Abstract
Recognition memory and anxiety were examined in nulliparous (NP: 0 litters) and multiparous (MP: 5–6 litters) middle-aged female rats (12 months old) to assess possible enduring effects of multiparity at least 3 months after last litter was weaned. MP females performed significantly better than NP females on the non-spatial memory task, object recognition, and the spatial memory task, object placement. Anxiety as measured on the elevated plus maze did not differ between groups. Monoaminergic activity and levels were measured in prefrontal cortex, CA1 hippocampus, CA3 hippocampus, and olfactory bulb (OB). NP and MP females differed in monoamine concentrations in the OB only, with MP females having significantly greater concentrations of dopamine and metabolite DOPAC, norepinephrine and metabolite MHPG, and the serotonin metabolite 5-HIAA, as compared to NP females. These results indicate a long-term change in OB neurochemistry as a result of multiparity. Brain-derived neurotrophic factor (BDNF) was also measured in hippocampus (CA1, CA3, dentate gyrus), and septum. MP females had higher BDNF levels in both CA1 and septum; as these regions are implicated in memory performance, elevated BDNF may underlie the observed memory task differences. Thus, MP females (experiencing multiple bouts of pregnancy, birth, and pup rearing during the first year of life) displayed enhanced memory task performance, but equal anxiety responses, as compared to NP females. These results are consistent with previous studies showing long-term changes in behavioral function in MP, as compared to NP, rats, and suggest that alterations in monoamines and a neurotrophin, BDNF, may contribute to the observed behavioral changes.
Keywords: reproductive experience, multiparity, recognition memory, monoamines, BDNF
Rats with reproductive experience (pregnancy, birth and pup rearing) demonstrate persistent behavioral changes which can outlast the period of maternal care, the most well-known of which is a prolonged memory for and maternal response towards pups, months after their initial exposure (Bridges, 1975). In addition to the display of ‘traditional’ maternal behaviors, further behavioral alterations occur that may be essential for offspring care. Specifically, parous females (females who have given birth and lactated; see Kinsley et al., 1999) have a reduced fear response as compared to nulliparous females (NP: females with no mating or reproductive experience), which may be necessary to accept and care for formerly fear-inducing pups (Fleming and Luebke, 1981). Similarly, reproductive experience alters anxiety and the stress response. Parous females have significantly decreased c-fos mRNA expression in the hypothalamus, medial amygdala and lateral septum after restraint stress in comparison to NP females (da Costa et al., 1996; Wartella et al., 2003). Parous females also have decreased anxiety-like behavior as measured in the open field (Wartella et al., 2003) and on the elevated plus maze (Neumann, 2001; Lonstein, 2005). Furthermore, recent work indicates that multiparity continues to decrease anxiety as measured on the elevated plus maze up to 18 months after last weaning (Love et al., 2005), indicating effects on behavior throughout the female’s lifespan.
When tested less than three months after last parturition, females with prior reproductive experience show enhanced spatial memory (Kinsley et al., 1999; Pawluski et al., 2006a) and foraging ability (Lambert et al., 2005) as compared to NP females, which may allow dams to spend less time and energy away from pups while searching for food. Interestingly, parous females (primiparous and multiparous) continue to outperform nulliparous females on a dry-land version of the Morris water maze at 6, 12, 18 and 24 months of age (Gatewood et al., 2005), and are significantly faster than NP females to find a baited food well at 13 months of age (Love et al., 2005), long past their last reproductive experience. However, one recent study indicated that, compared to NP females, primiparous but not multiparous females have significantly better spatial memory on the radial arm maze (Pawluski et al., 2006b). Thus, while parity reliably enhances spatial memory, whether multiple reproductive experiences further benefits memory warrants further investigation.
The mechanisms underlying the enduring effects of parity on anxiety and memory remain largely unexplored. Previous research has focused primarily on structural and synaptic changes in the hippocampus (Tomizawa et al., 2003; Kinsley et al., 2006), neurogenesis in the olfactory bulb (Shingo et al., 2003), and monoaminergic neurotransmitters in the medial preoptic area of the hypothalamus (Lonstein et al., 2003). In the current study, we have chosen to focus primarily on three regions associated with memory task performance: the hippocampus, prefrontal cortex, and olfactory bulb. Based upon abundant literature indicating a role in memory task performance, two neurochemical systems (monoaminergic neurotransmitters and brain-derived neurotrophic factor, BDNF) were examined in the above regions as possible means by which reproductive experience could affect memory task performance, likely through altered release or activity.
Specifically, memory task performance is affected by monoaminergic activity in the hippocampus (Lemon and Manahan-Vaughan, 2006; O’Carroll et al., 2006), prefrontal cortex (Marrs et al., 2005; Moore et al., 2005; Lapiz and Morilak, 2006), and olfactory bulb (Fleming and Rosenblatt, 1974; Pissonnier et al., 1985; Dickinson and Keverne, 1988; Levy et al., 1990; Calamandrei et al., 1992; Guan and Dluzen, 1994). If elevations in monoamines during pregnancy and lactation remain long-term in the aforementioned brain regions, then these neurochemicals may underlie the effects of parity on memory task performance.
BDNF is found throughout the brain and has many roles, including neuronal maintenance into adulthood (Allen and Dawbarn, 2006). In particular, hippocampal BDNF is of importance due to its role in learning and memory (Alonso et al., 2002; Tyler et al., 2002), particularly spatial memory (Radecki et al., 2005), as well as the well-accepted cellular substrate of learning and memory, long-term potentiation (Ying et al., 2002; Pang and Lu, 2004). Cholinergic input from the septum seems to modulate spatial memory (Smith and Pang, 2005); these same neurons transport BDNF protein from hippocampus to septum (Sobreviela et al., 1996). Furthermore, BDNF protein levels are naturally elevated during proestrus and estrus (Scharfman et al., 2003), and changes in BDNF mRNA and protein have been reported in ovariectomized rats after acute (Gibbs, 1999) and chronic (Zhou et al., 2005) estradiol treatment (for review see Scharfman and MacLusky, 2006). If multiparity exerts long-term changes on hippocampal (or septal) BDNF protein, it is possible that BDNF may underlie any positive effects of parity on spatial memory.
In this study, age-matched nulliparous (NP: virgin) and multiparous (MP: 5–6 litters) female rats were compared on a non-spatial memory task, object recognition (OR), and a spatial memory task, object placement (OP). To date, these two tasks have not been used to examine memory changes due to reproductive experience, and the effects of parity as defined here (5–6 litters) have yet to be assessed in relation to memory ability. In addition, anxiety was assessed on the elevated plus maze (EPM) to determine any anxiolytic effect of multiparity. Monoaminergic levels and activity, and BDNF, were measured in a variety of brain regions as possible mechanisms underlying possible behavior differences. Results show that 1) multiparity is associated with better memory, but does not affect anxiety; 2) multiparity significantly alters monoaminergic concentration and activity in the OB; and 3) MP females have elevated BDNF protein levels in CA1 hippocampus and septum as compared to NP females.
