Abstract
Core symptoms of autism include deficits in social interaction, impaired communication, and restricted, repetitive behaviors. The repetitive behavior domain encompasses abnormal motoric stereotypy, an inflexible insistence on sameness, and resistance to change. In recent years, many genetic mouse models of autism and related disorders have been developed, based on candidate genes for disease susceptibility. The present studies are part of an ongoing initiative to develop appropriate behavioral tasks for the evaluation of mouse models relevant to autism. We have previously reported profiles for sociability, preference for social novelty, and resistance to changes in a learned pattern of behavior, as well as other functional domains, for 10 inbred mouse strains of divergent genetic backgrounds. The present studies extend this multi-component behavioral characterization to several additional strains: C58/J, NOD/LtJ, NZB/B1NJ, PL/J, SJL/J, SWR/J, and the wild-derived PERA/EiJ. C58/J, NOD/LtJ, NZB/B1NJ, SJL/J, and PERA/EiJ demonstrated low sociability, measured by time spent in proximity to an unfamiliar conspecific, with 30% to 60% of mice from these strains showing social avoidance. In the Morris water maze, NZB/B1NJ had a persistent bias for the quadrant where the hidden platform was located during acquisition, even after nine days of reversal training. A particularly interesting profile was found for C58/J, which had low social preference, poor performance in the T-maze, and overt motoric stereotypy. Overall, this set of tasks and observational methods provides a strategy for evaluating novel mouse models in behavioral domains relevant to the autism phenotype.
Keywords: autism, Morris water maze, reversal learning, sociability, social preference, spectrum disorders, stereotypy, T-maze
1. Introduction
Autism is a neurodevelopmental disorder diagnosed on the basis of an aberrant behavioral phenotype, rather than by a physiological biomarker or specific neuropathology. Core symptoms typically emerge early in life, and include deficient social interaction, impaired verbal communication, and stereotyped repetitive or ritualistic behavior, including abnormal motoric responses, resistance to change in routines or schedules, and unusual or obsessional interests [1]. There is significant co-morbidity between autism and mental retardation [15,35], which can include severe language deficits. While the etiology of autism is not well understood, support for a strong genetic component is evident from the 80% - 90% concordance between monozygotic twins [14,32], and association analyses have identified candidate genes for autism susceptibility (e.g. [8,9,43]).
Given the high heritability of autism, the investigation of relevant genetic mouse models can provide a powerful approach to determine the underlying neuropathology and etiology of the clinical disease. One challenge for the evaluation and validation of mouse models has been the ability to assess behavior in mice that reflects autism symptomatology. We have developed a set of behavioral measures for characterizing mouse models of the autism phenotype [30,31,33], based on clinical observations of autistic children and findings from research with human subjects. For example, children with autism have deficiencies in social responses. One study reported that autistic children spend less time in close proximity to other children and are less likely to focus attention on another child, in comparison to typically-developing children [27]. More recently, Jahr et al. [19] found significantly reduced frequency of spontaneous social contact in autistic children, both high-functioning or with mild mental retardation. These deficits in social approach can be modeled in mice by using social choice tasks, in which mice are presented with a choice between spending time in the proximity of another mouse, or remaining alone [7,30,31,33,42]. Inbred strain distributions using social choice tasks have shown that levels of social approach and preference are dependent upon genetic background, with some strains (AKR/J, C57BL/6J, C3H/HeJ, FVB/NJ) demonstrating high affiliation, and other strains (A/J, BALB/c, BALB/cByJ, BTBR T+tf/J, 129S1/SvImJ) having low preference or even avoidance [6,7,30,31,33,42].
Restricted, repetitive behavior is also a core diagnostic indicator of autism [1]. The domain of repetitive behavior can include abnormal or stereotyped motor responses. We use systematic home cage observations to detect the occurrence of unusual motoric responses in mice. Repetitive behavior can also encompass resistance to change a learned response, compulsions, obsessions, and other persistent behavioral patterns, which may be related to deficits in executive function. For example, repetitive behavior in autistic adults was found to correlate with impaired cognitive flexibility and response inhibition [26]. Children with autism have been reported to have deficient performance in a spatial-reversal task, another measure for the ability to change a learned pattern of behavior [11]. To model this task, we have used reversal learning in the Morris water maze or T-maze tasks [31]. In our previous 10-strain comparison, mice from the BTBR T+tf/J strain had a selective deficit in reversal learning in the Morris water maze, but normal performance in reversal learning in a T-maze task, suggesting that measures of quadrant selectivity in the water maze is a more sensitive measure of cognitive flexibility. Deficits in reversal learning have been reported in genetic mouse models relevant to autism, including the fragile X syndrome-model mouse [2,22,38,46] and Relnrl/+ mice [5], as well as in mice with prenatal exposure to an inflammatory challenge [28].
The present study reports the behavioral phenotype of an additional 7 inbred mouse strains (C58/J, NOD/LtJ, NZB/BINJ, PERA/EiJ, PL/J, SJL/J, and SWR/J), and provides a second assessment of social approach in 4 strains previously evaluated (AKR/J, C57BL/6J, DBA/2J, and FVB/NJ). Strains were also tested for acquisition and reversal learning. Control measures were taken of general health, home cage behaviors, neurological reflexes, olfactory ability, activity, motor coordination, and anxiety-like behavior, to identify potential confounding factors. Subjects were male mice, to reflect the approximately 4:1 higher incidence of autism in males [14,15,32].
2. Materials and methods
2.1. Animals
Sets of male mice (n = 17-22 mice) from 10 inbred strains, AKR/J, C57BL/6J, C58/J, DBA/2J, FVB/NJ, NOD/LtJ, NZB/BINJ, PL/J, SJL/J, and SWR/J, were purchased from the Jackson Laboratory (JAX; Bar Harbor, ME). Due to limitations on available mice, the PL/J and SJL/J mice arrived in 2 separate cohort groups. Mice were 3 to 4 weeks of age upon arrival at the University of North Carolina animal facility in Chapel Hill, NC. An additional set of PERA/EiJ mice (11 males), matched in age to the other inbred strains, was derived from breeding pairs obtained from JAX. Mice were housed separately by strain, with 3 or 4 animals per plastic tub cage, and given free access to water. Purina 5058 chow was provided ad libitum, except when mice were under food restriction for appetitive tasks. The housing room had a 12-hr light/dark cycle (lights off at 7:00 p.m.). Testing methods were designed to minimize pain and discomfort in the mice. All procedures were conducted in strict compliance with the policies on animal welfare of the National Institutes of Health and the University of North Carolina (stated in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animal Resources, National Research Council, 1996 edition). All procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
2.2. Test procedures
Order of testing for most strains was: 1) home cage observations at age 3-4 weeks (initiated at least 5 days following arrival); 2) general health and neurological reflexes at age 4-5 weeks; 3) open field locomotion and rotarod at age 5-6 weeks; 4) social behavior test at age 6-7 weeks; 5) olfactory test with buried food at age 7-8 weeks; and 6) elevated plus-maze at age 8-10 weeks. The 4 inbred strains included in the previously published strain distribution from our laboratory [31] were not re-assessed in every test. Only the 7 new inbred strains were evaluated in the Morris water maze task for reversal learning at age 3-6 months. 4 of these strains were unable to perform the cued visible platform task on the water maze, and were assessed for T-maze learning and reversal. Across the entire study, mice were periodically observed for the emergence of abnormal behaviors in the home cage or during testing. Unless otherwise indicated, testing was conducted during the light phase of the light cycle, under fluorescent laboratory lighting (180-205 lux for activity and water maze tests, 320-340 lux for social approach and elevated plus maze tests, and 420 lux for the T-maze test).
2.3. Control measures
Several tests were conducted to aid in the interpretation of the results from the social approach and learning tasks, and to identify possible confounding factors. Observational measures were taken of home cage behavior, general health, and neurological reflexes, using direct scoring by a single observer. Exploration in a novel environment was used to assess activity levels (Versamax system, Accuscan Instruments). Motor coordination was assayed with an accelerating rotarod (Ugo-Basile, Stoelting Co., Wood Dale, IL), measuring maximum time for remaining at the top of the rotating barrel. For this test, records were taken of latency to fall off the barrel or to passively rotate, or invert off, by clinging to the barrel. Anxiety-like behavior was evaluated in the elevated plus maze test. Olfactory ability was assessed using a buried-food procedure following food deprivation. These procedures have previously been described in detail [31].
