Abstract
Resequencing genes in individuals at extremes of the population distribution constitutes a powerful and efficient strategy to identify sequence variants associated with complex traits. An excess of sequence variants at one extreme relative to the other that is not due to chance or to population stratification constitutes evidence for genetic association and implies the presence of functionally significant sequence variants. Recently, we reported that non-synonymous sequence variants in Niemann–Pick type C1-like 1 (NPC1L1), an intestinal cholesterol transporter, were significantly more common among individuals with low cholesterol absorption than in those with high cholesterol absorption. To determine whether sequence variations identified in individuals with low cholesterol absorption affect protein function, we performed studies in cultured cells and in families. Expression of the mutant proteins in Chinese hamster ovarian-K1 cells revealed that a majority (14 of 20) of the variants identified in low absorbers were associated with very low levels of NPC1L1 protein. In two extended families, mean cholesterol absorption levels, as measured using stable isotopes, were significantly lower in family members with the sequence variants than in those without the variant. These data indicate that the excess of sequence variations in individuals with extreme phenotypes reflects an enrichment of functionally significant variants. These findings are consistent with in silico predictions that some sequence variations found in healthy individuals are as deleterious to protein function as mutations that, in other genes, cause monogenic diseases. Such sequence variations may explain a significant fraction of quantitative phenotypic variation in humans.
INTRODUCTION
The uptake of cholesterol from the intestine is a major source of sterols in humans. Individuals consuming Western diets ingest ∼400 mg of dietary cholesterol each day, and an additional ∼1000 mg of cholesterol enters the intestine via the bile (1). On average, ∼50% of the cholesterol entering the intestine is absorbed, but the fraction ranges from as little as 29% to as much as 80% among normal individuals (2). The wide variation in efficiency of cholesterol absorption is largely attributable to heritable factors (3,4). Plasma levels of the plant sterols, campesterol and sitosterol, which provide an index of cholesterol absorption (5), are strongly correlated between parents and offspring, but not between spouses (4). The heritability of plasma levels of plant sterols is significantly higher than that of plasma cholesterol levels (over 80 versus 50%) (3,4). Furthermore, plasma levels of these plant sterols were significantly more highly correlated between identical twins than between fraternal twins (3,4).
The molecular mechanisms of cholesterol absorption have not been fully defined, and the factors underlying the wide variation in fractional cholesterol absorption are poorly understood. Cholesterol is solubilized into mixed micelles by bile acids in the proximal small intestine and taken up into enterocytes, where it is esterified with fatty acids by acyl-cholesterol acyltransferase. The esterified cholesterol is then packaged into large, triglyceride-rich lipoproteins called chylomicrons, which are secreted from the basolateral surfaces of enterocytes into the lymphatic system. Variation in the efficiency of any of these processes may contribute to differences in the efficiency of cholesterol absorption.
The uptake of cholesterol into the enterocyte is facilitated by the polytopic membrane protein, Niemann–Pick type C1-like 1 (NPC1L1) (6). Mice lacking NPC1L1 (Npc1l1−/−) have markedly reduced absorption of cholesterol and plant sterols (6). NPC1L1 is the target of ezetimibe (7), a drug that blocks cholesterol absorption, and several studies have reported that sequence variations in NPC1L1 are associated with responsiveness to ezetimibe (8–11), and statins (12).
Recently, we examined the contribution of sequence variation in NPC1L1 to variation in cholesterol absorption in humans (13). Plasma levels of plant sterols (sitosterol and campesterol) and cholesterol precursor sterols (lathosterol) were measured in 3557 individuals from a population-based study (The Dallas Heart Study) (14). The campesterol:lathosterol ratio was used as a surrogate marker of cholesterol absorption. High rates of cholesterol absorption result in reduced endogenous cholesterol synthesis, which is reflected in lower plasma levels of lathosterol. Conversely, low absorption of cholesterol results in increased cholesterol synthesis, and thus higher lathosterol levels. Thus, a high campesterol:lathosterol ratio is indicative of efficient sterol absorption, whereas a low ratio indicates a low fractional absorption of sterols (15). A significantly higher number of missense sequence variations in NPC1L1 were found in individuals with a low fractional cholesterol absorption than in those with a high fractional cholesterol absorption, whereas the prevalence of synonymous sequence variants was similar between the two groups (13). Alleles associated with low cholesterol absorption were present in 6% of the African-Americans, and in ∼2% of whites and Hispanics. Analysis of the entire Dallas Heart Study revealed that plasma levels of low-density lipoprotein cholesterol (LDL-C) were significantly lower among African-Americans with sequence variants associated with low cholesterol absorption than those without the variants, even after removing those individuals in the extremes in which the variants were first identified.