Methods
Cohort 1
Subjects
Sixteen 11-month-old Fisher-344 rats (nulliparous: NP = 8; multiparous: MP = 8) were obtained from the NIA colony at Harlan, Inc (Indianapolis, IN) and double-housed under a 12:12 light:dark cycle (lights on at 0500 h) with water and food (Harlan Teklad Rodent Chow) available ad libitum. Upon arrival in our facility, MP females were approximately one month past last weaning of pups, and had at least 5 litters throughout the first year of life. There were no weight differences between the groups. Experiments began after a two-week acclimation period to their home cage, during which all subjects were handled daily by the experimenter. All testing occurred between 1000 and 1500 hours. All procedures used were approved by the IACUC at Hunter College of the City University of New York.
Following behavioral testing and prior to sacrifice at 13 months of age, one 13-month old NP female died and her blood and tissue were therefore unavailable for hormonal or neurochemical analyses. All other females were sacrificed by immediately after completion of all behavior tasks (see below) by decapitation following light anesthesia by carbon dioxide. Brains were removed and blocked just behind the prefrontal cortex (PFC; removed and frozen on dry ice) and just anterior to the cerebellum (discarded), then quickly frozen in dry ice and stored at −70°C to prevent degradation of the samples until brain neurochemical analysis (see below).
Object recognition and placement tasks
Rats were habituated to the object recognition (OR) and object placement (OP) tasks as described previously (Bisagno et al., 2002; Luine et al., 2003). Briefly, each task consisted of a sample trial (T1) and a recognition/retention trial (T2). Rats were given four days of exposure to the OR task with increasingly larger inter-trial delays (1 min, 10 min, 1 hour, 2 hour) between T1 and T2; delays of 2 and 4 hours were used for testing. During T1, two identical objects were placed at one end of an open field (70 × 70cm × 30cm high); the amount of time the rat explored both objects was recorded for 3 min. During T2, at the end of the inter-trial delay, one object was replaced; exploration of the old and new object was recorded. Following habituation, OR was tested over two days, with a 2 hour inter-trial delay followed by a 4 hour inter-trial delay; these tests were used for data analysis
Rats were subsequently given four days of habituation to the OP task with increasingly larger inter-trial delays (10 min, 40 min, 1 hour, 2 hour) between T1 and T2; delays of 2 and 4 hours were used for testing. T1 was identical to the OR task; during T2, the same two objects were again presented, with one object moved to a new location. Following habituation to OP, testing occurred over two test days, with a 2 hour inter-trial delay followed by a 4 hour inter-trial delay; these tests were used for data analysis. Throughout habituation and testing on both tasks, novel objects were presented each day. Exploration of the objects was defined as any time in which the subject sniffed at, whisked at, or looked at the objects from no more than 2 cm away. Females were approximately 12 months old at completion of OR and OP tasks.
Elevated Plus Maze
Elevated plus maze (EPM) protocol was adapted from Pellow et al.(1985) and consisted of two open arms (50cm × 10cm) and two closed arms (50cm × 10 cm × 40cm high) elevated 40cm above the floor. The walls extended from a central neutral area (10 × 10cm) not counted in exploration or entries; subjects were placed in the neutral area facing one of the open arms and given five minutes to explore the maze. The number of entries into open and closed arms, and duration of entries into the open and the closed arms, were recorded. A total of three paws inside of an arm were used as criteria for entry. Time spent in the central area was not recorded. All subjects were tested in the EPM three weeks after completion of OR and OP tasks and sacrificed immediately following completion of the EPM task.
Radioimmunoassay
Serum hormones (testosterone and progesterone) were measured as described in Sharfman et al (2003, 2005) utilizing Coat-a-Count kits from Diagnostic Products Corporation (DPC: testosterone, catalog #TKTT1; progesterone, catalog #TKPG1), according to the kit instruction. Briefly, to each 50μl sample, 1ml of 125I-testosterone or 125I-progesterone was added, vortexed briefly, then incubated overnight at 4°C. The following day, the liquid was poured off and bound isotope was counted in a Wizard 1470 Automatic Gamma Counter (PerkinElmer Life Sciences, Wellesley, MA). Amount of testosterone and progesterone was calculated by comparison with the standard curve using log-logit transformation of the data. The minimum detectable concentrations of hormone, defined as being greater than the 95% upper confidence limit for the zero standard, were 10pg/ml for testosterone and 0.1 ng/ml for progesterone. The intra- and inter-assay coefficients of variation were below 10% for both assays.
Serum estradiol was measured using a #TKE21 kit (Coat-a-Count from DPC) with prior ether extraction (see Scharfman et al., 2003) to separate estradiol from the binding proteins present in rat, but not human, serum. Aliquots (100 μl) of serum were extracted with 2 × 1ml anhydrous ethyl ether from a freshly opened can of anesthetic ether; the extracts were transferred to 12 × 75 mm glass tubes and dried under a stream of air. Equal volumes of serum from the kit standards were extracted in parallel, to correct for procedural losses. The dry extracts from both samples and standards were redissolved by addition of 1ml of the kit I125 estradiol solution and vortexed thoroughly. The assay was then conducted as per kit instructions, and concentrations determined as in testosterone and progesterone assays. The minimum detectable concentration of estradiol was 2 pg/ml. The intra- and inter-assay coefficients of variation were below 10%.
Monoamine and metabolite analysis
Frozen brains were sliced in half sagitally. One hemisphere was sectioned into 300μm sections through the hippocampus using a cryostat at −4°C. The frozen PFC and hippocampal sections were sampled with a 500μm-diameter cannula (4–6 tissue punches from each animal in the PFC, and 10–12 tissue punches from each animal in CA1 and CA3). Monoamines and metabolites were measured through high performance liquid chromatography (HPLC) with electrochemical detection (E.C.): dopamine (DA) and two metabolites 3,4-dihydroxy-phenylacetic acid (DOPAC) and homovanillic acid (HVA); norepinephrine (NE) and metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG); serotonin (5HT) and metabolite 5-hydroxy indole acetic acid (5-HIAA). The procedure was as described previously (Bisagno, Ferguson & Luine, 2002). To each tissue sample, 60μl sodium acetate buffer (pH 5.0) with α-methyl-dopamine as an internal standard was added. The samples were frozen, thawed, the supernatant drawn off, and the pellet re-suspended in 100μl (PFC: 4–6 tissue punches) or 200μl (CA1/CA3: 10–12 tissue punches) of 2.0N NaOH for protein analysis using Bio-Rad reagent (Bio-Rad Laboratories, Hercules, CA). The supernatant containing the monoamines and metabolites was injected into a Waters Associates chromatographic system (Waters 2690), consisting of an automated refrigerated injector, pump, C-18 reverse-phase column (Novapak three micron), and an ESA Coulochem III detector (-150mV to 0.50V potential). The mobile phase, described elsewhere (Luine and Hearns, 1990), contained 3% acetonitrile and peak sharpness was increased by the addition of 100% methanol (99.5% mobile: 0.5% methanol). Millennium software (Waters Associates) was used to run the chromatography system, in which concentrations of transmitters and metabolites were calculated by reference to standards using peak integration. Monoamine concentrations are expressed as pg/μg protein.