2.4. Sociability and preference for social novelty
The social behavior apparatus, previously described in detail [31,33], was designed to assess whether subject mice prefer to spend time in the proximity of stranger mice. The apparatus was a rectangular, 3-chambered box fabricated from clear polycarbonate (42.5 cm W × 22.2 cm H; center chamber, 17.8 cm L; side chambers, 19.1 cm L). Dividing walls had retractable doorways allowing access into each chamber. Photocells were embedded in each doorway to allow automatic quantification of entries and duration in each chamber of the social test box.
2.4.a. Habituation
The test mouse was first placed in the middle chamber and allowed to explore for 10 min, with the doorways into the 2 side chambers open. Each of the 2 sides contained an empty wire cage (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH). Measures were taken of time spent in each of the side chambers and number of entries into each side chamber by the automated measurement system. The habituation phase was given immediately before the sociability test.
2.4.b. Sociability
After the habituation period, the test mouse was enclosed in the center compartment of the social test box, and an unfamiliar mouse (stranger 1; an adult C57BL/6J male) was enclosed in 1 of the wire cages and placed in a side chamber. The location for stranger 1 alternated between the left and right sides of the social test box across subjects. Following placement of stranger 1, the doors were re-opened, and the subject was allowed to explore the entire social test box for a 10-min session. Measures were taken of the amount of time spent in each chamber and the number of entries into each chamber by the automated testing system. In addition, a human observer scored time spent sniffing each wire cage, using a computer keypad and software [20].
2.4.c. Preference for social novelty
At the end of the 10-min sociability test, each mouse was further tested in a third 10-min session to quantitate preference for spending time with a new stranger. A new unfamiliar mouse was placed in the wire cage that had been empty during the prior 10-min session. The test mouse had a choice between the first, already-investigated mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). Measures were taken as described above.
2.5. Water maze test
The Morris water maze task was based on the standard methods for spatial learning in rodents [29,37,48]. The water maze consisted of a large circular pool (diameter = 122 cm) partially filled with water (45 cm deep, 24-26°C), located in a room with numerous visual cues. To allow detection by an automated tracking system (Noldus Ethovision), overhead fluorescent lighting was used for dark-pigmented strains, while halogen lighting directed at the ceiling was used for the albino strains (NOD/LtJ, PL/J, SJL/J, and SWR/J). Mice were tested for their ability to find an escape platform (diameter = 12 cm) on 3 different components: ability to find a cued visible platform, acquisition in the hidden (submerged) platform test, and reversal learning with the hidden platform moved to the opposite quadrant. In each case, the criterion for learning was an average latency of 15 sec or less to locate the platform across a block of 4 consecutive trials per day. In addition, at the end of the hidden platform acquisition and reversal learning phases, mice were given 1-min probe trials with the platform removed. In these probe trials, spatial learning could be demonstrated by higher levels of swimming in the quadrant where the platform had been located in the training trials, versus swimming in the other quadrants of the pool.
In the visible platform test, each animal was given 4 trials per day, across 3 days, to swim to an escape platform cued by a patterned cylinder extending above the surface of the water. For each trial, the mouse was placed in the pool at 1 of 4 possible locations (randomly ordered), and then given 60 sec to find the cued platform. If the mouse found the platform, the trial ended, and the animal was allowed to remain 10 sec on the platform before the next trial began. If the platform was not found, the mouse was placed on the platform for 10 sec, and then given the next trial. Measures were taken of latency to find the platform, swimming distance, and swimming velocity, via an automated tracking system (Noldus Ethovision). Only groups that were able to reach criterion with a cued visible platform were given further tests with the hidden platform.
The following week, mice were trained on the hidden platform test. Using the same procedure as described above, each animal was given 4 trials per day, for up to 9 days, to learn the location of the submerged platform. At the end of the day that the group met the 15-sec criterion for learning, or else on day 9 of testing, mice were given a 1-min probe trial in the pool with the platform removed. Selective quadrant search was evaluated by measuring percent of time spent in each quadrant of the pool. In the week following the acquisition phase, mice were tested for reversal learning, using the same procedure. In this phase, the hidden platform was located in a different quadrant in the pool, diagonal to its previous location. On the day that the criterion for learning was met, or else on day 9 of testing, the platform was removed from the pool, and the group was given a probe trial to evaluate reversal learning.
2.6. T-maze acquisition and reversal learning
The T-maze procedure was used with 4 strains (C58/J, PL/J, SJL/J, SWR/J) that did not meet criterion for learning in the cued platform task in the water maze. The T-maze was constructed of black Plexiglas, with a wire mesh floor, 41 cm stem section, and a 91.5 cm arms section. Each section was 11.2 cm wide, with 19 cm walls. Mice were first reduced to around 85% of their free-feeding body weight before starting the appetively-motivated task. The food-deprivation regimen involved giving the mice a limited number of pellets in their home cage each day. Since animals were group-housed, subjects which appeared to be losing too much weight were given supplemental feeding in a separate cage, as necessary. Mice were habituated to the T-maze and shaped to obtain food from cups recessed into the ends of the arms across 5 days, with 1 acclimation trial per day. 10 training trials per day were then initiated. For each mouse, 1 arm was designated as the correct arm. 1 reinforcer (Noyes sucrose pellet, 20 mg., Research Diets, Inc., New Brunswick, NJ) was available in the designated arm for each trial. The reinforced arm was on the left side for half of the mice, and on the right side for the other half. At the beginning of each test session, the mouse was placed in the start box at the bottom of the T-maze stem. The start box door was opened, and the mouse was given a choice between entering either arm. If the mouse made the correct choice, it was given time to consume the sugar pellet, and then guided back into the start box for the next trial. For each successive trial, the reward was always placed in the same arm. Latency to enter an arm, number of errors in arm selection, and number of days to criterion were recorded by a human observer. The criterion for task acquisition was an average of 80% correct responses across 3 days of testing. When the group average was at criterion, the mice were further tested using a reversal procedure. For the C58/J strain, the group average did not meet criterion across 10 days of acquisition. However, 5 mice out of the 20 tested did meet the criterion for learning. These 5 mice were further evaluated for reversal learning.
2.7. Statistical analysis
Each inbred strain was tested separately in the behavioral assays. Therefore, data from each strain were analyzed separately, using within-strain comparisons relevant to the behavioral parameter(s) of the specific task. Data from the social approach tests were analyzed using within-strain repeated measures ANOVA, with the factors of chamber side (e.g., stranger 1 side or the opposite side) and test (sociability or preference for social novelty). Separate within-strain repeated measures ANOVA were used to determine effects of side for each test. Within-strain repeated measures ANOVA were also used to compare time spent in each quadrant of the water maze during the probe trials. For all comparisons, significance was set at p < 0.05.
3. Results
3.1. Control measures
Mice were evaluated across several domains of function, including motor coordination, olfactory ability, level of activity, and anxiety-like behavior, in order to detect overt deficiencies or alterations in behavior that could impact testing in the social or reversal learning assays. General observations of the 11 inbred strains indicated that the mice appeared healthy, without physical impairment or signs of illness, such as poor coat condition (Table 1). Simple measures of corneal responses and visual placement did not reveal the overt visual deficits that were evident during later testing in the Morris water maze. Findings from the olfactory assay indicated that none of the inbred strains was anosmic, although only around half of the C58/J and NZB/B1NJ mice located the buried food. The NOD/LtJ strain had the lowest latencies to find the buried food, with a group average of less than 3 min. This strain was also characterized by poor performance on the rotarod. The young C57BL/6J mice had the highest latencies for remaining on the rotarod.
Table 1.