The excess of missense sequence variations among low absorbers may reflect an increased prevalence of defective NPC1L1 alleles in this group, occult differences in the genetic ancestries of the high and low absorbers (population stratification) or chance fluctuations in allele frequencies. Chance fluctuations in allele frequencies were excluded by replication of the findings in a second sample of high and low absorbers drawn from the same population, and analysis of 2000 ancestry informative SNPs revealed no evidence of population stratification (13). Taken together, these findings are consistent with the hypothesis that the non-synonymous sequence variations we identified in individuals with low campesterol:lathosterol ratios impair NPC1L1 function, resulting in a reduced cholesterol absorption and lower plasma levels of LDL-C.
To directly evaluate this hypothesis, we examined the effects of the sequence variations in NPC1L1 on protein expression in cultured Chinese hamster ovarian (CHO) cells. Here we show that a large proportion of these sequence variations destabilized the expressed protein, resulting in significantly lower steady-state levels of NPC1L1 in the cells. These analyses were complemented by studies in the families of individuals with non-synonymous sequence variations that were associated with very low NPC1L1 levels in heterologous cells. We found a significant reduction in cholesterol absorption in the subjects with the NPC1L1 variants compared with their unaffected family members.
RESULTS
Sequence variations associated with low cholesterol absorption destabilize NPC1L1
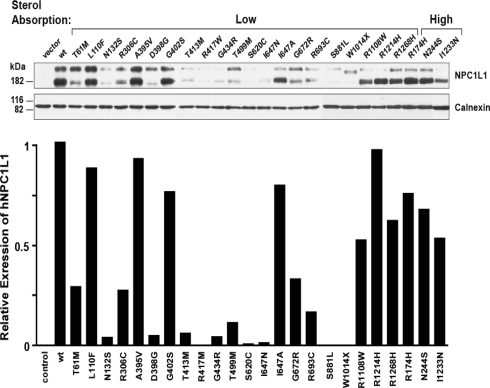
Transfection of constructs expressing wild-type NPC1L1 into CHO-K1 cells resulted in robust expression of a protein with an apparent molecular weight of 175 kDa, which is significantly greater than that with 145 kDa predicted from the amino acid sequence (Fig. 1). Treatment of the expressed protein with PNGase F reduced its apparent molecular weight to ∼150 kDa (Supplementary Material, Fig. S1), indicating that NPC1L1 is glycosylated. Endonuclease H did not reduce the apparent molecular weight of the expressed protein, indicating that almost all of the NPC1L1 present in these cells have exited the endoplasmic reticulum and traversed the trans-Golgi. A higher molecular weight band was also consistently observed in cells transfected with NPC1L1, but not in mock transfected cells.
Figure 1.
Expression of wild-type and mutant NPC1L1 in CHO-K1 cells. Plasmids expressing human NPC1L1 were transfected into CHO-K1 cells. After 48 h, cells were collected and lysed by passage through a 23G needle. Cell membranes were pelleted by ultracentrifugation, resuspended in SDS buffer and size-fractionated on 8% polyacrylamide gels. Proteins were then transferred to Hybond-C extra nitrocellulose filters (Amersham Biosciences) and visualized by immunoblotting with antibodies against hNPC1L1 and calnexin as described in Materials and Methods. The X-ray film was scanned using a UMAX PowerLook III scanner and the intensity of each band was calculated using the computer program Scion Image.
Of the 20 alleles found in the low cholesterol absorbers, 14 were associated with markedly reduced expression of NPC1L1 (65–100% decrease) in CHO-K1 cells (Fig. 1). This finding suggests that the amino acid changes resulting from these nucleotide substitutions reduced the stability of the expressed protein. The reduction in protein expression from these alleles was consistent across at least three replicate transfections, using two independent clones for each construct (not shown). The 14 sequence variants associated with low NPC1L1 levels were distributed throughout the protein, and the mechanism(s) by which they perturb protein stability is not known. Expression levels of three alleles found in high absorbers were comparable with that of the wild-type protein (Fig. 1).