BDNF analysis
From the other half of each brain, the entire hippocampus and septum were dissected out and stored individually at −70°C until analysis of BDNF via enzyme-linked immunosorbent assay (ELISA). The tissue was weighed and placed into 1000μl of Lysis buffer (Promega). The solution was homogenized using a mechanical homogenizer from IKA (Eurostar power-control 6000). Following centrifugation for 30 minutes at 13,000rpm, the supernatant was removed (pellet was discarded). Seven serially-diluted standards were made from the BDNF standard in the Elisa kit (Chemicon, Intl #CYT306) ranging from 0–500 pg/ml, and 100μl of each was added to the microplate. For samples, 25μl of each sample was mixed with 75μl of diluent. Each standard and sample was measured in duplicate. After addition to the microplate, the samples were incubated overnight at 4°C.
On the second day, the wells were washed 4 times with wash buffer; 100μl of biotinylated mouse anti-BDNF antibody (1:1000) was added to each well and incubated for 2.5 hours at room temperature. Samples were washed with buffer 4 times; 100μl of streptavidin-HRP conjugate (1:1000) was added to each well and incubated for 1 hour at room temperature. Samples were washed 4 times; 100μl of TMB/E solution (at room temperature) was added to each well and incubated at room temperature for 15 minutes. 100μl of stop solution was then added to each well and the microplate was immediately placed into microplate reader (Bio-Tek EL312, interfaced with Dell Optiplex GS computer running KC Junior software) and measured at 450nm. Optical density units were converted to nanograms of BDNF using the generated standard curve. Concentrations are expressed as ng/g wet weight.
Statistical analysis
All data analysis was conducted using SPSS™ software (SPSS Inc, Chicago, Illinois). Data from the OR and OP tasks were analyzed as follows. Data from the sample trial (T1) of both tasks were analyzed with a two-way ANOVA (group × delay). Data from the recognition/retention trial (T2) of both tasks (exploration ratio: time with new object/time with old + time with new) were analyzed with a two-way ANOVA (group × delay). Data from the EPM were analyzed with a two-way ANOVA (group × entries) for number of entries into open arms and duration of entries into open arms.
Data from each RIA were analyzed with a t-test. Monoamines, metabolites and turnover ratios in each brain region (CA1, CA3, PFC) were analyzed with a two-way ANOVA (group × neurochemical). Monoamine analysis was carried out on data from seven MP and six NP females, as data from one animal in each group were statistical outliers (over 2 standard deviations from the mean) and therefore excluded. BDNF levels were analyzed separately in hippocampus and septum using a two-way ANOVA (group × region). Post hoc analysis of monoamines and BDNF used independent-samples t-tests. For all statistical tests, p < 0.05 for significance.
Cohort 2
Subjects
A second cohort of fourteen 12-month-old Sprague-Dawley rats (NP = 6; MP = 8) were obtained after use at Helen Hayes Hospital (West Haverstraw, NY). The MP females had been used solely for breeding for 6 months prior to this study (4 with four litters, 2 with 3 litters, and 2 with two litters), and the NP females were housed in pairs and had no history of behavioral or reproductive use. These subjects were used to measure monoamine and metabolite concentration in the olfactory bulb (OB), as well as BDNF concentration in subregions of the hippocampus.
All subjects were sacrificed by decapitation following light anesthesia by carbon dioxide. Brains were immediately removed, placed into dry ice, and stored at −70°C to prevent degradation of the samples. Each brain was then divided into 6–7 thick sections based upon anatomical markings (sections made at olfactory bulb (OB), prefrontal cortex, anterior to optic chiasm, posterior to optic chiasm, just anterior to the hypothalamus, and just posterior to the hypothalamus; see Luine et al., 1974).
Monoamine analysis
Brains sections containing the OB were sampled with a 500μm-diameter cannula (4–6 tissue punches were obtained from each animal). Tissue punches were taken under a dissecting microscope, on a microscope stage maintained at −4°C. Monoamine and metabolite concentrations were measured in the same manner as described for Cohort 1.
BDNF analysis
A 500μm-diameter cannula was used to sample tissue from three subregions of the hippocampal formation: CA1, CA3, and dentate gyrus (DG). Tissue punches were taken under a dissecting microscope, on a microscope stage maintained at −4°C. BDNF levels using the ELISA kit and procedure described above for Cohort 1 was used, with minor modifications. As the tissue punches contained less tissue than the entire hippocampus, it was homogenized in 250μl of Lysis buffer (Promega) to prevent dilution and the full 100μl of sample specified in the kit was added to each well. As tissue weight could not be obtained, total protein levels were measured by Bradford assay (see da Silva and Arruda, 2006). BDNF concentration is expressed as ng/g protein.
Statistical analysis
Monoamine concentrations in the OB were examined using a two-way ANOVA (group × neurochemical) for monoamines, metabolites, and turnover ratios. BDNF protein levels were analyzed using a two-way ANOVA (group × region). For all statistical tests, post-hoc analysis was carried out using independent-samples t-tests, and p < 0.05 for significance.
Results
Object Recognition
The OR task was administered at 2- and 4-hour inter-trial delays. In the sample trials (T1), no significant differences were found between groups (Figure 1A) indicating equal overall exploration between NP and MP females. In the recognition trial (T2), there was a significant group effect (F1,28 = 6.80, p < 0.02), as well as a significant group × delay interaction (F1,28 = 4.08, p < 0.05). MP females had significantly higher exploration ratios than did NP females (approximately 0.85 and 0.37, respectively) at the 2 hour (p < 0.001), but not the 4 hour delay (Figure 1B). At the 4 hour inter-trial delay, NP and MP females had similar exploration ratios, (approximately 0.54 and 0.60, respectively) indicating similar exploration of both objects by both groups.
Figure 1. Effect of parity on object recognition.
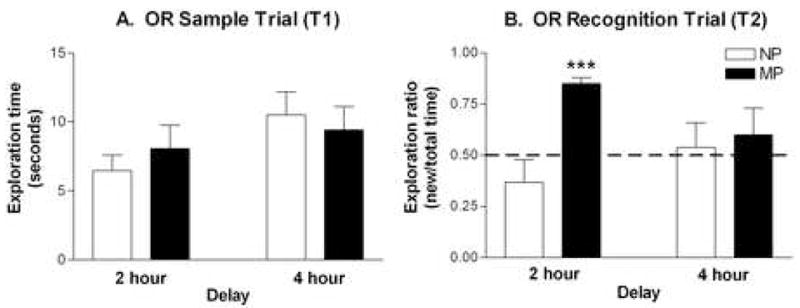
(A) Mean exploration times (± SEM) during the sample trial (T1) are shown for NP (□; n = 8) and MP (■; n = 8) females. No significant differences by two-way ANOVA. (B) Mean exploration ratios (± SEM) during the recognition trial (T2) are shown for NP and MP females. The exploration ratio is calculated as [time with new object/time with old object + time with new object]. Dashed line at 0.50 indicates chance performance on the task (equal time exploring old and new objects). ***p < 0.001.