Physical characteristics, vocalizations, home cage behavior, sensory reflexes, olfactory ability, and a motor test. Data shown are means ± SEM for weight and latency measures, percent of cages for home cage measures, and percent of mice per strain for other measures.
| AKR/J | C57BL/6J | C58/J | DBA/2J | FVB/NJ | NOD/LtJ | NZB/BINJ | PERA/EiJ | PL/J | SJL/J | SWR/J | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical Characteristics | |||||||||||
| Body weight (g) | 21.6±0.6 | 17.5±0.5 | 16.7±0.7 | 16.6±0.5 | 21.8±0.3 | 21.2±0.3 | 15.2±0.4 | 14.6±0.5 | 17.0±0.3 | 18.0±0.2 | 16.5±0.2 |
| Poor coat condition | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Piloerection | 0% | 5% | 5% | 6% | 35% | 25% | 30% | 18% | 0% | 14% | 0% |
| Vocalization during handling or reflex test | 70% | 5% | 0% | 67% | 6% | 60% | 5% | 64% | 0% | 31% | 5% |
| Home cage (% cages) | |||||||||||
| Nest building | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Huddling | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 80% | 100% |
| Aberrant responses | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Reflexes (% normal) | |||||||||||
| Corneal | 100% | 100% | 95% | 83% | 100% | 80% | 100% | 100% | 100% | 100% | 90% |
| Visual placing | 90% | 90% | 100% | 100% | 100% | 80% | 100% | 100% | 100% | 100% | 100% |
| Vibrissae orienting | 100% | 100% | 100% | 83% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Preyer reflex | 100% | 100% | 100% | 94% | 82% | 75% | 100% | 100% | 100% | 100% | 100% |
| Olfaction test | |||||||||||
| Uncovered buried food (% mice) | No test | 90% | 50% | No test | 92% | 95% | 53% | 82% | 95% | 73% | 95% |
| Latency to find (sec) | No test | 228±60 | 532±92 | No test | 275±70 | 161±45 | 569±84 | 348±96 | 246±47 | 410±73 | 277±44 |
| Motor coordination | |||||||||||
| Rotarod latency to fall (sec) | 204±19 | 237±9 | 171±15 | 130±12 | 138±13 | 34±3 | 214±13 | 185±20 | 199±24 | 145±10 | 114±14 |
| Inverting on rotarod (Trial 2) | 45% | 50% | 65% | 72% | 94% | 50% | 55% | 13% | 60% | 64% | 15% |
3.1.a. Observational measures
Initial home cage observations did not reveal deficiencies in nest building or huddling in any of the inbred strains at the beginning of the study. However, the use of a multi-component testing battery allowed frequent and repeated observations of the same set of mice across several weeks or months of testing, thus optimizing the detection of repeated or aberrant responses. Starting around 6 weeks of age, mice from the C58/J strain were observed to have spontaneous stereotyped responses. During the social approach tests, 35% of the C58/J mice demonstrated hyperactivity, back-and-forth running, and repeated jumping in the 3-chambered test box. These same responses were found in 60% of the C58/J mice during habituation to the T-maze. By the end of the study, a distinctive, persistent back-flipping behavior was observed in all 5 home cages of C58/J mice (with 4 mice per cage). Observations of another strain, PL/J, indicated that 30% of the mice had seizure-like responses during the social approach tests (see [21]).
3.1.b. Activity in an open field
Mice were also evaluated for exploration in a novel environment (Figure 1). Mice from the NOD/LtJ strain had the highest levels of horizontal activity, distance traveled, and rearing, in comparison to the other strains. While none of the strains appeared to be markedly hypoactive, both the DBA/2J and NZB/B1NJ spent low amounts of time in the center region of the activity chamber, suggesting that these strains might have more anxiety-like behavior, especially in comparison to FVB/NJ, SJL/J, or PL/J mice. It is notable that the mean values of the C58/J strain for horizontal activity, distance traveled, fine movement, or rearing, were not higher than the mean values observed in the closely-related C57BL/6J strain. In particular, the measure for fine movements, which might reflect continuous sniffing or other repetitive, non-ambulatory responses, was not increased in the C58/J strain. Thus, the activity measures did not provide evidence for the emergence of hyperactivity or stereotypy in the C58/J mice.
Figure 1. Exploration in a novel open field in 11 inbred mouse strains.
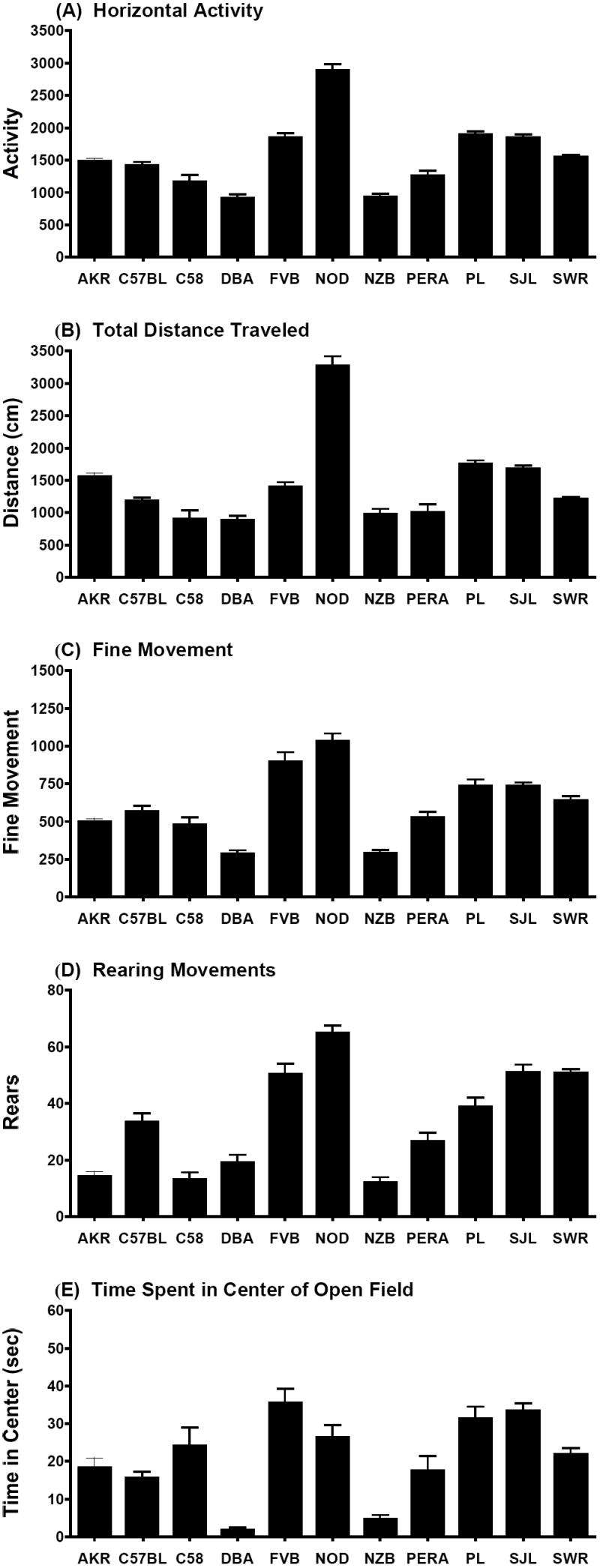
Activity was assessed by a 5-min trial in an open field chamber. Low time in the center (E), as observed in DBA/2J (DBA) and NZB/B1NJ (NZB), may reflect anxiety-like behavior in these strains. Data shown are mean percent + SEM. AKR/J (AKR), C57BL/6J (C57BL), C58/J (C58), FVB/NJ (FVB), NOD/LtJ (NOD), PERA/EiJ (PERA), PL/J (PL), SJL/J (SJL), SWR/J (SWR).
3.1.c. Elevated plus maze
A potential explanation for lower sociability scores in some inbred mouse strains could be higher levels of anxiety-like behavior, leading to less exploration and avoidance of novelty. Therefore, the inbred strains were assessed for anxiety-like behavior on an elevated plus maze (Figure 2). Mice from the PL/J strain spent almost 30% of the total arm time on the open arms, while FVB/NJ and SWR/J had around 23% open arm time. In contrast, both AKR/J and NZB/B1NJ mice had very low open arm times, suggesting an anxiety-like phenotype in these strains. The measure for total arm entries indicated that all of the strains showed exploration of the plus maze, with NOD/LtJ again having the highest level of activity, in comparison to the other strains.
Figure 2. Elevated plus maze performance in 10 inbred mouse strains.