Sequence variations I647N and R693C destabilize NPC1L1 and decrease cholesterol absorption
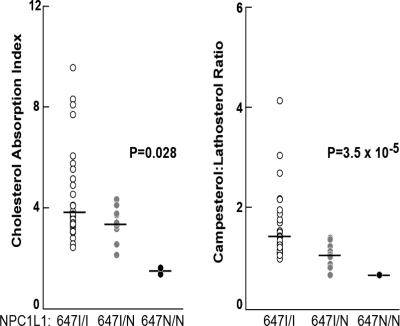
By screening family members of probands with sequence variations in NPC1L1, we identified two large extended pedigrees in which multiple individuals carried an NPC1L1 allele that encoded an unstable NPC1L1 protein. In family DHS14, two individuals were homozygotes for the I647N substitution, nine were heterozygotes and 26 were homozygotes for the wild-type allele. The sequence variant showed a statistically significant monotonic gene dosage effect on cholesterol absorption (Fig. 2) assessed by the area under the curve of deuterated cholesterol (P = 0.028) and the campesterol:lathosterol ratio (P = 3.5 × 10−5).
Figure 2.
Cholesterol absorption and plasma campesterol:lathosterol ratios in a family segregating the I647N mutation in NPC1L1. Individuals were given 90 mg deuterated cholesterol (25D3, 26D3) in 9 ml medium-chain triglyceride oil (Novartis), and fasting blood samples were drawn before and 24, 48, 72 and 96 h after cholesterol ingestion. Plasma levels of native cholesterol, hexadeuterated cholesterol, campesterol and lathosterol were determined using gas-chromatography and mass spectrometry. The cholesterol absorption index was calculated as the area under the curve of the deuterated cholesterol:native cholesterol ratio plotted against time. P-values were calculated using the Jonckheere–Terpstra test. I, isoleucine; N, asparagines.
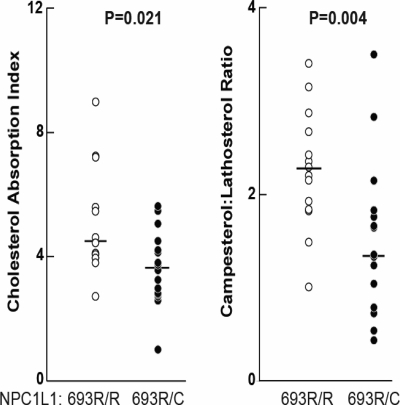
The second family, DHS9, included 16 individuals who were heterozygotes for the R693C substitution and 14 other family members who were homozygotes for the wild-type allele. Cholesterol absorption was significantly lower in R693C heterozygotes than in their family members who did not share this allele (Fig. 3) as determined from the cholesterol absorption index (P = 0.021) or the campesterol:lathosterol ratio (P = 0.004).
Figure 3.
Cholesterol absorption and plasma campesterol:lathosterol ratios in a family segregating the R643C mutation in NPC1L1. For each individual in the family, the cholesterol absorption index and campesterol:lathosterol ratio were determined as described in the legend to Figure 2. P-values were calculated using the Wilcoxon rank sum test. R, arginine; C, cysteine.
DISCUSSION
The major finding of this study is that the majority of the non-synonymous sequence variations in NPC1L1 identified by resequencing individuals with plasma campesterol:lathosterol levels at the extremes of the population distribution had severe adverse effects on the expressed protein. Of 20 non-synonymous sequence variants identified only in low absorbers, 14 markedly reduced expression of recombinant NPC1L1 when expressed in CHO-K1 cells. Measurements of cholesterol absorption in two large extended families confirmed that two of these mutations (I647N and R693C) that were associated with decreased NPC1L1 expression were also associated with reduced uptake of dietary cholesterol. Previously, we showed that 6% of African-Americans and 2% of whites and Hispanics carry one of these sequence variants (13). Therefore, missense sequence variations that destabilize NPC1L1 and markedly reduce the levels of this protein are common in the population and lead to reduced cholesterol absorption and lower levels of plasma LDL-C.