Object Placement
As in OR, the OP task was administered at 2- and 4- hour inter-trial delays. In the sample trial (T1), there was a significant effect of delay (F1,28 = 9.66, p < 0.01). Both groups spent significantly greater time exploring the objects during the sample trial prior to the 4 hour delay than prior to the 2 hour delay (Figure 2A). However, there was no significant group effect (F1,28 = 0.36, p > 0.10), or group × delay interaction (F1,28 = 0.16, p > 0.10), indicating equal exploration between groups at each delay.
Figure 2. Effect of parity on object placement.
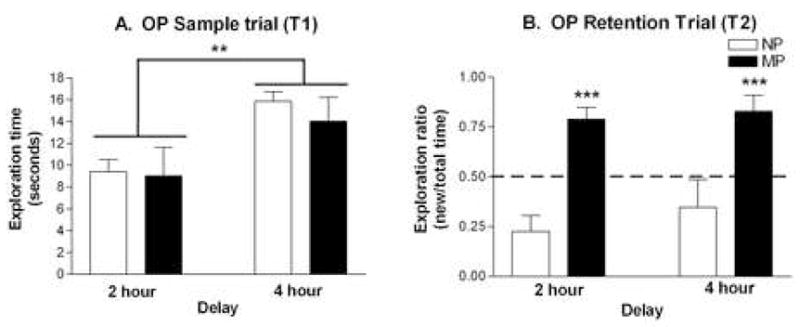
(A) Mean exploration times (± SEM) during the sample trial (T1) is shown for NP (□; n = 8) and MP (■; n = 8) females. Data were analyzed by two-way ANOVA (group × delay). (B) Mean exploration ratios (± SEM) during the recognition trial (T2) is shown for NP and MP females. The exploration ratio is calculated as [time with new object/time with old object + time with new object]. Dashed line at 0.50 indicates chance performance on the task (equal time exploring old and new objects). **p < 0.01; ***p < 0.001.
In the recognition trial (T2), there was a highly significant group effect (F1,28 = 30.69, p < 0.001), but no significant effects of delay, or group × delay interaction (Figure 2B). Thus, at both inter-trial delays, MP females had significantly higher exploration ratios (2 hour: 0.79, 4 hour: 0.83) than did NP females (2 hour: 0.23, 4 hour: 0.35).
Elevated Plus Maze
A two-way ANOVA (group × entries) revealed no significant differences in entries into the open arms (NP: 4.13 ± 0.77; MP: 5.38 ± 0.84) or duration of entries into open arms (NP: 69.94 ± 18.64; MP: 77.62 ± 15.56) between NP and MP females.
Serum gonadal hormones
No significant differences were present between NP and MP females in serum estradiol, testosterone, or progesterone levels (Table 1).
Table 1. Serum gonadal hormone levels in nulliparous and multiparous females.
Entries are mean serum hormone levels ± SEM for NP (n = 7) and MP (n = 8). Data were analyzed by t-test for each hormone.
| Group | Estradiol (pg/ml) | Testosterone (pg/ml) | Progesterone (ng/ml) |
|---|---|---|---|
| Nulliparous (NP) | 24.14 ± 4.88 | 13.29 ± 3.29 | 13.71 ± 1.73 |
| Multiparous (NP) | 21.38 ± 4.50 | 34.13 ± 17.02 | 9.75 ± 2.45 |
Brain monoamines and metabolites
Monoamine and metabolite concentrations (DA and metabolites DOPAC and HVA, NE and metabolite MHPG, 5HT and metabolite 5-HIAA) were measured in three brain regions: CA1, CA3 hippocampus, and PFC (Table 2). In each brain region, data was analyzed by a two-way ANOVA (group × neurochemical). There were no effects of group, or group × neurochemical interactions in PFC (F1,11 = 1.03, p > 0.50), in CA1 (F1,11 = 12.30, p > 0.20), or in CA3 (F1,11 = 1.07, p > 0.50). Concentrations of monoamines, metabolites, and turnover ratios (data not shown) did not significantly differ between NP and MP females in these brain regions.
Table 2. Effect of parity on monoamines and metabolites in three brain regions.
Entries are mean concentration ± SEM for NP (n = 6) and MP (n = 7) females. Concentrations given are in pg/μg total protein. DA, dopamine; DOPAC, 3,4-dihydroxy-phenylacetic acid; HVA, homovanillic acid; NE, norepinephrine; MHPG, 3-methoxy-4-hydroxyphenylglycol; 5HT, serotonin; 5-HIAA, 5-hydroxy indole acetic acid. Data were analyzed by two-way ANOVA (group × neurochemical) with no significant differences in any brain region (PFC, CA1, CA3).
| NT | Group | PFC | CA1 | CA3 |
|---|---|---|---|---|
| DA | NP | 1.32 ± 0.13 | 0.64 ± 0.54 | 0.96 ± 0.27 |
| MP | 1.10 ± 0.15 | 0.35 ± 0.13 | 0.87 ± 0.10 | |
| DOPAC | NP | 0.82 ± 0.10 | 0.51 ± 0.20 | 0.92 ± 0.27 |
| MP | 0.77 ± 0.13 | 0.26 ± 0.06 | 0.75 ± 0.09 | |
| HVA | NP | 0.72 ± 0.09 | 1.36 ± 0.47 | 1.40 ± 0.44 |
| MP | 0.71 ± 0.12 | 0.72 ± 0.18 | 1.29 ± 0.21 | |
| NE | NP | 3.42 ± 0.46 | 2.54 ± 0.52 | 6.09 ± 0.94 |
| MP | 3.54 ± 0.39 | 2.84 ± 0.34 | 6.10 ± 0.54 | |
| MHPG | NP | 4.39 ± 0.90 | 15.35 ± 1.76 | 6.55 ± 0.98 |
| MP | 3.00 ± 0.38 | 17.05 ± 4.00 | 4.97 ± 0.83 | |
| 5HT | NP | 5.44 ± 0.71 | 2.77 ± 0.88 | 3.54 ± 0.33 |
| MP | 4.87 ± 0.63 | 2.64 ± 0.43 | 3.14 ± 0.24 | |
| 5-HIAA | NP | 5.50 ± 0.83 | 4.21 ± 0.75 | 4.90 ± 0.33 |
| MP | 6.32 ± 1.40 | 4.54 ± 0.69 | 4.92 ± 0.28 |
A second cohort of NP and MP females (without behavior experience; see Method) was used to measure monoamines, metabolites and turnover ratios in the olfactory bulb (OB) (Figure 3). A two-way ANOVA (group × neurochemical) revealed a significant overall effect of group (F1,12 = 58.62, p < 0.05). Compared to NP females, MP females had significantly higher levels of DA (p < 0.01) and the metabolite DOPAC (p < 0.001; Figure 3A); significantly higher levels of NE (p < 0.001) and metabolite MHPG (p < 0.001; Figure 3C); and the 5HT metabolite 5-HIAA (p < 0.01; Figure 3E).
Figure 3. Effect of parity on monoamines, metabolites, and turnover ratios in the OB.