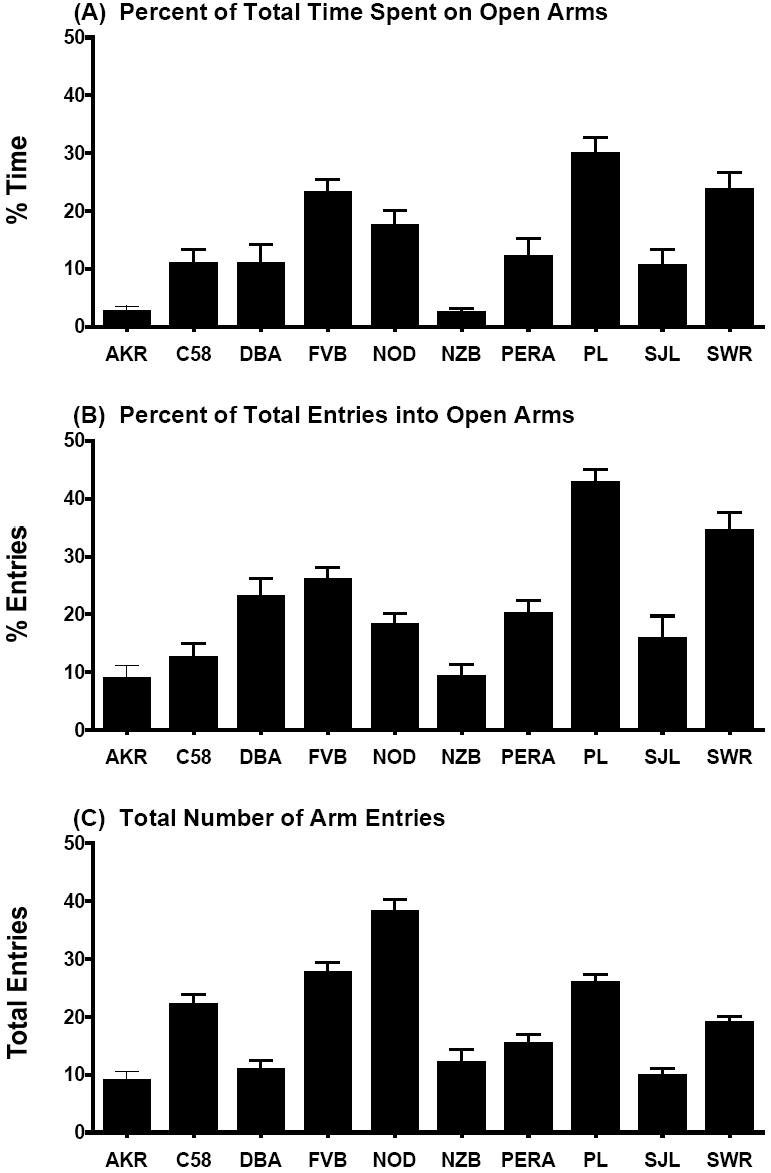
Mice from the AKR and NZB strains had very low percent time in the open arms (A), an index of anxiety-like behavior. Data shown are mean percent + SEM for each strain during one 5-min test.
3.2. Social approach tests
3.2.a. Time spent in each side
In the present study, 5 of the 11 strains (AKR/J, C57BL/6J, FVB/NJ, PL/J, SWR/J) demonstrated significant preference for spending time in the side containing the stranger mouse, versus time in the side with the empty cage (Figure 3A). Within-strain repeated measures ANOVA indicated significant interactions between side and test (sociability or social novelty) for all of these groups [with F values ranging from 11.39 to 102.65; p values from .0007 to <.0001]. C58/J, NOD/LtJ, NZB/B1NJ, PERA/EiJ, and SJL/J did not show social preference. Repeated measures ANOVA revealed significant side × test interactions in only 2 of these strains, NOD/LtJ [F(1,19)=15.91, p=.0008] and SJL/J [F(1,21)=8.22, p=0.0092]. In replication of previous findings from our research group [30,31,33], significant sociability was observed in the AKR/J, C57BL/6J, and FVB/NJ strains. However, the DBA/2J group did not have a preference for spending time with the stranger mouse, in contrast to the first 2 sets of DBA/2J mice tested in our laboratory. Because of these discrepant results, a second set of DBA/2J mice were tested. This next set also failed to demonstrate significantly more time in the stranger side, in comparison to the side with the empty cage. 2 strains, FVB/NJ and SWR/J, showed tail rattling and aggressive “boxing” responses, directed toward the stranger mice, during the social approach test. These latter results suggest that the high levels of social approach in these strains might be attributable to tendencies for aggression, rather than affiliation.
Figure 3. Time spent in each side during the tests for (A) sociability and (B) preference for social novelty.
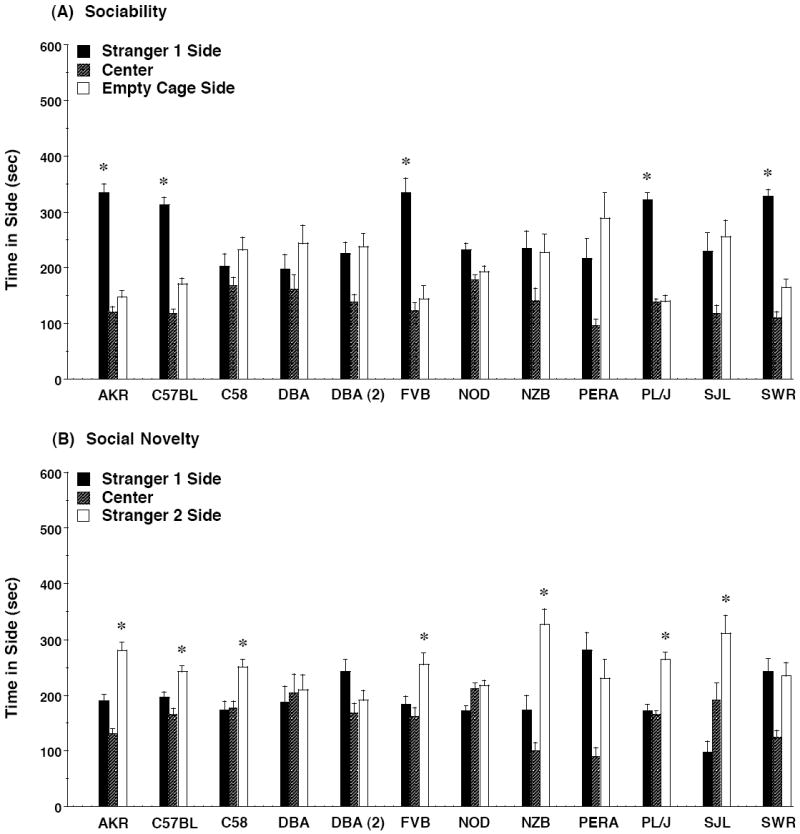
Data shown are mean + SEM for each strain. 2 separate sets of DBA/2J mice (DBA and DBA (2)) were tested. No preference for the social stimuli (stranger 1 side) was observed in 6 of the 11 strains, including wild-derived PERA/EiJ (A). 1 mouse from the PERA/EiJ strain died, leaving 10 mice in the group. * p<0.05, within-strain comparison, stranger 1 side different from empty cage side (A) or stranger 2 side (B).
As shown in Figure 3B, 7 of the 11 inbred strains demonstrated significant social novelty preference, measured as spending more time in the side containing stranger 2 (the less-familiar conspecific), in comparison to stranger 1. The present study replicated our previous findings of significant social novelty preference in C57BL/6J and FVB/NJ [30,31,33]. Unlike the previous group, the AKR/J mice also had significant preference for proximity to stranger 2. And, also unlike previous groups in our laboratory, both the first and second sets of DBA/2J mice failed to demonstrate social novelty preference.
3.2.b. Time spent sniffing the wire cages
The measure for time spent sniffing at the wire cage containing the stranger, versus the empty wire cage, serves as an index of more direct social interest in the choice task. In the present study, within-strain repeated measures ANOVA indicated significant side × test interactions for all groups [with F values ranging from 13.23 to 123.32; p values from 0.002 to <0.0001] except PERA/EiJ, the only wild-derived strain included in the distribution. Further within-strain comparisons indicated that all of the inbred strains spent significantly more time sniffing the cage containing stranger 1 (Figure 4A), indicating that the sniff measure is a more sensitive evaluation of social approach in this test. Overall, only the A/J strain (as reported in [31]) has failed to demonstrate significant sociability with the measure for sniffing. In the test for social novelty preference, only the 7 strains that had significant preference for stranger 2 with the measure for time in side (Figure 3B) also had significant preference with the measure for time spent sniffing the wire cages (Figure 4B).
Figure 4. Time spent sniffing each wire cage during the tests for (A) sociability and (B) preference for social novelty.