The sequence variations examined in this study were identified by sequencing NPC1L1 in individuals with campesterol:lathosterol ratios in the upper or lower 10% of the population (13). Comparison of the numbers of sequence variants found in individuals with trait levels at opposite ends of the distribution provides a simple, unbiased strategy to detect functionally significant sequence variations. A limitation of this approach is that some sequence variants may occur in individuals with extreme phenotypes purely by chance. Furthermore, sequence variants will be enriched at the extremes of the trait distribution even if they have modest effects on trait levels. Therefore, further studies are required to assess the functional consequences of individual sequence variants. As a first step towards functional characterization of the missense sequence changes in NPC1L1, we expressed the recombinant mutant proteins in CHO-K1 cells. The finding that 14 of the 20 amino acid substitutions reduce the levels of NPC1L1 protein expressed suggests that these mutations act primarily by preventing the formation of a stable protein, rather than by altering specific residues required for cholesterol transport. This finding is consistent with a large-scale in silico analysis, which indicated that a majority of mutations responsible for monogenic diseases cause loss of protein structure stability (16). Since we did not measure protein stability directly, we cannot exclude the possibility that some of the mutations decrease protein expression by reducing transcription, RNA stability or protein translation.
Previously we determined that the sequence variations found in low absorbers altered residues that showed strong evolutionary conservation: 12 of the 20 residues were completely conserved from humans to bony fish (13). In contrast, the amino acids altered by sequence variations in the high absorbers, or in both groups, were much more variable among species. Interestingly, the sequence variations that resulted in unstable proteins altered both highly conserved and poorly conserved residues. Of the 14 sequence variations associated with reduced protein expression, eight were in completely conserved residues. Mutations in the other four completely conserved residues had little effect on protein levels. These amino acid substitutions may have more specific effects on NPC1L1 function, such as substrate binding or translocation. Alternatively, their association with low cholesterol absorption may be spurious.
The sequence variants associated with low cholesterol absorption were individually too rare to allow meaningful statistical analysis of any single variant in the Dallas Heart Study. Accordingly, we ascertained family members of probands for more direct estimates of cholesterol absorption using the single-isotope-labeled method of Wang et al. (17). In 67 individuals from two large extended pedigrees, cholesterol absorption was significantly lower in individuals with sequence variants in NPC1L1 (I647N or R693C) than in those who did not have these variants. Very similar results were obtained using the plasma campesterol:lathosterol ratio. This finding confirms that the low campesterol:lathosterol ratios in individuals with sequence variants in NPC1L1 reflect low fractional absorption of cholesterol in these individuals.
Pharmacological blockade of cholesterol absorption is an effective strategy for LDL-lowering. Ezetimibe, the most commonly used inhibitor of cholesterol absorption, reduces cholesterol absorption by binding NPC1L1 (7) and is associated with reduction in plasma level of LDL-C of 18% (18). Previously, we found that heterozygotes for the non-synonymous variants in NPC1L1 identified in the low campesterol:lathosterol group had a mean reduction in LDL-C of 9%, which is consistent with the loss of one NPC1L1 allele. The finding that a majority of these sequence variants resulted in very low levels of NPC1L1 protein in cultured cells is thus congruent with the magnitude of the reduction in LDL-C observed in vivo.
The cholesterol-lowering efficacy of ezetimibe varies widely among individuals. Since ezetimibe targets NPC1L1, variation in NPC1L1 may contribute to individual variation in responsiveness to ezetimibe. Sequence variations in NPC1L1 have been associated with responsiveness to ezetimibe in several small studies (8–11). Wang et al. (8) sequenced the exons of NPC1L1 in non-responders to ezetimibe and identified a compound heterozygote for two non-synonymous sequence variations in the gene. Since these variants were not identified in any other individuals, their relationship to ezetimibe responsiveness could not be determined. However, one of these variants (I1233N) was subsequently identified in an individual with a high campesterol:lathosterol ratio and is therefore unlikely to be a loss-of-function allele. These authors also identified a common NPC1L1 haplotype that was associated with a greater reduction in plasma LDL-C, with ezetimibe in 101 dyslipidemic subjects (10). Simon et al. (9) identified a 3-SNP haplotype that was associated with responsiveness to ezetimibe in two independent studies: carriers of the minor alleles had a significantly improved response to ezetimibe compared with non-carriers. One of the SNPs in this haplotype, g.1679C>G (L272L), was subsequently reported to be associated with increased response to ezetimibe in two small cohorts of hyperlipidemic individuals (11). In each of these studies, none of the SNPs in NPC1L1 was associated with baseline lipid levels. This is consistent with our finding that none of the 32 common sequence variations in NPC1L1 is associated with baseline levels of plasma plant sterols, cholesterol or LDL-C (13). Thus, sequencing individuals with extreme phenotypes and analysis of common sequence variants at a locus have provided complementary insights into the genetic architecture of a complex trait.
