development. 2004;131:2769–2779. doi: 10.1242/dev.01157. [DOI] [PubMed] [Google Scholar]
- Wiland A. M., Denu J. M., Mourey R. J., Dixon J. E. Purification and kinetic characterization of the mitogen-activated protein kinase phosphatase rVH6. J Biol. Chem. 1996;271:33486–33492. doi: 10.1074/jbc.271.52.33486. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R., Doolen S. Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Zhu C. B., Carneiro A. M., Dostmann W. R., Hewlett W. A., Blakely R. D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol. Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu C. B., Hewlett W. A., Feoktistov I., Biaggioni I., Blakely R. D. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]