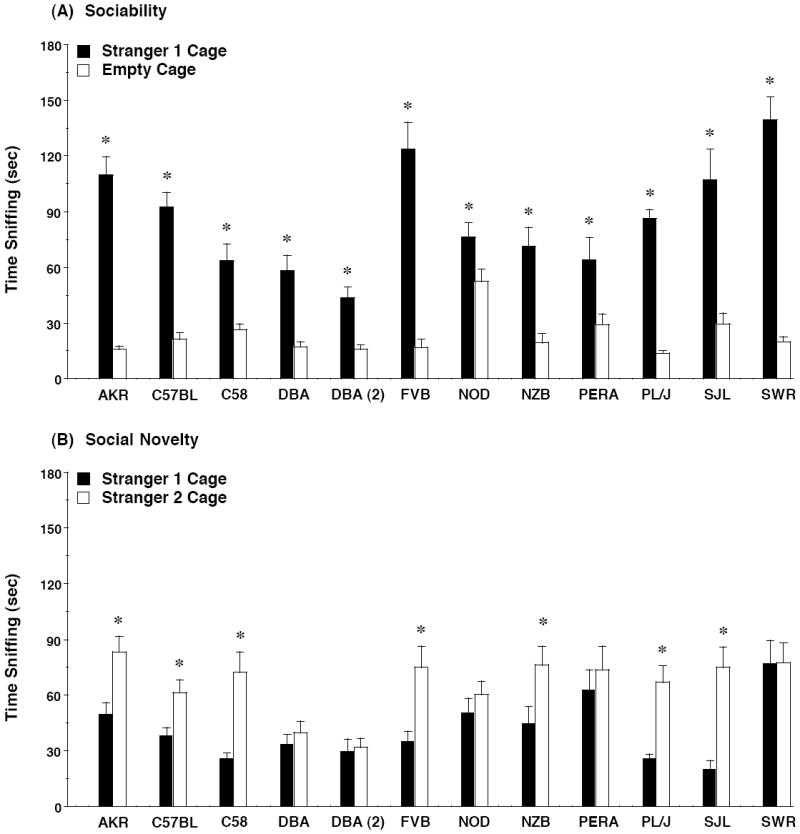
All strains demonstrated a significant preference for the wire cage containing an unfamiliar mouse (stranger 1) versus an empty cage (A). Data shown are mean + SEM for each strain. * p<0.05, within-strain comparison, stranger 1 side different from empty cage side (A) or stranger 2 side (B).
3.2.c. Entries into each side
As previously reported [30,31,33], the measure for entries into each side was the least sensitive index of social approach in the choice tests (Figure 5), with the majority of strains not demonstrating side preferences. However, within-strain repeated measures ANOVA indicated that 4 of the strains which showed significant sociability for time in side, AKR/J, C57BL/6J, FVB/NJ, and PL/J, also had significant side × test interactions with the measure for entry [with F values ranging from 5.27 to 14.10; p values from 0.0316 to 0.0013]. A significant side × test interaction was also found for the NOD/LtJ strain [F(1,19)=5.27, p=0.0333]. The entry measure provided evidence that all of the inbred strains tested in the present study explored the social test box. Therefore, lack of sociability or preference for social novelty in some strains could not be attributed to simple hypoactivity. The relatively high numbers of entries found in the C58/J mice, in contrast to the moderate levels of activity seen in the open field, might reflect the emergence of hyperactive responses in this strain.
Figure 5. Entries into each side during the tests for (A) sociability and (B) preference for social novelty.
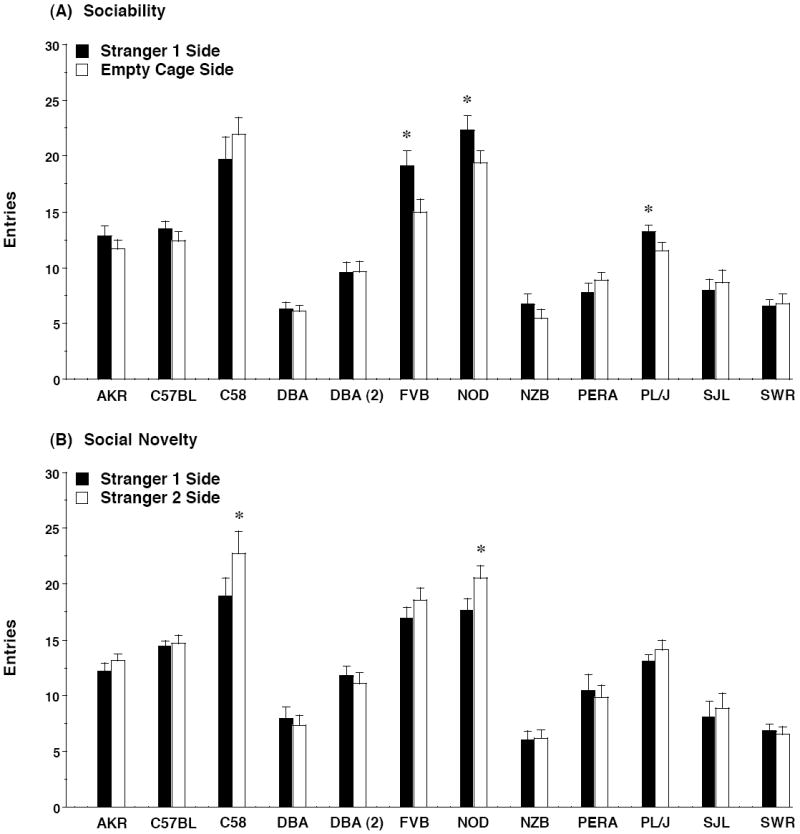
Data shown are mean + SEM for each strain. * p<0.05, within-strain comparison, stranger 1 side different from empty cage side (A) or stranger 2 side (B).
3.2.d. Within-strain sociability and social avoidance
A remaining question was the extent of intrastrain variability in levels of social approach. General positive social approach within a strain should be reflected in positive difference scores, calculated as time spent in the side with stranger 1 minus time spent in the empty cage side. In the present study, 90% to 95% of the mice from the 5 strains which had significant sociability (AKR/J, C57BL/6J, FVB/NJ, PL/J, and SWR/J) had positive difference scores, reflecting positive social approach. On the other hand, the 6 strains which did not have significant sociability varied from 40% (DBA/2J; group 2) to 70% (NOD/LtJ) of individual mice with a positive difference score.
For the 6 strains that did not show significant sociability, Figure 6 presents levels of social approach in subsets of mice that had positive difference scores (Figure 6A) or negative scores (Figure 6B), and the number and percentage of subjects for each subset. By this division, we can show that approximately half of the mice from each of these inbred strains had significant social approach, and about half had (in almost every case) significant social avoidance. Therefore, in the present study, strains characterized by a lack of sociability were not failing to distinguish between the social and non-social novel stimuli. Rather, these strains included a substantial number of mice that avoided proximity to the unfamiliar conspecific, without concomitant avoidance of the novel object (the empty wire cage).
Figure 6. Time spent in each side during the test for sociability in mice with (A) positive difference scores or (B) negative difference scores.
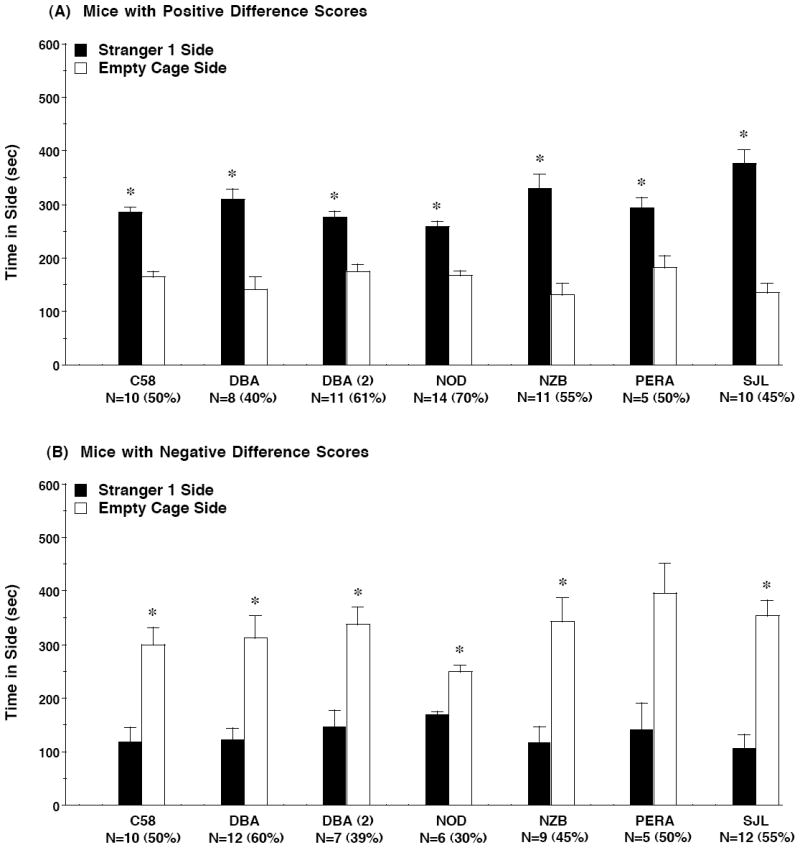
Data were taken from the 6 strains which did not have significant social approach, as shown in Figure 3A. Difference scores were calculated as time spent in side containing stranger 1 minus time spent in the empty cage side. Data shown are mean + SEM. N values indicate number of mice from each group, followed by percent of each group, having either positive or negative difference scores. For all of these strains, only 1 or 2 subjects had difference scores falling between 30 and -30 sec (indicating a 30 sec or less difference between time spent in either side). The exception was the first set of DBA/2J mice, which included 6 subjects with difference scores between 30 and -30 sec (inclusive). * p<0.05, within-strain comparison, stranger 1 side different from empty cage side.