Of the ∼25 000 genes in the human genome, approximately 2000 have been associated with monogenic diseases. Thus, for the significant majority of human genes, no monogenic disease-causing mutation has been identified. Yue et al. (16) noted three possible differences between proteins associated with monogenic diseases and those that are not. First, the absence of disease-causing mutations in a gene may reflect intrinsic robustness of the protein to amino acid substitution. Robustness to mutation is unlikely to account for the lack of mutations in a large proportion of genes, since nonsense mutations that totally abolish gene function can arise in any gene. Alternatively, mutations in a gene may lead to early fetal death and thus never be recognized as disease-causing. However, data from knockout mice suggest that only a small proportion of genes (10–15%) are essential for viability (19,20). A third possibility is that for some genes, even mutations that disrupt the function of the expressed protein do not result in a severe phenotype. The identification of two apparently healthy adults who are homozygous for mutations that prevent expression of stable NPC1L1 protein indicates that even chronic deficiency of this protein is not associated with significant adverse clinical sequelae. Rather, mutations that severely impair NPC1L1 function result in modest, quantitative phenotypic variation. This finding is consistent with our previous observations with other genes involved in lipid metabolism (21,22) and may be widely applicable: several non-synonymous sequence variations present in apparently healthy individuals appear to be at least as deleterious to protein function as mutations found in monogenic diseases (16). These sequence variations may explain a significant fraction of quantitative phenotypic variation in humans.
MATERIALS AND METHODS
Study subjects
The study protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. Participants in the Dallas Heart Study who had sequence variations in NPC1L1 associated with low plasma campesterol:lathosterol ratios were eligible for the study. Eligible individuals were contacted by telephone, and two had large extended families in the Dallas area that were willing to participate in the study.
Measurement of cholesterol absorption
Subjects were seen at home or in the outpatient clinic of the General Clinical Research Center in Parkland Hospital, Dallas, TX, USA. Initial blood samples were drawn after an overnight fast to measure plasma lipoprotein levels and assess thyroid status and liver function (thyroid-stimulating hormone, alkaline phosphatase, aspartate and alanine transaminase, creatinine and albumin). Two days after the initial blood draw, a second fasting blood sample was drawn and cholesterol absorption was measured using a single isotope-labeled cholesterol tracer (17). Subjects were given 90 mg deuterated cholesterol (25D3, 26D3) (Medical Isotopes, Inc.) in 9 ml medium-chain triglyceride oil (Novartis). Blood samples were drawn 24, 48, 72 and 96 h after cholesterol ingestion. Plasma was separated by centrifugation at 2500g for 10 min at 4°C, separated into aliquots (200 µl) and stored frozen at −80°C.
Measurements of the isotopic cholesterol in plasma
The amount of deuterated cholesterol in the plasma was assayed by gas-chromatography mass spectrometry as described previously (23). The mass spectrometer was run in selected ion-monitoring mode. Native cholesterol was measured at m/z 458, and the deuterated cholesterol was measured at m/z 464. In each sample, the concentration of deuterated cholesterol was normalized to the concentration of native cholesterol, and the area under the curve of the deuterated cholesterol plotted against time was calculated by numerical integration using the trapezoidal method, exactly as described (17).