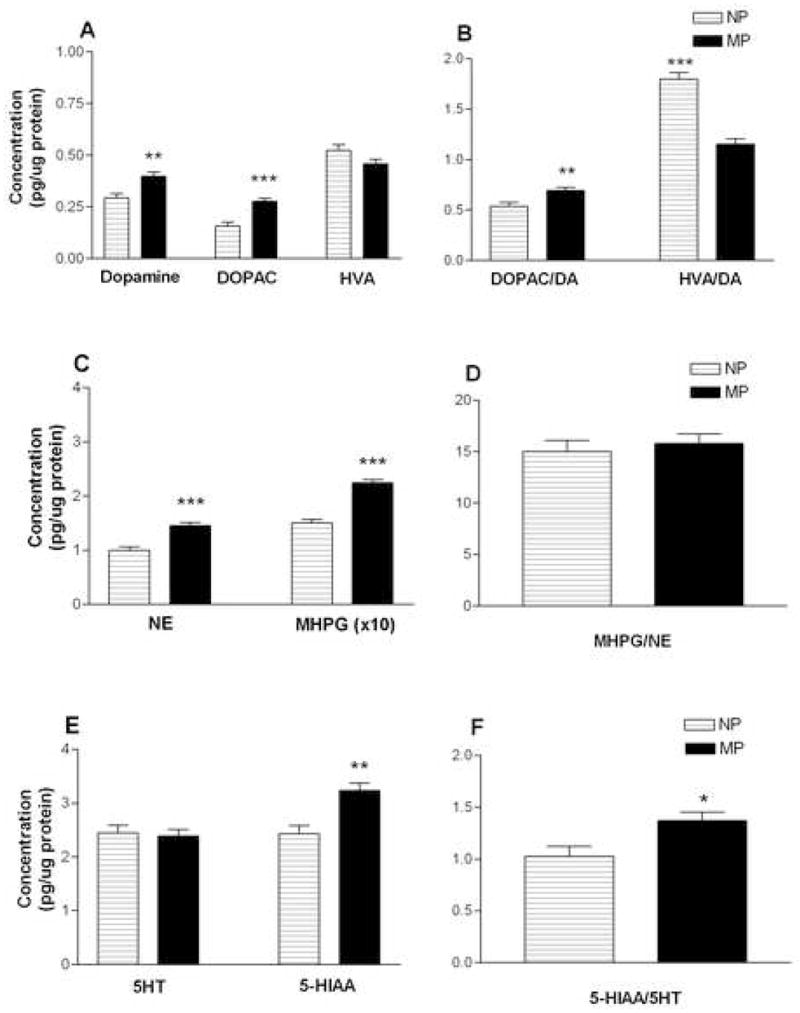
Mean concentration (± SEM) of monoamines, metabolites and turnover ratios for NP (□; n = 6) and MP (■; n = 8) females. Concentrations are pg/μg total protein. DA, dopamine; DOPAC, 3,4-dihydroxy-phenylacetic acid; HVA, homovanillic acid; NE, norepinephrine; MHPG, 3-methoxy-4-hydroxyphenylglycol; 5HT, serotonin; 5-HIAA, 5-hydroxy indole acetic acid. Data were analyzed by two-way ANOVA (group × neurochemical). *p < 0.05; **p < 0.01; ***p < 0.001
Turnover ratios (metabolite/monoamine) were calculated as a measure of monoaminergic activity. Compared to NP females, MP females had significantly higher turnover ratios of DOPAC/DA (p < 0.01; Figure 3B) and 5-HIAA/5HT (p < 0.05; Figure 3F). In contrast, as compared to NP females, MP females had a significantly lower turnover ratio of HVA/DA (p < 0.001; Figure 3B) and did not differ in turnover ratio of MHPG/NE (Figure 3D). Combined, results indicate that parity significantly enhanced both concentrations and activities in the DA, NE and 5HT monoaminergic systems in the OB, with different patterns of activity for the DA metabolites DOPAC and HVA.
Brain BDNF
Concentrations of BDNF in the hippocampus and septum of NP and MP females are shown in Figure 4. Two-way ANOVA (group × region) revealed a significant effect of group (F1,26 = 4.81, p < 0.05), and a significant group × region interaction (F1,26 = 4.06, p < 0.05). BDNF concentration did not significantly differ in the hippocampus (Figure 4A), but MP females had significantly greater BDNF protein in the septum than NP females (p < 0.05; Figure 4A). Thus, parity enhanced BDNF concentration in the septum of MP females, but not in the hippocampus.
Figure 4. Effect of parity on BDNF expression in hippocampus and septum.
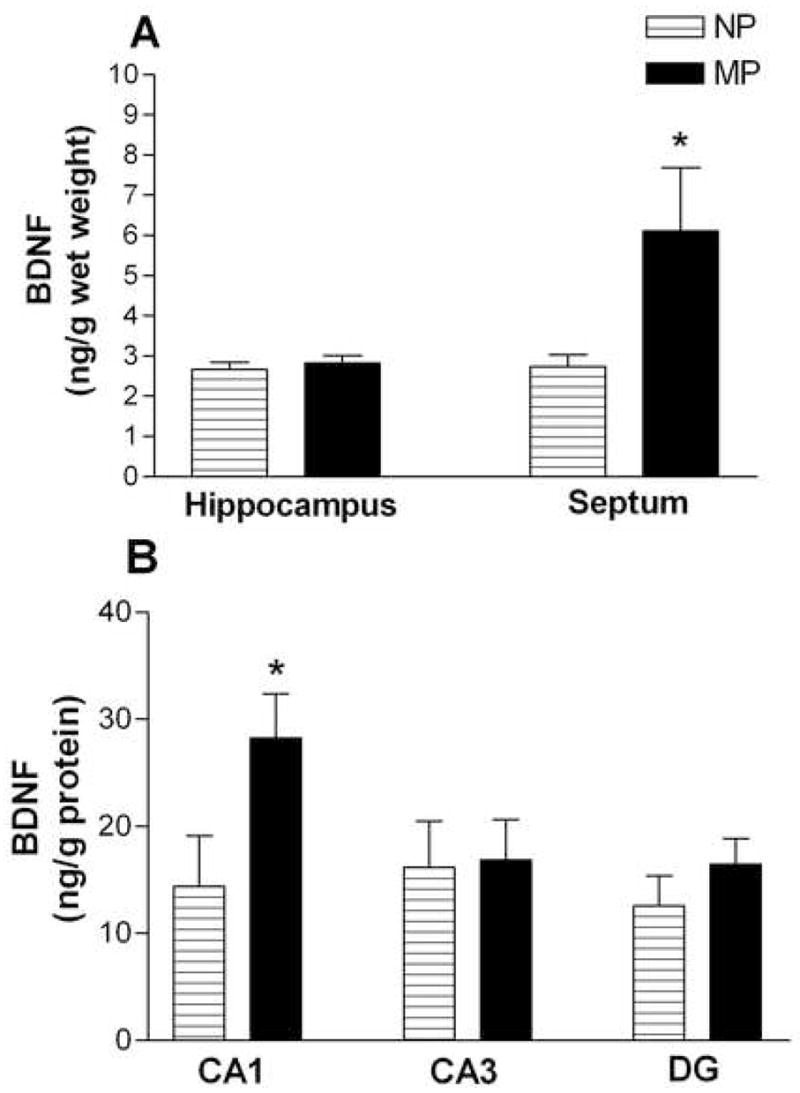
(A) Cohort 1. Mean BDNF concentration (± SEM) in whole hippocampus (left) and septum (right) for NP (□; n = 7) and MP (■; n = 8). Concentrations given are in ng/g wet weight. (B) Cohort 2. Mean BDNF concentration (± SEM) in three hippocampal subregions: CA1 (left), CA3 (middle), and dentate gyrus (DG; right) of NP (□̶; n = 6) and MP (■; n = 8) females. Concentrations given are ng/g protein. Data from each cohort were analyzed by two-way ANOVA (group × region). * p < 0.05.