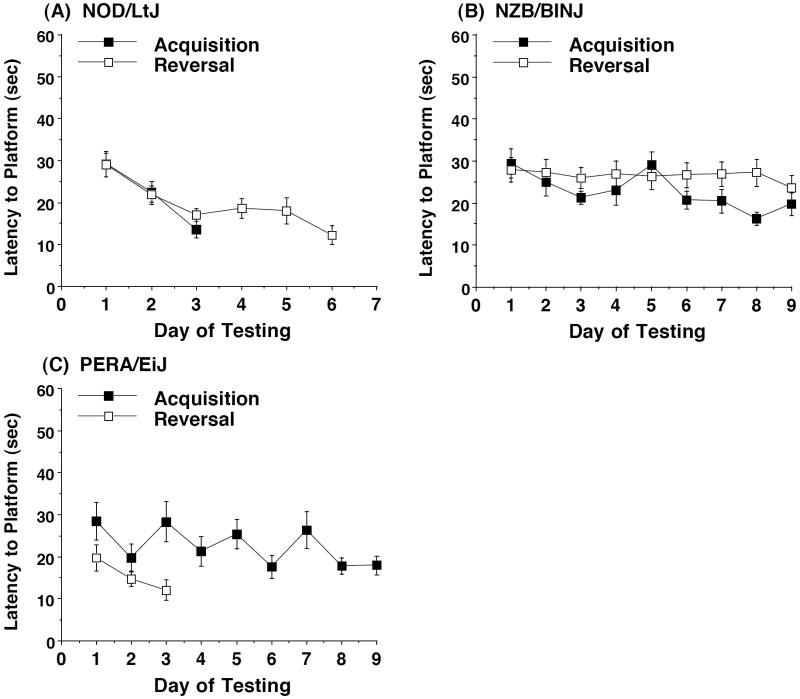
3.3. Reversal learning in the Morris water maze
Mice were first tested for their ability to locate a cued visible platform in the water maze. Mice from the PL/J, SJL/J, and SWR/J strains failed to meet the criterion for learning (a group average latency of 15 sec or less to reach the platform) in this task, and the C58/J mice had markedly poor swimming ability. Therefore, these strains were not further tested in the water maze. Mice from 3 strains (NOD/LtJ, NZB/B1NJ, and PERA/EiJ) met the learning criterion in the visible platform task, and were then tested for acquisition and reversal in the hidden platform task (Figure 7). Only the NOD/LtJ mice reached criterion for learning. The NZB/B1NJ mice closely approached this criterion on day 8 of acquisition (group latency of 16.3 sec). Mice from the PERA/EiJ strain also closely approached this latency during acquisition (17.8 sec on day 8). These latencies did not reflect limitations in swim speed, since the NZB/B1NJ mice reached an average group latency of 12.3 sec, and the PERA/EiJ mice 9.5 sec, on day 3 of the visible platform test (data not shown). The 3 inbred strains demonstrated different patterns of reversal learning: the NOD/LtJ mice took twice as many trials to reach criterion during reversal, versus acquisition, while the PERA/EiJ mice had more rapid learning during the reversal phase. The NZB/B1NJ mice had minimal decreases in latency to find the hidden platform across the 9 days of testing for reversal learning.
Figure 7. Acquisition and reversal in the Morris water maze task for 3 inbred strains.
Mice were given up to 9 days to reach criterion for learning, set at a group average latency of 15 sec or less to find the hidden platform. The location of the hidden platform was changed to a new quadrant for the reversal task. Only NOD/LtJ (A) met the learning criterion during acquisition, while both NOD/LtJ and PERA/EiJ (C) met criterion during reversal. Data shown are mean (± SEM) of 4 trials per day. 1 mouse from the NOD/LtJ strain died during the reversal phase, leaving a total of 16 subjects in this group.
On the final day of testing during each phase (acquisition and reversal), mice were given a 1-min probe trial in the pool without the platform in place. Measures were taken of percent time spent in each quadrant of the pool (Figure 8). All 3 strains demonstrated significant quadrant selectivity following acquisition, although the PERA/EiJ mice did not have a clear preference for the target quadrant [NOD/LtJ, F(3,48) = 26.51, p<0.0001; NZB/B1NJ, F(3,54)=10.89, p<0.0001; PERA/EiJ, F(3,27)=4.31, p=0.0132]. As observed for the latency measure, the 3 strains had different patterns of results for the probe trial following reversal learning. Only the NOD/LtJ mice had a selective preference for the quadrant where the platform had been relocated [F(3,45)=4.24, p=0.0101]. NZB/B1NJ mice retained the bias for the quadrant where the platform had been located during acquisition [F(3,54)=5.93, p=0.0014], and mice from the PERA/EiJ strain failed to show quadrant selectivity after reversal learning [F(3,27)=1.76, p=0.1796].
Figure 8. Selective quadrant search on the Morris water maze.
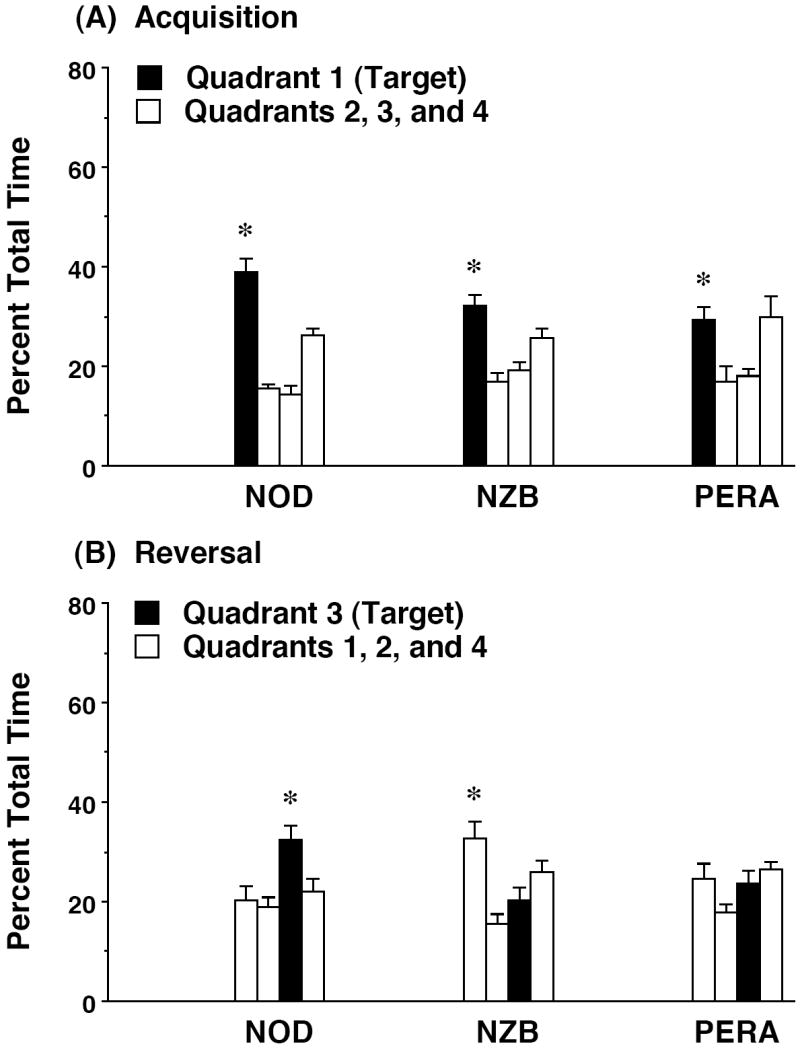
Each mouse was given a 1-min probe trial with the escape platform removed. Target (black bars) indicates the quadrant where the platform had been located during training trials. NZB/B1NJ was the only strain to demonstrate a persistent bias for quadrant 1 following reversal (B). * p<0.05, within-strain repeated measures ANOVA, significant main effect of quadrant.