Construction of pcDNA3.1-hL1 plasmid and hNPC1L1 sequence variant plasmids
Complementary DNA from human intestine was used as template to amplify hNPC1L1 cDNA (GenBankTM accession number AY437865) by PCR. Two PCR primers (5′-GCTCTAGACTTGGCTGTTCCTGAGGCCTGGC-3′ and 5′-CGGGATCCTCAGAACTGCCGCCCATTGTTGG-3′) were synthesized with XbaI and BamHI restriction sites at their 5′ ends. The gel-purified 4 kb PCR products were treated with XbaI and BamHI and cloned into pcDNA3.1(-) vector (Invitrogen). Resulting recombinant plasmid was verified by sequencing using T7 and gene-specific primers. Site-directed mutagenesis was used to generate plasmids expressing 20 mutations identified in the low cholesterol absorber group and three mutations found in the high cholesterol absorber group. (13).
Preparation of anti-hNPC1L1 antibody
DNA fragments encoding amino acids 29–253, 404–614 and 860–1100 of NPC1L1 were amplified by PCR and cloned into the bacterial expression vector pET-28a(+) (Novagen). The expressed proteins were purified using Ni-NTA beads (Qiagen) and verified by SDS–PAGE. A mixture of three purified peptides was used to immunize rabbits.
Cell culture and preparation of whole-cell membranes
CHO-K1 cells were maintained in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium containing 100 units/ml penicillin and 100 µg/ml streptomycin sulfate supplemented with 5% fetal calf serum. Cells were grown at 37°C in 8–9% CO2. For transfection, CHO-K1 cells were set up at a density of 7 × 105 cells/10 cm dish on day 0. On day 1, cells were transfected with 2 µg plasmid DNA/dish using FuGene 6 (Roche Pharmaceuticals). After 48 h, cells were collected and whole-cell membrane was prepared.
CHO-K1 cells from each dish were washed twice with PBS and resuspended in 1 ml TES buffer (20 mm Tris–HCL, 1 mm EDTA, 250 mm sucrose, pH 7.4). Cells were then passed through a 23G needle 30 times and centrifuged at 600g for 10 min. The supernatant was collected and centrifuged at 100 000g for 30 min to obtain the membrane pellet. The pellet was resuspended in loading buffer (3% SDS, 100 mm NaCl, 10 mm Tris–HCL, 1 mm EDTA, pH 6.8). Protein concentration was determined using a BCA kit (Pierce Biotechnology Inc.).
Immunoblot analysis
Thirty micrograms of membrane protein was size-fractionated on 8% SDS–PAGE gels. Protein was then transferred to Hybond-C extra nitrocellulose filters (Amersham Biosciences), immunoblotted using anti-hNPC1L1 serum from immunized rabbits (1:1000 dilution) and calnexin (Stressgen, Victoria, BC, Canada), incubated with HRP-conjugated goat anti-rabbit IgG (Sigma-Aldrich) and visualized using SuperSignal substrate system (Pierce Biotechnology Inc.).
Statistical methods
The cholesterol absorption index and plasma campesterol:lathosterol ratios were compared using the Wilcoxon rank sum test (to compare two genotype groups) and the Jonckheere–Terpstra test, a distribution-free test for ordered differences among classes (to compare three genotype groups).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from the Donald W. Reynolds Foundation, the W. M. Keck Foundation, the National Institutes of Health (HL 20948) and The Perot Family Fund.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tommy Hyatt and Fang Xu for technical assistance, Barbara Gilbert for help with the cholesterol absorption studies and Scott M. Grundy for helpful discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Grundy S.M. Absorption and metabolism of dietary cholesterol. Ann. Rev. Nutr. 1983;3:71–96. doi: 10.1146/annurev.nu.03.070183.000443. [DOI] [PubMed] [Google Scholar]
- 2.Bosner M.S., Lange L.G., Stenson W.F., Ostlund R.E., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 1999;40:302–308. [PubMed] [Google Scholar]
- 3.Boomsma D.I., Princen H.M., Frants R.R., Gevers Leuven J.A., Kempen H.J. Genetic analysis of indicators of cholesterol synthesis and absorption: lathosterol and phytosterols in Dutch twins and their parents. Twin Res. 2003;6:307–314. doi: 10.1375/136905203322296674. [DOI] [PubMed] [Google Scholar]
- 4.Berge K.E., von Bergmann K., Lutjohann D., Guerra R., Grundy S.M., Hobbs H.H., Cohen J.C. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 2002;43:486–494. [PubMed] [Google Scholar]