A second cohort of NP and MP females (without undergoing behavior testing; see Method) was used to measure BDNF concentration in three subregions of the hippocampus (CA1, CA3, DG). A two-way ANOVA (group × region) revealed no significant effect of group (F1,12 = 1.73, p > .05). However, there was a significant group × region interaction (F1,12 = 4.88, p < 0.05). BDNF concentration was significantly higher in the CA1 subfield of MP as compared to NP females (p < 0.05), but did not differ in either CA3 or DG (Figure 6B).
Discussion
The current study compared behaviors and neurochemicals in multiparous (MP) females, i.e. females having experienced 5–6 pregnancies, births, and lactation periods, with age-matched nulliparous (NP) females, i.e. virgin females with no mating or reproductive experience. Performance on tasks assessing memory, but not anxiety, was different. In addition, levels of monoamines and BDNF were different in some, but not all, brain areas examined.
Multiparity significantly enhanced memory performance
The current study presents novel findings that performance on a non-spatial task, OR, was better in MP as compared to NP females. At a 2 hour inter-trial delay, MP females spent over 80% of their time exploring the new object, whereas NP females spent approximately 40% of the time exploring the new object. Thus, NP females explored the new object at levels similar to chance, whereas MP females explored the new object significantly more than the old object, indicating a preference for the novel object. However, at a 4 hour inter-trial delay, NP and MP females did not significantly differ in performance; both groups explored the new object at levels similar to chance (approximately 50% of the time). Thus, multiparity was sufficient to enhance recognition memory at a short inter-trial delay (2 hour), but when the cognitive load was increased (i.e. inter-trial delay of 4 hours), effects of parity were no longer evident. These results are the first to report an enhancing effect of multiparity on non-spatial memory.
On the spatial memory task, OP, MP females outperformed NP females, spending approximately 80% of the time exploring the new object location in the recognition trial at both 2 and 4 hour inter-trial delays, while NP females spent less than 35% of the time exploring the new object location at both delays. Thus, NP females explored the new object location at levels similar to chance, whereas MP females explored the new object location significantly more than the old object, indicating a preference for the novel object location. These results are consistent with previous studies in which MP females had enhanced spatial memory on the radial arm maze while young (Kinsley et al., 1999; Pawluski et al., 2006a) and on the dry land maze throughout the lifespan (Gatewood et al., 2005).
Comparison of OP and OR results suggests that spatial memory may be more sensitive to the effects of multiparity than non-spatial memory. In both tasks, the time spent exploring objects during the sample trial was not significantly different between the two groups. This result suggests that performance differences on the recognition/retention trials (T2) between NP and MP females was not due to differences in exploratory drive, but may involve differences in memory processing.
Multiparity did not significantly alter anxiety
A significant increase in number and duration of entries into the open arm of the EPM is a reliable indicator of decreased anxiety (Pellow et al., 1985; Lonstein, 2005). In the current study, NP and MP females did not significantly differ in either measure of anxiety, indicating that anxiety-like behavior was not affected by previous reproductive experience. Previous work utilizing the EPM has indicated that anxiety is decreased during the post-partum period, when mothers are allowed to suckle their pups (Lonstein, 2005), and that reproductive experience earlier in life (less than 6 months old) decreases anxiety on the EPM at 10 and 14 months of age (Love et al., 2005). However, a recent study has indicated that on the EPM prior reproductive experience may be anxiogenic in middle-aged (10–11 month old), but not in young (3–4 month old) females (Byrnes and Bridges, 2006). The current findings indicate that multiparity is neither anxiolytic nor anxiogenic in middle aged females; as such, further exploration into the relationship between parity and anxiety, particularly in middle age, is required.
Multiparity did not affect serum hormone levels
NP and MP females did not significantly differ in measured levels of serum estradiol, testosterone, or progesterone, hormones that can affect memory and anxiety (Dohanich, 2002). While only examined on day of sacrifice, vaginal cytology indicated that the majority of females in both groups were in an estrus state, as evidenced by the presence of primarily cornified epithelial cells (data not shown). In Fisher rats, the onset of irregular estrous cycles begins around 12 months of age (Markowska, 1999), and is characterized by a prolonged cornified cell phase between 11–14 months of age (Sone et al., 2007). As estradiol and progesterone levels did not differ, and vaginal cytology appeared similar across both groups, prior reproductive experience did not appear to influence reproductive aging of the females. It is unlikely that performance differences between MP and NP females on the OR and OP tasks were due to differences in circulating gonadal hormone levels at time of testing.
Multiparity significantly altered monoaminergic function in olfactory bulb
Since monoamines are known to contribute to memory function (see Luine et al., 1998), we measured monoamine and metabolite concentrations in CA1, CA3 hippocampus and PFC. No significant differences in monoamine or metabolite levels, or in turnover ratios, were found between NP and MP females in PFC or hippocampus, indicating no effect of mulitparity on dopamine, norepinephrine or serotonin systems in these brain regions. In contrast, multiparity significantly affected monoamine systems in the OB; this study is the first to show large and enduring changes due to multiparity in this region.
Specifically, MP females had 30–75% higher levels of DA, DOPAC, NE, MHPG, and 5-HIAA than NP females. Additionally, turnover ratios of DOPAC/DA and 5-HIAA/5HT were elevated by 17% and 34%, respectively. In contrast, levels of the DA metabolite HVA did not significantly differ between NP and MP females, yet MP females had turnover ratios of HVA/DA that were 60% lower than in NP females. Combined, these results indicate greater intraneuronal synthesis of DA and metabolism to DOPAC, with less release into the synapse, corresponding with previous work in which parity increased DA and DOPAC levels in forebrain regions (Byrnes et al., 2001). Overall, the results indicate that while DA activity is decreased in the OB of MP females, synthesis and metabolism to DOPAC are elevated. Additionally, both levels and activity (as measured by conversion to MHPG) of NE are increased in the OB of MP females, as is activity of 5HT (as measured by conversion to 5-HIAA).