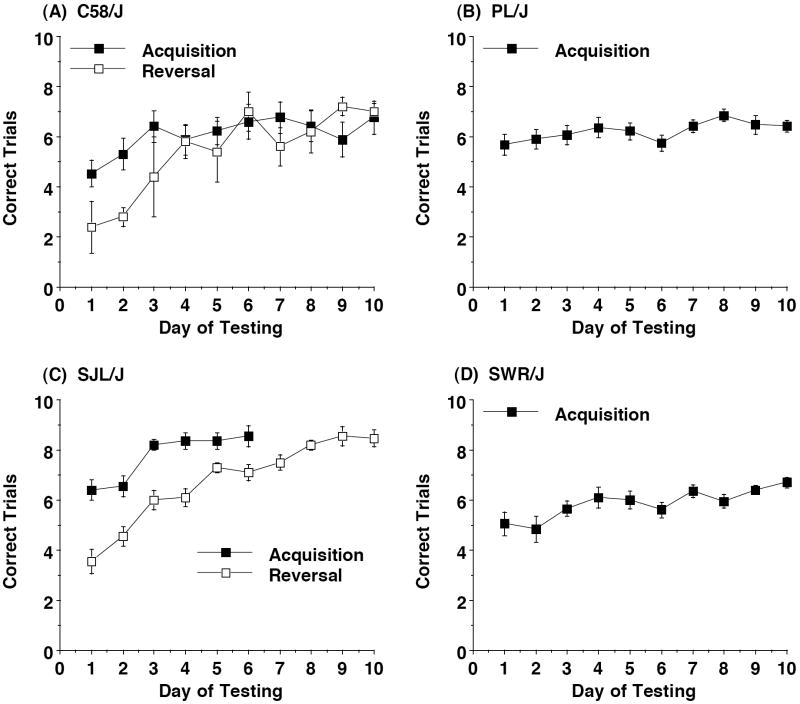
3.5. Reversal learning in an appetitive T-maze task
The T-maze task was used for the 4 strains (C58/J, PL/J, SJL/J, and SWR/J) that had poor performance in the visible platform test in the water maze. Overall, there were clear strain differences in performance of the T-maze task (Figure 9). The SJL/J mice had rapid learning during the acquisition phase, with the group meeting criterion for learning (80% correct responses across 3 days of testing) by day 5 (determined after compiling data from the 2 separate cohort groups, tested approximately 1 month apart). Slower learning was observed in the SJL/J mice during the reversal phase. In contrast, the C58/J mice had poor acquisition, with only 5 mice meeting criterion for learning. These 5 mice did not meet criterion during reversal learning. Only 4 PL/J mice and 1 SWR/J animal reached criterion during the initial phase of testing. We decided that these were too few subjects for further testing in reversal learning. Seizure-like responses in the PL/J mice may have interfered with performance in the task.
Figure 9. T-maze learning in 4 inbred strains.
Data are mean (± SEM) number of correct trials out of 10 trials per day. 5 correct trials out of 10 would be expected by chance. C58/J (A) showed poor acquisition, with only 5 mice reaching criterion for learning. These 5 mice received further testing for reversal learning. PL/J (B) and SWR/J (D) also showed poor acquisition, precluding further testing.
4. Discussion
In the present studies and previous work [31], we have phenotyped 17 inbred mouse strains, selected from the 40 strains chosen for the Mouse Phenome Project, an international collaborative effort to obtain mouse phenotypic data under standardized conditions [3]. In recent years, phenotypic datasets for measures of behavior and neurosensory function, as well as anatomy, blood chemistry, drug effects, and multiple other parameters, have been assembled into the Mouse Phenome Database (MPD), housed at JAX ([4]; http://www.jax.org/phenome). The inbred strains for our studies on mouse behavioral tasks relevant to autism were predominantly from the groups given highest priority for phenotyping by the Mouse Phenome Project.
Previous work has suggested that there is a continuum of social approach across inbred mouse strains, evidenced by varying degrees of sociability or social avoidance. The present study characterized 7 additional strains for social approach. Out of the total 17 strains we have tested (present study, [31]), 7 (or 41%) showed significant sociability, while a little more than half (10/17, or 59%) did not have significant social preference, as measured by time spent in each side. The overall lack of social preference in 6 of the strains tested in the present study was not due to a failure to distinguish between the side containing the stranger mouse, versus the side containing an empty wire cage. Rather, the non-significant sociability was due to a sizable percentage of the mice from each strain showing avoidance (to varying degrees) of the side with the unfamiliar conspecific. Therefore, the choice test can be utilized to detect mice from the same strain that are either sociable or asocial, despite identical genotypes. These findings are particularly interesting, given a recent report of differential gene expression in monozygotic twins discordant for severity of autism symptoms [18].
All of the mouse strains in the present study and in the previous distribution, except for A/J [30,31], demonstrated a preference for sniffing at the cage containing the stranger, versus an empty cage, suggesting that a preference for social olfactory stimuli is a relatively general trait in inbred mouse strains. Deficits in preference for sniffing in the social choice test may indicate fundamental alterations in social behavior, and could serve as a hallmark trait for genetic mouse models of the autism phenotype. Further assessments of direct social interaction, such as the tests of juvenile play and resident-intruder responses used to characterize Engrailed 2-null mice [10], could provide additional support for altered social behavior in mouse models relevant to autism.
A second index of social approach is the assay for social novelty preference. This test is based on 2 factors: the ability of the mouse to distinguish between 2 conspecifics, and preference for the more-novel stranger. Overall, out of 17 strains characterized by our group, 10 (59%, including AKR/J) demonstrated significant preference for social novelty. 5 of these strains did not have significant sociability, suggesting that the 2 assays are measuring different components of social behavior.
Phenotypes on behavioral tasks for modeling symptoms of autism can be confounded by abnormalities in general health, sensory abilities, motor functions, activity levels, and anxiety-like behavior. For example, motor deficiencies due to a mutation in dysferlin, observed in A/J mice [17], may be the underlying cause of apparent low social approach in this strain [30,31]. Olfactory information is a key element of social interaction in mice [13,24,25,36]. In the present study, the 4 strains with the highest latencies to find the buried food (C58/J, NZB/B1NJ, PERA/EiJ, and SJL/J) also failed to show significant social preference for the measure of time in each side. Since at least half of the mice from each strain were able to find the buried food, these results may be an indication of altered motivational valence for olfactory stimuli (appetitive or social) in these strains, rather than loss of olfactory function.
One puzzling result in the present study was the failure to show significant sociability or social novelty preference in 2 separate groups of DBA/2J mice. These findings are in contrast to results, reported by our laboratory, from 2 previous sets of DBA/2J mice [30,31,33]. All 4 groups were male mice (n = 10-20), purchased from JAX. One significant difference between the previous tests and the present study was that the location of the mouse behavior laboratory was changed to a newly constructed building. Altered housing conditions included a switch from micro-isolator, filter-top cages to a ventilated caging system (Tecniplast, Buguggiate, Italy).
Crabbe, Wahlsten, and colleagues [12,47,49,50] have provided compelling evidence that results from behavioral testing can significantly differ between laboratories, even when procedures, animal source, and other environmental factors are carefully controlled and standardized. Discrepancy of findings may be greater in tests related to anxiety-like behavior [47]. Some strain comparisons have suggested that DBA/2J mice are characterized by anxiety-related responses and high or intermediate-high emotional reactivity [16,41,45]. In the present study, the DBA/2J strain had the lowest level of time spent in the center of the activity chamber. One possibility is that the new housing environment for our mouse behavior laboratory is more stressful or, possibly, more impoverished in terms of olfactory stimuli (due to the ventilated caging system). DBA/2J may be more sensitive to these conditions than mice from the C57BL/6J and FVB/NJ strains, which had consistent levels of social approach across testing locations. Other researchers have found differences in the performance of DBA/2J mice in the Morris water maze task, dependent upon origin of the mice [37].