- 5.Miettinen T.A., Tilvis R.S., Kesaniemi Y.A. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism. 1989;38:136–140. doi: 10.1016/0026-0495(89)90252-7. [DOI] [PubMed] [Google Scholar]
- 6.Altmann S.W., Davis H.R., Jr, Zhu L.J., Yao X., Hoos L.M., Tetzloff G., Iyer S.P., Maguire M., Golovko A., Zeng M., et al. Niemann–Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Calvo M., Lisnock J., Bull H.G., Hawes B.E., Burnett D.A., Braun M.P., Crona J.H., Davis H.R., Jr, Dean D.C., Detmers P.A., et al. The target of ezetimibe is Niemann–Pick C1-Like 1 (NPC1L1) Proc. Natl Acad. Sci. USA. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Williams C.M., Hegele R.A. Compound heterozygosity for two non-synonymous polymorphisms in NPC1L1 in a non-responder to ezetimibe. Clin. Genet. 2005;67:175–177. doi: 10.1111/j.1399-0004.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 9.Simon J.S., Karnoub M.C., Devlin D.J., Arreaza M.G., Qiu P., Monks S.A., Severino M.E., Deutsch P., Palmisano J., Sachs A.B., et al. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics. 2005;86:648–656. doi: 10.1016/j.ygeno.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Hegele R.A., Guy J., Ban M.R., Wang J. NPC1L1 haplotype is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis. 2005;4:16. doi: 10.1186/1476-511X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisciotta L., Fasano T., Bellocchio A., Bocchi L., Sallo R., Fresa R., Colangeli I., Cantafora A., Calandra S., Bertolini S. Effect of ezetimibe coadministered with statins in genotype-confirmed heterozygous FH patients. Atherosclerosis. 2007;194:e116–e122. doi: 10.1016/j.atherosclerosis.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Chan D.C., Watts G.F., Wang J., Hegele R.A., van Bockxmeer F.M., Barrett P.H. Variation in Niemann–Pick C1-Like 1 gene as a determinant of apolipoprotein B-100 kinetics and response to statin therapy in centrally obese men. Clin. Endocrinol. 2007 doi: 10.1111/j.1365-2265.2007.03144.x. in press. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J., Pertsemlidis A., Fahmi S., Esmail S., Vega G., Grundy S., Hobbs H. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc. Natl Acad. Sci. USA. 2006;103:1810–1815. doi: 10.1073/pnas.0508483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victor R.G., Haley R.W., Willett D.L., Peshock R.M., Vaeth P.C., Leonard D., Basit M., Cooper R.S., Iannacchione V.G., Visscher W.A., et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am. J. Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen T.A., Tilvis R.S., Kesaniemi Y.A. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 16.Yue P., Li Z., Moult J. Loss of protein structure stability as a major causative factor in monogenic disease. J. Mol. Biol. 2005;353:459–473. doi: 10.1016/j.jmb.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Vanstone C.A., Parsons W.D., Jones P.J. Validation of a single-isotope-labeled cholesterol tracer approach for measuring human cholesterol absorption. Lipids. 2004;39:87–91. doi: 10.1007/s11745-004-1206-6. [DOI] [PubMed] [Google Scholar]
- 18.Knopp R.H., Gitter H., Truitt T., Bays H., Manion C.V., Lipka L.J., LeBeaut A.P., Suresh R., Yang B., Veltri E.P. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur. Heart J. 2003;24:729–741. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 19.Liao B.Y., Zhang J. Mouse duplicate genes are as essential as singletons. Trends Genet. 2007;23:378–381. doi: 10.1016/j.tig.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Wilson L., Ching Y.H., Farias M., Hartford S.A., Howell G., Shao H., Bucan M., Schimenti J.C. Random mutagenesis of proximal mouse chromosome 5 uncovers predominantly embryonic lethal mutations. Genome Res. 2005;15:1095–1105. doi: 10.1101/gr.3826505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z., Tuakli-Wosornu Y., Lagace T.A., Kinch L., Grishin N.V., Horton J.D., Cohen J.C., Hobbs H.H. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 2006;79:514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romeo S., Pennacchio L.A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H.H., Cohen J.C. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilund K.R., Yu L., Xu F., Vega G.L., Grundy S.M., Cohen J.C., Hobbs H.H. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler. Thromb. Vasc. Biol. 2004;24:2326–2332. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.