Previous research has indicated that as male and female rats age, levels of DA, NE and 5HT decrease throughout the brain: young rats (3–6 months old), as compared to aging rats (15 months or older), display higher monoamine levels in striatum, entorhinal cortex, hippocampus, frontal cortex (Luine et al., 1990; Tanila et al., 1994; Miguez et al., 1999), and cerebral cortex (Lee et al., 2001). By 15 months of age, significant decreases in the monoamine metabolites DOPAC, MHPG and 5-HIAA are observed in the OB of male rats (Dluzen, 1996) with corresponding decreases in olfactory-based memory (Guan and Dluzen, 1994). Thus, age-related activity changes in monoaminergic systems of the OB affect olfactory ability. Multiparity may maintain monoamine concentrations that ordinarily decrease with age, as higher concentrations of DA, NE, and metabolites DOPAC, MHPG and 5-HIAA were found in the OB of MP as compared to NP females.
Elevated monoamines may aid in offspring recognition, which is critically dependent upon the OB (Fleming and Rosenblatt, 1974; Brennan and Keverne, 1997), particularly NE projections throughout (Pissonier et al., 1985; Dickinson and Keverne, 1988; Levy et al., 1990; Calamandrei et al., 1992; Calamandrei and Keverne, 1994). Exposure to an odor-rich environment (such as a litter of pups) enhances olfactory memory and OB neurogenesis (Rochefort et al., 2002), which is also increased during pregnancy when prolactin increases (Shingo et al., 2003). Additionally, oxytocin, which is elevated during lactation, aids in social memory of conspecifics by male rats through actions in the NE system in the olfactory bulb (Dluzen et al., 1998; Dluzen et al., 2000). Little to no research has examined olfactory bulb makeup in relation to olfactory-based memory in females; what information exists focuses on the maternal period (i.e. Calamandrei et al., 1992; Calamandrei and Keverne, 1994). This study is the first to indicate a role for olfactory bulb monoamines in performance on non-social memory tasks in females with prior reproductive experience. It is also notable that changes in OB monoamines are so long-lasting after parturition, indicating a relatively permanent change in OB makeup.
It is important to note that differences in monoamines between NP and MP females were not found in Cohort 1 animals with behavioral experience, but only in Cohort 2 animals without behavior experience. However, as sampling was done in different brain regions between the two cohorts (hippocampus and prefrontal cortex for Cohort 1; olfactory bulb in Cohort 2), it is reasonable that these differences are due to parity, and not behavior experience.
Multiparity significantly elevated BDNF concentration in CA1 hippocampus and septum
BDNF protein levels did not significantly differ in whole hippocampus of NP and MP females. However, BDNF was 51% higher in the CA1 hippocampal subregion of MP females as compared to NP females. No significant differences in BDNF between the two groups were found in CA3 or DG. Expression of BDNF protein in CA1 is typically much lower than in CA3 or DG, regardless of gender or serum estradiol levels, but this pattern of expression occurs when an antibody is used that preferentially identifies BDNF in nerve terminals (Conner et al., 1997; Yan et al., 1997; Scharfman et al., 2003). The ELISA kit used in the current study depended on an antibody that may preferentially localize BDNF protein in pyramidal cell bodies and dendrites (see Tongiorgi et al., 2006). Using this antibody, there were significant effects of multiparity on BDNF expression in CA1 hippocampus.
BDNF levels were also 45% higher in the septum of MP females relative to NP females, indicating an increase in BDNF expression throughout the septum. The medial septum contains cholinergic neurons responsible for retrograde transport of BDNF protein from hippocampus to septum (Sobreviela et al., 1996). Treatment with estradiol and progesterone decreases hippocampal BDNF protein, but increases hippocampal BDNF mRNA (Gibbs, 1999), suggesting either an increase in BDNF protein degradation or transport away from hippocampus (Sohrabji et al., 1995), possibly through septal cholinergic neurons. Repeated exposure to estrogen during pregnancies could have increased retrograde transport of hippocampal BDNF protein. However, there are limitations to measuring protein levels alone, specifically that amount of synthesis and/or release of BDNF cannot be measured. To fully understand the effect of parity on BDNF in the hippocampus and septum, future studies should examine BDNF mRNA in these regions.
Additionally, it is possible that, as with the monoamine results, differences in hippocampal BDNF may have been due to the lack of behavioral experience in Cohort 2 animals, and not simply based upon the subregions sampled. However, the OR and OP tasks do not use a training paradigm, and as such traditional ‘learning’ does not occur (Ennaceur et al., 2005), which should eliminate possible confounds of behavioral experience between the two cohorts. As such, we believe that the differences in BDNF found between NP and MP females in the CA1 are in greater part due to parity, and not behavior experience.
Alternatively, MP females may have higher BDNF than NP females due to the physical aspect of rearing pups. New dams display a variety of physical behaviors such as nest building, crouching over pups, and pup retrieval, that were not displayed prior to pregnancy and parturition (Rosenblatt et al., 1988). Exercise in female rats (several days of voluntary wheel-running) significantly increases BDNF mRNA and protein in the hippocampus, particularly in the CA3 and DG (Berchtold et al., 2001; Berchtold et al., 2002; Cotman and Berchtold, 2002). Additionally, exercise and the subsequent increase in BDNF protein act together to enhance spatial memory as measured on the Morris water maze (Vaynman et al., 2004). If maternal behaviors, including nest building and retrieval of pups, increase physical activity in new dams in a manner similar to voluntary wheel running, then the recurring display of these behaviors with multiparity may increase BDNF levels in the hippocampus to a point that is beneficial to spatial memory.
We suggest here that BDNF in CA1 pyramidal cells and the septum may underlie memory task performance. This hypothesis is supported by the evidence to date that BDNF is important for LTP in CA1 hippocampus (Ying et al., 2002; Pang and Lu, 2004), that BDNF plays an important role in learning and memory (Alonso et al., 2002; Tyler et al., 2002; Alonso et al., 2005), and specifically is involved in spatial memory (Linnarsson et al., 1997; Mu et al., 1999; Mizuno et al., 2000; Radecki et al., 2005). This hypothesis is also consistent with a role for both CA1 and septum in memory performance. Lesions to the CA1 significantly impair performance on OR and OP tasks (Broadbent et al., 2004; Gulinello et al., 2006); OP in particular is dependent on an intact hippocampus and/or fornix (Ennaceur and Aggleton, 1994; Ennaceur et al., 1997; Mumby et al., 2002; Broadbent et al., 2004). Similarly, lesions to the dorsal hippocampus (containing CA1 and septal regions) impair performance on the Morris water maze to the same extent as lesions of whole hippocampus (Clark et al., 2005). Neurons in the medial and lateral septum fire with hippocampal neurons, and aid in processing spatial information (Zhou et al., 1999); lesions to the septum interfere with both hippocampal firing and spatial working memory (Leutgeb and Mizumori, 1999). The CA1 and septum are thus particularly important for spatial memory task performance, and to a lesser extent non-spatial memory (Ennaceur and Aggleton, 1994; Ennaceur et al., 1997; Broadbent et al., 2004). Current findings of elevated BDNF in the CA1 of MP females as compared to NP females likely contributed to enhanced performance on the object recognition and placement tasks.