In the present study, all 3 of the strains that were tested in the water maze had not shown significant sociability with the time in side measure, yet each strain had a different pattern of learning in the spatial task. Notably, PERA/EiJ demonstrated accelerated learning and NOD/LtJ showed slower learning during the reversal phase. An interesting result was observed with NZB/B1NJ, which had a persistent bias for the quadrant where the target platform had been located, even after 9 days of reversal training. We have not observed this failure to shift quadrant selectivity following reversal in any other of the 10 strains we have tested, although AKR/J, BTBR/T+tf/J, and 129S1/SvImJ (from the previous distribution), and PERA/EiJ (from the present study) all had significant quadrant preference following acquisition, but not following reversal. In the NZB/B1NJ strain, the probe tests revealed that the mice had formed a spatial bias for the first target quadrant, although the group had not reached the criterion for learning during acquisition and reversal. The results indicate that the probe test may provide an informative evaluation of spatial learning, even when criterion for finding the hidden platform has not been reached. Overall, these findings suggest that the reversal task in the water maze can be used to detect selective deficiencies in acquisition and reversal learning, and resistance to change a learned pattern of behavior, which may reflect symptoms observed in autism [1,11,26].
4 strains showed poor performance on the cued, visible platform task in the water maze. Of these strains, PL/J, SJL/J, and SWR/J are all homozygous for a retinal degeneration gene, Pde6brd1, which leads to blindness by the age of weaning [44]. The mice from the C58/J strain had an alarming tendency to swim downwards, necessitating rescue by the human observer. Wahlsten et al. [48] have reported that C58/J is susceptible to heavily waterlogged fur during testing in the water maze, which may be related to the markedly poor swimming skills. Due to the issues with visual and swimming abilities, these strains were evaluated for reversal learning in the T-maze test. Previous work has shown that having the gene for retinal degeneration does not preclude meeting the criterion for learning in the T-maze [31]. In the present study, C58/J, PL/J, and SWR/J all demonstrated poor learning during the initial phase of the T-maze task. The low performance in the PL/J strain may have been due, in part, to the seizure-like responses characteristic of this strain [21]. So far, only one strain, SJL/J, has shown rapid acquisition of the task, without meeting criterion for learning during reversal. The evaluation of reversal learning with other types of tests, such as discrimination procedures utilizing simple nose poke responses and auditory or olfactory stimuli, may help control for the significant interstrain variations in motor and visual abilities and rates of acquisition found in the present study.
The C58/J strain demonstrated a particularly interesting behavioral profile, with lack of sociability by the time in side measure, poor learning acquisition, and aberrant stereotyped behavior in the home cage, social test box, and T-maze. This repetitive behavior involved hyperactivity, repeated jumping, and a distinctive back-flipping response using the wire lid of the home cage. The responses were remarkably similar in form to stereotypy reported for deer mice housed in standard cages [39,40]. Enriched housing can reduce the levels of stereotypy and delay the emergence of aberrant motor responses in the deer mice, as well as in other animal models of repetitive behavior [23]. It is possible that the standard, ventilated caging system used to house mice in the present study might have contributed to the high levels of spontaneous stereotypy in the C58/J strain.
Overall, this strain distribution has provided evidence for a continuum of social approach across mice with different genotypes. The data may be valuable for the selection of appropriate background strains for the development of genetic mouse models of autism, for evaluation and validation of behavioral phenotypes relevant to the clinical disorder, and for interpretation of deficits in social approach. In addition, our research group is currently investigating the relationship between different mouse strain phenotypes and gene expression in selected regions of brain (e.g. [34]). Correlational analyses from these studies may reveal the sets of genes that are important for social behavior, task acquisition, and reversal learning in mice, and suggest candidate genes underlying selected endophenotypes associated with the symptomatology of autism.
Acknowledgments
This work was supported by NIH STAART grant U54 MH66418 and NICHD grant P30 HD03110, and by the NIMH Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeven AT, Oostra BA, Reyniers E, de Boulle K, D’Hooge R, Cras P, van Velzen D, Nagels G, Martin J-J, de Deyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 3.Bogue MA, Grubb SC. The Mouse Phenome Project. Genetica. 2004;122:71–74. doi: 10.1007/s10709-004-1438-4. [DOI] [PubMed] [Google Scholar]
- 4.Bogue MA, Grubb SC, Maddatu TP, Bult CJ. Mouse Phenome Database (MPD) Nucleic Acids Res. 2007;35:D643–649. doi: 10.1093/nar/gkl1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- 6.Brodkin ES. BALB/c mice: Low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, Anckarsater H, Rastam M, Smith CJ, Silverman JM, Hollander E, Leboyer M, Gillberg C, Verloes A, Betancur C. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B Neuropsychiatr Genet. 2007;144:484–491. doi: 10.1002/ajmg.b.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 11.Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. J Genet Psychol. 2003;164:29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- 12.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 13.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 14.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 15.Fombonne E. Epidemiological trends in rates of autism. Mol Psychiatry. 2002;7(Suppl 2):S4–6. doi: 10.1038/sj.mp.4001162. [DOI] [PubMed] [Google Scholar]
- 16.Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 17.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet. 2004;13:1999–2010. doi: 10.1093/hmg/ddh212. [DOI] [PubMed] [Google Scholar]
- 18.Hu VW, Frank BC, Heine S, Lee NH, Quackenbush J. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics. 2006;7:118. doi: 10.1186/1471-2164-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahr E, Eikeseth S, Eldevik S, Aase H. Frequency and latency of social interaction in an inclusive kindergarten setting: A comparison between typical children and children with autism. Autism. 2007;11:349–363. doi: 10.1177/1362361307078134. [DOI] [PubMed] [Google Scholar]
- 20.Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, Walker CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1998;20:525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitami T, Ernest S, Gallaugher L, Friedman L, Frankel WN, Nadeau JH. Genetic and phenotypic analysis of seizure susceptibility in PL/J mice. Mamm Genome. 2004;15:698–703. doi: 10.1007/s00335-004-3007-7. [DOI] [PubMed] [Google Scholar]
- 22.Kooy RF, D’Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, Willems PJ. Transgenic mouse model for the fragile X syndrome. Am J Med Genet. 1996;64:241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebenauer LL, Slotnick BM. Social organization and aggression in a group of olfactory bulbectomized male mice. Physiol Behav. 1996;60:403–409. doi: 10.1016/s0031-9384(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 26.Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- 27.McGee GG, Feldman RS, Morrier MJ. Benchmarks of social treatment for children with autism. J Autism Dev Disord. 1997;27:353–364. doi: 10.1023/a:1025849220209. [DOI] [PubMed] [Google Scholar]
- 28.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 31.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 33.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for rapid quantitation of autism-like social deficits in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 34.Nadler JJ, Zou F, Huang H, Moy SS, Lauder J, Crawley JN, Threadgill DW, Wright FA, Magnuson TR. Large-scale gene expression differences across brain regions and inbred strains correlate with a behavioral phenotype. Genetics. 2006;174:1229–1236. doi: 10.1534/genetics.106.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 36.Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 38.Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 39.Powell SB, Newman HA, McDonald TA, Bugenhagen P, Lewis MH. Development of spontaneous stereotyped behavior in deer mice: effects of early and late exposure to a more complex environment. Dev Psychobiol. 2000;37:100–108. [PubMed] [Google Scholar]
- 40.Powell SB, Newman HA, Pendergast JF, Lewis MH. A rodent model of spontaneous stereotypy: initial characterization of developmental, environmental, and neurobiological factors. Physiol Behav. 1999;66:355–363. doi: 10.1016/s0031-9384(98)00303-5. [DOI] [PubMed] [Google Scholar]
- 41.Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 42.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M, Hijimans C, Staal WG, Baird G, Bolton PF, Rutter ML, Weisblatt E, Green J, Aldred C, Wilkinson JA, Pickles A, Le Couteur A, Berney T, McConachie H, Bailey AJ, Francis K, Honeyman G, Hutchinson A, Parr JR, Wallace S, Monaco AP, Barnby G, Kobayashi K, Lamb JA, Sousa I, Sykes N, Cook EH, Guter SJ, Leventhal BL, Salt J, Lord C, Corsello C, Hus V, Weeks DE, Volkmar F, Tauber M, Fombonne E, Shih A. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Jackson Laboratory. Genetic background effects: Can your mice see? JAX/Notes. 2002;485:2. [Google Scholar]
- 45.Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- 46.Van Dam D, D’Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, Oostra BA, Kooy RF, De Deyn PP. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res. 2000;117:127–136. doi: 10.1016/s0166-4328(00)00296-5. [DOI] [PubMed] [Google Scholar]
- 47.Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 49.Wahlsten D, Metten P, Crabbe JC. A rating scale for wildness and ease of handling laboratory mice: results for 21 inbred strains tested in two laboratories. Genes Brain Behav. 2003;2:71–79. doi: 10.1034/j.1601-183x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 50.Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]