In conclusion, these results show enduring changes in brain function and chemistry in rats experiencing several previous pregnancies. Furthermore, these changes persist many months after last reproductive experience, indicating possible semi-permanent or permanent changes in cognitive function and neurochemistry. More research into the factors contributing to these changes, particularly the relationship between multiparity and hippocampal BDNF, may lead to a greater understanding of amelioration of certain kinds of neurodegenerative and age-related losses in brain function.
Acknowledgments
The authors thank S. Attalla, J. Berberena, L. Jacome, G. Mohan and C. Saenz for assistance in behavior testing, D. McCloskey for assistance in ELISA procedures, and C. Kinsley for advice and help. This work was part of the dissertation thesis of AHM; input given by Drs. V. Quinones-Jenab, C. Harding and C. Kinsely is greatly appreciated. This research was supported by S06-GM-60654, NS 37562 (NIH) and a Research Centers in Minority Institutions award (RR-03037) from the National Center for Research Resources of NIH which supports the infrastructure of the Biopsychology program at Hunter College. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 2006;110:175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Alonso M, Bekinschtein P, Cammarota M, Vianna MR, Izquierdo I, et al. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learn Mem. 2005;12:504–510. doi: 10.1101/lm.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Cotman CW. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res. 2002;68:511–521. doi: 10.1002/jnr.10256. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol Behav. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM, Bridges RS. Reproductive experience alters anxiety-like behavior in the female rat. Horm Behav. 2006;50:70–76. doi: 10.1016/j.yhbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Byrnes JJ, Bridges RS. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacol Biochem Behav. 2001;68:481–489. doi: 10.1016/s0091-3057(01)00449-x. [DOI] [PubMed] [Google Scholar]
- Calamandrei G, Keverne EB. Differential expression of Fos protein in the brain of female mice dependent on pup sensory cues and maternal experience. Behav Neurosci. 1994;108:113–120. doi: 10.1037//0735-7044.108.1.113. [DOI] [PubMed] [Google Scholar]
- Calamandrei G, Wilkinson LS, Keverne EB. Olfactory recognition of infants in laboratory mice: role of noradrenergic mechanisms. Physiol Behav. 1992;52:901–907. doi: 10.1016/0031-9384(92)90369-d. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 1996;742:177–184. doi: 10.1016/s0006-8993(96)00962-6. [DOI] [PubMed] [Google Scholar]
- da Silva MA, Arruda MA. Mechanization of the Bradford reaction for the spectrophotometric determination of total proteins. Anal Biochem. 2006;351:155–157. doi: 10.1016/j.ab.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Dickinson C, Keverne EB. Importance of noradrenergic mechanisms in the olfactory bulbs for the maternal behaviour of mice. Physiol Behav. 1988;43:313–316. doi: 10.1016/0031-9384(88)90193-x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE. Age-related changes in monoamines within the olfactory bulbs of the Fischer 344 male rat. Mech Ageing Dev. 1996;91:37–45. doi: 10.1016/0047-6374(96)01774-5. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Ebner K, Landgraf R. Oxytocin induces preservation of social recognition in male rats by activating alpha-adrenoceptors of the olfactory bulb. Eur J Neurosci. 2000;12:760–766. doi: 10.1046/j.1460-9568.2000.00952.x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Landgraf R. Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neurosci Lett. 1998;254:161–164. doi: 10.1016/s0304-3940(98)00691-0. [DOI] [PubMed] [Google Scholar]
- Dohanich G. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Farbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 265–327. 2. [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res. 2005;159:247–266. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974;86:221–232. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, et al. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav Brain Res. 1994;61:87–90. doi: 10.1016/0166-4328(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, et al. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, et al. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav. 2005;84:799–806. doi: 10.1016/j.physbeh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Chang CK, Liu IM, Chi TC, Yu HJ, et al. Changes in endogenous monoamines in aged rats. Clin Exp Pharmacol Physiol. 2001;28:285–289. doi: 10.1046/j.1440-1681.2001.03439.x. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Mizumori SJ. Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations. J Neurosci. 1999;19:6661–6672. doi: 10.1523/JNEUROSCI.19-15-06661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F, Gervais R, Kindermann U, Orgeur P, Piketty V. Importance of beta-noradrenergic receptors in the olfactory bulb of sheep for recognition of lambs. Behav Neurosci. 1990;104:464–469. doi: 10.1037//0735-7044.104.3.464. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Dominguez JM, Putnam SK, De Vries GJ, Hull EM. Intracellular preoptic and striatal monoamines in pregnant and lactating rats: possible role in maternal behavior. Brain Res. 2003;970:149–158. doi: 10.1016/s0006-8993(03)02315-1. [DOI] [PubMed] [Google Scholar]
- Love G, Torrey N, McNamara I, Morgan M, Banks M, et al. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- Luine V, Bowling D, Hearns M. Spatial memory deficits in aged rats: contributions of monoaminergic systems. Brain Res. 1990;537:271–278. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs W, Kuperman J, Avedian T, Roth RH, Jentsch JD. Alpha-2 adrenoceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology. 2005;30:1500–1510. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- Miguez JM, Aldegunde M, Paz-Valinas L, Recio J, Sanchez-Barcelo E. Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. J Neural Transm. 1999;106:1089–1098. doi: 10.1007/s007020050225. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, et al. Cognitive impariment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behavioural Brain Research. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res. 2001;133:143–152. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- O’Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RG. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learning and Memory. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006b;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006a;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pissonnier D, Thiery JC, Fabre-Nys C, Poindron P, Keverne EB. The importance of olfactory bulb noradrenalin for maternal recognition in sheep. Physiol Behav. 1985;35:361–363. doi: 10.1016/0031-9384(85)90309-9. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Smith HR, Pang KC. Orexin-saporin lesions of the medial septum impair spatial memory. Neuroscience. 2005;132:261–271. doi: 10.1016/j.neuroscience.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Pagcatipunan M, Kroin JS, Mufson EJ. Retrograde transport of brain-derived neurotrophic factor (BDNF) following infusion in neo- and limbic cortex in rat: relationship to BDNF mRNA expressing neurons. J Comp Neurol. 1996;375:417–444. doi: 10.1002/(SICI)1096-9861(19961118)375:3<417::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone K, Yamamoto-Sawamura T, Kuwahara S, Nishijima K, Ohno T, et al. Changes of estrous cycles with aging in female F344/n rats. Exp Anim. 2007;56:139–148. doi: 10.1538/expanim.56.139. [DOI] [PubMed] [Google Scholar]
- Tanila H, Taira T, Piepponen TP, Honkanen A. Effect of sex and age on brain monoamines and spatial learning in rats. Neurobiol Aging. 1994;15:733–741. doi: 10.1016/0197-4580(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, et al. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Domenici L, Simonato M. What is the biological significance of BDNF mRNA targeting in the dendrites? Clues from epilepsy and cortical development. Mol Neurobiol. 2006;33:17–32. doi: 10.1385/MN:33:1:017. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, et al. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, et al. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou TL, Tamura R, Kuriwaki J, Ono T. Comparison of medial and lateral septal neuron activity during performance of spatial tasks in rats. Hippocampus. 1999;9:220–234. doi: 10.1002/(SICI)1098-1063(1999)9:3<220::AID-HIPO3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]


